Significance
Early adversity has profound and lasting effects on neurodevelopment and emotional behavior. Under typical environmental conditions, prefrontal cortex connections with the amygdala are immature during childhood and become adult-like during adolescence. Rodent models show that maternal deprivation accelerates this development as an ontogenetic adaptation to adversity. Here, we demonstrate that, as in the rodent, children who experienced early maternal deprivation exhibit early emergence of mature amygdala–prefrontal connectivity. Evidence suggests that the adult-like neural phenotype, which is mediated by cortisol levels, confers some degree of enhanced emotion regulation, as maternally deprived youths with adult-like phenotypes are less anxious than their counterparts with immature phenotypes. Accelerated amygdala–prefrontal development may serve as an ontogenetic adaptation in the human in response to early adversity.
Keywords: fMRI, emotion regulation, stress, neurodevelopment
Abstract
Under typical conditions, medial prefrontal cortex (mPFC) connections with the amygdala are immature during childhood and become adult-like during adolescence. Rodent models show that maternal deprivation accelerates this development, prompting examination of human amygdala–mPFC phenotypes following maternal deprivation. Previously institutionalized youths, who experienced early maternal deprivation, exhibited atypical amygdala–mPFC connectivity. Specifically, unlike the immature connectivity (positive amygdala–mPFC coupling) of comparison children, children with a history of early adversity evidenced mature connectivity (negative amygdala–mPFC coupling) and thus, resembled the adolescent phenotype. This connectivity pattern was mediated by the hormone cortisol, suggesting that stress-induced modifications of the hypothalamic–pituitary–adrenal axis shape amygdala–mPFC circuitry. Despite being age-atypical, negative amygdala–mPFC coupling conferred some degree of reduced anxiety, although anxiety was still significantly higher in the previously institutionalized group. These findings suggest that accelerated amygdala–mPFC development is an ontogenetic adaptation in response to early adversity.
Even brief exposure to stressful experiences early in life can have life-long impact on brain development and socioemotional functioning. Adverse or deprived caregiving is an example of a highly potent early life stressor in altricial species. Animal studies of maternal deprivation have demonstrated long-term effects on socioemotional and brain development (1–5), with particular influences on frontoamygdala circuitry. The timing of stress exposure and cellular properties of this circuitry may render it particularly vulnerable to early adversity (6). Consistently, rodent and nonhuman primate models show that the amygdala is highly susceptible to early environmental adversity due to its early structural development and readiness to respond to stressors (7–9). Human studies of early adverse caregiving have demonstrated structural volume abnormalities in the amygdala that were associated with increased trait anxiety and emotion dysregulation (10, 11) and increased amygdala reactivity to emotional stimuli (12).
Abnormally rapid brain development following early adversity may be a response that reprioritizes developmental goals to match the demands of an adverse early environment. Early life caregiving adversity in rodents alters amygdala–medial prefrontal cortex (mPFC) circuits that in adulthood serve to regulate the activity of the amygdala (13, 14), perhaps through accelerating development of the circuitry. For example, maternal deprivation results in the early emergence of adult-like fear learning based in frontoamygdala circuitry (5) and earlier emergence of amygdala function (8, 15) and structural maturation (16). Maternal separation has also been associated with increased development of neurons in mPFC (17). Such changes can lead to adult-like fear extinction learning and mPFC-mediated down-regulation of the amygdala, even though as a group, these stressed animals can be more fearful and anxious (18). Maternal absence acts to accelerate amygdala–prefrontal functional development via premature elevations of glucocorticoids (8, 19), suggesting that maternal absence acts on amygdala-related circuitry through alterations of the hypothalamic–pituitary–adrenal (HPA) axis, consistent with the established close relationship between the amygdala and HPA axis (20). Whether a similar neurohormonal process explains affective development in humans following early maternal absence is currently unknown.
Whereas the hypothesis of accelerated development has not been tested in humans, the conservation of frontoamygdala phenotypes across species would predict a common mechanism through which early life stress influences neuroaffective development. Such changes are important to understand given affective behaviors, including emotion regulation and fear learning, that rely on the amygdala and its connections with mPFC (21–27). In typical human development, childhood and adolescence is a period of large change in frontoamygdala phenotypes (28, 29), with amygdala–mPFC connectivity being markedly immature during childhood (28). The transition from childhood into adolescence has been characterized by a developmental shift in amygdala–mPFC functional connectivity. Specifically, adolescents and adults exhibit inverse (negative) correlations between amygdala and mPFC in response to emotional stimuli, a pattern of connectivity that has been characterized as indexing top-down inhibitory connections (24, 27, 28). Children exhibit a positive amygdala–mPFC coupling phenotype (28) that is associated with greater emotional reactivity, which is typically characteristic of young children. This shift from an immature (positive) amygdala–mPFC coupling phenotype to a mature (negative) coupling phenotype parallels age-related attenuation of amygdala signal and mediates maturation of emotional behavior (28). The goal of the current study was to examine childhood amygdala–mPFC phenotypes following early life adversity with the hypothesis that early life adversity would accelerate development of amygdala–mPFC circuitry. We examined the effects of early adversity among previously institutionalized (PI) children who have a history of early life maternal deprivation.
