ABSTRACT
INTRODUCTION
The information provided by pharmaceutical sales representatives has been shown to influence prescribing. To enable safe prescribing, medicines information must include harm as well as benefits. Regulation supports this aim, but relative effectiveness of different approaches is not known. The United States (US) and France directly regulate drug promotion; Canada relies on industry self-regulation. France has the strictest information standards.
METHODS
This is a prospective cohort study in Montreal, Vancouver, Sacramento and Toulouse. We recruited random samples of primary care physicians from May 2009 to June 2010 to report on consecutive sales visits. The primary outcome measure was “minimally adequate safety information” (mention of at least one indication, serious adverse event, common adverse event, and contraindication, and no unqualified safety claims or unapproved indications).
RESULTS
Two hundred and fifty-five physicians reported on 1,692 drug-specific promotions. “Minimally adequate safety information” did not differ: 1.7 % of promotions; range 0.9–3.0 % per site. Sales representatives provided some vs. no information on harm more often in Toulouse than in Montreal and Vancouver: 61 % vs. 34 %, OR = 4.0; 95 % CI 2.8–5.6, or Sacramento (39 %), OR = 2.4; 95 % CI 1.7–3.6. Serious adverse events were rarely mentioned (5–6 % of promotions in all four sites), although 45 % of promotions were for drugs with US Food and Drug Administration (FDA) “black box” warnings of serious risks. Nevertheless, physicians judged the quality of scientific information to be good or excellent in 901 (54 %) of promotions, and indicated readiness to prescribe 64 % of the time.
DISCUSSION
“Minimally adequate safety information” did not differ in the US and Canadian sites, despite regulatory differences. In Toulouse, consistent with stricter standards, more harm information was provided. However, in all sites, physicians were rarely informed about serious adverse events, raising questions about whether current approaches to regulation of sales representatives adequately protect patient health.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2411-7) contains supplementary material, which is available to authorized users.
KEY WORDS: health policy, patient safety, primary care, health services research
INTRODUCTION
Pharmaceutical sales representatives (PSRs), and the free samples they provide, represent the largest spending category for pharmaceutical promotion.1 Despite widespread belief by physicians to the contrary, PSRs have been shown to influence prescribing.2,3 Greater exposure to promotion is associated with higher prescribing volume and costs, and lower quality prescribing.4 A 2009 United States (US) survey found that 85 % of physicians see PSRs, and PSRs are their first information source for most newly prescribed drugs.5 In a Canadian physician survey, the most frequent reason for seeing PSRs was to obtain information.6
Patient health may be negatively affected if PSRs fail to inform physicians of a medicine’s harmful effects. For example, a memo from Merck advised PSRs to avoid discussing cardiac risks of rofecoxib.7 The research evidence on the content of PSR messages is sparse, but points to frequent inaccuracies and omissions.8 A long-term French survey (2000 to 2005) found that PSRs failed to mention adverse effects around 70 % of the time, and nearly one-third of promotions discussed unapproved indications or doses.9
Regulation of promotion differs between countries. In the US, PSRs are regulated by the Food and Drug Administration (FDA), and are subject to “fair balance” provisions requiring harm as well as benefit information in all components of promotion. PSRs cannot promote unapproved indications, but may provide reprints on these uses at physicians’ request. In 2004, France introduced an eight-page Sales Visit Charter, which prohibits samples, food, gifts, and invitations to participate in studies. PSRs must provide physicians with approved product information. In Canada, regulation of PSRs is largely delegated to the industry association, Rx&D. The Rx&D ethical code requires consistency with approved product information and current medical thinking. The federal regulatory agency, Health Canada, can exercise its legislative authority if necessary,10 but rarely does so in practice.
We ask whether the national regulatory differences described above affect how often PSRs provide safety information. We hypothesize that “minimally adequate safety information” (defined below) is provided more often in Toulouse, with stricter information standards, than in Vancouver, Montreal or Sacramento, and that harm is mentioned more often in Sacramento than Canadian sites, reflecting US “fair balance” provisions. This is a “real life” observational study, in which we recruited primary care physicians who see PSRs to report on consecutive sales visits.
METHODS
Primary care physicians were enrolled in a prospective cohort study between May 1, 2009 and June 30, 2010. Each physician saw PSRs as usual, and completed a questionnaire about the next eight consecutively promoted drugs at office PSR visits, either immediately after the visit or, if not possible, later on the same day.
Four urban areas were chosen for comparability and practicality: Vancouver, Montreal, Sacramento, and Toulouse. Vancouver and Montreal share a national system of regulation of promotion, but differ in provincial per capita drug costs, reimbursement, and medical culture.
