Abstract
High-performance ion chromatography and inductively coupled plasma–mass spectrometry methods have been applied to estimate the content of Cd, Co, Cu, Fe, Mn, Zn, and Ni in whole blood, plasma, and urine of obese and nonobese children. The study was conducted on a group of 81 Polish children of age 6–17 years (37 males, 44 females). Obese children were defined as those with body mass index (BMI) >95th percentile in each age–gender-specific group. Statistical testing was done by the use of nonparametric tests (Kruskal–Wallis's and Mann–Whitney's U) and Spearman's correlation coefficient. Significant correlations appeared for control group in plasma (Mn–Cd, Ni–Co), urine (Cu–Co), and blood (Fe–Cu), while for obese patients in plasma (Cd–Mn, Ni–Cu, Ni–Zn) and urine (Fe–Cd, Co–Mn). Sex criteria did not influence correlations between metals' content in plasma and urine of obese patients. Metals' abundance was correlated in non-corresponding combinations of body fluids. Rare significant differences between content of metals according to sex and the type of body fluids were discovered: Zn in plasma from obese patients of both sexes, and Zn, Co, and Mn in blood, Mn in plasma from healthy subjects. Negative correlations between BMI and Zn in blood, Cu in plasma, and Fe in urine were discovered for girls (control group). Positive correlation between Co content in plasma and BMI was discovered for obese boys. The changes in metals' content in body fluids may be indicators of obesity. Content of zinc, copper, and cobalt should be monitored in children with elevated BMI to avoid deficiency problems.
Keywords: Metals, Body fluids, Childhood obesity, BMI, IC, ICP-MS
Introduction
Childhood obesity has become a global epidemic with important consequences for the health of future generations [1]. Obesity is a common endocrine and metabolic disorder in children, teenagers (over 20 % of population of young people in Poland), and adults. It is a disease characterized by the increase in the body mass above the normal level because of excessive filling of already existing fat cells with triglycerides (hypertrophy) and/or forming new cells (hyperplasia). It is a very common change in a phenotype with a grave prognosis and severe negative medical and social effects [2]. The World Health Organization claimed that more than 10 % of the world's adult population was obese in 2008. In 2011, more than 40 million children under the age of 5 were overweight [3]. Obesity is associated with an increase in mortality and morbidity, including type II diabetes mellitus, cardiovascular, orthopedic, and respiratory disease [1, 4]. However, studies of Lloyd et al. [5] do not fully support the view that childhood obesity is an independent risk factor for adult metabolic syndrome or type II diabetes. Moreover, according to the authors, those who are at the lower end of the body mass index (BMI) range in childhood but continue to be obese during adulthood seem to be at particular risk of metabolic syndrome.
Recently, our knowledge on abundance of trace elements in human tissues and fluids has increased significantly due to improvements in analytics. Analytical methods have become useful in exploring the relationship between basic composition of body fluids and tissues, pathological conditions, and general nutritional status in humans. Many authors [6–10] studied the content of essential elements and toxic metals in human tissues, organs, and body fluids. However, the literature on trace elements status in obesity in children and adults is scarce. The authors concentrate mainly on iron [11], selenium, zinc [12], and copper [13]. Little is known about the content of nickel, cadmium, cobalt, or manganese in body fluids of obese children. Very recently, Hasbahceci et al. [14] have described the reverse relationship between BMI and boron level in blood of obese adults.
Published research reveals that dietary deficiencies for certain mineral and trace elements may increase the absorption of toxic metals by certain tissues. Zinc deficiency has shown to increase cadmium deposition in the liver, while iron and copper deficiencies increase cadmium intake in the kidneys [15]. Cadmium affects the metabolism of zinc and copper. Some animal studies have demonstrated that cadmium exposure causes an increase in the urinary excretion of some essential metals, such as copper, zinc, and iron [16, 17]. In one experiment, the adverse effects of increased cadmium intake in animals were prevented by the supplementation of zinc and copper [18]. There is substantial experimental evidence that the apparent clinical toxicity of certain metals may actually be the secondary result of deficiencies for essential trace elements, such as zinc, copper, and iron [19]. Therefore, it is important to know and measure disorders of trace element status in humans because both the alterations in the content and their mutual interactions may play an important role in the pathogenesis of obesity.
Our current studies aim to provide information concerning the content of both essential and nonessential metals in plasma, whole blood, and urine of obese and nonobese children. Authors decided to analyze these three types of body fluids in order to check how obesity influences the distribution of selected metals in the whole blood and plasma, and how urine reflects the possible differences in levels of metals in both studied groups. Mutual correlations of metals and various body fluids and associations with BMI are described as well. It seems that it is the first time high-performance ion chromatography (HPIC) method was used to carry out simultaneous, precise, accurate, fast, and sensitive determination of Cd, Co, Cu, Fe, Mn, Zn, and Ni in blood, plasma, and urine of obese and nonobese children. In order to compare the IC results and, whenever it was needed, to assure lower detection limit, inductively coupled plasma–mass spectrometry (ICP-MS) was applied. Both methods of measurements were performed in two independent analytical laboratories.
Methods
Subjects
Diagnostic tests were performed in children undergoing treatment at the Department of Pediatric Endocrinology and Diabetology, Medical University of Lublin, Poland. Informed consent was obtained from the parents. The study was approved by the ethics Committee of the Medical University of Lublin.
Tests were performed in 81 children who constituted two groups: obese (29 girls and 27 boys, age 6 to 17 years, the average age 12.8 years) and the control group (15 girls and 10 boys, age 8 to 17 years, the average age 13.5 years). The authors are conscious of the fact that increasing the number of patients would improve statistical analysis; however at the present stage of research, it was impossible to include new children. Despite the relatively small sample size, the authors decided to present some interesting results to the scientific community. In the obese group of patients, the overall mean height was 162.6 cm for boys and 154.6 cm for girls, weight was 82.3 kg for boys and 72.2 kg for girls, respectively. Boys had a higher mean BMI (37.7 kg/m2) than girls (29.7 kg/m2). For the control group, the mean values were 161.6 cm, 52.8 kg, and 20.13 kg/m2 for boys and 160.1 cm, 52.9 kg, and 20.4 kg/m2 for girls. BMI was calculated using the formula (weight in kilogram) / (height in meter)2. Other parameters were as follows: average blood pressure (systolic/diastolic) in obese group was 116.81 ± 10.11/75.67 ± 10.11 mmHg; in the control group 110.00 ± 12.06/75.00 ± 10.30 mmHg; average total cholesterol in serum 174.45 ± 22.17 mg/dL (obese), 150 ± 24.25 mg/dL (controls); average high-density lipoprotein (HDL) in serum 50.88 ± 4.55 mg/dL (obese), 40.25 ± 3.18 mg/dL (controls); average low-density lipoprotein in serum 100.38 ± 12.51 mg/dL (obese), 100.12 ± 7.18 mg/dL (controls); average triglyceride level in serum 110.69 ± 24.16 mg/dL (obese), 90.34 ± 18.19 mg/dL (controls); and average concentration of glucose in serum 87.62 ± 2.23 mg/dL (obese) and 75.25 ± 4.09 mg/dL (controls). Children did not take any mineral supplements or drugs before the samples for analyses were collected. They were not on a special diet prior the tests.
