Significance
The potential impacts of climate change on human health are possibly large and not yet well understood, especially for vector-borne diseases. This study provides projections of how climate change may affect the population of a West Nile virus mosquito vector across the southern United States. Using a climate-driven mosquito population model, we simulate vector abundance under base and future climate. Under future climate, many locations exhibit a lengthening of the mosquito season with a decrease in summer populations. These impacts are not uniform geographically and vary with local temperature and precipitation conditions. The results imply that disease-transmission studies and vector-control programs must be targeted locally to maximize their effectiveness.
Keywords: disease, insect, ecology
Abstract
Climate change will affect the abundance and seasonality of West Nile virus (WNV) vectors, altering the risk of virus transmission to humans. Using downscaled general circulation model output, we calculate a WNV vector's response to climate change across the southern United States using process-based modeling. In the eastern United States, Culex quinquefasciatus response to projected climate change displays a latitudinal and elevational gradient. Projected summer population depressions as a result of increased immature mortality and habitat drying are most severe in the south and almost absent further north; extended spring and fall survival is ubiquitous. Much of California also exhibits a bimodal pattern. Projected onset of mosquito season is delayed in the southwestern United States because of extremely dry and hot spring and summers; however, increased temperature and late summer and fall rains extend the mosquito season. These results are unique in being a broad-scale calculation of the projected impacts of climate change on a WNV vector. The results show that, despite projected widespread future warming, the future seasonal response of C. quinquefasciatus populations across the southern United States will not be homogeneous, and will depend on specific combinations of local and regional conditions.
Projections of disease-related climate change impacts are currently limited and constitute a key research priority (1). During its expansion across North America, West Nile virus (WNV) led to major epidemics during the summers of 2002–2004 (2) and is now endemic in most areas. Although host-bird species behavior and viral strain temperature tolerances are critical components of WNV dynamics (2), vector ecology is also a key element of the virus ecology. Although climate and meteorological factors can moderate mosquito vector population dynamics (3, 4) and infection rates (5), the projected impacts of climate change on WNV vectors are not yet known. From a human health perspective, a better understanding of this complex system will facilitate the implementation of more effective control measures and reduce virus transmission to human populations (6).
Studies relating climate to the incidence of other mosquito-borne diseases have been performed with varying success for malaria (7–9) and dengue fever (10, 11). The most effective way to study the complex feedbacks and impacts of climate change on vector populations is through dynamic modeling because it resolves some of the limitations associated with empirically based statistical techniques. These limitations include the lack of long-term and continuous mosquito surveillance data and the inappropriate extrapolation of projections from temperature and precipitation combinations outside the bounds of past measurements. Some of these issues, however, still manifest themselves in dynamic modeling because mosquito response to climate variables may change in time and between places as a result of evolutionary pressures. Limited test data from mosquito trapping and inaccuracies in climate change projections are limitations in both analysis techniques.
Few studies have quantified the connection between climate and WNV vectoring mosquito populations through process-based modeling (12). Hopp and Foley (11) explored the worldwide distribution of dengue fever through the container-inhabiting mosquito simulation model (CiMSIM) at a 1° grid cell resolution. CiMSIM is a dynamic life-table model driven with climate input to predict Aedes aegypti populations (13). Another process-based model, created by Ahumada et al. (14), simulates Culex quinquefasciatus mosquito populations in Hawaii or similar environments. Other region-based climate-driven models have been created to simulate vector dynamics by Gong et al. (15) for WNV-transmitting mosquito species in the Northeast United States and by Schaeffer et al. (16) for yellow fever in the Ivory Coast.
Recently, Morin and Comrie (17) created the Dynamic Mosquito Simulation Model (DyMSiM) to examine C. quinquefasciatus population dynamics across a wide range of environments and at different spatial scales. The model captured much of the seasonal and subseasonal vector dynamics in two contrasting environments: the humid climate of southern Florida and the arid climate of southeastern California. Although the model did not always replicate daily dynamics well, our focus in this study is larger scale (weekly–monthly) where the model performed better.
Because it feeds on avian and human hosts and it inhabits urban environments, this species is suspected to be a primary vector of WNV in many southern US states (18, 19). In this study, we use DyMSiM to discern the effects of climate change on C. quinquefasciatus populations across the southern United States, using a general circulation model (GCM) output run on guidelines established by the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report (AR4).
Because of a scientific consensus that climate change is occurring with associated human health consequences, public health research has focused on identifying and implementing effective mitigation and adaptive strategies (20–22). Challenges for public health responses to climate change include the need for location-specific assessment of risk (20, 21) and the lack of quantitative research (22). This study addresses both challenges by quantifying how projected climate change will affect the spatial and temporal dynamics of a WNV vector across a large spatial domain at a spatial resolution necessary for effective public health action.