We recruited physicians by selecting random samples from lists of primary care physicians practicing within each area. In Vancouver, we obtained a database of family physicians from the provincial college of physicians. In Montreal, the college of physicians, and in Toulouse, the physicians’ association (Union Régionale des Professionnels de Santé - Médecins Libéraux - Midi-Pyrénées), provided random samples from their lists of primary care physicians. In Sacramento, physicians at two large practice groups, Kaiser Permanente and University of California, Davis, do not see PSRs. We therefore developed a list of primary care physicians in independent physician associations (IPA). In each site, we drew random samples of physicians in blocks of 25 to contact over 1 year, using Salant and Dillman’s repeated contact methods to maximize the proportion of physicians successfully contacted.11 To minimize volunteer bias, physicians were reimbursed at a rate commensurate with a brief consultation (US $28–$36 per questionnaire).
Physicians were assessed for eligibility and invited to participate. Informed consent was obtained on enrollment. Physicians were informed that the aim of the study was to compare the quality of information provided by PSRs in the three included countries. To be included, physicians had to see PSRs, work ≥ 20 clinical hours/week, and serve > 50 % primary care patients. Physicians belonging to advocacy groups on promotion (e.g., No Free Lunch), and pharmaceutical company employees were excluded.
The questionnaire (Appendix 1; available online) was developed in English and translated into French. It was adapted from instruments used in France,9 and Australia and Malaysia,12 and was pilot tested in Victoria, British Columbia (n = 15 physicians, 41 promotions). Following revisions and translation, written and on-line versions were tested for comprehension and timing of completion. Physicians reported on presence/absence of information elements; what was said; visit characteristics; documentation, food, samples, gifts and invitations, key messages; information quality and likelihood to prescribe. Each promoted drug was the focus of a separate questionnaire.
The primary outcome measure was presence of “minimally adequate safety information,” defined a priori as mention of ≥ 1 approved indication, ≥ 1 serious adverse event (SAE), ≥ 1 common non-serious adverse event (AE), ≥ 1 contraindication (CI) and no unapproved indications or unqualified safety claims (e.g., “this drug is safe”). Required items are a subset of elements identified by a random sample of Canadian physicians as needed in an “ideal detail.”13 To allow for brief interactions, we only included those elements also required by the US FDA in television ads. We checked national product information for SAE and CI; if none were mentioned, we omitted the relevant requirement. We planned to combine Vancouver and Montreal for secondary analyses if the primary outcome did not differ. Physicians provided demographic and practice characteristic information on enrollment.
A small proportion of observations on sales visits had missing data (< 1 % in total). Data were assumed to be missing at random. Multiple imputation methods were applied and all observations retained in the analysis.
Ethics approval was obtained from the University of British Columbia behavioural ethics committee, the Ethics Committee of the Centre de recherche du Centre hospitalier de l’Université de Montréal (CR-CHUM), University of California at Davis, and the Union Régionale des Professionnels de Santé - Médecins Libéraux - Midi Pyrénées.
ANALYSIS
The unit of analysis was a drug-specific promotion in which a PSR stated the name of a prescription-only drug and made at least one claim. Sample size calculations were based on a 20 % estimate of promotions with “minimally adequate safety information.” We judged a difference of ≥ 10 % between sites to be the minimum level potentially affecting clinical practice. This was an estimate, as we found no relevant empirical research. Allowing for extra variability due to clustering of observations per physicians, we aimed to enroll 65 physicians per site (1,664 promotions in total).
In order to assess consistency with approved product information, two independent coders compared physician reports with national drug compendia: the Compendium of Pharmaceuticals and Specialties in Canada; Physician’s Desk Reference in the US; and Thériaque in France. Differences were resolved through discussion, with adjudication by a pharmacologist if consensus could not be reached.
We used general estimation equations (GEE) to adjust for multiple responses from the same physician. Covariates included in multivariate regression models are: physician demographics, practice and sales visit characteristics, previous prescribing of the drug, and whether the drug’s label has boxed warnings.