Samples
The studied material consisted of 243 samples of human whole blood, plasma, and urine. Samples taken from healthy and obese patients were pretreated and analyzed in the same way. The blood and urine for the evaluation of trace metals in all the subjects were collected in the morning, before meal. For plasma samples, blood was centrifuged and separated immediately after centrifuging (10 min at 3,000×g). Commercially available anticoagulant-treated tubes (EDTA-treated) were used. Samples were frozen and stored at −40 °C until mineralization procedure. All the samples were stored and transported in polypropylene containers.
One milliliter of each type of sample was divided in two parts (each 0.5 mL) in order to have two independent solutions prior to the mineralization procedure. A microwave-assisted high-pressure digestion system (UniClever BM-1, Plazmotronika, Poznań, Poland) was used. Each time an acidic digestion with 99 % nitric acid (Sigma- Aldrich, Germany) water solution was applied (1 mL of HNO3:9 mL of deionized H2O). The conditions of the mineralization procedure had been previously optimized [20]. The obtained solutions were poured into volumetric flasks (PTFE) and when it was necessary they were tenfold diluted with deionized water (18 MΩ cm) before final analysis.
Multielemental Analysis of Body Fluids by HPIC and ICP-MS
After the mineralization, each sample was analyzed at least in triplicate using IC and ICP-MS techniques. Chromatographic analyses were performed on a Dionex DX-500 ion chromatograph (Dionex, Sunnyvale, CA, USA). The detailed procedure of the standard solutions preparation, operating HPIC conditions, and validation of the applied method have been already described in the previous papers [9, 10]. The limits of detection (LOD) values (in microgram per milliliter) were as follows: 0.022 (Cd), 0.026 (Co), 0.048 (Cu), 0.009 (Fe), 0.006 (Mn), 0.056 (Zn), and 0.006 (Ni) [20].
ICP-MS studies were conducted at the Warsaw University of Technology, Chemistry Faculty, Warsaw, Poland. ICP-MS was carried out with Agilent 7500a ICP Mass Spectrometer (Agilent Technologies, Tokyo, Japan) using 10 ng/mL of 89Y and 209Bi as internal standards. Sample introduction system consisted of concentric nebulizer 0.4 mL/min (Sea-Spray Nebulizer, Glass Expansion, Pocasset, MA USA) attached to commercial Scott's spray chamber (Agilent Technologies), quartz torch with platinum shield and quartz bonnet, Pt sampling, and skimmer cones. LODs values (in microgram per milliliter) were as follows: 0.0001 (Cd), 0.0002 (Co), 0.0005 (Cu), 0.0008 (Fe), 0.0005 (Mn), 0.0005 (Zn), and 0.0004 (Ni).
Since ICP-MS enables lower LODs than HPIC, metals could be determined thoroughly by ICP-MS. However, the investigations described here had started by the use of HPIC (when ICP-MS was not available). Measurements from these two analytical techniques improve the quality of the presented analytical data. It is known that both HPIC and ICP-MS offer several advantages over conventional methods of metals' determination. HPIC can be considered as being complementary rather than competitive modern analytical technique for determinations of metals thus was included in the research.
Analytical quality control included analysis of certified reference materials SeronormTM Trace Elements Whole Blood L-3 (ref. 210305) and SeronormTM Trace Elements Urine L-2 (ref.210705) (Billingstad, Norway). The obtained results agreed with the recommended reference values. Recoveries were calculated as a ratio of determined value to certified one (expressed in percent). Recoveries for a 95 % confidence level were as follows: 100 ± 3 % (Fe), 102 ± 4 % (Cu), 98 ± 9 % (Ni), 99 ± 2 % (Zn), 94 ± 7 % (Co), 91 ± 8 % (Cd), and 89 ± 9 % (Mn).
Statistical Analysis
The data collected were entered into Statistica 9.0 (Statsoft, Inc., USA) and checked for accuracy. At the first stage of statistical evaluation of data, normality was checked by the use of Lillefors' and Shapiro–Wilks' tests. In case of all variables, the p values of particular tests were lower than 0.05 which proved that results did not come from a normally distributed population. The differences between metal concentration in the groups of studied patients (obese vs nonobese children) and body fluids (i.e., blood, plasma, urine), as well as correlation between variables (i.e., metals), were tested with the use of nonparametric tests (Kruskal–Wallis' and Mann–Whitney's U) and Spearman's correlation coefficient. The use of multidimensional techniques was inappropriate due to relatively small sample size.
Results
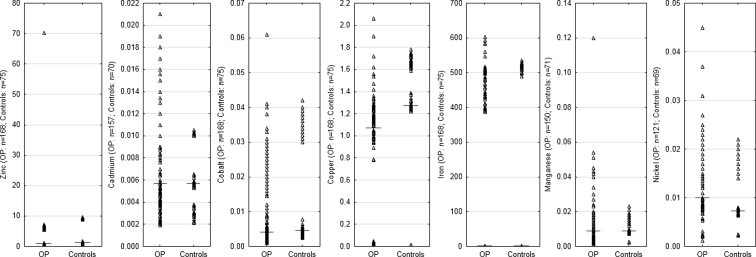
Figure 1 presents scatter plot of body fluids metal concentrations for control (healthy children) and studied group (obese patients). Due to the abnormal distribution of analytical results, Spearman's correlation coefficient was used for the evaluation of metal content according to groups of the patients and the type of body fluid. The correlation coefficient was always tested on three levels of significance: p = 0.001, p = 0.01, and p = 0.05. To avoid publicizing digits much lower than 0.001, we decided to introduce in the entire manuscript (omitting Table 2) the following notation: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05. The data indicated that significant values of correlation coefficient appeared in the control group in plasma (Mn–Cd, −0.47*; Ni–Co, 0.48* ), urine (Cu–Co, −0.49*), and blood (Fe–Cu, −0.74***) as well as in obese group in plasma (Cd–Mn, 0.42**; Ni–Cu, −0.69*; Ni–Zn, −0.91***) and urine (Fe–Cd, 0.30*; Co–Mn, −0.34*). None of statistically significant correlations was found in the blood of obese patients.