Results and Discussion
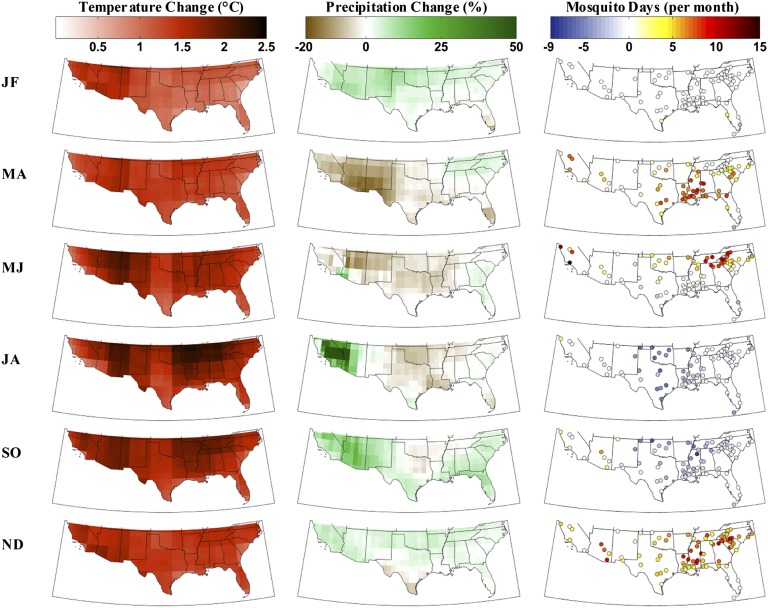
Our results show that projected changes in temperature, precipitation, and evaporation modify modeled water/habitat availability, life-cycle development, and the number of days with mosquitoes, summarized as mosquito days per month (MDM) (Fig. 1). During the cooler seasons there are widespread increases in MDM that follow latitudinal and elevational gradients. This pattern occurs despite drying conditions in many locations, indicating that temperature is the controlling climate variable. Anthropogenic responses to drying conditions like lawn watering, however, could replenish habitats. During late spring and summer, widespread decreases in MDM throughout much of the country, particularly Texas and the lower Midwest, suggest that precipitation limits mosquitoes. This finding contradicts the often-held assumption that projected warmer conditions always favor mosquitoes and clarifies some of the uncertainty in complex feedbacks involving climate and climate change influences on vectors and virus transmission, enabling more targeted public health action by using location-specific knowledge of vector responses to climate (23–25). Most notably, these results reveal that the relative importance of individual climate variables for mosquito population development and maintenance are strongly dependent on time and location.
Fig. 1.
Projected climate change (1970–1999 to 2021–2050) from the AR4 GCM ensemble and resulting changes in mosquito days per month (see text), averaged to 2-mo periods (JF, January–February; MA, March–April; MJ, May–June; JA, July–August; SO, September–October; ND, November–December). There is widespread warming throughout the study region, with the most intense warming occurring during the summer in the Southwest and lower Midwest. Precipitation changes are more variable in space and in time. The greatest drying occurs during spring and summer in the central United States. The drying extends to the Southwest during spring; however, precipitation is enhanced during the summer and fall. Precipitation changes in the eastern United States are relatively limited, with some drying in Florida and the southeast and small increases throughout the rest of the region. Higher spring and fall temperatures increase the number of days with mosquitoes across much of the United States, except in the Southwest during spring where severe drying inhibits population development. Decreased precipitation during summer and early fall decreases the number of days with mosquitoes across the central United States.
The analysis illustrates the sensitivity of mosquito populations to the complex effects of projected climate change at broad and local scales. For example, drying in high rainfall areas may have little or no effect on mosquito populations if precipitation is still sufficient to create and maintain immature mosquito habitat. Similarly, increased precipitation in an arid region may have no effect on mosquito populations if it is insufficient to support container-breeding mosquito populations, as exemplified in southeastern California during the summer. Several studies have even suggested that drought conditions can lead to increased vector populations (26, 27) and vector infection rates (5) caused by increased contact between vectors and avian hosts. Because climate change is temporally and spatially heterogeneous, the sensitivity of mosquito populations to climate change is dependent on local climatic context. The seasonal timing of precipitation (28, 29) and the intensity of events (30) are also important factors mitigating WNV vector population development and infection. Consequently, the impacts on mosquito populations can vary considerably between locations within a region.
