Abstract
Pancreatic stellate cells (PSC) are a subset of pancreatic cancer-associated fibroblasts. These cells provide prosurvival signals to tumors; however, little is known regarding their interactions with immune cells within the tumor microenvironment. We hypothesized that factors produced by human PSC could enhance myeloid-derived suppressor cell (MDSC) differentiation and function, which promotes an immunosuppressive microenvironment. Primary PSC cell lines (n = 7) were generated from human specimens and phenotypically confirmed via expression of vimentin, α-smooth muscle actin (α-SMA), and glial fibrillary acidic protein (GFAP). Luminex analysis indicated that PSC but not human fetal primary pancreatic fibroblast cells (HPF; negative controls) produced MDSC-promoting cytokines [interleukin (IL-6), VEGF, macrophage colony-stimulating factor (M-CSF)] and chemokines (SDF-1, MCP-1). Culture of peripheral blood mononuclear cells [peripheral blood mononuclear cell (PBMC), n = 3 donors] with PSC supernatants or IL-6/granulocyte macrophage colony-stimulating factor (GM-CSF; positive control) for 7 days promoted PBMC differentiation into an MDSC (CD11b+CD33+) phenotype and a subpopulation of polymorphonuclear CD11b+CD33+CD15+ cells. The resulting CD11b+CD33+ cells functionally suppressed autologous T-lymphocyte proliferation. In contrast, supernatants from HPF did not induce an MDSC phenotype in PBMCs. Culture of normal PBMCs with PSC supernatants led to STAT3 but not STAT1 or STAT5 phosphorylation. IL-6 was an important mediator as its neutralization inhibited PSC supernatant-mediated STAT3 phosphorylation and MDSC differentiation. Finally, the FLLL32 STAT3 inhibitor abrogated PSC supernatant-mediated MDSC differentiation, PSC viability, and reduced autocrine IL-6 production indicating these processes are STAT3 dependent. These results identify a novel role for PSC in driving immune escape in pancreatic cancer and extend the evidence that STAT3 acts as a driver of stromal immunosuppression to enhance its interest as a therapeutic target.
Introduction
An estimated 227,000 deaths per year worldwide are caused by pancreatic cancer (1). This malignancy is the fourth leading cause of cancer-related death in the United States with dismal 5-year survival rates of less than 5% that have remained unchanged over the last 40 years (1). Its inherent aggressive biology coupled with vague early symptomatology often results in presentation only after the tumor invades surrounding tissues or metastasizes to distant organs. Therefore, an improved understanding of pancreatic cancer biology may catalyze novel treatment approaches to improve survival.
An emerging hallmark of cancer is the ability to evade immune recognition (2). This is accomplished in part via secretion of factors produced by tumors and the stromal accessory cells including cytokines, chemokines, and growth factors. These inflammatory substances promote the differentiation of suppressive immune cells such as myeloid-derived suppressor cells (MDSC) and their trafficking into the tumor microenvironment (2, 3). MDSCs are a heterogeneous population of immature myeloid cells that mobilize from the bone marrow and become activated to inhibit tumor-specific immune responses (4). Specifically, MDSC can suppress the ability of cytotoxic lymphocytes, such as T and natural killer cells to eliminate tumors through depletion of nutrients required by lymphocytes, generation of oxidative stress, and a variety of other mechanisms. A greater understanding of the factors regulating MDSC expansion, their effects on lymphocytes, and their role in the tumor microenvironment could lead to enhanced immune recognition of cancer or new therapeutic approaches.
Stromal cells within the pancreatic cancer microenvironment produce numerous factors that support the growth and survival of malignant cells (5). However, our understanding of how soluble factors from the stroma alter immune cell phenotype and function in the tumor microenvironment is far from complete. Pancreatic stellate cells (PSC) are an important cell type found within pancreatic stroma. These cells are characterized by vitamin A storing lipid droplets, production of extracellular matrix turnover, and synthesis of matrix metalloproteinases (MMP). PSC can become activated through injury, inflammation, and cancer resulting in a loss of the vitamin A stores and increase in extracellular matrix proteins and MMPs (6). Activated stellate cells also acquire a myofibroblast like phenotype, expressing markers such as vimentin, glial fibrillary acidic protein (GFAP), and α-smooth muscle actin (α-SMA; 7). Pancreatic cancer cells can drive PSC into an activated state, which influences pancreatic cancer growth and survival through the secretion of an array of factors (8). However, to our knowledge, no studies to date have explored potential interactions between PSC and altered immune phenotype and function present in patients with advanced pancreatic cancer.
The current study set out to characterize the interactions between PSC and immunosuppressive cells that may be present within the tumor microenvironment. We hypothesized that soluble factors produced by PSC might enhance MDSC differentiation and function, thereby promoting immunosuppression. PSC isolated from primary human pancreatic cancer specimens were characterized for their ability to produce soluble factors and mediate expansion of MDSC in vitro. PSC produced predominantly cytokines that were T-helper(TH)2 in nature or those factors that have a documented role in promoting MDSC differentiation. Consistent with these observations, supernatants from PSC were an effective stimulus promoting in vitro differentiation of normal donor PBMC into functional MDSC that suppress autologous lymphocyte proliferation. Neutralization studies revealed an important role for interleukin (IL)-6 produced by stellate cells as a key factor contributing to STAT3 signaling and MDSC differentiation. Culture of PBMC in the presence of the STAT3 inhibitor, FLLL32, abrogated the ability of PSC to induce MDSC differentiation, showing this process was STAT3 dependent. Finally, we observed that FLLL32 treatment of PSC inhibited STAT3 phosphorylation and decreased IL-6 production and viability in stellate cells. These data are the first to show that PSC can promote expansion of immunosuppressive cell populations and delineate the molecular mechanisms responsible for this reciprocal interaction.
