Significance
The presence of biologically active monoterpenoid indole alkaloids (MIAs) on the leaf surfaces of medicinally important Catharanthus roseus has led to questions about the secretion processes involved and their prevalence within MIA-producing species of plants. This report shows that a transporter closely related to those involved in cuticle assembly in plants and belonging to the pleiotropic drug resistance family of ATP-binding cassette transporters is specialized for transport of the MIA catharanthine to the leaf surface from its site of biosynthesis in the leaf epidermis. The same transporter was also identified in plants from distinct geographical origins (Eurasian Vinca minor, African Tabernamontana elegans, Indian Rauvolfia serpentina, South American Cinchona ledgeriana, and North American Amsonia hubrichtii).
Keywords: plasma membrane ABC-PDR-alkaloid transporter, secretion to plant surfaces, gene duplication, metabolic diversification
Abstract
The Madagascar periwinkle (Catharanthus roseus) is highly specialized for the biosynthesis of many different monoterpenoid indole alkaloids (MIAs), many of which have powerful biological activities. Such MIAs include the commercially important chemotherapy drugs vinblastine, vincristine, and other synthetic derivatives that are derived from the coupling of catharanthine and vindoline. However, previous studies have shown that biosynthesis of these MIAs involves extensive movement of metabolites between specialized internal leaf cells and the leaf epidermis that require the involvement of unknown secretory processes for mobilizing catharanthine to the leaf surface and vindoline to internal leaf cells. Spatial separation of vindoline and catharanthine provides a clear explanation for the low levels of dimers that accumulate in intact plants. The present work describes the molecular cloning and functional identification of a unique catharanthine transporter (CrTPT2) that is expressed predominantly in the epidermis of young leaves. CrTPT2 gene expression is activated by treatment with catharanthine, and its in planta silencing redistributes catharanthine to increase the levels of catharanthine–vindoline drug dimers in the leaves. Phylogenetic analysis shows that CrTPT2 is closely related to a key transporter involved in cuticle assembly in plants and that may be unique to MIA-producing plant species, where it mediates secretion of alkaloids to the plant surface.
The single cell epidermal layer of plant leaves synthesizes a protective wax layer and a variety of other complex metabolites that regulate internal and external physiological processes in response to biotic and abiotic environmental factors (1, 2). The young leaf epidermis (LE) of Madagascar periwinkle (Catharanthus roseus) is a biochemical hive of biosynthetic activity involved in the assembly of monoterpenoid indole alkaloids (MIAs), pentacyclic terpenes, flavonoids, and secretory lipids as illustrated by transcript analysis (2) of this specialized cell type. Catharanthus leaves also produce low levels of the dimeric MIAs vinblastine and vincristine, which have been used in cancer chemotherapy for the past 40 y (3). The low levels of these anticancer drugs, which are derived from the coupling of catharanthine and vindoline, have been attributed to the specialized role of several leaf cell types in assembly of different parts of this pathway, with the LE being involved in the downstream elaboration of MIAs and in uncharacterized secretion mechanisms that traffic these molecules in the living plant (2, 4–7). The discovery that catharanthine accumulates entirely in the wax exudates on the leaf surface and that vindoline is found within specialized internal leaf cells that preferentially express the terminal pathway reactions (7) showed that spatial separation of these precursors accounted for the low levels of dimeric MIAs occurring in the plant.
Although the mechanism of export of catharanthine is unknown, several different alkaloid transporters have recently been reviewed (8, 9) that include a multidrug and toxin extrusion transporter involved in transport of nicotine to tobacco plant vacuoles (10), ATP-binding cassette (ABC) transporters involved in alkaloid transport in Coptis japonica (11), and assorted multidrug transporters in plants (12) and in yeast (13). The present study characterizes CrTPT2, a member of the pleiotropic drug resistance (PDR) family of ABC transporters that is predominantly expressed in the epidermal cell layers of younger leaves, as the catharanthine efflux transporter responsible for exporting this MIA to the surface of C. roseus leaves.
Results
Expression Analysis of CrTPT2 in C. roseus.
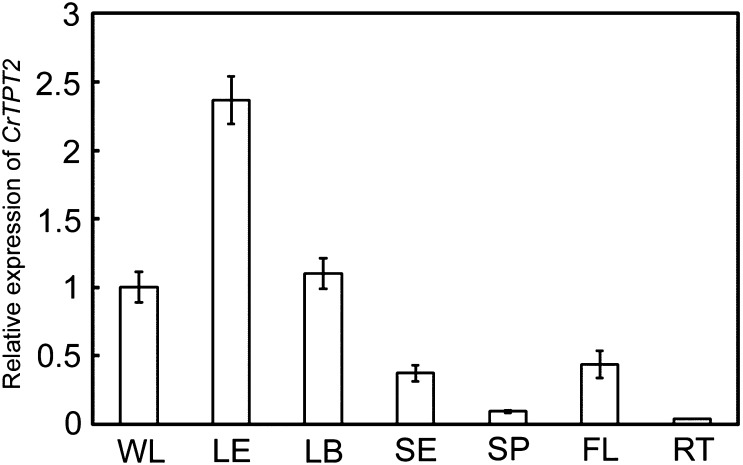
The specialization of the LE of Catharanthus for MIA biosynthesis (2, 4–7) coupled with the preferred secretion of catharanthine to the leaf surface (7) suggested that MIA-specific transporters might be present within these cells. Inspection of a LE-enriched transcript database that was shown to preferentially express MIA pathway genes (2) identified several putative catharanthine transporters, including a typical full ABC transporter (CrTPT2) that contains two transmembrane domains and two nucleotide-binding domains belonging to the PDR subfamily. Treatment of 2-wk-old Catharanthus seedlings with catharanthine or methyl jasmonate increased CrTPT2 expression sixfold and fourfold, respectively, within 8 h, whereas neither salicylic acid nor indole-3-acetic acid triggered this response (Fig. S1A). Developmental studies with older plants showed that expression of CrTPT2 was restricted to younger leaves (Fig. S1B, leaf pairs 1 and 2) that are well known as primary sites of catharanthine and vindoline biosynthesis in C. roseus (2, 4–7). Further analyses of CrTPT2 expression in whole young leaves (WL), LE, young leaf base (LB), stem epidermis (SE), stem pith (SP), flowers (FL), and roots (RT) suggested that only above-ground tissues expressed this transporter, with expression being significantly enriched in the LE where catharanthine was synthesized (Fig. 1).