Results
In a sample of 41 PI youth and 48 comparison (never-institutionalized) youth, we used functional MRI (fMRI) to examine functional coupling between the amygdala and mPFC while participants viewed emotional faces. Participants completed two runs of the fMRI task. During the fear run, participants viewed singly presented fear faces (50% of the trials) interspersed with neutral faces (50% of the trials); during the happy run, they viewed happy faces (50% of the trials) interspersed with neutral faces (50% of the trials). The mean age was 10.8 (SD = 2.7) for the comparison group and 12.1 (SD = 3.3) for the PI group, and analyses treated age as a continuous variable. For purposes of visualization only, we grouped children (6.5–10.4 y) and adolescents (10.5–17.6 y). Youth who experience institutional care as a result of maternal deprivation endure suboptimal caregiving conditions in various ways that act as potent stressors early in life. For example, orphanages are often overcrowded with employees in charge of caring for many infants, resulting in an unstable caregiving environment with inconsistent attention to infants’ needs and poor potential for nurturing and attachment (30).
Amygdala–mPFC Functional Connectivity Development.
To examine the effects of early adversity on effective connectivity between the amygdala and mPFC, we performed a psychophysiological interaction (PPI) analysis for each participant and analyzed at the group level using a whole-brain ANOVA. The PPI analysis identified neural regions whose activity was more strongly coupled with the amygdala during task performance than during baseline fixation. A significant group × emotional run interaction was observed in bilateral mPFC (t = 11.36, cluster: 487 voxels, P < 0.01, corrected; peak voxel: −3, 27, −1), which included the anterior cingulate [Brodmann area (BA) 32, BA 24, BA 10]. Specifically, during the fear run PI participants exhibited stronger negative amygdala–mPFC coupling than comparison participants (SI Appendix for happy results). Given prior evidence for functionally significant changes in amygdala–mPFC connectivity across healthy development (28, 29), we tested whether early adversity altered the expected age-related changes.
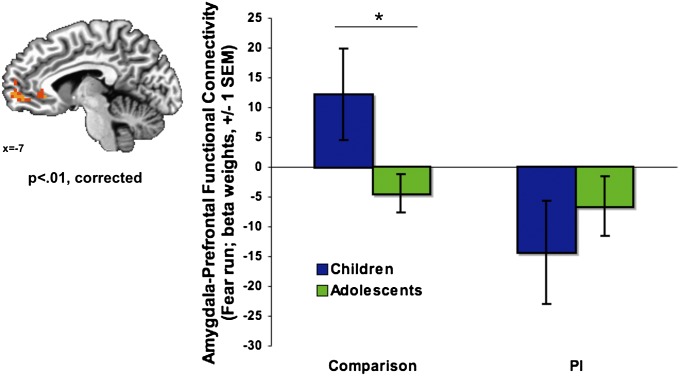
Comparison and PI participants exhibited different patterns of age-related change in amygdala–mPFC connectivity to the fear run (Fig. 1; group × age interaction: F(13, 94) = 3.53, P = 0.013; age modeled continuously), controlling for amygdala reactivity. Specifically, in the comparison group children showed positive (i.e., immature) amygdala–mPFC connectivity, and comparison adolescents exhibited negative (i.e., mature) amygdala–mPFC connectivity (t(52) = 2.40, P = 0.022). By contrast, in the PI group both children and adolescents exhibited the negative (i.e., mature) pattern of amygdala–mPFC coupling, and there was no difference in connectivity between PI children and PI adolescents (t(39) = −0.78, P = 0.439). There was also no difference in amygdala–mPFC connectivity between PI children and comparison adolescents (t(54) = 1.29, P = 0.203). Post hoc tests showed that none of these effects were the result of differences in connectivity during implicit baseline (P > 0.05). There were no group or age-related differences in mPFC activation, based on the PPI-defined functional cluster in mPFC (P > 0.05).
Fig. 1.
Mature amygdala–mPFC connectivity following maternal deprivation. (Left) A group × emotional run interaction was observed in the mPFC (P < 0.01, corrected), such that group differences emerged for the fear run. (Right) Unlike comparison children who showed immature (positive) amygdala–mPFC connectivity, PI children exhibited the mature pattern of negative amygdala–mPFC coupling, such that PI children resembled adolescents. *Post hoc analysis of age, independent of the whole-brain analysis. SEM = standard error of the mean.
Amygdala Reactivity.