Materials and Methods
Cell lines and reagents
Human PANC-1 cells purchased from American Type Culture Collection were cultured in Dulbecco’s Modified Eagle Medium (Gibco) with 10% FBS, 10 mmol/L L-glutamine, and antibiotics. A commercially available human fetal primary pancreatic fibroblast cell (HPF) line purchased from Vitro Biopharma was cultured in low serum MSC-GRO media with antibiotics (Vitro Biopharma). A commercially available normal human dermal fibroblast (NHDF) cell line purchased from Lonza was cultured in FGM 2 Bulletkit media (Lonza). These cell lines were not authenticated. Recombinant human IL-2, IL-6, granulocyte macrophage colony-stimulating factor (GM-CSF), and IFN-γ were purchased from Peprotech, Inc. The FLLL32 small-molecule STAT3 inhibitor was synthesized, purified, and evaluated for purity as previously described (9).
Stellate cell isolation and culture
Fresh tissue was obtained from patients undergoing surgical resection for pancreatic carcinoma at the Arthur G. James Comprehensive Cancer Center (Columbus, OH) under an Institutional review board-approved protocol following informed consent. Fresh tissue was dissected with a scalpel into 0.5 to 1 mm3 pieces, plated in 6-well 10 cm2 uncoated culture wells in Dulbecco’s Modified Eagle Medium with 10% FBS and antibiotics and incubated at 37°C. PSC typically grew out of the tissue in 2 to 3 weeks and were characterized by morphology. Following the second passage (14–20 days after dissection), supernatants were collected and stored at −80°C every 7 days when cells were 70% to 80% confluent. PSCs were maintained in culture with fresh media added twice weekly.
Peripheral blood mononuclear cell isolation and MDSC generation
Peripheral blood mononuclear cells (PBMC) were isolated from source leukocytes of healthy donors (American Red Cross) via density gradient centrifugation using Ficoll-Paque (Amersham, Pharmacia Biotech) as described (10, 11). PBMC from healthy donors (American Red Cross) were cultured in 10% FBS, 10 mmol/L L-glutamine, and 100 μg/mL penicillin/streptomycin in RPMI 1640 (Gibco). To generate functional MDSC, PBMC were cultured with 10 ng/mL of IL-6 and GM-CSF for 7 days as previously described by our group and others (10, 11). Briefly, cells were labeled with anti-CD33/66b magnetic microbeads (STEMCELL Technologies) and positively selected using an Easy Sep magnet. Isolated cells were washed twice before further studies. PBMC isolated from the same donor but not treated with cytokines were used as controls.
Immunofluorescence
PSC were cultured on chamber slides (Thermo Fisher Scientific Inc.) until 70% confluent (2–3 days). Cells were stained for myofibroblast markers as described (6, 12) using antibodies specific for vimentin (SP20 clone, Thermo Scientific), GFAP (abcam), and α-smooth muscle actin (1A4 clone, Sigma Aldrich). Alexa Fluor 488 goat anti-mouse (Invitrogen) and Alexa Fluor 488 goat anti-rabbit (Invitrogen) secondary antibodies were used and nuclei were stained using DAPI permamount (Vecta Shield). Staining was visualized by immunofluorescent microscopy and representative images from a minimum of 10 high powered fields were captured to characterize phenotypic properties of PSC cultures.
Analysis of cytokines and chemokines in PSC culture supernatants
A panel of 18 cytokines and chemokines was analyzed in supernatants from PSC cultures (day 7 of culture when 70% confluent) using commercially available, high-throughput Luminex Multiplex Cytokine Kits (Procarta Cytokine Assay Kit, Affymetrix). All samples were batch run in duplicate and quantified on the basis of a unique standard curve for each analyte. Supernatants from PSC cultures were also validated for the presence of IL-6 using commercial ELISA (R&D Systems, Inc.). Prostaglandin E2 (PGE2) supernatant levels were measured using an R&D parameter assay kit (R&D Systems, Inc.). Samples were run in duplicate per manufacturer’s recommendations.
Flow cytometry
Antibodies for MDSC or T-cell surface staining were as follows: mouse anti-human CD11b-PE or mouse immunoglobulin G (IgG)1 PE (isotype control), mouse anti-human CD33-APC or mouse IgG1-APC, mouse anti-human CD15-FITC or mouse IgM-FITC, mouse anti-human CD4-APC, or mouse anti-human CD8-APC (Beckman Coulter). Cells were incubated on ice for 30 minutes, washed, and fixed in PBS containing 1% formalin for flow cytometric analysis on a FACS calibur or LSRII flow cytometer (BD Biosciences).
T-cell suppression assay
Total CD3+ T cells were enriched from source leukocytes by negative selection with Rosette Sep reagents (STEMCELL Technologies, Inc.). T cells were labeled with 1 μmol/L CFSE (Invitrogen) and cultured with CD3/CD28 beads (Invitrogen) for 3 days. Cells were collected, stained for CD4+ or CD8+ T cell markers and fixed for flow cytometric analysis on a FACS Calibur. Cells were gated on CD4+ or CD8+ T cells and percent proliferation was determined on the basis of carboxyfluorescein succinimidyl ester (CFSE) dilution.