Fig. 1.
Scatter plot of body fluids metal concentrations [in milligram per liter] for control and obese patients (OP) subjects. Bars indicate medians
Table 2.
Statistical assessment of the differences of median concentration of metals according to the type of patient and the type of body fluid by the use of Mann-Whitney's U test
| Body Fluid |
Metal | Sex | Median Obesity (mg/L) |
Median Control group (mg/L) |
U | p value | No. Obesity |
No. Control group |
|---|---|---|---|---|---|---|---|---|
| Blood | Cd | All | 0.004 | 0.003 | 472.5 | 0.020122 | 56 | 25 |
| M | 0.004 | 0.003 | 83.5 | 0.080357 | 27 | 10 | ||
| F | 0.004 | 0.003 | 154.0 | 0.118305 | 29 | 15 | ||
| Co | All | 0.022 | 0.035 | 153.0 | <0.0000001 | 56 | 25 | |
| M | 0.021 | 0.037 | 43.0 | 0.001726 | 27 | 10 | ||
| F | 0.024 | 0.033 | 39.0 | 0.00001 | 29 | 15 | ||
| Cu | All | 1.180 | 1.680 | 50.0 | <0.0000001 | 56 | 25 | |
| M | 1.130 | 1.660 | 10.0 | 0.00002 | 27 | 10 | ||
| F | 1.190 | 1.700 | 15.0 | 0.000001 | 29 | 15 | ||
| Fe | All | 478.945 | 513.900 | 258.0 | 0.000006 | 56 | 25 | |
| M | 478.900 | 517.200 | 34.0 | 0.000588 | 27 | 10 | ||
| F | 478.990 | 510.650 | 101.5 | 0.004238 | 29 | 15 | ||
| Mn | All | 0.016 | 0.016 | 620.0 | 0.414604 | 56 | 25 | |
| M | 0.016 | 0.017 | 115.0 | 0.503324 | 27 | 10 | ||
| F | 0.015 | 0.015 | 148.0 | 0.086257 | 29 | 15 | ||
| Zn | All | 6.375 | 9.250 | 25.0 | <0.0000001 | 56 | 25 | |
| M | 6.360 | 9.190 | 0.0 | 0.000004 | 27 | 10 | ||
| F | 6.390 | 9.340 | 15.0 | 0.000001 | 29 | 15 | ||
| Ni | All | 0.009 | 0.007 | 224.0 | 0.000001 | 56 | 25 | |
| M | 0.008 | 0.007 | 64.0 | 0.015603 | 27 | 10 | ||
| F | 0.009 | 0.007 | 38.5 | 0.000009 | 29 | 15 | ||
| Plasma | Cd | All | 0.006 | 0.006 | 649.5 | 0.608517 | 56 | 25 |
| M | 0.006 | 0.006 | 90.0 | 0.127365 | 27 | 10 | ||
| F | 0.006 | 0.006 | 188.5 | 0.479450 | 29 | 15 | ||
| Co | All | 0.002 | 0.003 | 282.0 | 0.000019 | 56 | 25 | |
| M | 0.002 | 0.003 | 63.5 | 0.014984 | 27 | 10 | ||
| F | 0.002 | 0.003 | 73.0 | 0.000356 | 29 | 15 | ||
| Cu | All | 1.120 | 1.270 | 207.0 | <0.0000001 | 56 | 25 | |
| M | 1.140 | 1.270 | 43.0 | 0.001722 | 27 | 10 | ||
| F | 1.110 | 1.270 | 58.5 | 0.000086 | 29 | 15 | ||
| Fe | All | 1.115 | 1.460 | 311.0 | 0.00007 | 56 | 25 | |
| M | 1.090 | 1.460 | 92.0 | 0.145329 | 27 | 10 | ||
| F | 1.120 | 1.465 | 47.5 | 0.000027 | 29 | 15 | ||
| Mn | All | 0.005 | 0.009 | 525.5 | 0.075073 | 56 | 25 | |
| M | 0.004 | 0.009 | 98.5 | 0.217849 | 27 | 10 | ||
| F | 0.005 | 0.008 | 163.0 | 0.180821 | 29 | 15 | ||
| Zn | All | 0.930 | 1.340 | 0.0 | <0.0000001 | 56 | 25 | |
| M | 0.94 | 1.340 | 0.0 | 0.000004 | 27 | 10 | ||
| F | 0.870 | 1.320 | 0.0 | <0.0000001 | 29 | 15 | ||
| Ni | All | 0.002 | 0.002 | 56.5 | 0.151539 | 9 | 19 | |
| M | 0.002 | 0.002 | 8.0 | 0.133942 | 5 | 7 | ||
| F | 0.002 | 0.002 | 23.0 | 0.950396 | 4 | 12 | ||
| Urine | Cd | All | 0.013 | 0.010 | 178.0 | 0.000109 | 45 | 20 |
| M | 0.013 | 0.010 | 32.0 | 0.015341 | 20 | 8 | ||
| F | 0.012 | 0.010 | 59.0 | 0.003212 | 25 | 12 | ||
| Co | All | 0.004 | 0.005 | 540.0 | 0.101793 | 56 | 25 | |
| M | 0.004 | 0.005 | 124.5 | 0.731019 | 27 | 10 | ||
| F | 0.003 | 0.004 | 122.0 | 0.018360 | 29 | 15 | ||
| Cu | All | 0.015 | 0.020 | 526.5 | 0.075004 | 29 | 15 | |
| M | 0.020 | 0.020 | 102.0 | 0.262195 | 27 | 10 | ||
| F | 0.010 | 0.020 | 156.5 | 0.131537 | 29 | 15 | ||
| Fe | All | 0.120 | 0.150 | 357.0 | 0.000418 | 56 | 25 | |
| M | 0.120 | 0.150 | 84.5 | 0.085286 | 27 | 10 | ||
| F | 0.120 | 0.150 | 82.5 | 0.000779 | 29 | 15 | ||
| Mn | All | 0.005 | 0.003 | 34.0 | <0.0000001 | 38 | 21 | |
| M | 0.005 | 0.003 | 1.5 | 0.000120 | 22 | 7 | ||
| F | 0.006 | 0.002 | 19.0 | 0.000093 | 16 | 14 | ||
| Zn | All | 0.430 | 0.660 | 111.0 | <0.0000001 | 56 | 25 | |
| M | 0.450 | 0.580 | 9.5 | 0.000019 | 27 | 10 | ||
| F | 0.420 | 0.715 | 45.5 | 0.000021 | 29 | 15 | ||
| Ni | All | 0.016 | 0.017 | 644.0 | 0.568820 | 56 | 25 | |
| M | 0.015 | 0.016 | 90.0 | 0.127002 | 27 | 10 | ||
| F | 0.019 | 0.019 | 45.5 | 0.369336 | 29 | 15 |
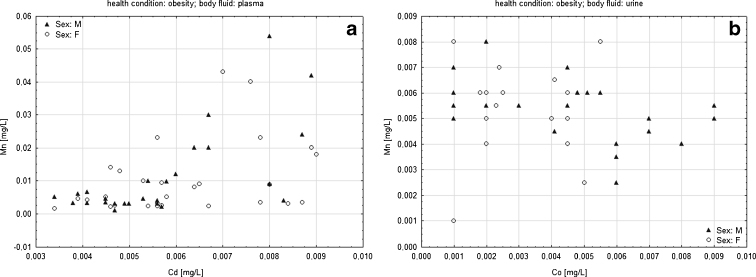
To analyze the possible influence of sex on correlation between variables in two groups of patients and body fluids, suitable categorized scatter plots were created. However, the aim was to present general trends in the graphic form and this is why only selected scatter plots are presented in Fig. 2.