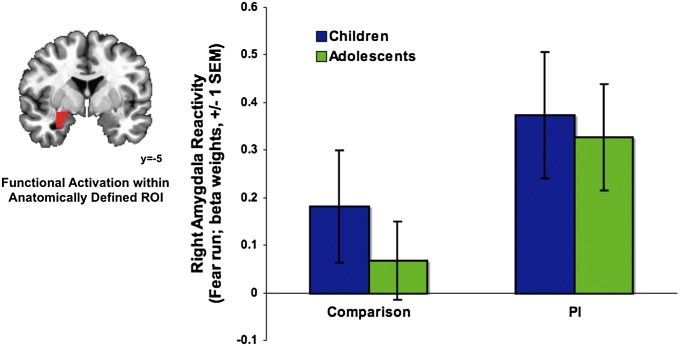
A significant group × emotional run interaction was observed in the right amygdala (t = 18.74, cluster: 636 voxels, P < 0.01, corrected; peak voxel: −1, 2, 2). Specifically, PI participants exhibited heightened amygdala reactivity in response to fear faces relative to the comparison group [Fig. 2; t(93) = −2.50, P = 0.014]. There was no group difference to happy faces [t(93) = 1.36, P = 0.18]. See SI Appendix for results on additional regions.
Fig. 2.
Elevated amygdala reactivity following maternal deprivation. (Left) A group × emotional run interaction was observed in the right amygdala (P < 0.01, corrected), such that PI participants exhibited elevated amygdala reactivity relative to the comparison group during the fear run. (Right) Beta weights were derived from a right amygdala mask based on the intersection of the functional region of interest (ROI) and a structural ROI.
Associations Between Amygdala–mPFC Connectivity and Anxiety.
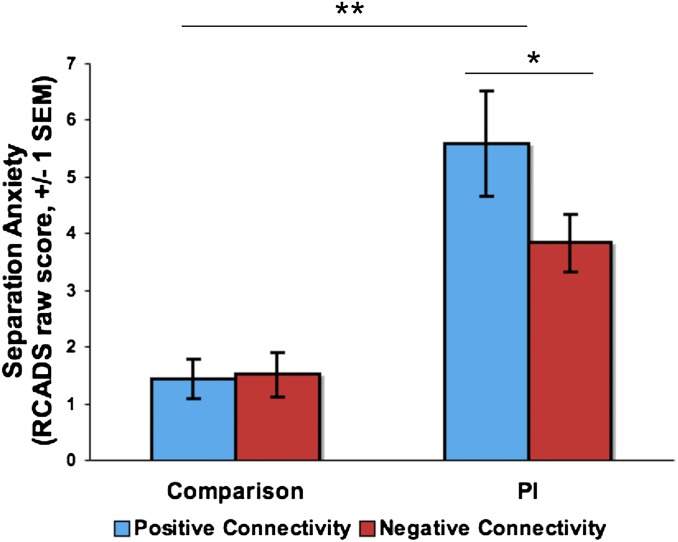
To examine the behavioral correlates of the mature versus immature patterns of amygdala–mPFC coupling, we assessed developmentally normative separation anxiety using the Revised Children’s Anxiety and Depression Scale (RCADS) parent report (31). A univariate general linear model (GLM) tested for effects of early adversity and connectivity valence (binary coding for positive or negative amygdala–mPFC PPI) on anxiety, controlling for age and amygdala reactivity (Fig. 3). A main effect of group was observed, such that PI participants had higher anxiety than comparison participants (F(1,90) = 35.7, P < 0.0001). Of primary interest to the present study was a significant group × connectivity valence (positive, negative) interaction [F(1,90) = 4.3, P = 0.04]. Although PI participants exhibited higher anxiety overall, individual differences within the PI group were associated with anxiety; PI youth with negative amygdala–mPFC connectivity had lower anxiety than PI youth with positive connectivity (F(1,40) = 4.18, P = 0.048). The group × connectivity interaction was also significant when connectivity was included as a continuous measure (F(1,90) = 4.33, P = 0.041). Amygdala and mPFC activation were not associated with anxiety (P = 0.90, 0.20, respectively). The relationship between connectivity and anxiety in the PI group was also observed when separation anxiety was measured with the Screen for Child Anxiety Related Emotional Disorders (32).
Fig. 3.
Connectivity phenotype and separation anxiety. Maternal deprivation was associated with elevated separation anxiety. However, more mature (negative) amygdala–mPFC coupling within the PI sample was associated with lower anxiety relative to immature (positive) amygdala–mPFC coupling, controlling for age and amygdala reactivity.
Cortisol and Amygdala–mPFC Connectivity.