Immunoblot analysis
Lysates from PBMCs were assayed for protein expression by immunoblot analysis with antibodies to STAT1 (BD Biosciences), pSTAT1, STAT3, pSTAT3, STAT5, pSTAT5 (Cell Signaling Technology), or β-actin (Sigma). Following incubation with the appropriate horseradish-peroxidase-conjugated secondary antibodies, immune complexes were detected using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
IL-6 neutralization assay
To block the effects of IL-6, cultured PSC supernatants or recombinant IL-6 were pretreated with 5μg/mL of anti-IL-6 antibody (clone 6708, R&D Systems) for 30 minutes before addition to PBMC as described (13). Phosphorylated-STAT3 and MDSC differentiation were assessed as described above.
Statistical analysis
Results were expressed as mean ± SD of at least 2 to 3 matched donors. Mixed effects regression models were used in all of the analyses with a random effect for the experiment. Bonferroni adjustments were used within each set of experiments to account for multiplicity.
Results
Characterization of pancreatic stellate cells
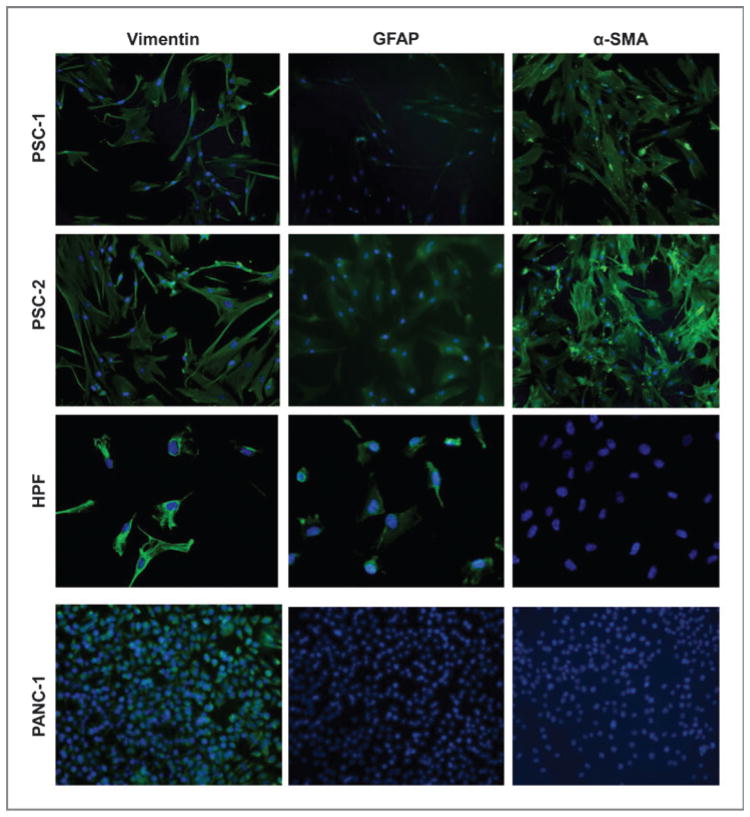
Pancreatic stellate cell lines were generated from surgical specimens of patients with pancreatic adenocarcinoma (Fig. 1A). Immunohistochemical analysis confirmed stellate cells were present within the stromal portion of each pancreatic tumor specimen by positive staining for α-SMA (Fig. 1B). A total of 7 lines were established, which typically remained viable for several months of in vitro culture. We confirmed that the cell lines cultured had a phenotype consistent with PSC by immunofluorescent staining for myofibrolastic markers vimentin, GFAP, and α-SMA (14). In contrast, the human pancreatic cancer cell line, PANC-1, was positive for vimentin but lacked the expression of the stellate markers GFAP and α-SMA and therefore served as a negative control (Fig. 2). HPF, a normal fetal human pancreatic fibroblast cell line, expressed vimentin and GFAP but were negative for α-SMA and used as a negative control fibroblast line.
Figure 1.
α–SMA-positive PSC are present in patient with pancreatic tumors. A, characteristics of patient tumors from which primary PSC lines were derived. B, representative IHC analysis of α-SMA staining in human pancreatic cancer specimens from which PSC-2 and PSC-4 were derived. Arrows indicate components of the tumor (T; black) and stroma (S; blue) showing positive α-SMA staining.
Figure 2.
Pancreatic stellate cells are positive for GFAP and α-SMA. Immunofluorescence analysis of representative primary PSC lines grown on chamber slides. Cells were stained with 4′, 6-diamidino-2-phenylindole (DAPI; blue) and antibodies (green) for vimentin, GFAP, and α-SMA. The human PANC-1 cell and HPF line were negative controls for α-SMA staining. Slides were visualized at ×40 magnification.