Fig. 2.
Categorized scatter plots of a Mn vs. Cd (plasma) and b Mn vs. Co (urine) according to sex in obese patients
Figure 2 reveals that males and females are mixed which suggests that sex criterion does not influence the correlation between metal content in plasma, and in urine separately in obese patients. Similar mixed layout was observed in case of all scatter plots; however, categorized scatter plots of Mn vs Cd (plasma) and Mn vs Co (urine) were selected as an example. More detailed correlation analysis was performed in the next stage. The aim of it was to explore the character of correlation between metals' content in all possible combinations of the types of body fluids in both studied groups. To maximize the clarity in Table 1, only statistically significant coefficients were included.
Table 1.
Spearman's correlation coefficient matrix of metals occurring in all possible combinations of the types of body fluids in the groups of studied patients (values presented above and below diagonal of the matrix present correlation coefficients for control group and obese patients, respectively)
| Control group (n = 25) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood | Plasma | Urine | |||||||||||||||||||||
| Cd | Co | Cu | Fe | Mn | Zn | Ni | Cd | Co | Cu | Fe | Mn | Zn | Ni | Cd | Co | Cu | Fe | Mn | Zn | Ni | |||
| Obesity (n = 56) | Blood | Cd | 1.00 |
−0.40 (*) |
|||||||||||||||||||
| Co | 1.00 |
0.41 (*) |
0.40 (*) |
||||||||||||||||||||
| Cu | 1.00 |
−0.74 (***) |
−0.62 (**) |
||||||||||||||||||||
| Fe | 1.00 | ||||||||||||||||||||||
| Mn | 1.00 |
−0.42 (*) |
|||||||||||||||||||||
| Zn | 1.00 |
−0.46 (*) |
|||||||||||||||||||||
| Ni | 1.00 | ||||||||||||||||||||||
| Plasma | Cd | 1.00 |
−0.47 (*) |
0.48 (*) |
|||||||||||||||||||
| Co |
0.29 (*) |
1.00 |
0.47 (*) |
||||||||||||||||||||
| Cu | 1.00 | ||||||||||||||||||||||
| Fe |
−0.32 (*) |
1.00 | |||||||||||||||||||||
| Mn |
0.42 (**) |
1.00 |
−0.56 (**) |
||||||||||||||||||||
| Zn |
−0.45 (***) |
1.00 | |||||||||||||||||||||
| Ni |
0.75 (*) |
−0.69 (*) |
−0.91 (***) |
1.00 | |||||||||||||||||||
| Urine | Cd |
−0.29 (*) |
1.00 | ||||||||||||||||||||
| Co |
0.28 (*) |
1.00 |
−0.49 (*) |
||||||||||||||||||||
| Cu |
0.30 (*) |
0.38 (**) |
1.00 | ||||||||||||||||||||
| Fe |
0.30 (*) |
1.00 | |||||||||||||||||||||
| Mn |
−0.34 (*) |
−0.34 (*) |
1.00 | ||||||||||||||||||||
| Zn |
0.30 (*) |
1.00 | |||||||||||||||||||||
| Ni |
0.27 (*) |
−0.29 (*) |
1.00 | ||||||||||||||||||||
***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05
Besides the correlations mentioned previously for corresponding types of body fluids, several statistically significant correlations were found in both groups in non-corresponding combinations of body fluids. In the group of obese patients, directly proportional correlations were found between Ni (blood) and Co (plasma), Ni (blood) and Ni (plasma), Cu (blood) and Cu (urine), Co (plasma) and Cu (urine), Mn (plasma) and Co (urine), Fe (plasma) and Zn (urine), and Mn (plasma) and Ni (urine), while inversely proportional between Co (blood) and Fe (plasma), Ni (blood) and Zn (plasma), Zn (blood) and Cd (urine), and Zn (plasma) and Mn (urine). Several directly and inversely proportional correlations were also found for metals' content in non-corresponding types of body fluids in the control group.