RESULTS
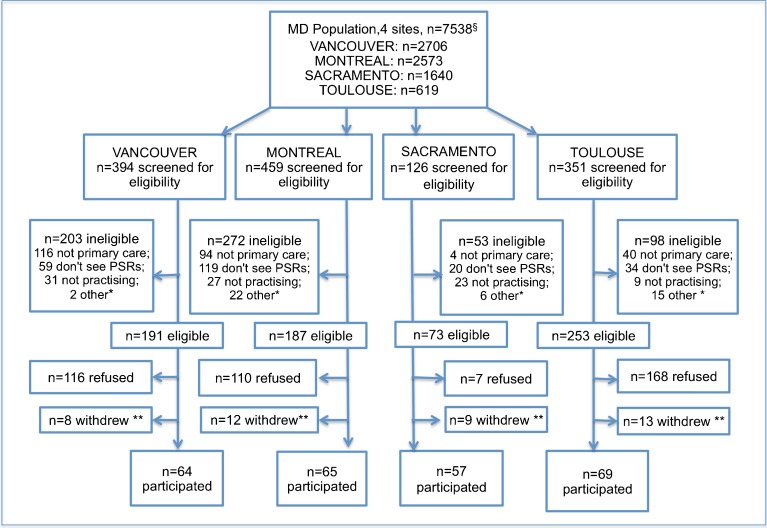
Figure 1 describes physician recruitment and study participation. We attempted to reach 1,966 physicians in the four sites, 1,330 (68 %) of whom were successfully contacted. Nearly half (47 %) of those contacted were ineligible, including 19 % with hospital or referral practices and 17 % who did not see PSRs. Among the 704 eligible physicians, 255 (36 %) participated. Participation was highest in Sacramento and lowest in Toulouse.
Figure 1.
Physician recruitment. Vancouver and Montreal primary care physicians lists from B.C. and Quebec Colleges of Physicians. Toulouse list from the Union Régionale des Professionnels de Santé - Médecins Libéraux - Midi-Pyrénées. Of the 4,380 licensed physicians in Sacramento County, an estimated 37 % are primary care physicians (U.S. physician workforce data. < http://bhpr.hrsa.gov/healthworkforce/allreports.html >). * “other” includes maternity leave, sick leave, deceased, and unspecified. ** physicians who withdrew before filling in any questionnaires.
We compared a random sample of non-participants (n = 100 per site) with study samples in the three North American sites (non-participant data unavailable for Toulouse). Mean graduation year differed by 2 years or less in each site. The sex ratio was identical in Vancouver and did not differ significantly in the other two sites.
Table 1 describes physician characteristics. There were differences between sites in demographics, practice characteristics, and sales visit frequency. Thirty-three percent of physicians received industry funding, half of whom (17 %) were on speakers’ bureaus or advisory boards. Most sales visits were one-to-one and 55 % lasted > 5 min. Free samples were provided for 75 % of promoted drugs in Vancouver, 57 % in Sacramento and Montreal and 4 % in Toulouse. Food accompanied nearly one fourth of promotions in Vancouver and Sacramento, but was rarely provided in Toulouse (0.2 %).
Table 1.
Characteristics of Physicians and Promotional Interactions
| Participating physicians | Vancouver (n = 64) | Montreal (n = 65) | Sacramento (n = 57) | Toulouse (n = 69) | Total (n = 255) |
|---|---|---|---|---|---|
| Male | 63 % | 46 % | 68 % | 78 % | 63 % |
| Graduation yr (mean ± sd) | 1986 ± 12 | 1983 ± 9 | 1991 ± 9 | 1986 ± 10 | 1986 ± 10 |
| Solo practice | 16 % | 20 % | 23 % | 36 % | 24 % |
| Fee-for-service | 96 % | 62 % | 56 % | 91 % | 77 % |
| Patients/week (mean ± sd) | 166 ± 55 | 96 ± 47 | 103 ± 59 | 110 ± 35 | 119 ± 57 |
| PSR visits ≥ twice a week | 59 % | 38 % | 84 % | 88 % | 67 % |
| Medical faculty affiliation* | 38 % | 28 % | 43 % | 17 % | 31 % |
| Any pharmaceutical industry funding† | 30 % | 34 % | 24 % | 42 % | 33 % |
| Drug-specific promotions | N = 418 | N = 423 | N = 445 | N = 406 | N = 1692 |
| Number of unique brands | 112 | 120 | 135 | 150 | 342 |
| Previously prescribed | 74 % | 74 % | 73 % | 70 % | 73 % |
| Sales visit ≤ 5 min | 49 % | 39 % | 56 % | 36 % | 45 % |
| One-to-one session | 76 % | 80 % | 78 % | 96 % | 82 % |
| Free samples provided | 75 % | 57 % | 57 % | 4 % | 49 % |
| Lunch or food provided | 23 % | 9 % | 24 % | 0.2 % | 14 % |
| Invited to an event | 10 % | 19 % | 9 % | 8 % | 12 % |
| Invited to participate in study | 1 % | 2 % | 0 | 5 % | 2 % |
*Preceptor or clinical instructor for students, interns and/or residents
†Industry funding was for study participation (n = 50), advisory boards (n = 30), speaker’s bureaus (n = 24), travel expenses (n = 19), unrestricted educational grants (n = 12), research (n = 6), other (n = 7)
In total, the 255 physicians observed 1,692 drug-specific promotions. In 73 % of promotions, physicians had previously prescribed the drug. Table 3 (available on-line, Appendix 2) presents an overview of the most frequently promoted drugs. The ten top drugs were discussed in 27–29 % of interactions per site. Two-thirds of these brands were among the top 20 per country in promotional spending in 2009–2010.14
Provision of Safety-Related Information
Table 2 compares the frequency of safety-related information per site. “Minimally adequate safety information” was provided in 5/412 (1.2 %) of promotions in Vancouver and 7/423 (1.7 %) in Montreal, adjusted OR = 0.7 (95 % CI 0.1–5.7). As the primary outcome did not differ, we combined the sites in subsequent analyses. Overall, “minimally adequate safety information” was rare: 28/1,692 (1.7 %), and differed little between sites (range, 0.9 % in Sacramento to 3.0 % in Toulouse). The Sacramento-Toulouse difference was marginally significant ( p = 0.03).