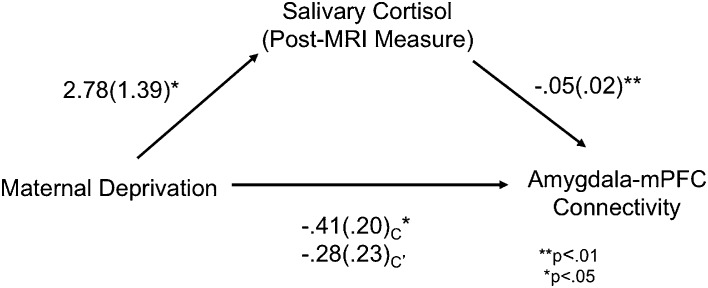
Salivary cortisol was collected immediately before and after MRI scanning, between 8:45 AM and 7:00 PM; there were no group differences in the time of day at which the samples were collected (pre-MRI: P = 0.96; post-MRI: P = 0.89). For the pre-MRI measure, the comparison group had a mean cortisol level of 8.19 nmol/L (SD = 8.32), and the PI group had a mean cortisol level of 8.59 nmol/L (SD = 8.32). For the post-MRI measure, the comparison group had a mean cortisol level of 7.34 nmol/L (SD = 5.72), and the PI group had a mean cortisol level of 10.07 nmol/L (SD = 7.01). Post-MRI cortisol was higher in the PI group than the comparison group (t(84) = 1.99, P = 0.049). There was no group difference in pre-MRI cortisol. We used mediational analysis to test the extent to which variance in cortisol explained variance in amygdala–mPFC connectivity. Results revealed that post-MRI cortisol level mediated the relationship between maternal deprivation and amygdala–mPFC connectivity (Fig. 4). Hierarchical regression was used to test the mediation while controlling for the time at which the cortisol sample was collected for each participant. Step one demonstrated that maternal deprivation significantly related to salivary cortisol (B = 2.784, t = 2.003, P = 0.048, adjusted R2 = 0.030). Step two demonstrated that maternal deprivation significantly related to amygdala–mPFC connectivity (B = −0.406, t = −1.993, P = 0.049, adjusted R2 = 0.031). Step three demonstrated that salivary cortisol significantly related to amygdala–mPFC connectivity (B = −0.046, t = −2.636, P = 0.01, adjusted R2 = 0.078). Step four demonstrated that maternal deprivation was less related to amygdala–mPFC connectivity when controlling for salivary cortisol level (B = −0.275, t = −1.217, P = 0.227, adjusted R2 = 0.062; Sobel test Z = −1.61, P = 0.05, one tailed). These results show that cortisol statistically mediated the association between maternal deprivation and amygdala–mPFC development, such that altered cortisol levels among children with a history of maternal deprivation may contribute to changes in the development of connections between the amygdala and mPFC.
Fig. 4.
Cortisol mediates effects of maternal deprivation on amygdala–prefrontal connectivity. Regression analyses revealed that post-MRI salivary cortisol level mediated the relationship between maternal deprivation and amygdala–mPFC functional connectivity.
Discussion
The present study examined age-related amygdala–prefrontal functional phenotypes in a cross-sectional sample of children and adolescents who experienced maternal deprivation and a typically developing comparison group. The maternally deprived youth were subsequently adopted into stable families, thus restricting the period of maternal deprivation to early life in this sample. Findings revealed aberrant frontoamygdala development following maternal deprivation, such that PI children and adolescents exhibited amygdala hyperreactivity and an altered trajectory of amygdala–prefrontal connectivity. Specifically, comparison children showed an immature pattern of connectivity (positively coupled amygdala–mPFC activity), whereas children with a history of maternal deprivation displayed the mature pattern of connectivity (negatively coupled amygdala–mPFC activity), such that they resembled the adolescent phenotype. The present study replicates earlier findings of amygdala hyperactivity in a PI sample (12) and provides support for the hypothesis that maternal deprivation instantiates earlier maturation of amygdala–mPFC connectivity.
Our interpretation that the observed mature-like negative amygdala–mPFC connectivity in PI children evidences accelerated development is supported by rodent models that have demonstrated causal relationships between maternal absence and acceleration of amygdala–mPFC phenotypes (5, 15, 17). Research in normative human development has shown that regulatory connections between the amygdala and mPFC manifest as positive coupling in childhood and shift to a mature pattern of negative coupling during adolescence (28). Here, we hypothesize that the mature pattern of amygdala–mPFC connectivity observed in PI children may result from early amygdala hyperactivity facilitating development of connections with mPFC. Because the youngest participants in the current study were 6 y old and by this age all participants showed an amygdala response to fear faces, we cannot say whether there is earlier emergence of amygdala reactivity following stress. Moreover, the developmentally normative pattern of high amygdala response to the fear condition, present in the youngest participants of both the comparison and PI groups, may mask group differences in amygdala reactivity at young ages. However, group differences in amygdala responsivity were present for the fear but not for the happy condition, and strong amygdala signal to fear has been associated with a more mature response (12, 33), suggesting that amygdala function, even in young PI children, is atypical. Prior evidence from the animal literature shows that early acceleration of amygdala development is followed by a maintenance of elevated amygdala response (8, 34); thus, the amygdala hyperactivation observed in the present sample may indicate accelerated development early in life. However, we cannot definitively conclude that accelerated amygdala function follows maternal deprivation in humans because even the youngest children in the present sample are likely past the developmental window that would allow us to observe that acceleration (i.e., before age 4). The current findings motivate additional research, particularly studies that use longitudinal design and examination of younger participants, to characterize the developmental trajectory of amygdala phenotypes. A hypothesis generated from the current study is that premature amygdala function instantiates a developmental cascade that promotes the emergence of mature connectivity with the mPFC.