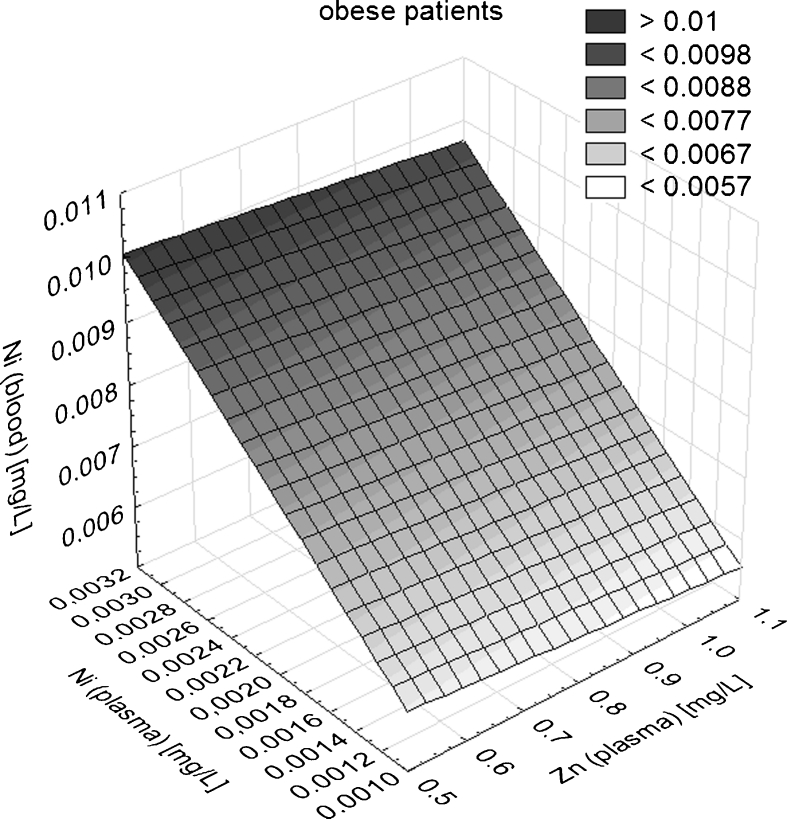
Figure 3 presents mutual dependence of Ni and Zn content in the body fluids (see Table 1 for the most significant correlations) found in obese patients. At the next stage, the differences between median values of analyzed metals according to various criteria were tested. The possible role of sex as a discriminating factor was checked again. The median content of heavy metals in both sexes in the control group and obese patients (with and without taking into consideration the type of body fluid) was tested by the use of Mann–Whitney U test on p = 0.05. Statistically significant differences between the content of metals in both sexes were not found (p > 0.05) in neither the obese nor the control group. That means that sex does not play any important role as a discriminating factor when the type of body fluid is not taken into consideration. On the other hand, when the type of body fluid was taken into consideration, uncommon statistically significant differences between content of metals according to sex were found. In obese patients, the median concentration of Zn in plasma of boys was higher (0.94 mg/L) than girls (0.87 mg/L). In the control group, the median concentration of Co and Mn in blood as well as Mn in plasma was higher for boys (0.037, 0.017, and 0.009 mg/L) than for girls (0.033, 0.015, and 0.008 mg/mL). On the contrary, Zn median concentration in blood and urine was higher for girls (9.34 and 0.71 mg/L, respectively) than for boys (9.19 and 0.58 mg/L, respectively). This is why in the consecutive testing both discriminating criteria (the type of patient and the type of body fluid) were taken into consideration (Table 2).
Fig. 3.
Example of mutual dependence of Ni and Zn concentrations in non-corresponding body fluids of obese patients
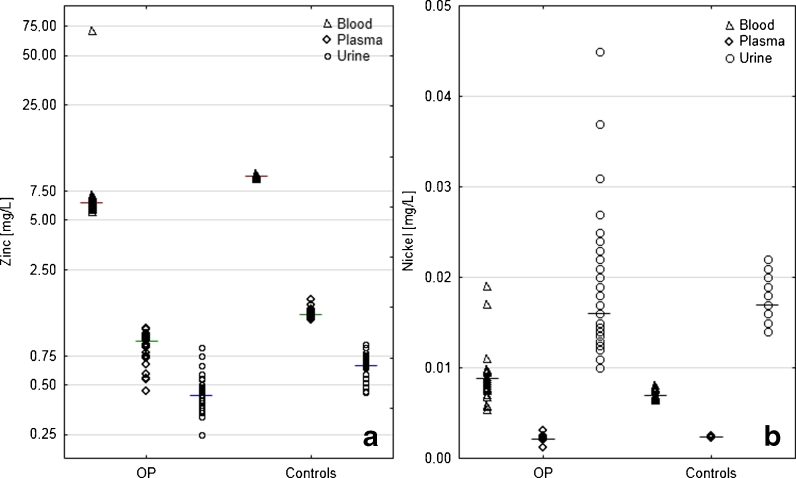
In the majority of cases, there was a difference found between median concentration of the given metal in various types of body fluids (blood vs plasma vs urine) in obese and healthy groups (Table 2). To identify the pairs of body fluids with statistical difference in the metal content, a multiple comparison Kruskal–Wallis' test was applied (p = 0.05). In the majority of cases, the difference in the median concentration was found in all combinations, i.e., blood vs plasma, plasma vs urine, and blood vs urine. No differences were found for median concentration of Cu in plasma and blood and Mn in urine and plasma of obese patients.
Figure 4 presents the example of differences between the content of specific metals (Zn and Ni) in particular matrices for both groups. To maintain the brevity, only Zn and Ni were chosen leaving out the correlations between other metals. This choice is justified by highly statistically significant correlation coefficient between Zn and Ni content in various body fluids (Table 2). Zinc is the element most often described in relation to obesity; however, it is difficult to find a simultaneous analysis of its content in plasma, blood, and urine. On the other hand, nickel is generally omitted in studies as a nonessential element therefore little is known about changes in its concentration in obesity, and especially in body fluids in obese children.
Fig. 4.
Differences between contents of Zn (a—logarithmic scale) and Ni (b) in three types of body fluids of obese and healthy children. Bars indicate medians
Correlation Between BMI and Content of Studied Metals
Only few statistically significant correlations (p ≤ 0.05) between BMI and content of metals in studied body fluids were discovered, and most of them were inversely proportional. Negative correlations between BMI and Zn in blood (r = −0.66), Cu in plasma (r = −0.87), and Fe in urine (r = −0.78) were found for boys in the control group. Only one negative correlation was found (BMI vs Co in plasma: r = −0.59) for girls in the control group. However, positive correlation between Co content in plasma and BMI (r = 0.40) was found for the obese boys.
Discussion
The consequences of changes in trace elements (mainly deficiency) in children are much more important for the health of the public than is generally realized. Zinc, in particular, has been the element of greatest interest to many researchers. It takes part in the metabolism of hormones involved in the pathophysiology of obesity. Zinc plays a major role in the stabilization of insulin hexamers and the storage of hormone in the pancreas [21]. It is also an efficient antioxidant [22]. Zinc is of critical importance in certain metabolic pathways, acting as a cofactor for numerous enzymes in the metabolism of carbohydrates, proteins, and lipids. Zinc also plays a catalytic and structural role in tissue formation and hormone receptor activation [23]. Few studies about the role of Zn in obesity were published [12, 24]. Some demonstrated a reduction in the concentration of zinc in plasma in obesity. According to literature [25], zinc concentrations in plasma and erythrocytes were significantly lower in the obese group. Urinary zinc excretion was significantly higher in the same group. The results seem to indicate that zinc nutritional status in obese individuals is altered. Low zinc content was reported in lymphomonocytes in obese children [26] and in the serum and hair in obese adults [27]. Low serum zinc levels in obese patients have been correlated with an increased urine excretion, suggesting that low levels in plasma are indicative of the metabolic complications in obesity [28]. Our study confirms the significantly lower value of Zn content in obese children than in the blood of healthy children. The decrease was observed both in plasma and in urine.