Table 2.
Frequency of Safety Information Provision in the Four Sites
| Van/Mon N = 841 | Sac N = 445 | Toul N = 406 | Total N = 1692 | Toul vs. Van/Mon Adjusted OR (95 % CI) | Toul vs. Sac Adjusted OR (95 % CI) | Sac vs. Van/Mon Adjusted OR (95 % CI) | |
|---|---|---|---|---|---|---|---|
| Minimally adequate safety information* | 12 (1.4 %) | 4 (0.9 %) | 12 (3.0 %) | 28 (1.7 %) | 2.9 (1.0–8.8) NS | 7.3 (1.2–44) p = 0.03 | 0.2 (0.04–1.3) NS |
| Any harm information† | 282 (34 %) | 173 (39 %) | 246 (61 %) | 701 (41 %) | 4.0 (2.8–5.6) p < 0.001 | 2.4 (1.7–3.6) p < 0.001 | 1.4 (1.0–2.0) p = 0.04 |
| Written prescribing information | 391 (46 %) | 207 (47 %) | 293 (72 %) | 891 (53 %) | 2.7 (1.9–3.9) p < 0.001 | 3.7 (2.4–5.6) p < 0.001 | 1.1 (0.8–1.5) NS |
| No oral or written harm information | 334 (40 %) | 162 (36 %) | 43 (11 %) | 539 (32 %) | 0.15 (0.1–0.2) p < 0.001 | 0.20 (0.1–0.3) p < 0.001 | 0.8 (0.6–1.8) NS |
| Specific safety information | |||||||
| Serious adverse events§ | 45/830 (5 %) | 26/439 (6 %) | 22/383 (6 %) | 93/1652 (6 %) | 1.1 (0.5–2.3) NS | 0.8 (0.3–1.8) NS | 1.1 (0.5–2.2) NS |
| Contraindications§ | 117/831 (14 %) | 69/404 (17 %) | 155/391 (40 %) | 341/1626 (21 %) | 4.6 (3.0–7.1) p < 0.001 | 4.0 (2.4–6.7) p < 0.001 | 1.1 (0.7–1.8) NS |
| Non-serious adverse events | 185 (22 %) | 113 (25 %) | 146 (36 %) | 444 (26 %) | 2.2 (1.5–3.3) p < 0.001 | 1.2 (0.8–1.8) NS | 1.6 (1.1–2.3) p < 0.001 |
| Potential to compromise safety | |||||||
| Unapproved indications | 109 (13 %) | 44 (10 %) | 65 (16 %) | 218 (13 %) | 1.3 (0.9–2.1) NS | 2.0 (1.1–3.5) p = 0.02 | 0.8 (0.5–1.3) NS |
| Unqualified safety claims | 60 (7 %) | 21 (5 %) | 59 (15 %) | 140 (8 %) | 2.5 (1.4–4.2) p = 0.001 | 3.5 (1.7–7.1) p < 0.001 | 0.6 (0.3–1.3) NS |
All odds ratios are adjusted for physician sex, # years in practice, remuneration, practice size, frequency of PSR visits, industry funding, medical faculty affiliation, sales visit duration, one-to-one or group, if drug was previously prescribed, and drug safety warnings. Results not adjusted for multiple comparisons
Van/Mon combined results of Vancouver and Montreal sites; Sac Sacramento; Toul Toulouse
*Minimally adequate safety information is defined as: at least one approved indication AND at least one non serious adverse event AND at least one serious adverse event (among drugs with SAE) AND at least one contraindication (among drugs with contraindications) AND no unqualified safety claims or unapproved indications
†Any harm information is defined as at least one mention of either a serious adverse event, a non-serious adverse event or a contraindication
‡Defined as promotions without a single mention of harm reported and with no written prescribing information (approved product information or alternative)