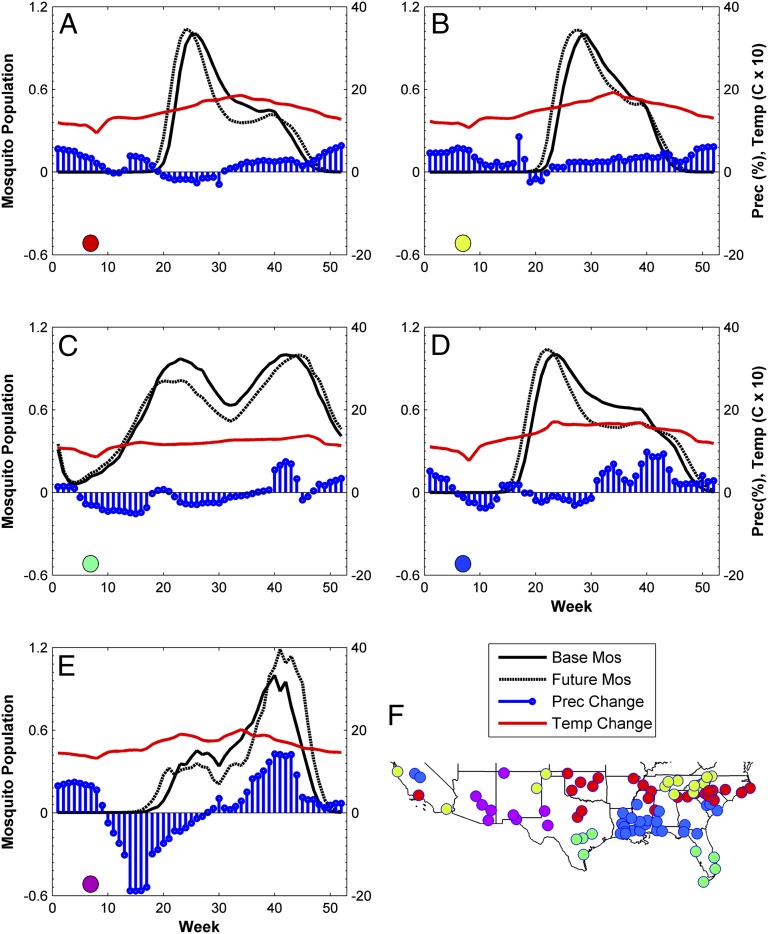
To generalize beyond local variation, temporal patterns of change across the study locations are regionalized for summary purposes into five geographic zones based on similar changes in their mosquito seasons (Fig. 2). Time series of climate variables and mosquito populations averaged by region reveal the factors responsible for population change (Fig. 2). Whereas most locations are clustered in their respective geographic zones, some are distant. Although two locations have different climates, similar changes in temperature and precipitation can produce a similar response in mosquito population dynamics. Additionally, California’s complex topography produces spatially heterogeneous climates, some of which may be similar (in a mosquito population-related sense) to climate regimes further east. It is also possible for areas experiencing different changes in climate to have similar changes in population dynamics. For example, increased spring rains in an arid environment and warmer spring temperatures in a moist environment could both create similar increases in spring mosquito populations. All locations experience an extended season, with mosquito populations rising earlier in the spring and lasting later into the fall as a result of elevated temperatures under projected climate change. Although rising temperatures could influence WNV ecology by extending the season during which mosquitoes are active, it could also influence vector competence. Warmer temperatures increase viral replication, which can shorten the extrinsic incubation period, increase virus infection and dissemination within the mosquito, and result in increased transmission rates (2, 31–33). Thus, warmer fall conditions may benefit the ecology of WNV by allowing the virus to circulate within the avian and mosquito populations for a greater period.
Fig. 2.
Regionally averaged time series of projected climate change and mosquito populations (relative to the base maximum population) under base and future climate scenarios (A–E), with a map of locations (F). Increasing temperatures extend the duration of mosquito season in all regions. Extreme heat combined with projected decreases in precipitation result in fewer mosquitoes during summer. This trend is most severe in southern locations and almost absent in the northern and eastern locations where more mild temperatures prevail and precipitation remains steady or increases. In the southwestern United States change in population strongly follows that of precipitation because this region is water-stressed and mosquitoes often must rely on seasonal rains for habitat.
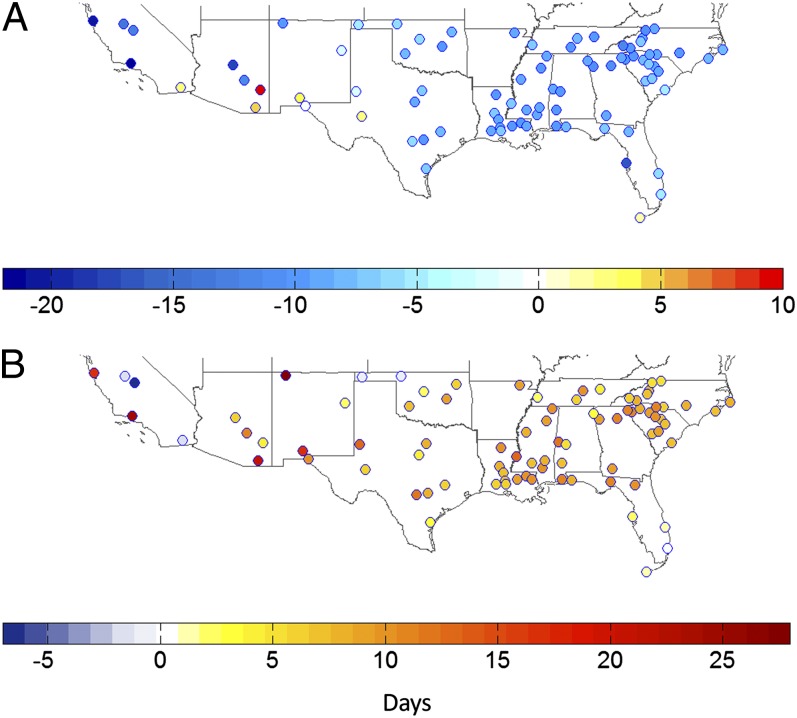
Changes in the start date (Fig. 3A) and end date (Fig. 3B) of the mosquito season between baseline (1970–1999) and future (2021–2050) climate conditions also suggest a lengthening of vector activity. Warmer temperatures during the cool season are likely responsible for the earlier and larger spring and early summer populations. Studies have shown that climate during the previous fall, winter, and spring are good predictors of current-year vector populations (3, 34). During an exceptionally warm California winter, vector population and reproductive activity increased compared with previous years (35). As stated previously, increased mosquito activity during cooler parts of the year extends the time of virus circulation in the avian and mosquito population. Warmer winter temperatures, for example, have been associated with WNV-related declines in crow populations (36).