When neuronal phenotypes deviate from typical development, there is the possibility that the circuitry functions in an atypical fashion, exhibiting a deviation from typical functional development. Alternatively, although present at a developmentally atypical time, the circuitry could serve the same affect–regulatory function as it does under typical conditions despite earlier emergence. To test these possibilities, we examined individual differences in trait anxiety, which we (28) and others (24) have shown to correlate with this circuitry (with negative connectivity correlating with lower trait anxiety). Consistent with prior research (35), youth with a history of maternal deprivation exhibited higher anxiety than the comparison group in the present study. However, despite higher levels of anxiety at the group level, the current findings suggest that mature amygdala–mPFC phenotypes mitigated anxiety within the PI group, as observed in nonstressed samples (24, 28). That is, PI participants with negative (i.e., mature) amygdala–mPFC connectivity showed lower separation anxiety relative to PI peers with positive connectivity, controlling for age and amygdala reactivity. Thus, it may be that the early emergence of negative connectivity between the amygdala and mPFC serves as a developmental adaptation to heightened amygdala reactivity. Even PI youth with mature amygdala–mPFC connectivity had higher anxiety than the comparison youth, but this behavioral group difference may persist as a result of powerful bottom-up reactivity with which regulatory circuitry has to contend. This interpretation of the data requires further testing, as there may be alternative explanations for the association between connectivity phenotype and anxiety. Although prior work has also observed the relationship between connectivity valence and anxiety in healthy controls (28), this finding was limited to the PI group in the current study. The present comparison sample was older in age than our previous sample and thus had lower, and less variable, separation anxiety, which may have precluded the ability to detect an association between connectivity and anxiety in the comparison group. It is notable that amygdala connectivity, but not amygdala or mPFC reactivity, was associated with anxiety, suggesting that it may be particularly important to examine frontoamygdala connectivity when studying behavioral phenotypes related to early life stress.
On the one hand, negative (mature) amygdala–mPFC coupling in childhood may be a source of proximal resilience for children with a history of adversity (i.e., lower relative anxiety). However, there may be later costs associated with premature maturation of negative amygdala–mPFC functional connectivity. Previous research has demonstrated that a neotenized brain (one showing slower cortical development) has been associated with optimized behavioral outcomes in adulthood (e.g., higher IQ) (36), perhaps because a prolonged period of immaturity allows for more opportunity to learn from the environment and to increase adult efficiency (37, 38). Although the positive amygdala–mPFC coupling observed early in typical development is not well understood, it may serve an important function that is abbreviated or missed following early life stress, which could have consequences for later circuit optimization. The juvenile period in mice (childhood equivalent) has been found to be a period of mPFC immaturity, and this immaturity was critical for mPFC-mediated learning of cues that served anxiolytic function (i.e., safety signals) in adulthood (39). This finding demonstrates the important function of cortical immaturity in the juvenile period. Maternal presence may help to maintain a neotenized state of the human cortex to extend the period of neuronal plasticity.
Mediation analyses indicated that cortisol contributes to the association between maternal deprivation and the development of amygdala–mPFC connectivity. Prior research suggests that developmental changes in cortisol (40, 41) contribute to brain development through interactions with receptors that regulate genes involved in neuromaturational processes (42, 43). Some of these maturational changes are specific to amygdala–mPFC circuitry, instantiated either through direct administration of glucocorticoids or via maternal absence (which elevates glucocorticoid levels) (5, 8, 15). We observed higher levels of cortisol, the primary glucocorticoid in humans, in the PI group, consistent with past findings in similar samples (44). Thus, although speculative at this point, the current findings are consistent with the hypothesis that maternal deprivation influences amygdala–mPFC development via HPA axis activity, providing a potential mechanistic framework for understanding how early environments influence subsequent affective development. Post-MRI cortisol, but not pre-MRI cortisol, contributed to the relationship between maternal deprivation and amygdala–mPFC connectivity. Cortisol measures collected at home for a subsample of participants were used to inform the extent to which the scanning environment could have been associated with changes in cortisol from baseline (SI Appendix). Given that at-home cortisol estimates did not differ from cortisol levels at the scanner for either group, the results do not support the idea that the scan increased cortisol reactivity. Similarly, prior studies indicate that the MRI experience does not act as a stressor for developmental populations (45). However, it may be that comparison participants displayed expected decreases in diurnal cortisol, whereas cortisol in PI participants failed to attenuate during the experience in the scanning environment, as has been shown in prior work examining children in low-quality daycare (46).