§Denominators are promotions of drugs with at least one serious adverse event in labeling (for serious adverse events) or at least one contraindication in labeling (for contraindications)
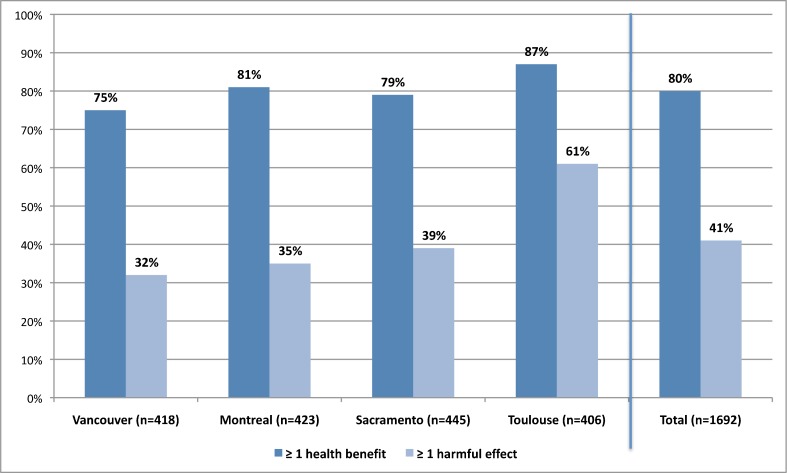
There were more promotions with at least one mention of harm (Fig. 2) in Toulouse than in Vancouver/Montreal (61 % vs. 34 %; adjusted OR = 4.0; 95 % CI 2.8–5.6) or Sacramento (61 % vs. 39 %; adjusted OR = 2.4 (95 % CI 1.7–3.6). Sacramento and Vancouver/Montreal differed by 5 % (39 % vs. 34 %; OR = 1.4; 95 % CI 1.0–2.0, p = 0.04).
Figure 2.
Drug-specific promotions with any mention of health benefits versus any mention of harm.
For drugs with listed SAE (n = 1,652 promotions, 97.6 % of total), mention of SAE did not differ between sites: 5–6 % of promotions/site. In Toulouse, physicians reported unqualified safety claims more often than in Vancouver/Montreal (15 % vs. 7 %; OR = 2.5, 95 % CI 1.4–4.2) or Sacramento (15 % vs. 5 %; OR = 3.5, 95 % CI 1.7–7.1).
PSRs discussed indications in 93 % of promotions. In 13 % of promotions, unapproved uses were mentioned. The only significant difference was for Toulouse (16 %) vs. Sacramento (10 %): OR = 2.0 (95 % CI 1.1–3.5), p = 0.02.
In a combined analysis of all sites, no measured physician or visit characteristic was associated with provision of “minimally adequate safety information.” Some versus no information on harm was provided more often in longer visits than those ≤ 5 min: 49.9 % vs. 31.1 %; adjusted OR 2.5 (95 % CI 1.8–3.5).
If physicians have not yet prescribed a drug, they may be less aware of its safety profile. “Minimally adequate safety information” did not differ for drugs not previously prescribed: 1.8 % vs. 1.6 % for previously prescribed drugs. Similarly, mention of SAE did not differ (6.3 % vs. 5.4 %), p = 1.0, nor did contraindications (22.8 % vs. 19.4 %), p = 0.3. PSRs mentioned any harm more often for drugs not yet prescribed: 47.9 % vs. 39.0 %, adjusted OR = 2.3 (95 % CI 1.2–4.6), p = 0.01.
Safety Profile of Promoted Medicines
In the US, a “black box” label warning indicates serious or life-threatening risks. Canada also has boxed warnings in labeling; France does not. Many promotions were for drugs with a US FDA black box, n = 765 (45 %) or a Canadian boxed warning, n = 892 (53 %); 962 (57 %) had either one. In 549 (57 %) of these 962 promotions, no harm was mentioned. PSRs made fewer unqualified safety claims for drugs with boxed warnings: 7 % vs. 10 % for other drugs, adjusted OR = 0.6 (95 % CI 0.4–0.8), p = 0.002, and mentioned SAE more often: 7 % vs. 4 % (n = 1,652 with SAE), adjusted OR = 2.1 (95 % CI 1.3–3.4), p = 0.003.