PSC secrete growth factors that promote MDSC expansion
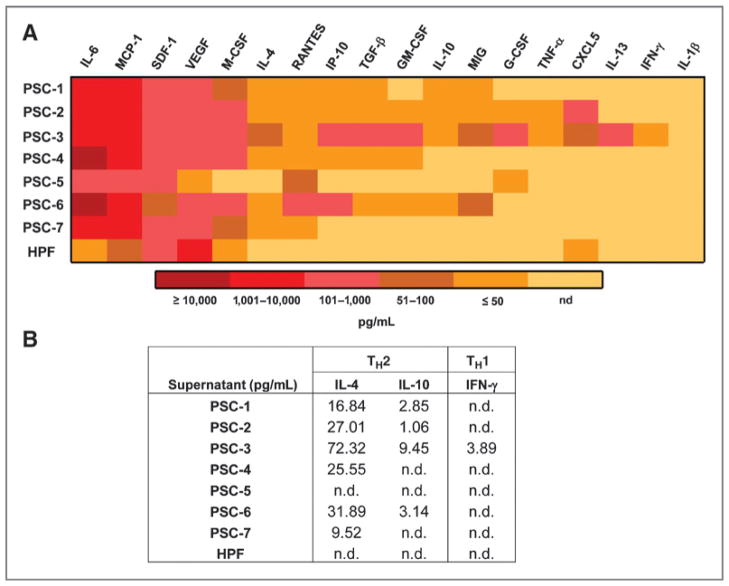
PSC secrete many factors that can support the growth and survival of tumor cells (15). However, it was of particular interest to further investigate whether PSC secrete soluble factors, which play a role in modulating immune cell differentiation and function. To our knowledge, a comprehensive analysis of immunomodulatory factors derived from primary human PSC has not been conducted. Supernatants from several patient-derived PSC lines (n = 7) were assessed using a Luminex platform designed to assess profiles of cytokines involved in TH1/TH2 responses, as well as MDSC differentiation and chemotaxis. Supernatants were collected following the third passage in culture when cells were 70% confluent. Overall, these PSC supernatants had low or no expression of several notable proinflammatory cytokines including, TNF-α, IFN-γ, and IL-1β. In contrast, a majority of PSC lines secreted detectable IL-4, indicating a TH2 bias in cytokine expression (Fig. 3B). Remarkably, high levels of several other cytokines known to regulate myeloid cell differentiation including IL-6, VEGF, and macrophage colony-stimulating factor (M-CSF) were also observed (Fig. 3A). Of this class of cytokines, IL-6 in particular, was expressed in nanogram range levels or above, in all 7 PSC lines. In contrast, PGE2, also known to promote MDSC differentiation (16) was present at variable levels and only supernatants from 3 of 7 PSC lines (Supplementary Fig. S1). Expression of 2 chemokines known to play a role in MDSC chemotaxis was also evident across all PSC lines (SDF-1, MCP-1). However, other chemokines including CXCL5, MIG, IP-10, and RANTES were expressed with a high degree of variability across individual PSC lines. The negative control fibroblast cells, HPF, secreted very low or undetectable levels of most cytokines and chemokines produced by patient-derived PSC. These Luminex data were further confirmed by ELISA for IL-6 as a representative factor expressed at high levels in the PSC supernatants (data not shown).
Figure 3.
PSC secrete inflammatory cytokines. Culture supernatants from the 7 primary PSC lines and the pancreatic fibroblast line, HPF, were collected when cells were 70% confluent. A, a panel of cytokines and chemokines were quantitated via Luminex analysis. Data are presented as a heat map for expression of soluble factors in supernatants from each cell line. B, chart indicates the levels of TH2 (IL-4 and IL-10) and TH1 (IFN-γ) cytokines observed in the Luminex bioplex (n.d., not detectable).
PSC supernatants differentiate PBMCs into cells with an MDSC phenotype that functionally suppress autologous T-cell proliferation
Elevated percentages of circulating MDSCs in patients with gastrointestinal (GI) malignancy have been reported as a poor prognostic factor (17). We therefore investigated whether soluble factors produced by PSC could promote differentiation of immune effector cells into an MDSC phenotype. For these experiments, PBMCs from normal human donors were cultured in the presence of PSC supernatants (5 or 10%) or IL-6 and GM-CSF (positive control) and stained for markers consistent with an MDSC phenotype after 7 days. PSC supernatants promoted the differentiation of PBMC into CD11b+ CD33+ cells (Fig. 4B, all P values < 0.01 for comparisons to medium), and a subpopulation of polymorphonuclear CD11b+ CD33+CD15+ cells (Fig. 4C, 5% and 10% PSC P < 0.05, IL-6/GM-CSF not significantly different from medium). In contrast, there was no evidence for induction of a prominent CD11b+ CD33+CD14+ monocytic MDSC subpopulation by either IL-6/GM-CSF or PSC supernatants (data not shown). In all experiments, supernatants from HPF or NHDF were used as negative controls, and when cultured with PBMC did not induce an MDSC phenotype (Fig. 4B).
Figure 4.
PSC supernatants differentiate PBMC into cells with an MDSC phenotype. Normal donor PBMC were cultured for 7 days with 5 or 10% PSC supernatants, HPF and NHDF supernatants (negative controls), or 10 ng/mL each of IL-6 and GM-CSF (positive control) and stained for MDSC markers. A, representative dot plots of CD33+ and CD11b+ staining after 7 days of culture with PSC supernatants. Summary of generation of CD11b+CD33+ (B) and CD11b+ CD33+CD15+ (C) MDSC subsets. Error bars represent data from PBMC of 3 separate donors cultured with PSC supernatants or IL-6/GM-CSF to generate MDSC in vitro. *, statistically significant compared with media alone at P value < 0.05.