Fig. 1.
Real-time PCR analysis for CrTPT2 expression in WL, LE, LB, SE, SP, FL, and RT. Results were normalized to 60S ribosomal RNA and are shown relative to the level in WL. The error bars represent standard deviations from three technical replicates.
CrTPT2 Functions as a Catharanthine Transporter.
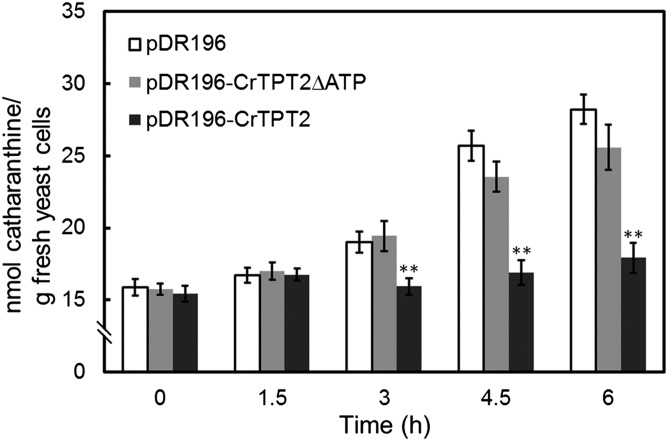
To examine whether CrTPT2 functions as a catharanthine transporter, we expressed it in the yeast strain AD12345678 lacking eight major yeast ABC transporter genes that confer multidrug resistance (13). Transient expression of this gene in yeast and in onion epidermal cells suggested its plasma membrane localization (Fig. S2 A and B). Yeast cells expressing CrTPT2 accumulated <18 nmol of catharanthine per gram of cells (Fig. 2 and Fig. S2C; pDR196–CrTPT2) compared with the almost 30 nmol found in cells expressing the empty vector (EV; Fig. 2 and Fig. S2C; pDR196). The efflux of catharanthine by CrTPT2 was abolished when both ATP- and GTP-binding motifs were deleted (Fig. 2 and Fig. S2C; pDR196–CrTPT2ΔATP), suggesting that transport of this MIA was ATP-dependent. Expression of CrTPT2 as a GFP fusion in yeast remained as functionally active as a catharanthine efflux transporter as cells expressing CrTPT2, whereas CrTPT2ΔATP fused with GFP (Fig. S2C; pDR196–GFP–CrTPT2ΔATP) abolished catharanthine transport, but the deletions did not change the yeast plasma membrane localization of the transporter (Fig. S2A).
Fig. 2.
CrTPT2 functions as an ATP-dependent catharanthine efflux transporter in yeast cells. EV control (pDR196), CrTPT2 (pDR196–CrTPT2), and truncated CrTPT2 (pDR196–CrTPT2ΔATP) expressing yeast cells were incubated in half-strength synthetic dropout medium supplemented with 0.3 mM catharanthine. The error bars represent standard deviations from three biological replicates, and asterisks indicate statistically significant differences compared with pDR196. **P < 0.01.
The CrTPT2 efflux transporter was highly specific for the transport of catharanthine compared with other MIAs (Fig. S3A). However, the low accumulation levels observed (Fig. S3A Inset; see PDR196) for vindoline, tabersonine, and strictosidine in yeast cells might not be sufficiently high to measure accurate transport of these MIAs (Fig. S3A Inset). To address these transport efficiency issues with live cells, vesicles prepared from pDR196 and pDR196–CrTPT2 yeast cells were used for in vitro transport studies. The results obtained indicate that, whereas tabersonine and strictosidine accumulate to similar levels as catharanthine in pDR196 expressing vesicles, only catharanthine was exported by CrTPT2-expressing yeast vesicles (Fig. S3B). To examine whether CrTPT5 has the same function of catharanthine transport because of its sequence similarity to CrTPT2, we expressed it in yeast cells. The results showed that, whereas CrTPT5 slowed yeast growth rates compared with the strain expressing EV or CrTPT2 (Fig. S4A), it was not able to transport catharanthine (Fig. S4B). The slow growth of the CrTPT5-expressing yeast strain suggests that CrTPT5 is active and is affecting its growth in a manner that remains to be determined.
Virus-Induced Gene Silencing of CrTPT2 in C. roseus.