Recent interest in determining copper content has been aroused by the observation of metabolic anomalies present in a number of diseases (such as obesity) and the interest in the role that deficiency/excess of minerals may play in these disease processes [29]. Cu is a component of antioxidant enzymes that act to protect the body against the action of free radicals, especially in cardiovascular diseases. An imbalance in the metabolism of Cu might trigger hypercholesterolemia and disorders in oxidative stress [30]. Increased Cu–Zn superoxide dismutase activity has been verified in obese children [31] as well as total circulating Cu [32]. Remarkable observations have been made on Cu disorders associated with lipoprotein oxidation. Early detection of these Cu disorders could play an important role in determining treatment and prevention strategies. Erythrocytes are the preferred biomarker of Cu since these cells are more independent of inflammatory and hormonal processes. There is some evidence suggesting that erythrocytes are more stable in their Cu content, unlike plasma which may be influenced by diet and circadian rhythms [33]. In the work of Lima et al. [34], the copper concentrations in plasma in the overweight and obese male groups were significantly higher than those in the control group. According to Hatano et al. [35], Cu concentration in erythrocytes was lower than in plasma. The results found by the authors suggest that excess weight associated with lipid metabolism disorders might predispose to changes in Cu concentrations in plasma in obesity, which is more evident in males, indicating a possible mechanism of this mineral, contributing to peroxidation or acting as an antioxidant. In the present study, the median Cu concentration in the blood in obese children was lower, but in plasma slightly higher than in the control group. In urine, there were no significant differences between the two groups. Iron has received a great deal of attention and has been found to be low in obese patients. However, the etiology of the hypoferremia in obesity has remained uncertain [36]. According to the studies of Pinhas-Hamiel et al. [37], overweight and obese children demonstrated an increased prevalence of iron deficiency. However, the authors pointed at the use of only serum samples as the limitation of their study because it did not include the information about possible factors, which could decrease iron content in serum (i.e., infections or inflammations). In the present study, the median concentration of Fe in blood in obese children was lower than in controls. The same decrease was observed in plasma in comparison with the control group and in urine.
Little is known about the influence of cobalt concentration on obesity in general. In the study of Tascilar et al. [38], Co content in serum was determined in 34 obese children (in obese it was significantly lower than in controls). Cited studies suggest that Co may have an effect on human glucose metabolism. In the present study, Co concentration in blood of obese children was lower than in controls. The same decrease was observed in plasma in comparison with the control group. In urine, the difference was not significant.
Unfortunately, we were not able to find any research on the determination of Ni in obese children, except the study by Tascilar et al. [38]. The cited authors determined Ni (in microgram per liter) in serum of obese children (0.78 ± 0.68) and in control group (1.17 ± 0.65, p = 0.051). It seems that Ni was not studied in matrices other than serum. Studies on animals suggest that nickel may also alter glucose homeostasis by affecting insulin secretion [39, 40]. Thus, more studies are needed on the effect of Ni on obesity in children. Studies on manganese in obesity are equally scarce. Animal studies confirmed that the deficiency of Mn incurred worsened glucose tolerance and serum HDL degradation [41].
Although the interactions between essential and nonessential trace elements have been studied for many decades, the effect of these interactions on obesity is not often studied in practice [42]. Toxic metals may contribute to obesity by influencing various aspects of metabolism, such as by substituting for essential elements or by inducing oxidative stress [43]. An excess of one element may decrease the bioavailability of another. On the other hand, deficiency in one trace element may impair the absorption of another, e.g., copper deficiency impairs iron absorption, intermediate to high levels of manganese interfere with iron metabolism, excess copper decreases zinc absorption, and cadmium interacts with iron to decrease its bioavailability. There is also evidence for a direct cobalt–iron antagonism; cobalt is more efficiently absorbed in iron deficiency. It has been reported that high dietary calcium intake reduces zinc absorption and balance in humans [44]; on the other hand low-calcium intake predicts excess body weight and fat in children and adolescents [45].
The outcome of an interaction may be a modification of toxic effects or, in the case of essential elements, a deficiency, or an excess [46]. In order to better judge the susceptibility to trace element toxicity and to determine deficiency and excess, a better understanding of basic interactions is needed [47]. The complex interactions between zinc, copper, or cadmium can be partly explained by their interactions with metallothioneins which may cause tissue deposition of certain metals and play an important role in the uptake of toxic metals [48]. The problem is more complicated because the trace element content in human body is influenced by many factors (e.g., age, sex, diseases, drugs, diet supplements, nutritional habits). If possible, each element should be studied in more than one biofluid. The present study confirms the inversely proportional correlation between Mn–Cd in plasma, Cu–Co in urine, and Fe–Cu in blood of healthy children, and Ni–Cu, Ni–Zn in plasma and Co–Mn in urine of obese children. Proportional correlations have been found in plasma between Ni and Co in healthy, and between Cd and Mn in obese children. Fe and Cd also correlated positively in urine in obese children. It was also found that sex criteria did not influence the correlations between metal content in plasma and urine in the group of obese patients (Fig. 2). When the type of body fluid was taken into consideration, rare statistically significant differences between metal content and sex were found (Table 2).
There were a surprisingly large number of correlations between elements in all three types of body fluids (Table 1). In the group of patients with obesity, directly proportional correlations were discovered between Ni (blood) and Co (plasma), Ni (blood) and Ni (plasma), Cu (blood) and Cu (urine), Co (plasma) and Cu (urine), Mn (plasma) and Co (urine), Fe (plasma) and Zn (urine), and Mn (plasma) and Ni (urine), while inversely proportional between Co (blood) and Fe (plasma), Ni (blood) and Zn (plasma), Zn (blood) and Cd (urine), and Zn (plasma) and Mn (urine). It seems that such correlations between so many elements and matrices have not been shown before. Their interpretation requires more studies, since some can be also age-dependent, e.g.,. like serum Zn and Cu [49].
As far as BMI is concerned, several reports have shown an association between serum zinc and copper levels and BMI [50]. Studies of Padilla et al. [43] suggest that the association of some heavy metals (like cadmium or cobalt) with BMI remains unclear. For example, according to the authors of the cited study, cadmium and cobalt were negatively associated with BMI. However, the amounts were assessed only in urine samples and such samples reflect only recent exposures. The results of this study revealed that BMI correlated negatively with Zn in blood, Cu in plasma, and Fe in urine in boys in the control group. Only one negative correlation was found (BMI vs Co in plasma) in girls in the control group. However, positive correlation between Co content in plasma and BMI was found in boys in the obese group. Our findings correlate with the study by Nead et al. [51], which showed that the prevalence of iron deficiency increases as BMI increases from normal weight to a risk for overweight and to overweight. It seems that this is the first study to examine the relationship between Cd, Co, Mn, or Ni concentrations in body fluids of children and BMI.