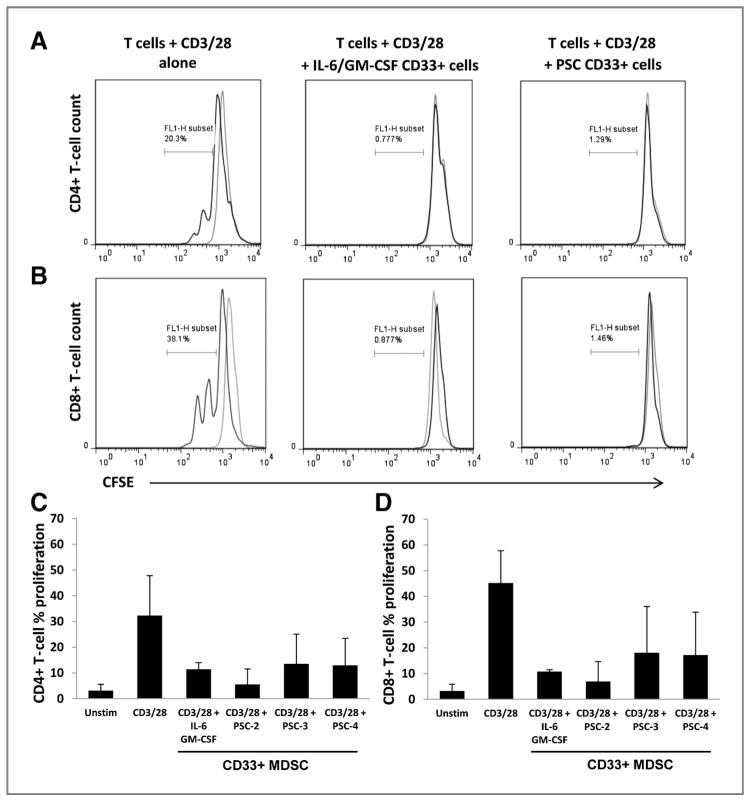
To confirm the CD11b+CD33+ cells generated via stimulation with PSC supernatants were functionally suppressive, PBMC were cultured with IL-6/GM-CSF or PSC supernatants for 7 days. CD33+ cells were then isolated via magnetic separation and cultured with donor matched, autologous CFSE-labeled T cells and stimulated with CD3/CD28 beads for 3 days. T-cell proliferation (CFSE dilution) was assayed by flow cytometry. A dramatic reduction of T-cell proliferation was observed when CD33+ cells generated from both IL-6/GM-CSF and using PSC supernatants from 3 different stellate lines were cocultured with CD3/CD28 stimulated, matched autologous donor CD4+ and CD8+ T cells (Fig. 5A–D). These data were reproducible using matched (autologous) MDSC and T-cell cultures from 2 separate donors.
Figure 5.
PSC generated CD33+ MDSC suppress autologous T-cell activation and proliferation. MDSC were generated by culture of PBMC with IL-6/GM-CSF or primary PSC supernatants for 7 days. CD33+ cells were then isolated by magnetic bead selection and cocultured with CFSE-labeled, donor-matched autologous T-cells ± CD3/CD28 activation beads. After 3 days, the percentage of T-cell proliferation was determined by flow cytometric analysis of CFSE dilution. Representative histograms of CFSE staining from unstimulated (gray lines) or CD3/CD28 activated (black lines) CD4+ (A) and CD8+ T cells alone (B), or in culture with CD33+ cells. Summary of inhibition of CD4+ (C) and CD8+ T-cell proliferation (D). Error bars represent SD of independent experiments using autologous CD33+ cells and T cells from 2 separate donors with each PSC supernatant.
PSC supernatants modulate MDSC differentiation via IL-6 and STAT3 signal transduction
Numerous transcription factors play a role in regulating the differentiation of immature myeloid cells into MDSC (3). The fact that PSC produce copious amounts of IL-6 and other cytokines (e.g., VEGF, M-CSF; Fig. 3A and B) led us to focus on the role of the STAT3 signal transduction pathway in promoting PSC-mediated differentiation. Culture of PBMCs with PSC supernatants resulted in rapid phosphorylation of STAT3 at levels comparable with cells stimulated with IL-6 (Fig. 6A). In contrast, cultures of PBMCs with PSC supernatants did not lead to increased phosphorylation of other STAT proteins including STAT1 or STAT5 (Supplementary Fig. 2A and 2B). These data were consistent with Luminex data showing PSC supernatants lacked high levels of canonical factors known to activate these other STAT proteins (Fig. 3A).
Figure 6.
PSC supernatants modulate MDSC differentiation via IL-6 and STAT3 signal transduction. A, PBMC were cultured with PSC supernatants or relevant cytokines for 20 minutes and analyzed for levels of total STAT3 or phosphorylated STAT3 (pSTAT3). B, recombinant IL-6 (10 ng/mL) or 10% PSC supernatants were preincubated for 30 minutes with 5μg/mL of anti-IL-6–neutralizing antibody and added to normal donor PBMCs. pSTAT3 was analyzed by immunoblot after 20 minutes. C, in a similar manner, the effect of neutralizing IL-6 on PSC-supernatant—induced MDSC generation was also determined by incubating cells for 7 days (changing and neutralizing medium/cytokines/PSC supernatants on days 3 and 5) and analysis of CD11b+CD33+ MDSC by flow cytometry. D, PBMC were cultured with the FLLL32 STAT3 inhibitor (6 μmol/L) or DMSO (vehicle) for 7 days with 10% PSC supernatants or IL-6/GM-CSF (positive control) and analyzed for CD11b+CD33+ cells by flow cytometry. Representative IHC analysis of pSTAT3 staining in a representative human pancreatic tumor tissue (E) or normal pancreatic tissue (F) specimen. Arrows indicate components of the tumor (T; black) and stroma (S; blue) showing positive pSTAT3 staining. Error bars represent SD from independent experiments with PBMC from 3 separate donors. *, statistically significant compared with DMSO control at P value < 0.05.