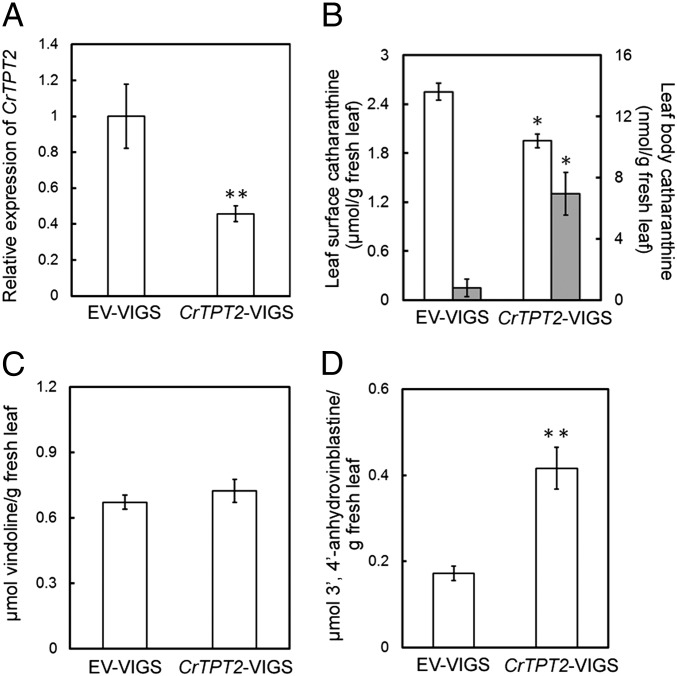
Virus-induced gene silencing (VIGS) has been useful for identifying genes involved in MIA biosynthesis (14, 15). VIGS of CrTPT2 in 24-mo-old plants triggered a 60% decline of transcript levels in emerging leaves compared with those found in EV control (Fig. 3A), whereas a 50% and 66% decline was observed in emerging leaf pairs 1 and 2 of CrTPT2-silenced seedlings (Fig. S5A). The leaf surface catharanthine levels in emerging leaves of 24-mo-old plants declined by ∼25% to 1.8 μmol/g fresh weight in CrTPT2–VIGS plants compared with EV controls, whereas catharanthine levels remaining within leaves rose from 0.7 nmol/g fresh leaf weight in control plants to >7 nmol/g fresh leaf weight in CrTPT2–VIGS–silenced plants (Fig. 3B). Remarkably, silencing of CrTPT2 also reduced surface leaf catharanthine levels of leaf pairs 1 and 2 by 30–50% in CrTPT2–VIGS seedlings compared with EV controls (Fig. S5B). Vindoline levels remained the same in EV–VIGS and CrTPT2–VIGS plants and seedlings (Fig. 3C and Fig. S5C), whereas 3′,4′-anhydrovinblastine increased by 30% in CrTPT2–VIGS seedlings compared with EV–VIGS seedlings (Fig. S5D), and they were 300% higher in CrTPT2–VIGS plants compared with EV–VIGS plants (Fig. 3D).
Fig. 3.
Virus-induced gene silencing (VIGS) of CrTPT2 in 24-mo-old Catharanthus plants. (A) Expression of CrTPT2 in EV–VIGS and CrTPT2–VIGS plants by real-time PCR. (B) Accumulation of catharanthine in leaf surfaces extracted with chloroform (white box) and in the leaf body extracted by methanol after chloroform extraction (grey box) in control (EV-VIGS) and CrTPT2-VIGS plant. (C) Accumulation of vindoline in control (EV–VIGS) and CrTPT2–VIGS plants. (D) Accumulation of 3′,4′-anhydrovinblastine in control (EV–VIGS) and CrTPT2–VIGS plants. The error bars represent SEs from 10 biological replicates of VIGS mature plants, and asterisks indicates statistically significant differences of transcript or MIA levels in CrTPT2–VIGS compared with EV–VIGS plants. *P < 0.05; **P < 0.01.
Discussion
The majority of MIAs are derived from the assembly of tryptamine and the monoterpene secologanin to form the central intermediate strictosidine, and the pathway is highly regulated by development-, environment-, organ-, and cell-specific controls (16) that are poorly understood at the molecular level. Remarkably, the biosynthesis of MIAs in underground tissues seem to occur entirely within the same protoderm and cortical cell types in Catharanthus root tips (17), whereas the pathway appears to be compartmented in multiple cell types in above-ground organs such as stems, leaves, and flowers (4). The 2-C-methyl-d-erythritol-4-phosphate pathway together with geraniol-10-hydroxylase and iridoid synthase have been localized to biochemically specialized internal phloem parenchyma (IPAP) cells (18–22), whereas the terminal loganic acid-O-methyltransferase and secologanin synthase reactions in secologanin biosynthesis are expressed exclusively within the epidermis of young leaves and stems (2). Assuming that the remaining reactions involved in the secologanin pathway occur in IPAP cells, it is possible that a loganic acid transporter would be involved in the translocation of this iridoid between IPAP and epidermal cells. The expression of tryptophan decarboxylase, strictosidine synthase, strictosidine β-glucosidase, tabersonine 16-hydroxylase, and 16-hydroxytabersonine-16-O-methyltransferase appears to be localized to the epidermis of leaves, stems, and flower buds, whereas the last three steps in vindoline biosynthesis are expressed within leaf mesophyll cells or in specialized idioblasts and laticifers (16). The secretion of catharanthine to the surface of Catharanthus leaves compared with vindoline accumulating in specialized cells within the leaf mesophyll (7) provided some logical explanations for the complex intercellular compartmentation observed for elaboration of MIAs in this plant species. These data have also been used to suggest that biochemical specialization for MIA biosynthesis could have evolved in the epidermal cells in above-ground organs of some plant families together with secretion systems to deliver MIAs to plant surfaces where their biological activities might be the most effective (7).