Fig. 3.
Changes in start date (A) and end date (B) of the mosquito season (days earlier/later than base case). Most locations experience a 1- to 2-wk earlier start and 1- to 2-wk later end to the mosquito season because of increases in temperature, with a few notable exceptions. Some southern-tier western locations experience a later seasonal start date because of the dry conditions during spring and early summer, which are exacerbated under climate change. Southern Florida experiences little change because this area is capable of sustaining mosquito populations throughout the year. Drier summer and fall conditions trigger a premature end to the mosquito season in northwestern Oklahoma. Extremely dry and hot summer conditions in inland California cause an earlier end to the mosquito season, whereas the coastal locations experience a later decline because of a second population spike during the fall, when the rains return and temperatures are sufficient for mosquito development.
Increased temperatures and extended seasonality of mosquito activity could also signify an expansion in the range of WNV vectors. Elevated summer temperatures in British Columbia, Canada, for example, have been associated with the expansion of WNV in this area (37). Under projected climate change, modeled Culex pipiens habitat suitability has been shown to increase further north in Canada (38). Climate change could also facilitate the introduction or reintroduction of new vectors and diseases, especially in conjunction with globalization as exemplified with dengue fever (39). Although we discuss the relevance of changing vector dynamics for WNV transmission, the complicated ecology of WNV prevents projections of changes in infection risk based on vector populations alone.
During the summer, mosquito populations under projected future climate conditions often decline because of decreased precipitation and increased evaporation and immature mortality at high temperatures (Fig. 4). This decline may not decrease WNV transmission risk, however, as it has been suggested that birds and mosquitoes can be forced into greater contact by congregating around scarce water sources serving as mosquito habitat, thereby increasing transmission (28, 29). In addition, vector species that rely on more permanent water sources may be less affected by drying conditions and thereby gain a larger role as disease vectors. Even within a local area microclimate, seasonality and surrounding vegetation can influence mosquito species composition (40).
Fig. 4.
Number of summer days (June 1 to September 30) with future mosquito population less than base mosquito population. In most locations a summer decrease in mosquito population is projected because of increased immature mortality at high temperatures and decreased larval and pupal habitat because of enhanced evaporation. The central and Gulf states experience the longest summer population dip because of the greater length and intensity of projected summer warming along with considerable drying. Locations further north and at higher elevations (especially along the Appalachians) experience a shallow dip because damaging high temperatures are less frequent and eastern locations receive greater precipitation under future climate. In the western United States, extremely dry summers limit sustained mosquito populations under both base and future climate conditions.
Although these results provide an important new understanding of the potential effects of climate change on WNV vector ecology, they have some important limitations. DyMSiM has been evaluated successfully across a range of environments, but not for all of the study locations because suitable validation data are not available. Improved integration between model output and mosquito observations is required to advance validation and develop location-specific predictions of mosquito population dynamics. The model also does not account for species interactions or for the effects of human interventions, such as pesticide use, water storage, or large-scale irrigation. Additionally, GCM precipitation projections, especially for summer convective precipitation, have much greater uncertainty than those for temperature, although climate models are improving in accuracy (41, 42). This study also focuses predominantly on large-scale climate circulation patterns simulated by GCMs, and therefore these results represent projected changes over large regions and are not predictions of local mosquito populations, which are affected by a variety of local environmental variables. Differences in land use and microclimates, for example, can cause differences in dynamics even across small scales.
In this study, precipitation and temperature are found to work interdependently to influence future mosquito population dynamics. In the southcentral and southwestern United States, water scarcity is often the dominant limiting variable, causing mosquito populations to be sensitive to changes in precipitation and vulnerable to larval/pupal habitat drying at higher temperatures. The southeastern United States generally receives sufficient precipitation to support mosquito populations throughout the year, making temperature the dominant variable influencing population dynamics.
Overall, the seasonal response of C. quinquefasciatus population dynamics to projected climate change across the southern United States varies geographically; however, some general trends emerge. Projected mosquito-season length increases by several weeks at the start and end of the current season in many locations. Most areas are also projected to experience a decrease in mosquitoes during the summer. Changes are sometimes subtle and in contrast to intuitive assumptions. This finding is especially true in locations that are water-stressed or experience strongly seasonal precipitation.