To gain further insight as to the importance of IL-6 in PSC as a key upstream factor driving MDSC differentiation, we neutralized IL-6 from supernatants of PSC via a commercially available blocking antibodies and assessed signaling and MDSC differentiation of immune cells treated with IL-6–depleted PSC supernatants. IL-6 neutralization inhibited a majority of STAT3 phosphorylation in response to PSC supernatants (Fig. 6B). Neutralizing IL-6 within PSC supernatants led to a reduction in the ability of PSC supernatants to differentiate normal PBMCs into MDSC (range 0.65–0.7-fold reduction; Fig. 6C) that approached (P = 0.1 for PSC1, n = 3 PBMC donors) or reached statistical significance (P = 0.019 for PSC2, n = 3 PBMC donors). These data support a role for IL-6 as a soluble factor present within PSC supernatants that supports MDSC differentiation.
Previous work from our laboratory and others has shown that IL-6–induced STAT3 activation plays a role in regulating MDSC expansion (9, 18, 19). In addition to IL-6, several other cytokines elevated in PSC supernatants have potential to act in a redundant manner via STAT3 signal transduction. Thus, we were interested in determining whether MDSC induced by PSC supernatants was a STAT3-dependent process. Culture of normal donor PBMC with 10% PSC supernatant or IL-6 and GM-CSF in the presence of the small-molecule STAT3 inhibitor, FLLL32, for 7 days and led to a significant reduction in the number of MDSC generated from PBMC as compared with dimethyl sulfoxide (DMSO) controls (Fig. 6D, P = 0.0001 and P = 0.0004, respectively). These data suggest STAT3 activating factors present in PSC supernatants may play a unique role in modulating a suppressive immune phenotype in the pancreatic cancer microenvironment.
STAT3 inhibition inhibits the survival of PSC and IL-6 production
Because high levels of IL-6 and other soluble factors were secreted in an autocrine manner from PSC, we postulated that these factors might facilitate a positive feedback loop that contributes to PSC survival. Indeed, STAT3 activation is essential for the survival of malignant cells derived from various anatomic sites (20). Furthermore, immunohistochemical (IHC) analysis of representative primary pancreatic tumors from patients revealed phosphorylated STAT3 present within the nucleus of both malignant cells and surrounding stroma (Fig. 6E). This was in contrast to normal pancreatic tissue, which was negative for pSTAT3 (Fig. 6F). However the role of STAT3 in regulating the biology of stromal PSC has not been examined. For these studies, the effects of STAT3 inhibition on PSC viability and cytokine secretion were evaluated. PSC were plated in fresh media in the presence of DMSO (vehicle) or the FLLL32 STAT3 inhibitor for 48 hours. By 48 hours, PSC treated with DMSO showed restoration of basal pSTAT3 levels. However pSTAT3 expression remained absent at this time point in cells treated with FLLL32 (Fig. 7A). Concurrently, PARP cleavage was evident in lysates from FLLL32-treated PSC at the 24-hour time point, indicating the cells were undergoing apoptosis. Cytotoxicity was confirmed in these PSC cultures by light microscopy, which confirmed a reduction of adherent viable cells after a 48-hour treatment with FLLL32 (Fig. 7B). Finally, treatment with FLLL32 reduced the autocrine production of IL-6 in PSC supernatants as compared with supernatants from DMSO-treated cells (Fig. 7C). These data indicate that STAT3 inhibition in PSC controls cell survival and regulates the secretion of IL-6.
Figure 7.
STAT3 inhibition induces pancreatic stellate cell apoptosis. A, PSC were cultured with 6 μmol/L FLLL32 for various time points (3, 6, 12, 24, 48 hours). Immunoblot analysis of pSTAT3 and PARP expression. Total STAT3 protein was also measured as a loading control. B, representative light microscopy images of PSC after 24 and 48 hours of culture with FLLL32 or DMSO control. C, IL-6 concentrations in the PSC supernatants were assessed by ELISA. Error bars represent SD from independent experiments with 2 separate primary PSC lines.
Discussion
Patients with pancreatic cancer often display profound immune dysfunction characterized by augmented systemic production of proinflammatory cytokines and suppressor cells (21, 22). The presence of these factors is also evident within the tumor microenvironment and is postulated to represent at least one factor limiting the efficacy of immune-based therapy in this disease. However, the role of the pancreatic cancer stroma in driving this process is not fully understood. A number of studies have shown that proinflammatory cytokines and MDSC are elevated in patients with pancreatic cancer (11, 17, 23). More recently, MDSC have emerged as an independent prognostic indicator in pancreatic, breast, esophageal, and gastric cancers, validating their clinical relevance (17, 24, 25). Elevated levels of CD15+ MDSC subsets have been shown to correlate with poor prognosis and radiographic progression in patients with GI malignancies (25). Therefore, delineating mechanisms leading to MDSC expansion in cancer represents an area of great interest. It is relatively well-established that soluble factors such as IL-6, GM-CSF, M-CSF, and VEGF can promote MDSC expansion (10, 11, 16). However, this study represents the first report showing that soluble factors (in particular, IL-6) produced by stellate cells from patients with pancreatic cancer promote the differentiation of immune cells into functional MDSC. Indeed, the ability of PSC to produce and secrete these factors in the tumor microenvironment could fuel the generation of immunosuppressive cells, which prevent innate or adaptive immune responses against the tumor. On the basis of these data, targeting stellate cells within the tumor microenvironment could reduce MDSC levels and amplify the effect of immunotherapy.