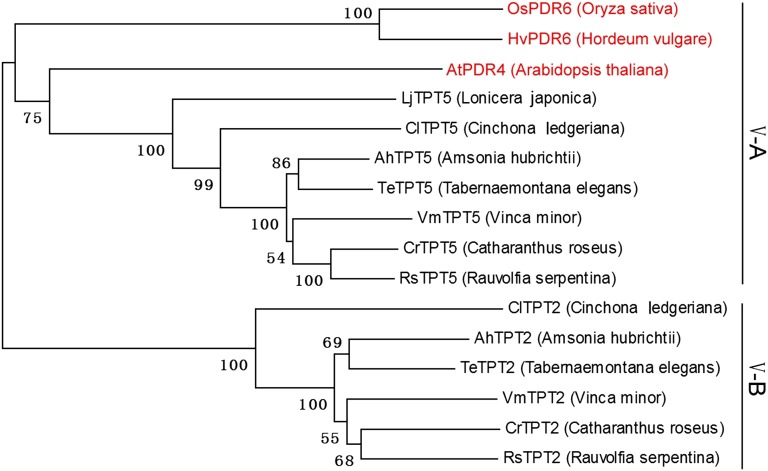
The present study identifies a unique member of the PDR subfamily V of ABC transporters, CrTPT2, which is involved in the secretion of catharanthine to the surface of Catharanthus leaves. Phylogenetic analysis of the 15 and 23 PDR transporters found in Arabidopsis thaliana and in Oryza sativa (rice), respectively, organized them into five clusters or subfamilies (9) (Fig. S6). Remarkably two Catharanthus PDR transporters (CrTPT2 and CrTPT5) both clustered in subfamily V together with unique Arabidopsis (AtPDR4), rice (OsPDR6), and barley (HvPDR6) PDR genes known to be involved in cuticle assembly (23, 24). Bioinformatic analysis of a large, publicly available annotated 454 C. roseus sequence library (PhytoMetaSyn; www.phytometasyn.ca/; refs. 25 and 26) led to the identification of CrTPT2 and CrTPT5 belonging to this subfamily that are 67.5% identical in their amino acid sequences (Fig. S6). Further bioinformatic analyses of large annotated 454 sequence libraries (PhytoMetaSyn; www.phytometasyn.ca/; refs. 25 and 26) produced from Vinca minor, Tabernaemontana elegans, Amsonia hubrichtii, Rauvolfia serpentina, and Cinchona ledgeriana that are active in MIA biosynthesis also contained two PDR transporters in subfamily V, whereas a single subfamily V PDR transporter could be found in Lonicera japonica (PhytoMetaSyn; www.phytometasyn.ca/; refs. 25 and 26) that makes the iridoid secologanin, but not MIAs. Phylogenetic analysis of these genes divided subfamily V into common subfamily V-A PDRs that are more closely related to AtPDR4, OsPDR6, and HvPDR6 known to be involved in cuticle formation and subfamily V-B PDRs that are specifically associated with five geographically separate species (Eurasian V. minor, African T. elegans, North American A. hubrichtii, Indian R. serpentina, and South American C. ledgeriana) specialized for MIA biosynthesis (Fig. 4). This observation raises the possibility that gene duplication of a cuticle-forming ABC transporter (23, 24) in MIA pathway-expressing species was a key evolutionary event in the acquisition of a novel physiological role associated with the secretion of MIAs in this family of plants. The existence of surface MIAs in members of MIA-producing species has been attributed to their role as possible feeding deterrents, but more studies should be dedicated to identifying their biological roles.
Fig. 4.
Comparative phylogenetic analysis of cluster V genes from MIA-producing species (C. ledgeriana, A. hubrichtii, T. elegans, V. minor, and C. roseus), iridoid-producing species (L. japonica), Arabidopsis, rice, and barley. The full-length PDR protein sequences were aligned by using the muscle program, and the neighbor-joining method was used to construct a phylogenetic tree with 10,000 bootstrap replicates. The GenBank accession nos. are as follows: CrTPT2 (KC511771), CrTPT5 (KC511772), VmTPT2 (KC511773), VmTPT5 (KC511774), AhTPT2 (KC511775), AhTPT5 (KC511776), TeTPT2 (KC511777), TeTPT5 (KC511778), RsTPT2 (KC511779), RsTPT5 (KC511780), ClTPT2 (KC511781), ClTPT5 (KC511782), LjTPT5 (KC511783), HvPDR6 (AB534899), and OsPDR6 (AJ535049).
When the functionally active CrTPT2 gene was expressed in yeast, it behaved as an ABC efflux catharanthine transporter (Fig. 2), in contrast to the Cjmdr1 gene from C. japonica that functions as a plasma membrane influx pump for berberine in xylem tissues of rhizomes (9, 11). Although the Coptis transporter appears to be involved in the mechanism of translocation of berberine from the site of biosynthesis in the root to the rhizome, CrTPT2 mediates the secretion of catharanthine and perhaps other MIAs to the leaf surface from the site of biosynthesis in leaf epidermal cells. Remarkably, the CjMDR1 transporter is the most similar to AtPGP4 (AtABCB4), which is part of the AtABCB family of auxin efflux carriers, that appears to be involved in auxin-transport processes associated with lateral root and root hair development (27). These results show that different classes of ABC transporters involved in functions common to plants have been evolved by natural selection for transporting alkaloids.