These results suggest that climate change will modify seasonal mosquito population levels across the United States, with possible consequences for vector ecology and public health policy. These findings also explain statistical associations between climate variables, such as warmer temperatures and heavy precipitation, and WNV infection rates in human populations (26, 43). Location-explicit knowledge of the ecology of WNV is also critical for predictive and preventative purposes (44). Public health interventions must account for site-specific changes and characteristics to implement the most effective control strategies to prevent WNV transmission to humans (20, 21).
These results also highlight the need for future research on WNV ecology under climate change in the United States. Most of the locations in this study also harbor other important WNV vectors whose response to climate change may differ from that of C. quinquefasciatus. Furthermore, additional work is needed on the avian response to climate change and how it may impact the ecology of WNV from a human health perspective. Finally, the implications of changing land cover and land use need to be examined as they can make an area more or less resistant to drying and alter the magnitude of the mosquito population by allowing more water to collect. Urban infrastructure (27), agriculture and water sources (45), and wetlands (46) have all been reported to influence vector populations or WNV transmission. With a more complete understanding of WNV ecology and more knowledge about the response of the individual components to climate change, more effective measures can be used to limit viral transmission to humans.
Materials and Methods
Observed climate data for each location were obtained through the US Historical Climatology Network (USHCN) (http://cdiac.ornl.gov/epubs/ndp/ushcn/ushcn.html) at the National Climatic Data Center. Site-selection requirements included locations within the habitat range of C. quinquefasciatus (47), sufficient historic climate data at each site to train the weather generator, and spatial representation across the southern United States. We consider only C. quinquefasciatus within our study range, although genetic introgression with C. pipiens has been reported along the northern part of our study area (48, 49) and the possibility exists that these hybrids may have different temperature tolerances and diapause behavior that could affect their population dynamics. Climate records for the years 1970–1999 were selected if at least 98% complete with no more than 1 mo of data completely missing. Eighty-four locations met these requirements (Table S1). Daily maximum temperature, minimum temperature, and total precipitation were obtained for the years 1970 through 1999 at each location. These data were used to train the statistical weather generator.
GCM data were acquired through the IPCC Data Distribution Centre (www.mad.zmaw.de/IPCC_DDC/html/ddc_gcmdata.html). We obtained monthly averaged temperature and total monthly precipitation for the years 1970–1999 and 2021–2050 under the A2 climate change scenario in the IPCC AR4. We collected these data for 16 of the models used in the AR4 (Table S2).
Weather generators provide a useful and practical method for downscaling monthly gridded GCM output to daily point data. The Long Ashton Research Station–Weather Generator 4 (LARS-WG) is a stochastic weather generator developed by M. A. Semenov (50, 51) at Rothamsted Research in the United Kingdom (www.rothamsted.bbsrc.ac.uk/mas-models/larswg.php). LARS-WG can generate a daily time series of weather data of specified length for a chosen site. A random seed allows the user to generate a number of different time series that have the same statistical properties as the original observed data but that represent different weather scenarios characteristic of the location. The weather generator requires input of historic climate information from the selected location to calculate site analysis statistics. In this study we used the USHCN climate data (1970–1999) to train the model independently at each location.
To produce time series that incorporate the results of climate change scenarios, we calculated the GCM projected change in temperature and precipitation at the monthly level at each location. For each month, changes in temperature (absolute) and precipitation (proportion) for each location were calculated as the average difference between the modeled base values (1970–1999) and the projected future values (2021–2050). These calculations were performed for each GCM separately and then averaged to generate a GCM ensemble mean. By incorporating the GCM ensemble changes into the site analysis statistics in LARS-WG, local weather time series with adjusted statistics consistent with projected site-specific future climate conditions were generated. For each location we produced 50 base and 50 future yearly weather scenarios representing the range of atmospheric variability reported in the station data. This approach has been used widely for calculating agricultural climate change impacts using crop models (52, 53).
We chose to use the GCM-derived 1970–1999 conditions as our base climate scenario instead of the raw station data to keep the changes between present and future GCM runs comparable. Using the differences between the raw station data for the present and GCM data for the future is not appropriate because they would include not only differences because of modeled climate change but also validation differences, a known issue with GCM precipitation data. Furthermore, the station data represent information for a particular site and the GCM data represent a grid box.
DyMSiM is a discrete, deterministic model (17) that uses Euler’s method of integration. The model requires the input of daily average temperature (C°), precipitation (cm), amount of impermeable land cover per area (cm2), amount of permanent water per area (cm2), and latitude. The model includes options for container depth, irrigation, and infiltration rates of permeable surface cover, but these options were not used in this study. We focus only on climate factors, which include temperature and precipitation, and thus land cover remains constant in time and space. Because containers and shallow surface puddles are the main breeding sources, impermeable land cover is set to 2,500 cm2 and permanent water is excluded.