We also examined whether the medicine was subject to a safety advisory in the US, Canada or European Union from January 2008 to May 2009, as PSRs might mention emergent safety concerns. Thirty medicines (305 promotions) were subject to advisories. SAE in advisories were mentioned in 18/247 (7 %) of promotions for 27 drugs; contraindications in 10/169 (6 %) of promotions for 15 drugs.
Physicians reported the PSR’s key message for each promotion. For rosiglitazone, withdrawn in Europe and restricted in North America in late 2010 due to cardiac risks, nearly all were claims of safety, including: “Avandia is safe even in patients with heart disease, as long as they don’t have heart failure” (Montreal); “Avandia is not as dangerous as the public makes it out to be” (Sacramento); “New studies indicate safety” (Vancouver).
Information on Costs
Reimbursement status (public or private) was mentioned in nearly half of promotions in all sites, 775 (46 %; range 43–48 % per site), and the cost to consumers in 581 (34 %; range 27–44 % per site). Costs were compared with another therapy in 332 promotions (20 %; 15–27 % per site) and cost-effectiveness claimed in 14 % (range 13–16 % per site).
Physicians’ Judgments
We asked physicians to judge the scientific quality of information provided. Most ratings were positive: 57 % ‘good’ or ‘excellent’; 33 % ‘fair’; 10 % ‘poor’ or ‘very poor’ (n = 1,669 responses). Physicians rated information quality more highly if harm was mentioned: 68 % ‘good’ or ‘excellent’ versus 50 % with no harm, an 18 % difference (95 % CI 14 % to 23 %), p < 0.0001. We also asked how likely physicians were to start or increase prescribing a drug after the sales visit (Fig. 3; available on-line, Appendix 2). Physicians said they were ‘somewhat’ or ‘very likely’ nearly 2/3 of the time: range 62 % (Toulouse) to 66 % (Sacramento).
DISCUSSION
This is the largest sample to date assessing information quality in PSR promotions to family physicians, and the only study to use identical data collection methods over the same time period in different jurisdictions. Our results suggest a serious lack of information on harmful effects of promoted medicines. In this sample in four cities, PSRs rarely provided information defined a priori, based on a physician survey and regulatory standards, to be “minimally adequate safety information” (1.7 % of promotions). Information on health benefits was provided twice as often as information on harm, with not a single harmful effect mentioned in over half of promotions in the three North American sites.
It might be expected that if physicians were unfamiliar with a medication, PSRs would provide safety information to help ensure appropriate use. Information on serious harm was no more frequent for drugs not previously prescribed. Similarly, serious harm was rarely mentioned for drugs with boxed warnings or subject to recent safety advisories.
Despite these omissions, physicians judged information quality positively and expressed willingness to increase prescribing nearly two-thirds of the time. This raises serious concerns about the basis for such prescribing decisions, given that an understanding of a medicine’s health effects requires knowledge of both benefit and harm.
It seems unlikely that PSRs had too little time to provide “minimally adequate safety information,” as this measure required less information than the audio portion of 60-second US television ads, and most sales visits were over 5 min. Physicians frequently reported mention of listed costs, reimbursement status, cost-effectiveness, and health benefits. This strongly suggests that time was available to discuss safety.
One or more health benefits were discussed nearly twice as often as any harm, 80 % versus 41 % of interactions. In all three countries, promotions that fail to include information on harm are inconsistent with national laws. In information for physicians on its website, Health Canada describes “messages which emphasize only product benefits without including safety information” as potential legal violations.15 Similarly, the US FDA’s website explains that efficacy information with no risk information violates US regulations.16 In France, the Sales Visit Charter states that PSRs must mention AE, precautions and contraindications (Section II.1).17
In Toulouse, non-serious AE and contraindications were mentioned more often, and product information provided more often, suggesting an influence from stricter regulatory standards. However, SAE did not differ and unqualified safety claims were more frequent, suggesting an imperfect situation from a patient safety perspective. Physicians in Toulouse rarely received food or free samples, but nevertheless expressed willingness to prescribe at a rate similar to other sites. Industry funding, mainly for study participation, was common in Toulouse (42 % of participating physicians). Fugh-Berman and Ahari highlight “finely titrated doses of friendship,” as key to PSR sales success.18 This type of influence could explain the similar stated propensity to prescribe.