The suppression of CrTPT2 expression by VIGS in developing seedlings and in mature plants triggered a decline in the leaf-surface catharanthine levels and increased catharanthine levels within leaves that, in turn, triggered an increase in catharanthine–vindoline dimers within leaves (Fig. 3). This result supports the role of CrTPT2 as an efflux transporter and suggests that creation of CrTPT2 knockouts in Catharanthus could lead to commercially useful plants that accumulate high levels of anticancer alkaloids such as vinblastine and vincristine. The catharanthine efflux transport activity of CrTPT2 and its preferential expression in the LE at stages of development when MIA biosynthesis is active helps to explain how catharanthine accumulates on the leaf surfaces of C. roseus, Catharanthus ovalis, and Catharanthus trichophyllus (7). With this discovery, we expect that other transporters remain to be identified that may control the export of posttabersonine MIA intermediates from the leaf epidermis to specialized internal leaf cells where vindoline is assembled. Similarly, the trafficking of iridoid precursors from specialized IPAP cells, where they are biosynthesized to the LE, likely involve other uncharacterized transporters where intermediates are elaborated into secologanin for assembly of MIAs.
Conclusions
The surfaces of plants have been well characterized for the diversity of their small molecule chemistry that includes a range of wax mixtures containing unusual fatty acids, terpenes and phenols (1, 2, 28–30). In the case of alkaloids, one study suggested that traces of soluble pyrolizidine alkaloids in Scenecio jacobea (31) and more recently that all of the catharanthine in C. roseus (7) could be found on their leaf surfaces, respectively. The presence of such diverse plant surface chemistries that include MIAs may expand the range of metabolites with biological activity present on the surface that are likely to protect plants against the majority of animal and insect herbivores, fungi, bacteria, and other organisms.
Methods
Molecular Cloning of CrTPT2 and CrTPT5 in C. roseus.
The C. roseus (L.) G. Don, Little Delicata plants were grown under a 16-h-light/8-h-dark cycle in the greenhouse at 25 °C. Leaves from the youngest leaf pair (LP1; 1 g) were harvested for RNA extraction by using TRIzol reagent (Invitrogen). After reaction of RNA with AMV Reverse Transcriptase (Promega) mix, the generated cDNAs were used directly as PCR template for cloning full-length CrTPT2 cDNA with specific primers (sense, 5′-CACTTCCCATCATCACACAACAT-3′; antisense, 5′-ATCGGCTATGTTAGTTCCTGTCTT-3′) and CrTPT5 cDNA with specific primers (sense, 5′-TATTCACTTCATTTCGTTTGAAG-3′; antisense, 5′-TGGGGAGGACAACACAGATA-3′).
Cultivation of Catharanthus Seedlings and CrTPT2 Expression Analysis.
Catharanthus seeds were sterilized and cultivated on wet sterile filter paper for 12 d (3 d in the dark and 9 d of a 16-h-light/8-h-dark cycle at 25 °C). Approximately 20 of the 12-d-old seedlings were transferred to 25-mL sterile water/250-mL flask on a shaker (120 rpm) with the same photoperiod, and they were submitted to various chemical treatments (from 1,000× DMSO stocks) after 24 h. Seedlings were harvested at the indicated times for CrTPT2 transcript analysis. The expression levels of CrTPT2 were analyzed with CFX Manager Software (Bio-Rad) and normalized to 60S ribosomal RNA by using the 2-ΔΔCt method. Primers were designed based on sequences from CrTPT2 and Catharanthus 60S ribosomal RNA: CrTPT2-forward, 5′-CAAAGGTGGACTTACGAACA-3′; CrTPT2-reverse, 5′-CATCCAGCCCTGAAGTGGG-3′; and 60S-fw, 5′-TCTTAGTTGGAATGTTCAGCACCTG-3′; 60S-rev, 5′-CAAGGTTGGAGCCCCTGCTCGTGTT-3′.
Functional Analysis of CrTPT2 in Yeast Cells.
CrTPT2 ORF was PCR-amplified with the primers 5′-GCACTAGTATGTTGTTGAATTCCTCTGC-3′ and 5′-GCGTCGACTCATCTCCTTTGGAAGTTGA-3′ to introduce Spe1 and Sal1 restriction sites and mobilized to pDR196 yeast expression vector digested with Spe1/Sal1 to produce plasmid pDR196–CrTPT2. The truncated CrTPT2 missing the two ATP/GTP-binding motifs (18 nt of each) was produced by PCR to obtain three partial CrTPT2 sequences that included the N-terminal sequence with the ATG start codon to just before the first ATP-/GTP-binding motif (Nco1 site was introduced), C-terminal sequence just after the second ATP/GTP-binding motif (Nco1 site was introduced) to the stop codon TGA, and the sequence between two ATP/GTP-binding motifs (Nco1 sites were introduced at both ends). The ligation of the three partial sequences through the Nco1 sites produced the truncated CrTPT2 cDNA lacking the two ATP-/GTP-binding sites that was mobilized in the pDR196 yeast expression vector to produce plasmid pDR196–CrTPT2∆ATP. CrTPT5 ORF was PCR-amplified with the primers 5′-GCACTAGTATGTGGAATTCGGCGGAGAA-3′ and 5′-GCGTCGACCTAGCTTACTTTAAGTGAAAC-3′ to introduce Spe1 and Sal1 restriction sites and mobilized to pDR196 yeast expression vector digested with Spe1/Sal1 to produce plasmid pDR196–CrTPT5. The plasmids, pDR196, pDR196–CrTPT2, pDR196–CrTPT2∆ATP, and pDR196–CrTPT5, were transformed to the yeast strain AD12345678 (MATα, PDR1-3, ura3, his1, ∆yor1::hisG, ∆snq2::hisG, ∆pdr5::hisG, ∆pdr10::hisG, ∆pdr11::hisG, ∆ycf1::hisG, ∆pdr3::hisG, and ∆pdr15::hisG) by the lithium acetate method. The yeast transformants were precultured in 250-mL flasks containing 50 mL of synthetic dropout medium [without uracil (−uracil)], harvested at A600 = 1.0, and resuspended in 50-mL half-strength SD medium (−uracil) containing 0.3 μmol/mL catharanthine (from 0.3 mmol/mL DMSO stock). For experiments relating to Fig. 2 and Figs. S2C and S4B, the cells were incubated at 29 °C with shaking at 180 rpm, harvested at the indicated times by centrifugation, and washed twice with sterile Milli-Q water. Next, yeast cells were disrupted with acid-washed glass beads in methanol by TissueLyser (QIAGEN) for 15 min at 30 Hz. The samples were centrifuged, and supernatants were filtered for ultra performance liquid chromatography (UPLC) analysis (2).