Because some new metrics of measurements were used in this study that were not evaluated previously, we performed a validation analysis on model and trap data for Coachella Valley, CA and Pasco County, FL (Tables S3 and S4). The metrics had to be altered slightly, however, to account for the smaller population sizes and because the trap and model data behave differently. The validation data should be interpreted with caution, as they are limited in a number of ways. For example, trapping ceases or greatly decreases during the shoulder seasons when we were attempting to calculate season start/end and the trap data are very location-specific and do not fully represent an entire area. Still, these data are the best source of information to evaluate the model. In future work, improved validation analysis can be achieved by conducting mosquito trapping to enable location-specific predictions and further enhance model performance. Further improvement can be made by including more location-specific habitat sources, such as storm drains and pools, instead of focusing predominantly on containers and puddles.
We ran DyMSiM with climate data generated from LARS-WG for the 84 locations across the southern United States under each climate scenario. In the model, containers and puddles fill with water from precipitation and lose water through evaporation. The model output consists of two 50-y time series (base and future) of daily mosquito populations for each location. The first-year output was not included in the analysis because the model requires the initial year as a spin-up period.
To decipher geographic trends in the data, we mapped the change in MDM between the two climate scenarios. This process was done to determine if the effects of future climate would be unique at each location or if there were general trends associated with areas sharing a broad geographical region. Similar maps of temperature and precipitation change across the time periods were produced to assess their similarity with the MDM maps and determine the relative importance of each variable geographically. MDM was defined as the total number of days during each month that the daily modeled mosquito population was above 10. The results for each month represent the average number of mosquito days over the 49-y run at each location. During the validation analysis, MDM was calculated as the number of days during the month when the average population across the county was greater than 0.5. This number was adjusted because the trap numbers were much lower because of their small effective range. There is general agreement between model and trap MDM with the exception of January/February and March/April in Florida during 1997 (Table S3). It is difficult to tell if this is the result of an inaccurate model parameter or if there was some form of human intervention, such as pesticide spraying or elimination of breeding sources. The accuracy of the model during the other tests suggests the latter.
Because of noise in model output at the daily level, we used mean weekly mosquito populations for our time-series analysis. An individual week’s mosquito population at a location and time period is calculated as the mean mosquito population during that week, averaged across that week for all 49 y, in each of the base and future model runs. Time series of mean weekly temperature and total weekly precipitation were also produced. We calculated the change in the weekly mosquito population between the base and future climate conditions at each location as follows:
where CM is the change in week i’s mosquito population at site j, FM is the mosquito population during week i at location j under the future climate scenario, and BM is the mosquito population during week i at location j under the base climate scenario.
To identify overall patterns of change in mosquito population dynamics we performed an S-mode principal components (PCs) analysis on the population change data series across all sites. A similar method of regionalization has been used on climate data (54). We used the correlation matrix in our calculation to enable comparison of locations with high and low mosquito populations. The first five PCs were retained (based on variance explained and eigenvalues greater than 1) and each location was initially assigned to a PC based on its maximum loading. Once mapped, some locations were reassigned to the PC with the second- or third-highest loading to create contiguous groupings on the map. There was generally little difference between the strengths of the loadings when this was done. Time series of temperature, precipitation, and mosquito population were created by averaging all sites within each PC group.
The change in the season start date and season end date were calculated and mapped to identify geographic trends. To calculate the change in season, we first averaged the daily mosquito population across the 49 y of the base and future model runs to create two time series of data. Averaged daily populations were calculated rather than performing the analysis on each individual year, because it limited noise in the data and provided a clearer signal. For each dataset (base and future) we identified the dates at which the population first reached a percentage of the base maximum population. This process was repeated using a range of values from 7% to 25% of the base maximum population at 1% intervals for each time series. From this procedure we used the median date as the start date, thereby eliminating outliers or biases that could result from choosing an arbitrary percentage. Still, these dates are subject to our chosen parameters and represent qualitative estimates of season shift rather than hard predictions. The season end date was calculated in a similar way by identifying the last day at which the population was above each percentage of the base population maximum. A more detailed description of this procedure and an example is provided in the SI Materials and Methods and Tables S5–S7. For each location we also calculated and mapped the number of summer days when the future mosquito population was less than the base mosquito population (by at least two mosquitoes), with summer defined as June 1 to September 30. In the validation analysis the start/end dates are defined as the day when the mosquito population has increased for 2 consecutive days. It was not possible to perform this analysis with the same algorithm because of the small amount of trap data and because trapping was limited or ceased during the cooler times of the year when the start/end would normally occur. Once again the test on the 1997 Florida trap data shows considerable disagreement with the model; however, model and trap start/end dates for most other years and locations are within a week or a few days of each other (Table S4).
Supplementary Material
Acknowledgments
This research was supported in part by the National Oceanic and Atmospheric Administration Regional Integrated Sciences and Assessments program via the Climate Assessment for the Southwest program at the University of Arizona.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307135110/-/DCSupplemental.
References
- 1. Confalonieri U, et al. (2007) in Human Health in Climate Change 2007: Impacts, Adaptation and Vulnerability, eds Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (Cambridge Univ Press, Cambridge), pp 391–431.