The present study had some limitations to be addressed in future research. As with previous studies, the current examination of maternal deprivation should be interpreted within the context of a quasiexperimental design (which is why we needed to use a comparison group as opposed to a true control group). However, prior research including random assignment of care (35) and dose–response associations (10, 11, 47) strongly suggests that effects of maternal deprivation are causal in nature. In addition, some PI participants had current medication use (primarily psychostimulants) and were instructed to take medication as usual due to ethical considerations. Though it is important to note medication use in the present study, we conducted multiple analyses, including analyses that covaried for medication use, tested for differences between medicated and unmedicated participants, and excluded medicated participants, all of which ruled out the possible influences of medication on our findings (SI Appendix). In addition, prior research associating stimulant medications with normalized amygdala–prefrontal function (48) suggests that medication use would have reduced the chance of finding a group difference. The present investigation relied on a cross-sectional design, which can be influenced by interindividual variance and factors that limit our ability to characterize developmental trajectories. Whereas our results are consistent with early emergence of amygdala–mPFC phenotypes following early life stress, it is unclear whether these “mature” phenotypes maintain across the life span. Although the present data suggest that connectivity does not change with age in the PI group, we acknowledge the possibility that maternal deprivation incites early emergence of negative coupling that later changes to positive or nonsignificant coupling. Although cross-sectional data cannot define the trajectory of connectivity over time in the PI group, there is some evidence that prematurely developing phenotypes maintain throughout adulthood following early life stress (49–51). In addition, it is notable that the negative connectivity observed in PI adolescents is functioning in the same way as in mature adults, with negative connectivity corresponding to lower anxiety (52), providing further evidence of a mature phenotype in adolescence. Future longitudinal examinations of amygdala reactivity and connectivity will be necessary to better understand the developmental trajectory following early life stress. Lastly, PPI analyses rely on statistical correlations and thus cannot address questions of directionality in regional influences. Continued translational work including animal models (15, 53, 54) will inform the nature of directional influences between amygdala and mPFC to inform distinctions in the development of bottom-up versus top-down connections following early adversity.
The present study demonstrated an adult-like pattern of frontoamygdala connectivity among children and adolescents who experienced early life stress. In contrast to a shift from positive to negative amygdala–mPFC connectivity among comparison participants, PI children exhibited the early emergence of a mature pattern of negative amygdala–mPFC coupling such that they resembled comparison adolescents and adults in previous studies (21, 24, 28). This finding represents a marked change from typical frontoamygdala development, which holds important functional significance for long-term effects on emotional behavior following early life stress. Moreover, the present findings provide insight into the mechanisms underlying a potential relationship between early adversity and accelerated development of amygdala circuitry. Based on the neurodevelopmental nature and implication of frontoamygdala circuitry in many psychiatric disorders (55, 56), mapping the typical and atypical development of amygdala–prefrontal connections has critical implications for understanding the temporal nature of risk and resilience factors for psychopathology as well as informing developmental timing related to intervention.
Materials and Methods
Participants.
The present study focused on children and adolescents who experienced adverse caregiving (i.e., institutional care) early in life and who were later adopted into stable homes, as well as a comparison group. This population allows for the unique examination of an isolated period of adversity, thus limiting confounds such as subsequent adversities that often accompany naturally occurring stress. Participants consisted of 89 children and adolescents (41 PI participants and 48 comparison, never-institutionalized participants) (SI Appendix for demographic details). Data from 28 of the comparison participants were previously published (28). The mean age of the entire sample was 11.6 y (SD = 3.1; range = 6.5–17.6). Anxiety was measured among child and adolescent participants using the RCADS parent-report questionnaire (31). Due to the anticipated decline in separation anxiety with age that has been demonstrated in prior research (57, 58) and our previous finding of a relationship between connectivity valence and separation anxiety, we specifically examined the separation anxiety subscale of the RCADS. Based on prior work in rodents showing that frontoamygdala circuitry matures as the rat pup begins to leave the nest (59), we anticipated that changes in human amygdala–mPFC circuitry may relate to children’s affective ability to separate from the caregiver, leading to the examination of separation anxiety. Given evidence for the tight association between the HPA axis and amygdala development (8, 15) and early life stress (44, 60), we examined salivary cortisol as an index of HPA axis activity (SI Appendix). The protocol was approved by the Institutional Review Board at the University of California, Los Angeles. Participants provided informed consent or assent (parental informed consent for minors).
Procedures: fMRI Task Paradigm.
During the scan, participants completed two runs of an emotional faces task. The task consisted of a mixed design with one blocked variable [emotional run: fear (included fear and neutral faces) vs. happy (included happy and neutral faces)] and one event-related variable [stimulus type: emotional face (either fear or happy) vs. neutral face]. Facial expressions were selected as stimuli due to their utility as a probe of frontoamygdala circuitry (61). During the fear run, participants viewed fear faces interspersed with neutral faces; during the happy run, they viewed happy faces interspersed with neutral faces. The order of the fear run and the happy run was counterbalanced across participants, and the stimuli within each run were randomized and fixed across participants. To ensure that participants were paying attention, they were asked to press a button when they saw a neutral face. Female faces were selected from the Karolinska Directed Emotional Faces database (62). The probability of a neutral face was 50% on any given trial. Stimuli were jittered (variable intertrial interval ranging from 3,000 to 9,000 ms) and randomized based on a genetic algorithm (63) to allow for unique estimates of the hemodynamic response for each trial type. Each run contained 48 trials (24 neutral faces, 24 fear or happy faces). Each face remained on the screen for 500 ms.
fMRI Data Acquisition.