We had hypothesized that US “fair balance” requirements would lead to more frequent harm information in Sacramento than in the Canadian sites, and that unapproved indications would be mentioned more often in Sacramento. Neither proved to be the case. Our results suggest little influence from US/Canadian regulatory differences.
The limited differences observed between sites may reflect the near universal lack of monitoring of interactions by governments and self-regulatory agencies.
In previous studies of PSRs from 1975 to 1994 in Finland, Australia and the United States8 and 2000–2005 in France,9 AE were mentioned in 27–30 % of promotions, contraindications in 25–29 % and drug interactions in 25 %. In our study, AE (serious or non-serious) were mentioned in 29 %, contraindications in 20 % and interactions in 3 %. Thus, we found no indication of more complete harm information than in these older studies.
This study has some limitations. We cannot generalize our results to specialists or beyond the four included cities. We relied on physician recall, not recordings. However, physicians’ recall ultimately informs prescribing. Previous research found that physicians infrequently recalled hearing PSRs make false claims.19 We asked physicians to treat sales visits as per their usual practice, and did not distinguish spontaneously provided information from responses to questions. Physician recruitment rates (36 %) were similar to that in a study of Veterans Affairs psychiatrists on their interactions with PSRs (35 %).20 Sampling in Sacramento differed from the other sites, with a lower refusal rate among eligible physicians, but rates of reported safety information was similar to the other two North American sites, which argues against selection bias. Additionally, the most frequently reported drugs were those with highest promotional spending.
This is the first study to compare information provision by PSRs in different regulatory environments and the first to systematically focus on safety information. In all four sites, information on serious harm was usually lacking. Such omissions may threaten patient health. Unless regulatory oversight is improved to ensure balanced information, limits to PSR–physician interactions may be the most effective way to ensure that prescribing decisions are based on adequate information on harm as well as benefit.
Electronic Supplementary Material
(PDF 370 kb)
(DOCX 708 KB)
Acknowledgments
Author Contributions
BM had the primary responsibility for development of the study protocol and data collection tools, implemented the Vancouver study arm, oversaw data collection, wrote the plan for analysis, oversaw data cleaning, participated in data analysis, and drafted and revised the paper. She is the guarantor. JL was involved in protocol development and questionnaire design, plans for analysis, and drafting and revisions of the paper. JMS contributed to planning of data analysis, carried out statistical analysis, assisted in interpretation, and participated in drafting and revision of the manuscript. MD-B, MSW, GD and ER assisted in protocol development and instrument design, data interpretation and revisions of the manuscript; MD-B implemented the Montreal study arm; MSW the Sacramento study arm, and GD the Toulouse study arm. ER oversaw study administration in all four sites.
Barbara Mintzes had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of data analysis.
Acknowledgements: Contributors
We would like to thank the following people for their assistance with this study: Ken Bassett, Jim Wright, Alan Cassels, Aaron Tejani, Steve Morgan, Morris Barer, Florence Vandevelde, Line Guénette, Jean-Louis Montastruc, Les Toop, Dee Mangin, Libby Roughead, and Noordin Othman, for assistance with development of survey materials and study design; Theresa Lo, for her research on safety advisories; Chris Adlparvar for website development; Gisèle Foucault for translation; Aileen To and Jocelyne Gagne for administrative support; Lucy Lu, Kelp Watson, Amelia Daly, Neha Musini, Linda Lewis, Meghan Webb, Robert Renteria, Sara Pilote, Stacy Hayashi, Christine Dumolard, Marie Bounouh, Assia Meloua, Audrayanne Desjardins, and Jocelyne Bastien and Fabrice Amatulli, Union Régionale des Professionnels de Santé - Médecins Libéraux - Midi-Pyrénées, for assistance with recruitment of physicians; Anat Fisher, Herbert Fisher, Stephen Adams, Haithem Hamdi and Alexandra Laugerotte for coding of consistency with approved product information, and Pierre Biron for adjudication. Finally, we would like to extend our thanks to all of the physicians who participated in the study; without your help, this study would not have been possible.
Funders
This study was funded by the Institute of Health Services and Policy Research, Canadian Institutes of Health Research, and by Michael Smith Health Research Foundation. The funders had no involvement in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in preparation, review, or approval of the manuscript or decision to submit for publication.
Prior Presentation
Preliminary results of this study were presented in a poster at the 11th Annual International Society of Pharmacovigilance meeting in Istanbul, Turkey, in October 2011, and an oral presentation at the Canadian Cochrane Colloquium, Vancouver, B.C., February 2011.