- 2.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006;43(2):309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Reisen WK, et al. Impact of climate variation on mosquito abundance in California. J Vector Ecol. 2008;33(1):89–98. doi: 10.3376/1081-1710(2008)33[89:iocvom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Trawinski PR, Mackay DS. Meteorologically conditioned time-series predictions of West Nile virus vector mosquitoes. Vector Borne Zoonotic Dis. 2008;8(4):505–521. doi: 10.1089/vbz.2007.0202. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz MO, et al. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors. 2010;3(19) doi: 10.1186/1756-3305-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladeau SL, Marra PP, Kilpatrick AM, Calder CA. West Nile virus revisited: Consequences for North American ecology. Bioscience. 2008;58(10):937–946. [Google Scholar]
- 7.Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289(5485):1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- 8.Small J, Goetz SJ, Hay SI. Climatic suitability for malaria transmission in Africa, 1911–1995. Proc Natl Acad Sci USA. 2003;100(26):15341–15345. doi: 10.1073/pnas.2236969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebi KL, et al. Climate suitability for stable malaria transmission in Zimbabwe under different climate change scenarios. Clim Change. 2005;73(3):375–393. [Google Scholar]
- 10.Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet. 2002;360(9336):830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 11.Hopp JH, Foley JA. Worldwide fluctuations in dengue fever cases related to climate variability. Clim Res. 2003;25(1):85–94. [Google Scholar]
- 12.Hartley DM, et al. Effects of temperature on emergence and seasonality of West Nile virus in California. Am J Trop Med Hyg. 2012;86(5):884–894. doi: 10.4269/ajtmh.2012.11-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): Analysis of the literature and model development. J Med Entomol. 1993;30(6):1003–1017. doi: 10.1093/jmedent/30.6.1003. [DOI] [PubMed] [Google Scholar]
- 14.Ahumada JA, Lapointe D, Samuel MD. Modeling the population dynamics of Culex quinquefasciatus (Diptera: Culicidae), along an elevational gradient in Hawaii. J Med Entomol. 2004;41(6):1157–1170. doi: 10.1603/0022-2585-41.6.1157. [DOI] [PubMed] [Google Scholar]
- 15.Gong H, DeGaetano AT, Harrington LC. Climate-based models for West Nile Culex mosquito vectors in the Northeastern US. Int J Biometeorol. 2011;55(3):435–446. doi: 10.1007/s00484-010-0354-9. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffer B, Mondet B, Touzeau S. Using a climate-dependent model to predict mosquito abundance: Application to Aedes (Stegomyia) africanus and Aedes (Diceromyia) furcifer (Diptera: Culicidae) Infect Genet Evol. 2008;8(4):422–432. doi: 10.1016/j.meegid.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Morin CW, Comrie AC. Modeled response of the West Nile virus vector Culex quinquefasciatus to changing climate using the dynamic mosquito simulation model. Int J Biometeorol. 2010;54(5):517–529. doi: 10.1007/s00484-010-0349-6. [DOI] [PubMed] [Google Scholar]
- 18.Hayes EB, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinser M, Ramberg F, Willott E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: Blood meal analysis indicates feeding on both humans and birds. J Insect Sci. 2004;4(20) doi: 10.1093/jis/4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebi KL. Public health responses to the risks of climate variability and change in the United States. J Occup Environ Med. 2009;51(1):4–12. doi: 10.1097/JOM.0b013e31816fd67b. [DOI] [PubMed] [Google Scholar]
- 21.Frumkin H, Hess J, Luber G, Malilay J, McGeehin M. Climate change: The public health response. Am J Public Health. 2008;98(3):435–445. doi: 10.2105/AJPH.2007.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosking J, Campbell-Lendrum D. How well does climate change and human health research match the demands of policymakers? A scoping review. Environ Health Perspect. 2012;120(8):1076–1082. doi: 10.1289/ehp.1104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khasnis AA, Nettleman MD. Global warming and infectious disease. Arch Med Res. 2005;36(6):689–696. doi: 10.1016/j.arcmed.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael AJ, Lindgren E. Climate change: Present and future risks to health, and necessary responses. J Intern Med. 2011;270(5):401–413. doi: 10.1111/j.1365-2796.2011.02415.x. [DOI] [PubMed] [Google Scholar]
- 26.Landesman WJ, Allan BF, Langerhans RB, Knight TM, Chase JM. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7(3):337–343. doi: 10.1089/vbz.2006.0590. [DOI] [PubMed] [Google Scholar]
- 27.Deichmeister JM, Telang A. Abundance of West Nile virus mosquito vectors in relation to climate and landscape variables. J Vector Ecol. 2011;36(1):75–85. doi: 10.1111/j.1948-7134.2011.00143.x. [DOI] [PubMed] [Google Scholar]
- 28.Shaman J, Day JF, Stieglitz M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J Med Entomol. 2005;42(2):134–141. doi: 10.1093/jmedent/42.2.134. [DOI] [PubMed] [Google Scholar]