Conclusion
The study demonstrates that there are considerable differences between the content of some metals in body fluids of obese and nonobese children. These differences may be indicators of aberrations in physiology or pathology (like obesity). Our findings suggest that especially iron, zinc, copper, and cobalt levels should be monitored in children with elevated BMI in order to avoid deficiency problems. Increasing interest should be also directed towards interactions between different elements. A combination of blood analysis with that of plasma and urine can be more reliable than single-specimen analysis. In order to assess the levels of metals in relation to obesity, the best way (though not from an economic point of view) seems to be the correct analysis of all three types of biological fluids. The findings reflect various distributions of different metals in blood plasma and the whole blood of obese and nonobese children. The reliance exclusively on the analysis of the urine does not give complete information about the patient's condition. Both HPIC and ICP MS methods enable sensitive and accurate evaluation of the concentrations of metals on nanogram per liter or lower levels.
Acknowledgments
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23:2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 2.Burdkiewicz A, Janusz A. O kryteriach nadwagi i otyłości u dzieci i młodzieży. In: Jopkiewicz A, editor. Auksologia a promocja zdrowia. Kielce, Poland: PAN; 2000. pp. 207–220. [Google Scholar]
- 3.World Health Organization (WHO) Media Centre; Fact sheet N°311. Obesity and overweight. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed Mar 2013
- 4.Deckelbaum RJ, Williams CL. Childhood obesity: the health issue. Obes Res. 2001;9:239–243. doi: 10.1038/oby.2001.125. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes. 2012;36:1–11. doi: 10.1038/ijo.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum L, Hiu Shuen Chan I, Kwok-Kuen Cheung S, Goggins WB, Mok V, Lam L, Leung V, Hui E, Ng C, Woo J, Fung Kum Chiu H, Chung-Ying Zee B, Cheng W, Chan M-H, Szeto S, Lui V, Tsoh J, Bush AI, Wai Kei Lam C, Kwok T. Serum zinc is decreased in Alzheimer's disease and serum arsenic correlates positively with cognitive ability. Biometals. 2010;23:173–179. doi: 10.1007/s10534-009-9277-5. [DOI] [PubMed] [Google Scholar]
- 7.Zaichick S, Zaichick V. The effect of age and gender on 37 chemical element contents in scalp hair of healthy humans. Biol Trace Elem Res. 2009;134(1):41–54. doi: 10.1007/s12011-009-8456-0. [DOI] [PubMed] [Google Scholar]
- 8.Iyengar GV. Concentrations of 15 trace elements in some selected adult human tissues and body fluids of clinical interest from several countries: result from a pilot study for the establishment of reference values. Center, Juelich: Juelich Nuclear Res; 1985. [Google Scholar]
- 9.Błażewicz A, Dolliver W, Sivsammye S, Deol A, Randhawa R, Orlicz-Szczęsna G, Błażewicz R. Determination of cadmium, cobalt, copper, iron, manganese and zinc in thyroid glands of patients with diagnosed nodular goitre using ion chromatography. J Chromatogr B. 2010;878:34–38. doi: 10.1016/j.jchromb.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Błażewicz A, Orlicz-Szczęsna G, Prystupa A, Szczęsny P. Use of ion chromatography for the determination of selected metals in blood serum of patients with type 2 diabetes. J Trace Elem Med Biol. 2010;24:14–19. doi: 10.1016/j.jtemb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Bourgue SL, Komolova M, McCabe K, Adams MA, Nakatsu K. Perinatal iron deficiency combined with a high-fat diet causes obesity and cardiovascular dysregualtion. Endocrinology. 2012;153(3):1174–1182. doi: 10.1210/en.2011-1700. [DOI] [PubMed] [Google Scholar]
- 12.Bougle DL, Bureau F, Laroche D. Trace elements status in obese children: relationship with metabolic risk factors. e-SPEN, the European-Journal of Clinical. Nutr Metab. 2009;4:98–100. [Google Scholar]
- 13.Di Toro A, Marotta A, Todisco N, Ponticiello E, Collini R, Di Lascio R, et al. Unchanged iron and copper and increased zinc in the blood of obese children after two hypocaloric diets. Biol Trace Elem Res. 1997;57(2):97–104. doi: 10.1007/BF02778192. [DOI] [PubMed] [Google Scholar]
- 14.Hasbahceci M, Cipe G, Kadioglu H, Aysan E, Muslumanoglu M. Reverse relationship between blood boron level and body mass index in humans: does it matter for obesity? Biol Trace Elem Res. 2013;153:141–144. doi: 10.1007/s12011-013-9680-1. [DOI] [PubMed] [Google Scholar]
- 15.Fox MRS, Tao SH, Stone CL, Fry BEF. Effects of zinc, iron and copper deficiencies on cadmium in tissues of Japanese quail. Environ Health Perspect. 1984;54:57–65. doi: 10.1289/ehp.845457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashby SL, King LJ, Parke DVW. Effect of acute administration of cadmium on the disposition of copper, zinc, and iron in the rat. Environ Res. 1980;21:177–185. doi: 10.1016/0013-9351(80)90019-5. [DOI] [PubMed] [Google Scholar]
- 17.Chmielnicka J, Halatek T, Jedlinska U. Correlation of cadmium-induced nephropathy and the metabolism of endogenous copper and zinc in rats. Ecotoxicol Environ Sat. 1989;18:268–276. doi: 10.1016/0147-6513(89)90020-1. [DOI] [PubMed] [Google Scholar]
- 18.Bremner I, Campbell JK. Effect of copper and zinc status on susceptibility to cadmium intoxication. Environ Health Perspect. 1978;25:125–128. doi: 10.1289/ehp.7825125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdulla M, Chmielnicka J. New aspects on the distribution and metabolism of essential trace elements after dietary exposure to toxic metals. J. Biol Trace Elem Res. 1990;23:25–53. doi: 10.1007/BF02917176. [DOI] [PubMed] [Google Scholar]
- 20.Błażewicz A, Baj T, Świeboda R, Świątek Ł. Determination of heavy metals in aerial part of hyssop (Hyssopus officinalis L.) using high performance ion chromatography with the aid of a linear and non-linear weighted least-squares regression model. Pol J Environ Stud. 2007;16:191–198. [Google Scholar]