The supporting role of the tumor stroma in promoting cancer cell survival, growth, and metastasis has been well documented (26, 27). In particular, this dynamic relationship has been noted for pancreatic cancer in a number of studies. Apte and colleagues have shown that orthotopic pancreatic tumor xenografts grow larger when coinjected with human PSC (8). It has also been shown that cancer-associated fibroblasts are important for recruiting macrophages and supporting angiogenesis, enhanced tumor growth, and survival in pancreatic cancer (27, 28). Several factors within the pancreatic cancer microenvironment are conducive to promoting differentiation and recruitment of MDSC and other immunosuppressive cells. For example, IL-6 trans-signaling–dependent activation of STAT3/SOCS3 promotes pancreatic cancer in murine models (29, 30). In the present study, we show that PSC secrete nanogram quantities of IL-6, as well as high levels of M-CSF and VEGF that promote MDSC differentiation and chemotaxis (SDF-1, MCP—1; ref. 31–33). This mixture of factors is likely to synergize with other cancer-derived factors, such as GM-CSF that promote MDSC accumulation in the tumor microenvironment (34, 35) or HMGB1, which regulates a positive feedback loop between autophagy, IL-6, and pSTAT3 (36). The present study is aligned with these findings, as GM—CSF was not produced at high concentrations across all PSC lines, indicating its predominant source is likely the malignant cell. Data from the present study are also in agreement with prior studies showing cotransplantation of hepatic stellate cells can prevent rejection of islet allografts by promoting MDSC expansion (37). However, our data now provides novel evidence that pancreatic cancer stroma and malignant cells have opportunity to collaborate in driving immune suppression through the unique profile of soluble factors they produce in a STAT3-dependent manner.
The data from this study will provide greater insight into the signaling pathways, which mediate the biologic properties of activated PSC. Clearly, the exact factors or means through which tumors drive a quiescent stellate cell into an activated phenotype is not fully understood. It is hypothesized in the literature that proinflammatory factors, such as TGF-β and platelet-derived growth factor (PDGF) could be responsible due to these ligands’ ability to enhance PSC proliferation, collagen synthesis, and α-SMA expression (38, 39). Other studies have shown that ERK and mitogen-activated protein kinase pathways control the production of numerous inflammatory mediators in stellate cells (40). However, the mechanistic role for STAT3 in PSC is a novel finding. In this report, pancreatic stellate cells expressed constitutively phosphorylated STAT3 (data not shown), which was essential for both the survival and IL-6 secretion occurring from these stromal cells (Fig. 7). These data suggest that targeting IL-6, STAT3, or various other ligands within PSC could be a potential treatment modality for decreasing factors that support tumor growth and lead to a reduction in suppressive immune cell populations found within the tumor microenvironment.
Undoubtedly, there remain a number of controversial areas with regard to the origin and functional role of PSC in a tumor-bearing host. In fact, recent studies have shown that stellate cells can originate from the bone marrow in rodent models and can home to the pancreatic tumor site from the bone marrow (41, 42). Although the origin of these cells in humans remains debated, these observations leave open the possibility that the origin of primary cells grown out from patient specimens could be hematopoietic in nature. Regardless of their origin, our data show that these cells are indeed a component of the tumor stroma, based on positive staining for stromal elements such as vimentin, and the presence of well-characterized stellate cell markers, GFAP and α-SMA (Fig. 3). Another technically challenging issue is the difficulty in obtaining fresh primary, nonactivated pancreatic stromal cells that could serve as additional controls for these types of experiments. In our experience, procuring tissue suitable for outgrowth of normal, nonactivated stellate cells is quite difficult to obtain. Most likely, any pancreatic specimens that could be obtained as fresh surgical specimens would be from someone with an existing inflammatory condition (i.e., pancreatitis). These inflammatory conditions have also been to reported to promote activation of stellate cells, which produce large amounts of cytokines and growth factors (43). Regardless, because many patients with pancreatic adenocarcinoma have a long “latent” period before clinical presentation, even activated stellate cells in nonmalignant conditions could in theory promote early immune changes that drive carcinogenesis.
This study provides a clear rationale for targeting the pancreatic stroma to enhance immune-based treatment or endogenous immune recognition of cancer. This data also shows further complexity within the pancreatic stroma, which is composed of connective tissue, extracellular matrix, vasculature, and infiltrating hematopoietic cells (26). Therapies against molecules or pathways such as VEGF (44), sonic hedgehog (45), and notch (46) are currently being investigated as possible modalities for targeting both the pancreatic tumor and the surrounding stromal microenvironment (47, 48). Our study further suggests that the IL-6/STAT3 axis is also a relevant target due to its constitutive activation in the pancreatic cancer cells, immunosuppressive cells, and now in PSC within the stroma (49, 50).
In conclusion, the present study represents the first report documenting a relationship between PSC and MDSC. These data indicate that nonmalignant cells present within the pancreatic stroma may serve as a source of soluble, immunoregulatory factors like IL-6, which shape the cellular composition within the tumor microenvironment. Finally, our data point to a critical role for the STAT3 pathway as a key mediator of both immune suppressor cell differentiation and survival of PSC.
Supplementary Material
Acknowledgments
The authors thank The Ohio State University Comprehensive Cancer Center (OSUCCC) Analytical Cytometry and Biostatistics Shared Resources and Valerie Wright for technical assistance.