For substrate specificity assays, different MIAs (4–6 nmol/mL) were added into 2 mL of pDR196 or pDR196–CrTPT2 yeast cells (A600 = 1.0) in half-strength SD medium (−uracil). After 5-h incubation with shaking (180 rpm) at 29 °C, the yeast cultures were centrifuged, and the medium was collected for UPLC analysis. The amounts of substrates inside yeast cells were calculated by subtracting the MIA levels found in the medium at time 0 h minus that found at time 5 h. The transport activity was shown as the ratio of MIAs in pDR196 cells/pDR196–CrTPT2 cells. In vitro MIAs transport by yeast membrane vesicles was performed as described (32) with the following modifications. Yeast cell combined with breaking buffer (32), 5 mM Mg⋅ATP, and acid-washed glass beads were lysed by TissueLyser (QIAGEN) treatment for 5 min at 30 Hz. After harvesting membranes, the membrane pellet was washed two times with vesicle buffer (32) to remove Mg⋅ATP found outside the membrane, and the final pellet was suspended in transport buffer (32) without Mg⋅ATP to a protein concentration of ∼5 mg/mL to perform transport assays. Microsomal membrane proteins were quantified by the Bradford method. Although vesicles prepared by this method are a mixture of outside-in and inside-in membranes, only inside-in vesicles can hydrolyze ATP to drive membrane transport because the ATP inside the vesicles permeate the membrane. After the transport assay was performed at room temperature for 20 min, the vesicles were immediately loaded on a prewetted 0.45-μm filter (Millipore) and washed three times with transport buffer. The MIAs remaining within washed vesicles were extracted with methanol at room temperature and analyzed by UPLC.
Localization of GFP–CrTPT2 Fusion Protein in Yeast Cells and Onion Epidermal Cells.
The subcellular localization of CrTPT2 in yeast cells or onion epidermal cells was assessed with GFP–CrTPT2 fusion construct. The full-length cDNA fragment carrying the coding region of GFP was PCR-amplified by Phusion DNA polymerase (NEB) with the primers 5′-GCACTAGTATGGTGAGCAAGGGCGAGG-3′ and 5′- CGACTAGTTTACTTGTACAGCTCGTCC-3′ or 5′-CGACTAGTAAACTTGTACAGCTCGTCC-3′ (stop codon was mutated from TAA to TTT) to introduce the Spe1 site at both ends and cloned in pGEM-T easy vector. For yeast expression, the GFP fragment (with stop codon) was obtained by Spe1 digestion and ligated to pDR196 precut with Spe1 followed by dephosphorylation to produce pDR196–GFP, or the GFP fragment (without stop codon) was obtained by Spe1 digestion and ligated to pDR196–CrTPT2 or pDR196–CrTPT2∆ATP precut with Spe1 followed by dephosphorylation to produce pDR196–GFP–CrTPT2 and pDR196–GFP–CrTPT2∆ATP. For Agrobacterium tumefasciens-based onion epidermal transient expression, the Spe1-digested GFP fragments (with or without stop codon) were ligated to pBI121GD or pBI121GD–CrTPT2 (pBI121GD, binary vector pBI121 without GUS) precut with Xba1, followed by dephosphorylation to obtain pBI121GD–GFP and pBI121GD–GFP–CrTPT2. For each construct, the GFP orientation was confirmed by sequencing.
Yeast cells (strain AD12345678) were transformed with pDR196–GFP, pDR196–GFP–CrTPT2, and pDR196–GFP–CrTPT2∆ATP by the lithium acetate method. The transformants of pDR196–GFP, pDR196–GFP–CrTPT2, and pDR196–GFP–CrTPT2∆ATP obtained were grown in 50 mL of SD/−uracil medium at pH 6.0 until OD600 = 1.0. For transient expression in onion epidermal cells, pBI121GD–GFP and pBI121GD–GFP–CrTPT2 were transformed to Agrobacterium strain GV3101 to treat onion epidermal cells for GFP or GFP–CrTPT2 expression according to the method developed by Sun et al. (33)
GFP fluorescence was examined with a Nikon confocal microscope with a laser at an excitation wavelength of 488 nm and collection of emitted fluorescence after passage through a broad bandpass filter (500–550 nm). The resulting signal was amplified and digitalized, and the consistent picture was reconstituted by Nikon software.
VIGS of CrTPT2 in C. roseus.