Scanning was performed on a Siemens Trio 3.0 Tesla MRI scanner. A standard radiofrequency head coil was used. For each participant, an initial 2D spin echo image (TR = 4,000 ms, TE = 40 ms, matrix size 256 × 256, 4 mm thick, 0 mm gap) in the oblique plane was acquired to allow configuration of slices obtained in the structural and functional scans. A whole-brain high-resolution, T1*weighted anatomical scan [MPRAGE; 256 × 256 in-plane resolution, 256 mm field of view (FOV); 192 mm × 1 mm sagittal slices] was acquired for each participant for registration and localization of functional data to Talairach space (64). The task was completed during two functional scans and presented on a computer screen through MR-compatible goggles. T2*weighted echoplanar images were collected at an oblique angle of ∼30° (130 volumes per run, TR = 2,000 ms, TE = 30 ms, flip angle = 90°, matrix size 64 × 64, FOV = 192, 34 slices, 4-mm slice thickness, skip 0 mm, 24 observations per event type).
fMRI Data Analysis.
Functional imaging data were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI) software (65). Extensive steps were taken to ensure that motion artifact did not contaminate the data (66) (SI Appendix, Methods). Preprocessing of each individual’s images included slice time correction to adjust for temporal differences in slice acquisition within each volume, spatial realignment to correct for head motion, registration to the first volume of each run, spatial smoothing using a 6-mm Gaussian kernel [full width at half maximum (FWHM)], and transformation into the standard coordinate Talairach space (63) with parameters obtained from the transformation of each individual’s high-resolution anatomical scan. Talairached transformed images had a resampled resolution of 3 mm3. Time series were normalized to percent signal change to allow for comparisons across runs and individuals. The functional runs were concatenated before creating two individual-level models for each participant to model activation and functional connectivity. To examine whole-brain activation, each participant’s individual-level model included regressors for each of the stimulus conditions (fear, happy, neutral from the fear run, neutral from the happy run) and accuracy. Regressors were created by convolving the stimulus timing with the canonical hemodynamic response function. Six motion parameters were included as separate regressors. A GLM was performed to fit the percent signal change time series to each regressor. Linear and quadratic trends were modeled for each voxel’s time series to control for correlated drift.
The individual-level regression coefficients were submitted to random-effects, group level analyses. An ANOVA in AFNI modeled group (comparison, PI), emotional run (fear run, happy run), and stimulus type [emotional face (either fear or happy), neutral face]. We tested for main effects and interactions between group, emotion, and stimulus type. Correction for multiple comparisons was applied at the cluster level within a priori regions of interest following Monte Carlo simulations conducted in AlphaSim within AFNI. AlphaSim was conducted across the whole brain at 6 mm FWHM, 10,000 simulations, and an individual voxel threshold of 0.01. The minimum number of voxels necessary to achieve P < 0.01 was 57. To extract beta weights for visualization and to test for age-related changes in reactivity, a mask of the right amygdala was created based on overlapping voxels between the functional cluster identified in the ANOVA (from a group × emotional run interaction) and a structural mask of the right amygdala that was anatomically defined using the Talairach–Tournoux atlas implemented in AFNI (63). An independent samples t test was used to confirm group differences in reactivity for this specific cluster of the amygdala and for visualization purposes to identify the direction of the effect. Analyses were repeated using a mask of the functional cluster that extended beyond the right amygdala (cluster identified in the group × emotional run interaction); results replicated the findings with the combined functional–structural mask. For visualization purposes only, participants were grouped by age (6.5–10.4, 10.5–17.6 y). Age groups were selected based on prior work, demonstrating a shift in frontoamygdala circuitry at age 10 (28).
A PPI analysis (67) was conducted to examine task-dependent connectivity with the amygdala across the whole brain. That is, the PPI analysis tested the extent to which the amygdala covaried with other brain regions more during one condition than another (SI Appendix for details). Consistent with the analysis of amygdala reactivity, an ANOVA analysis was conducted in AFNI to model group (comparison, PI), emotional run (fear run, happy run), and stimulus type (emotional face, neutral face). Given evidence for a developmental shift in amygdala–mPFC connectivity during typical development, we sought to test for age-related changes in connectivity. To avoid nonindependence in statistical analyses (68), we first identified a group × emotional run interaction in mPFC in AFNI and then extracted beta weights for the identified cluster for the fear run. A univariate GLM was conducted in SPSS to test for effects of group, age (modeled continuously), and a group × age interaction in amygdala–mPFC connectivity.