Grant Support
This work was supported by NIH Grants 5T32CA009338-34 (T.A. Mace), K22CA134551 (G.B. Lesinski), The Valvano Foundation for Cancer Research (G.B. Lesinski), P30CA016058-36 (Caligiuri), and American-Italian Cancer Foundation Pancreatic Cancer Initiative grant (M. Bloomston). The project described was supported by Award Number UL1RR025755 from the National Center for Research Resources, funded by the Office of the Director, NIH (OD), and supported by the NIH Roadmap for Medical Research.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: T.A. Mace, A. Collins, J.R. Fuchs, T. Bekaii-Saab, M. Bloomston, G.B. Lesinski
Development of methodology: T.A. Mace, A. Collins, M. Bloomston
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): T.A. Mace, Z. Ameen, A. Collins, S. Wojcik, M. Mair, J. R. Fuchs, T.D. Eubank, M. Bloomston
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): T.A. Mace, Z. Ameen, G.S. Young, M. Bloomston, G.B. Lesinski
Writing, review, and/or revision of the manuscript: T.A. Mace, A. Collins, G. S. Young, W.L. Frankel, T. Bekaii-Saab, M. Bloomston, G.B. Lesinski
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): T.A. Mace, A. Collins, M. Bloomston, G. B. Lesinski
Study supervision: M. Bloomston, G.B. Lesinski
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao CY, Zhou JY, Yang L, Wang YD. Molecular mechanism of ciglitazone inhibiting the expression of extracellular matrix in human hepatic stellate cells. Zhonghua Gan Zang Bing Za Zhi. 2007;15:840–4. [PubMed] [Google Scholar]
- 6.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–33. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–90. doi: 10.4161/cc.19679. [DOI] [PubMed] [Google Scholar]
- 8.Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27:69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 9.Bill MA, Courtney N, Mace TA, Etter JP, Li C, Schwartz EB, et al. Structurally modified curcumin analogs inhibit STAT3 phosphorylation and promote apoptosis of human renal cell carcinoma and melanoma cell lines. PLoS One. 2012;7:e40724. doi: 10.1371/journal.pone.0040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, et al. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–79. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Burt AD. The diffuse stellate cell system. J Mol Histol. 2007;38:53–64. doi: 10.1007/s10735-007-9078-5. [DOI] [PubMed] [Google Scholar]
- 13.Li YY, Hsieh LL, Tang RP, Liao SK, Yeh KY. Interleukin-6 (IL-6) released by macrophages induces IL-6 secretion in the human colon cancer HT-29 cell line. Hum Immunol. 2009;70:151–8. doi: 10.1016/j.humimm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–87. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Erkan M, Reiser-Erkan C, Michalski CW, Kong B, Esposito I, Friess H, et al. The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr Mol Med. 2012;12:288–303. doi: 10.2174/156652412799218921. [DOI] [PubMed] [Google Scholar]
- 16.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, Yan C, Czader M, Foreman O, Blum JS, Kapur R, et al. Inhibition of PPARgamma in myeloid-lineage cells induces systemic inflammation, immunosuppression, and tumorigenesis. Blood. 2012;119:115–26. doi: 10.1182/blood-2011-06-363093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Du H, Li Y, Qu P, Yan C. Signal transducer and activator of transcription 3 (Stat3C) promotes myeloid-derived suppressor cell expansion and immune suppression during lung tumorigenesis. Am J Pathol. 2011;179:2131–41. doi: 10.1016/j.ajpath.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 22.Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, et al. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11:734–51. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. 2012;28:453–8. doi: 10.3892/or.2012.1812. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engels B, Rowley DA, Schreiber H. Targeting stroma to treat cancers. Semin Cancer Biol. 2012;22:41–9. doi: 10.1016/j.semcancer.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi O, Komaki R, Smith PD, Jurgensmeier JM, Ryan A, Bekele BN, et al. Combined MEK and VEGFR inhibition in orthotopic human lung cancer models results in enhanced inhibition of tumor angiogenesis, growth, and metastasis. Clin Cancer Res. 2012;18:1641–54. doi: 10.1158/1078-0432.CCR-11-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–17. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012;109:7031–6. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou HS, Hsieh CC, Yang HR, Wang L, Arakawa Y, Brown K, et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–19. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–95. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–41. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–60. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 41.Sparmann G, Kruse ML, Hofmeister-Mielke N, Koczan D, Jaster R, Liebe S, et al. Bone marrow-derived pancreatic stellate cells in rats. Cell Res. 2010;20:288–98. doi: 10.1038/cr.2010.10. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Masamune A, Kikuta K, Hirota M, Kume K, Satoh K, et al. Bone marrow contributes to the population of pancreatic stellate cells in mice. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1138–46. doi: 10.1152/ajpgi.00123.2009. [DOI] [PubMed] [Google Scholar]
- 43.Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:34–41. doi: 10.1111/j.1440-1746.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- 44.Taeger J, Moser C, Hellerbrand C, Mycielska ME, Glockzin G, Schlitt HJ, et al. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol Cancer Ther. 2011;10:2157–67. doi: 10.1158/1535-7163.MCT-11-0312. [DOI] [PubMed] [Google Scholar]
- 45.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumont AG, Yang Y, Reynoso D, Katz D, Trent JC, Hughes DP. Anti-tumor effects of the Notch pathway in gastrointestinal stromal tumors. Carcinogenesis. 2012;33:1674–83. doi: 10.1093/carcin/bgs221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almhanna K, Philip PA. Defining new paradigms for the treatment of pancreatic cancer. Curr Treat Options Oncol. 2011;12:111–25. doi: 10.1007/s11864-011-0150-8. [DOI] [PubMed] [Google Scholar]
- 49.Huang C, Huang R, Chang W, Jiang T, Huang K, Cao J, et al. The expression and clinical significance of pSTAT3, VEGF and VEGF-C in pancreatic adenocarcinoma. Neoplasma. 2012;59:52–61. doi: 10.4149/neo_2012_007. [DOI] [PubMed] [Google Scholar]
- 50.Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020–9. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.