A specific 388-bp sequence from the 3′ UTR of CrTPT2 was used for the construction of the CrTPT2 VIGS vector. A fragment generated from Catharanthus cDNA using Taq DNA polymerase (Fermentas) and specific primers 5′-TCAAAGCCTTCAACTTCCAA-3′ and 5′- TCCTTTTTGGTAGTTTGCATC-3′ was ligated to pGEM-T easy vector and digested with EcoR1, and the isolated insert was ligated to pTRV2 vector precut with EcoR1 followed by dephosphorylation to produce pTRV2–CrTPT2 vector. The DNA sequencing of cloned product was verified after mobilizing into pTRV2 vector.
Transformed Agrobacterium (pTRV1, pTRV2, and pTRV2–CrTPT2) was grown in 3 mL of LB medium containing 50 μg/mL kanamycin at 29 °C overnight. We used 0.5 mL of independent overnight cultures to inoculate 50 mL of LB medium containing 50 μg/mL kanamycin, 10 mM Mes, and 20 μM acetosyringone. Cultures were maintained at 29 °C overnight, harvested by centrifugation at 3,000 × g for 10 min, and resuspended in 5 mL of infiltration solution (10 mM MgCl2, 20 mM Mes, and 200 µM acetosyringone) for 3-h incubation with shaking at 29 °C. Agrobacterium harboring CrTPT2 and the pTRV2 EV were mixed at a ratio of 1:1(vol/vol) with Agrobacterium containing pTRV1 before infiltration. Young Catharanthus plants used for VIGS analysis were 2-wk-old seedlings. The stem just below the apical meristem was wounded with sterile toothpicks with the Agrobacterium mixture. Leaf pairs 1 and 2 were harvested for gene expression and MIA analysis after 2–3 wk. The 24-mo-old Catharanthus plants were grown under 16-h-light/8-h-dark cycles at 25 °C in the greenhouse. The same VIGS experiments were performed by inoculating the stem just below the lateral meristems of different branches occurring on the same plant with Agrobacterium harboring CrTPT2 or with the pTRV2 EV that had been mixed with Agrobacterium harboring pTRV1 vector. By using this approach, at least 60 inoculations with the same or different constructs could be made on the same plant. Three weeks after the initiation of the experiment, 10 biological replicates of leaf pair 1 from the CrTPT2–VIGS and EV control (EV–VIGS) experiments were collected for gene expression and MIA production analysis.
Supplementary Material
Acknowledgments
We thank the next-generation sequencing personnel at the McGill University-Genome Québec-Innovation Centre for skilled technical work; and Christoph Sensen, Mei Xiao, and Ye Zhang for dedicated bioinformatic support and large-scale gene annotation efforts that helped in the identification of ABC transporters from the Phytometasyn Web site. This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (to V.D.L.), an NSERC/Binational Agricultural Research and Development Fund/Agriculture Canada team grant, Canada Research Chairs (V.D.L.), Genome Canada, Genome Alberta, Genome Prairie, Genome British Columbia, the Canada Foundation for Innovation, the Ontario Ministry of Research and Innovation, the National Research Council of Canada, and other government and private-sector partners.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. KC511771 (CrTPT2), KC511772 (CrTPT5), KC511773 (VmTPT2), KC511774 (VmTPT5), KC511775 (AhTPT2), KC511776 (AhTPT5), KC511777 (TeTPT2), KC511778 (TeTPT5), KC511779 (RsTPT2), KC511780 (RsTPT5), KC511781 (ClTPT2), KC511782 (ClTPT5), and KC511783 (LjTPT5)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307504110/-/DCSupplemental.
References
- 1.Samuels L, Kunst L, Jetter R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu Rev Plant Biol. 2008;59:683–707. doi: 10.1146/annurev.arplant.59.103006.093219. [DOI] [PubMed] [Google Scholar]
- 2.Murata J, Roepke J, Gordon H, De Luca V. The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell. 2008;20(3):524–542. doi: 10.1105/tpc.107.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce HL, Alice Miller M. The evolution of cancer research and drug discovery at Lilly Research Laboratories. Adv Enzyme Regul. 2005;45:229–255. doi: 10.1016/j.advenzreg.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 4.St-Pierre B, Vazquez-Flota FA, De Luca V. Multicellular compartmentation of catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell. 1999;11(5):887–900. doi: 10.1105/tpc.11.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata J, De Luca V. Localization of tabersonine 16-hydroxylase and 16-OH tabersonine-16-O-methyltransferase to leaf epidermal cells defines them as a major site of precursor biosynthesis in the vindoline pathway in Catharanthus roseus. Plant J. 2005;44(4):581–594. doi: 10.1111/j.1365-313X.2005.02557.x. [DOI] [PubMed] [Google Scholar]
- 6.Levac D, Murata J, Kim WS, De Luca V. Application of carborundum abrasion for investigating the leaf epidermis: Molecular cloning of Catharanthus roseus 16-hydroxytabersonine-16-O-methyltransferase. Plant J. 2008;53(2):225–236. doi: 10.1111/j.1365-313X.2007.03337.x. [DOI] [PubMed] [Google Scholar]
- 7.Roepke J, et al. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc Natl Acad Sci USA. 2010;107(34):15287–15292. doi: 10.1073/pnas.0911451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shitan N, Yazaki K. Accumulation and membrane transport of plant alkaloids. Curr Pharm Biotechnol. 2007;8(4):244–252. doi: 10.2174/138920107781387429. [DOI] [PubMed] [Google Scholar]