Anxiety Analyses.
Developmentally normative anxiety was measured using the separation anxiety subscale of the RCADS. To test for effects of group and connectivity valence on anxiety, we conducted a univariate GLM in SPSS that modeled group, connectivity valence (i.e., whether a given participant’s amygdala–mPFC coupling was positive or negative), and a group × valence interaction, while controlling for age and amygdala reactivity.
Supplementary Material
Acknowledgments
The present research was supported by R01MH091864 (to N.T.) and P50MH078105 from the National Institute of Mental Health and a National Science Foundation Graduate Research Fellowship award (to D.G.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307893110/-/DCSupplemental.
References
- 1.Suchecki D, Nelson DY, Van Oers H, Levine S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: Effects of maternal deprivation. Psychoneuroendocrinology. 1995;20(2):169–182. doi: 10.1016/0306-4530(94)00051-b. [DOI] [PubMed] [Google Scholar]
- 2.Hofer MA. On the nature and consequences of early loss. Psychosom Med. 1996;58(6):570–581. doi: 10.1097/00006842-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Romeo RD, et al. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43(5):561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 2011;125(1):20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- 6.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: A key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21(11):471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22(5-6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatini MJ, et al. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27(12):3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 11.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tottenham N, et al. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118(2):389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 15.Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono M, et al. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156(4):1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 17.Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Caldji C, et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callaghan BL, Richardson R. The effect of adverse rearing environments on persistent memories in young rats: Removing the brakes on infant fear memories. Transcult Psychiatry. 2012;2:e138. doi: 10.1038/tp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. J Neurosci. 2008;28(26):6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 23.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hare TA, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Dev Psychopathol. 2008;20(4):1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- 26.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gee DG, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlman SB, Pelphrey KA. Developing connections for affective regulation: Age-related changes in emotional brain connectivity. J Exp Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: Research and policy. Dev Psychopathol. 2000;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- 31.Chorpita BF, Moffitt CE, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther. 2005;43(3):309–322. doi: 10.1016/j.brat.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Birmaher B, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Thomas KM, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 34.Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeanah CH, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 36.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 37.Lebel C, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: Heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32(44):15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu LH, et al. Relationships between brain activation and brain structure in normally developing children. Cereb Cortex. 2009;19(11):2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang E-J, Lin EW, Hensch TK. Critical period for acoustic preference in mice. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17213–17220. doi: 10.1073/pnas.1200705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiess W, et al. Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 42.Ojeda SR, Ma YJ. Glial-neuronal interactions in the neuroendocrine control of mammalian puberty: Facilitatory effects of gonadal steroids. J Neurobiol. 1999;40(4):528–540. doi: 10.1002/(sici)1097-4695(19990915)40:4<528::aid-neu9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 43.Walker E, Bollini AM. Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophr Res. 2002;54(1-2):17–23. doi: 10.1016/s0920-9964(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 44.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol. 2001;13(3):611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 45.Thomason ME. Children in non-clinical functional magnetic resonance imaging (FMRI) studies give the scan experience a “thumbs up”. Am J Bioeth. 2009;9(1):25–27. doi: 10.1080/15265160802617928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 47.Rutter M, O’Connor TG. English and Romanian Adoptees (ERA) Study Team Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Dev Psychol. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- 48. Posner J, et al. (2011) Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:828–837 .e3. [DOI] [PMC free article] [PubMed]
- 49.Rentesi G, et al. Early maternal deprivation-induced modifications in the neurobiological, neurochemical and behavioral profile of adult rats. Behav Brain Res. 2013;244:29–37. doi: 10.1016/j.bbr.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Vázquez DM, Neal CR, Jr, Patel PD, Kaciroti N, López JF. Regulation of corticoid and serotonin receptor brain system following early life exposure of glucocorticoids: Long term implications for the neurobiology of mood. Psychoneuroendocrinology. 2012;37(3):421–437. doi: 10.1016/j.psyneuen.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev Psychobiol. 2013 doi: 10.1002/dev.21138. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim MJ, et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 56.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 57.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gullone E, King NJ. Three-year follow-up of normal fear in children and adolescents aged 7 to 18 years. Br J Dev Psychol. 1997;15:97–111. [Google Scholar]
- 59.Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Dev Neurosci. 2012;34(2–3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burghy CA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whalen PJ, et al. Neuroscience and facial expressions of emotion: The role of amygdala–prefrontal interactions. Emotion Review. 2013;5:78–83. [Google Scholar]
- 62. Lundqvist D, Flykt A, Ohman A (1998) Karolinska Directed Emotional Faces (Psychology Section, Department of Clinical Neuroscience, Karolinska Institute, Stockholm)
- 63.Wager TD, Nichols TE. Optimization of experimental design in fMRI: A general framework using a genetic algorithm. Neuroimage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 64.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- 65.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 66.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 68.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.