Conflict of Interest
In 2010 Joel Lexchin was an expert witness for a law firm representing the family of a plaintiff who allegedly died from an adverse reaction from a product made by Allergan. He is currently on the Management Board of Healthy Skepticism Inc. Marie-Dominique Beaulieu is currently President of the College of Family Physicians of Canada (CFPC) (November 16 2012 to November 5 2013). She was not in this position when the study was conducted. None of the other authors have any conflicts of interest to declare.
REFERENCES
- 1.Gagnon MA, Lexchin J. The cost of pushing pills: a new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med. 2008;5(1):e1. doi: 10.1371/journal.pmed.0050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norris P, Herxheimer A, Lexchin J, Mansfield P. Drug Promotion: What We Know What We Have Yet to Learn. World Health Organisation and Health Action International. Geneva: 2005. Available at: http://apps.who.int/medicinedocs/pdf/s8109e/s8109e.pdf. Accessed January 24, 2013.
- 3.Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373–380. doi: 10.1001/jama.283.3.373. [DOI] [PubMed] [Google Scholar]
- 4.Spurling GK, Mansfield PR, Montgomery BD, Lexchin J, Doust J, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7(10):e1000352. doi: 10.1371/journal.pmed.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson BL, Silverman GK, Loewenstein GF, Zinberg S, Schulkin J. Factors associated with physicians’ reliance on pharmaceutical sales representatives. Acad Med. 2009;84:994–1002. doi: 10.1097/ACM.0b013e3181ace53a. [DOI] [PubMed] [Google Scholar]
- 6.Chalkley P. Targeting accessible physicians. Canadian Pharmaceutical Marketing. April 2009:29–30.
- 7.Waxman HA. The lessons of Vioxx—drug safety and sales. N Engl J Med. 2005;352:2576–8. doi: 10.1056/NEJMp058136. [DOI] [PubMed] [Google Scholar]
- 8.Lexchin J. What information do physicians receive from sales representatives? Can Fam Physician. 1997;43:941–945. [PMC free article] [PubMed] [Google Scholar]
- 9.Anon 15 ans d’observervation et un constat: rien à attendre de la visite médicale pour mieux soigner. La Revue Prescrire. 2006;26(272):383–389. [Google Scholar]
- 10.Government of Canada. Federal Food and Drugs Act and Regulations. Available at: http://www.hc-sc.gc.ca/fn-an/legislation/acts-lois/index-eng.php. Accessed January 24, 2013.
- 11.Salant P, Dillman DA. How to conduct your own survey. New York: Wiley; 1994. [Google Scholar]
- 12.Orthman N, Vitry AI, Roughead EE, Ismail SB, Omar K. Medicines information provided by pharmaceutical representatives: a comparative study in Australia and Malaysia. BMC Publ Health. 2010;10:743. doi: 10.1186/1471-2458-10-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strang DG, Gagnon M, Molloy DW, Darzins P, Etchells E, Bédard M, Davidson W. Development of a standardized, comprehensive ‘ideal drug detail’. Can J Clin Pharmacol. 2001;8(2):73–77. [PubMed] [Google Scholar]
- 14.Cegedim Strategic Data - CSD – Global Promotion Database, 2009–2010. www.cegedim.com.
- 15.Health Canada. Regulation of Health Product Advertising in Canada – Overview for Physicians. Ottawa: July 11, 2011. Available at: http://www.hc-sc.gc.ca/dhp-mps/advert-publicit/pol/overview-apercu-eng.php. Accessed January 24, 2013.
- 16.US Food and Drug Administration (FDA). Truthful Prescription Drug Advertising and Promotion (Bad Ad Program). Updated July 18, 2012. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/DrugMarketingAdvertisingandCommunications/ucm209384.htm#RecognizeReport. Accessed January 24, 2013.
- 17.Haute Autorité de la Santé (H.A.S.) Questions Réponses Rélatives à la Certification de la Visite Médicale. October 2009. Available at : http://www.has-sante.fr/portail/jcms/c_334342/referentiel-de-certification-de-la-visite-medicale. Accessed January 24, 2013.
- 18.Fugh-Berman A, Ahari S. Following the script: how drug reps make friends and influence doctors. PLoS Med. 2007;4(4):e150. doi: 10.1371/journal.pmed.0040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273:1296–8. doi: 10.1001/jama.1995.03520400066047. [DOI] [PubMed] [Google Scholar]
- 20.Sernyak M, Rosenheck R. Experience of VA psychiatrists with pharmaceutical detailing of antipsychotic medications. Psychiatr Serv. 2007;58:1292–1296. doi: 10.1176/appi.ps.58.10.1292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 370 kb)
(DOCX 708 KB)