- 9.Crouzet J, Trombik T, Fraysse AS, Boutry M. Organization and function of the plant pleiotropic drug resistance ABC transporter family. FEBS Lett. 2006;580(4):1123–1130. doi: 10.1016/j.febslet.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 10.Morita M, et al. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci USA. 2009;106(7):2447–2452. doi: 10.1073/pnas.0812512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shitan N, et al. Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci USA. 2003;100(2):751–756. doi: 10.1073/pnas.0134257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, He Z, Pandey GK, Tsuchiya T, Luan S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem. 2002;277(7):5360–5368. doi: 10.1074/jbc.M108777200. [DOI] [PubMed] [Google Scholar]
- 13.Decottignies A, et al. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem. 1998;273(20):12612–12622. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- 14.De Luca V, Salim V, Atsumi SM, Yu F. Mining the biodiversity of plants: A revolution in the making. Science. 2012;336(6089):1658–1661. doi: 10.1126/science.1217410. [DOI] [PubMed] [Google Scholar]
- 15.Liscombe DK, O’Connor SE. A virus-induced gene silencing approach to understanding alkaloid metabolism in Catharanthus roseus. Phytochemistry. 2011;72(16):1969–1977. doi: 10.1016/j.phytochem.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Facchini PJ, De Luca V. Opium poppy and Madagascar periwinkle: Model non-model systems to investigate alkaloid biosynthesis in plants. Plant J. 2008;54(4):763–784. doi: 10.1111/j.1365-313X.2008.03438.x. [DOI] [PubMed] [Google Scholar]
- 17.Laflamme P, St-Pierre B, De Luca V, De Luca V. Molecular and biochemical analysis of a Madagascar periwinkle root-specific minovincinine-19-hydroxy-O-acetyltransferase. Plant Physiol. 2001;125(1):189–198. doi: 10.1104/pp.125.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contin A, van der Heijden R, Lefeber AW, Verpoorte R. The iridoid glucoside secologanin is derived from the novel triose phosphate/pyruvate pathway in a Catharanthus roseus cell culture. FEBS Lett. 1998;434(3):413–416. doi: 10.1016/s0014-5793(98)01022-9. [DOI] [PubMed] [Google Scholar]
- 19.Burlat V, Oudin A, Courtois M, Rideau M, St-Pierre B. Co-expression of three MEP pathway genes and geraniol 10-hydroxylase in internal phloem parenchyma of Catharanthus roseus implicates multicellular translocation of intermediates during the biosynthesis of monoterpene indole alkaloids and isoprenoid-derived primary metabolites. Plant J. 2004;38(1):131–141. doi: 10.1111/j.1365-313X.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 20.Mahroug S, Burlat V, St-Pierre B. Cellular and sub-cellular organisation of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev. 2007;6:363–381. [Google Scholar]
- 21.Oudin A, et al. Spatial distribution and hormonal regulation of gene products from methyl erythritol phosphate and monoterpene-secoiridoid pathways in Catharanthus roseus. Plant Mol Biol. 2007;65(1-2):13–30. doi: 10.1007/s11103-007-9190-7. [DOI] [PubMed] [Google Scholar]
- 22.Geu-Flores F, et al. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature. 2012;492(7427):138–142. doi: 10.1038/nature11692. [DOI] [PubMed] [Google Scholar]
- 23.Bessire M, et al. A member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette transporters is required for the formation of a functional cuticle in Arabidopsis. Plant Cell. 2011;23(5):1958–1970. doi: 10.1105/tpc.111.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, et al. An ATP-binding cassette subfamily G full transporter is essential for the retention of leaf water in both wild barley and rice. Proc Natl Acad Sci USA. 2011;108(30):12354–12359. doi: 10.1073/pnas.1108444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facchini PJ, et al. Synthetic biosystems for the production of high-value plant metabolites. Trends Biotechnol. 2012;30(3):127–131. doi: 10.1016/j.tibtech.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Xiao M, et al. Transcriptome analysis based on next-generation sequencing of non-model plants producing specialized metabolites of biotechnological interest. J Biotechnol. 2013;166(3):122–134. doi: 10.1016/j.jbiotec.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Santelia D, et al. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2005;579(24):5399–5406. doi: 10.1016/j.febslet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 28.Usia T, Watabe T, Kadota S, Tezuka Y. Cytochrome P450 2D6 (CYP2D6) inhibitory constituents of Catharanthus roseus. Biol Pharm Bull. 2005;28(6):1021–1024. doi: 10.1248/bpb.28.1021. [DOI] [PubMed] [Google Scholar]
- 29.Kubo I, Matsumoto A. Secreted oleanolic acid on the cuticle Olea europaea (Oleaceae): A chemical barrier to fungal attack. Experienta. 1984;40:937–938. [Google Scholar]
- 30.Valant-Vetschera KM, Brem B. Chemodiversity of exudate flavonoids as highlighted by publications of Eckhard Wollenweber. Nat. Prod. Comm. 2006;1:921–926. [Google Scholar]
- 31.Vrieling K, Derridj S. Pyrrolizidine alkaloids in and on the leaf surface of Senecio jacobaea L. Phytochemistry. 2003;64(7):1223–1228. doi: 10.1016/j.phytochem.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, et al. Oxalate efflux transporter from the brown rot fungus Fomitopsis palustris. Appl Environ Microbiol. 2010;76(23):7683–7690. doi: 10.1128/AEM.00829-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun W, Cao Z, Li Y, Zhang H. A simple and effective method for protein subcellular localization using Agrobacterium-mediated transformation of onion epidermal cells. Biologia. 2007;62:529–532. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.