- 29.Shaman J, Harding K, Campbell SR. Meteorological and hydrological influences on the spatial and temporal prevalence of West Nile virus in Culex mosquitoes, Suffolk County, New York. J Med Entomol. 2011;48(4):867–875. doi: 10.1603/me10269. [DOI] [PubMed] [Google Scholar]
- 30.Koenraadt CJM, Harrington LC. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae) J Med Entomol. 2008;45(1):28–35. doi: 10.1603/0022-2585(2008)45[28:feoroc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39(1):221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- 32.Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007;7(4):629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4(6):e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh AS, Glass GE, Lesser CR, Curriero FC. Predicting seasonal abundance of mosquitoes based on off-season meteorological conditions. Environ Ecol Stat. 2008;15(3):279–291. [Google Scholar]
- 35.Reisen WK, et al. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J Med Entomol. 2010;47(2):230–237. doi: 10.1603/me09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaDeau SL, Calder CA, Doran PJ, Marra PP. West Nile virus impacts in American crow populations are associated with human land use and climate. Ecol Res. 2011;26(5):909–916. doi: 10.1007/s11284-010-0725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth D, et al. Members of the British Columbia West Nile Virus Surveillance Team West Nile virus range expansion into British Columbia. Emerg Infect Dis. 2010;16(8):1251–1258. doi: 10.3201/eid1608.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hongoh V, Berang-Ford L, Scott ME, Lindsay LR. Expanding geographical distribution of the mosquito, Culex pipiens, in Canada under climate change. Appl Geogr. 2012;33(1):53–62. [Google Scholar]
- 39.Brunkard JM, Cifuentes E, Rothenberg SJ. Assessing the roles of temperature, precipitation, and ENSO in dengue re-emergence on the Texas-Mexico border region. Salud Publica Mex. 2008;50(3):227–234. doi: 10.1590/s0036-36342008000300006. [DOI] [PubMed] [Google Scholar]
- 40.Bolling BG, Kennedy JH, Zimmerman EG. Seasonal dynamics of four potential West Nile vector species in north-central Texas. J Vector Ecol. 2005;30(2):186–194. [PubMed] [Google Scholar]
- 41.Gates WL, et al. An overview of the results of the Atmospheric Model Intercomparison Project (AMIP I) Bull Am Meteorol Soc. 1999;80(1):29–55. [Google Scholar]
- 42.Reichler T, Kim J. How well do coupled models simulate today’s climate? Bull Am Meteorol Soc. 2008;89(3):303–311. [Google Scholar]
- 43.Soverow JE, Wellenius GA, Fisman DN, Mittleman MA. Infectious disease in a warming world: How weather influenced West Nile virus in the United States (2001–2005) Environ Health Perspect. 2009;117(7):1049–1052. doi: 10.1289/ehp.0800487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabachnick WJ. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J Exp Biol. 2010;213(6):946–954. doi: 10.1242/jeb.037564. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Weng Q, Gaines D. Spatio-temporal analysis of the relationship between WNV dissemination and environmental variables in Indianapolis, USA. Int J Health Geogr. 2008;7(66) doi: 10.1186/1476-072X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson BJ, et al. The roles of mosquito and bird communities on the prevalence of West Nile virus in urban wetland and residential habitats. Urban Ecosyst. 2012;15(3):513–531. doi: 10.1007/s11252-012-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barr AR. The distribution of Culex p. pipiens and C.P. quinquefasciatus in North America. Am J Trop Med Hyg. 1957;6(1):153–165. doi: 10.4269/ajtmh.1957.6.153. [DOI] [PubMed] [Google Scholar]
- 48.Cornel AJ, et al. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40(1):36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- 49.Huang S, Molaei G, Andreadis TG. Reexamination of Culex pipiens hybridization zone in the Eastern United States by ribosomal DNA-based single nucleotide polymorphism markers. Am J Trop Med Hyg. 2011;85(3):434–441. doi: 10.4269/ajtmh.2011.10-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenov MA, Barrow EM. Use of a stochastic weather generator in the development of climate change scenarios. Clim Change. 1997;35(4):397–414. [Google Scholar]
- 51.Qian BD, Hayhoe H, Gameda S. Evaluation of the stochastic weather generators Lars-WG and AAFC-WG for climate change impact studies. Clim Res. 2005;29(1):3–21. [Google Scholar]
- 52.Barrow BM, Semenov MA. Climate-change scenarios with high spatial and temporal resolution for agricultural applications. Forestry. 1995;68(4):349–360. [Google Scholar]
- 53.Trnka M, Dubrovsky M, Semeradova D, Zalud Z. Projections of uncertainties in climate change scenarios into expected winter wheat yields. Theor Appl Climatol. 2004;77(3–4):229–249. [Google Scholar]
- 54.Comrie AC, Glenn EC. Principal components-based regionalization of precipitation regimes across the southwest United States and northern Mexico, with an application to monsoon precipitation variability. Clim Res. 1998;10(3):201–215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.