- 21.Wijesekara N, Chimienti F, Wheeler MB. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab. 2009;11(Suppl 4):202–214. doi: 10.1111/j.1463-1326.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 22.Prasad AS. Clinical, immunological, anti-inflammatory andantioxidant roles of zinc. Exp Gerontol. 2008;43:370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Vallee BL, Falchuk H. The biochemical basis of zinc physiology. Physiol Rev. 1993;73(1):79–117. doi: 10.2466/pr0.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Marreiro DN, Geloneze B, Tambascia MA, Lerário AC, Halpern A, Cozzolino SM. Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol Trace Elem Res. 2006;112:109–118. doi: 10.1385/BTER:112:2:109. [DOI] [PubMed] [Google Scholar]
- 25.Do Nascimento Marreiro D, Fisberg M, Franciscato Cozzolino SM. Zinc nutritional status in obese children and adolescents. Biol Trace Elem Res. 2002;86:107–122. doi: 10.1385/BTER:86:2:107. [DOI] [PubMed] [Google Scholar]
- 26.Marotta A, Todisco N, Di Toro A, Toraldo R, Ponte G, Perrone L. Zinc content of lymphocytes in obese children. Nutr Res. 1995;15:1411–1415. doi: 10.1016/0271-5317(95)02013-L. [DOI] [Google Scholar]
- 27.Chen MD, Lin PY, Lin WH, Cheng V. Zinc in hair and serum of obese individuals in Taiwan. Am J Clin Nutr. 1988;48:1307–1309. doi: 10.1093/ajcn/48.5.1307. [DOI] [PubMed] [Google Scholar]
- 28.Konukoglu D, Turhan MS, Ercan M, Serin O. Relationship between plasma leptin and zinc levels and the effect of insulin and oxidative stress on leptin levels in obese diabetic patients. J Nutr Biochem. 2004;15(12):757–760. doi: 10.1016/j.jnutbio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Uauy R, Olivares M, Gonzalez M. Essentiality of Cu in humans. Am J Clin Nutr. 1998;67(Suppl 5):952–959. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 30.Klevay LM. Cardiovascular disease from copper deficiency-a history. J Nutr. 2000;130(Suppl 25):489–492. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 31.Enderve O, Siklar Z, Kocaturk PA, Dallar Y, Kavas GO. Antioxidant superoxide dismutase activity in obese children. Biol Trace Elem Res. 2004;98(3):219–228. doi: 10.1385/BTER:98:3:219. [DOI] [PubMed] [Google Scholar]
- 32.Yakinci C, Paç A, Kuçukbay FZ, Tayfun M, Gui A. Serum zinc, copper and magnesium levels in obese children. Acta Paediatr Jpn. 1997;39(3):339–341. doi: 10.1111/j.1442-200X.1997.tb03748.x. [DOI] [PubMed] [Google Scholar]
- 33.Vitoux D, Arnaud J, Chappuis P. Are cooper, zinc, selenium in erythrocytes valuable biological indexes of nutrition and pathology? J Trace Elem Med Biol. 1999;13:113–128. doi: 10.1016/S0946-672X(99)80001-7. [DOI] [PubMed] [Google Scholar]
- 34.Lima SC, Arrais RF, Sales CH, Almeida MG, de Sena KC, Oliveira VT, et al. Assessment of copper and lipid profile in obese children and adolescents. Biol Trace Elem Res. 2006;114:19–29. doi: 10.1385/BTER:114:1:19. [DOI] [PubMed] [Google Scholar]
- 35.Hatano S, Nishi Y, Usui T. Copper levels in plasma and erythrocytes in healthy Japanese children and adults. Am J Clin Nutr. 1982;35(1):120–126. doi: 10.1093/ajcn/35.1.120. [DOI] [PubMed] [Google Scholar]
- 36.Menzie CM, Yanoff LB, Denkinger BI, McHugh T, Nancy G, Sebring NG, et al. Obesity-related hypoferremia is not explained by differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption. J Am Diet Assoc. 2008;108:145–148. doi: 10.1016/j.jada.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes (Lond) 2003;27(3):416–418. doi: 10.1038/sj.ijo.0802224. [DOI] [PubMed] [Google Scholar]
- 38.Tascilar ME, Ozgen IT, Abaci A, Serdar M, Aykut O. Trace elements in obese Turkish children. Biol Trace Elem Res. 2011;143:188–195. doi: 10.1007/s12011-010-8878-8. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Ahmad N, Husain MM, Srivastava RC. Involvement of nitric oxide in nickel-induced hyperglycemia in rats. Nitric Oxide. 2000;4:129–138. doi: 10.1006/niox.2000.0278. [DOI] [PubMed] [Google Scholar]
- 40.Bwititi PT, Ashorobi RB. Effects of chronic oral nickel chloride administration on glycaemia and renal function in normal and diabetic rats. Afr J Health Sci. 1998;5:198–201. [PubMed] [Google Scholar]
- 41.Davis CD, Ney DM, Greger JL. Manganese, iron, and lipid interactions in rats. J Nutr. 1990;120:507–513. doi: 10.1093/jn/120.5.507. [DOI] [PubMed] [Google Scholar]
- 42.O'Dell BL, Sunde RA. Bioavailability of and interactions among trace elements. In: Chandra RK, editor. Trace elements in nutrition of children. New York: Marcel Dekker; 1997. [Google Scholar]
- 43.Padilla MA, Elobeid M, Ruden DM, Allison DB. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99–02. Int J Environ Res Public Health. 2010;7(9):3332–3347. doi: 10.3390/ijerph7093332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood RJ, Zheng JJ. High dietary calcium intakes reduce zinc absorption and balance in humans. Am J Clin Nutr. 1997;65(6):1803–1809. doi: 10.1093/ajcn/65.6.1803. [DOI] [PubMed] [Google Scholar]
- 45.Major GC, Chaput JP, Ledoux M, St-Pierre S, Anderson GH, Zemel MB, et al. Recent developments in calcium-related obesity research. Obes Rev. 2008;9:428–445. doi: 10.1111/j.1467-789X.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 46.Goyer RA. Toxic end essential metal-metal interactions. Ann Rev Nutr. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- 47.Bárány E, Bergdahl IA, Bratteby LE, Lundh T, Samuelson G, Schütz A. Relationships between trace element concentrations in human blood and serum. Toxicol Lett. 2002;134:177–184. doi: 10.1016/S0378-4274(02)00187-X. [DOI] [PubMed] [Google Scholar]
- 48.Christie N, Costa M. In vitro assessment of the toxicity of metal compounds. IV Disposition of metals in cells: interactions with membranes, glutathione, metallothionein, and DNA. Biol Trace Elem Res. 1984;6:139–158. doi: 10.1007/BF02916931. [DOI] [PubMed] [Google Scholar]
- 49.Rükgauer M, Klein J, Kruse-Jarres D. Reference values for the trace elements copper, manganese, selenium and zinc in the serum/plasma of children, adolescent and adults. J Trace Elements Med Biol. 1997;11:92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 50.Chachera R. Assessment of obesity in children. Nutr Res. 1993;13:95–109. doi: 10.1016/S0271-5317(05)80287-8. [DOI] [Google Scholar]
- 51.Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114(1):104–108. doi: 10.1542/peds.114.1.104. [DOI] [PubMed] [Google Scholar]