Significance
Life History Theory posits a trade-off between mating and parenting effort, which may explain some of the observed variance in human fathers’ parenting behavior. The current study tested this hypothesis by measuring aspects of reproductive biology related to mating effort, as well as paternal nurturing behavior and the brain activity related to it. Both testosterone levels and testes volume were negatively correlated with paternal caregiving. In response to viewing pictures of one’s own child, brain activity in a key component of the reward and motivation system predicted paternal caregiving and was negatively related to testes volume. These results suggest that the biology of human males reflects a trade-off between mating effort and parenting effort.
Keywords: empathy, sperm competition
Abstract
Despite the well-documented benefits afforded the children of invested fathers in modern Western societies, some fathers choose not to invest in their children. Why do some men make this choice? Life History Theory offers an explanation for variation in parental investment by positing a trade-off between mating and parenting effort, which may explain some of the observed variance in human fathers’ parenting behavior. We tested this hypothesis by measuring aspects of reproductive biology related to mating effort, as well as paternal nurturing behavior and the brain activity related to it. Both plasma testosterone levels and testes volume were independently inversely correlated with paternal caregiving. In response to viewing pictures of one’s own child, activity in the ventral tegmental area—a key component of the mesolimbic dopamine reward and motivation system—predicted paternal caregiving and was negatively related to testes volume. Our results suggest that the biology of human males reflects a trade-off between mating effort and parenting effort, as indexed by testicular size and nurturing-related brain function, respectively.
In modern Western societies, paternal involvement is associated with reduced child mortality and morbidity (1, 2), as well as improved social, psychological, and educational outcomes (3, 4). Despite this finding, there is remarkable variation in paternal involvement (5) and father absence has increased precipitously over the last half of the 20th century (3), which raises the question: Why do some men choose not to invest in their children?
A branch of evolutionary theory known as Life History Theory posits a trade-off between mating and parenting effort (6–8). Given that organisms have finite amounts of energy to expend on reproduction, evolution optimizes the allocation of resources toward either mating or parenting so as to maximize fitness. Evidence in support of this trade-off is found throughout the animal kingdom, including humans (8). Although Life History Theory is traditionally invoked to explain differences between species, it has also been applied to explain individual differences within a species, including humans (9, 10). However, no study to date has investigated whether human anatomy and brain function reflect a trade-off between mating and parenting investment.
Several lines of research suggest that testosterone may bias males toward a mating strategy and away from a parenting strategy (11). In humans, low levels of testosterone are associated with reduced libido (12), and high levels predict mating success (13). Within married couples, testosterone levels are negatively correlated with relationship quality and high levels predict divorce (14) as well as polygyny (15). In contrast, a decrease in testosterone accompanies fatherhood in several species (11, 16), including humans (13, 17), where variation in testosterone is inversely related to paternal involvement (13, 15). Experimental manipulation of testosterone suggests that high testosterone is causally associated with both increased mating effort and decreased parenting effort (18). Thus, decreases in testosterone may suppress mating effort that might detract from investment in the infant. A decrease in testosterone might also both suppress impulsive aggression and promote empathic responding toward a highly vulnerable infant, who shapes parental behavior mainly through crying (19–22).
In addition to testosterone, both testicular size and function are related to mating strategies (reviewed in ref. 23). For example, among primates, monogamy and single-male polygyny are associated with smaller testes size compared with multimale, multifemale breeding systems. Presumably, female promiscuity selects for sperm competition and enlarged testes size in multimale, multifemale groups (24, 25). Intraspecific variation in testes size is related to reproductive success (26) and copulatory rates (27) in two different mammalian species, and one group claims to have shown that testes size predicts mating strategies in humans (28), although another group failed to replicate this finding (29). Although the testes produce testosterone, inter- and intraspecies differences in testes size likely reflect differences in sperm production more so than hormone production, because seminiferous tubules account for 70–80% of the volume of the testes and testes volume is more highly correlated with sperm count and quality than with testosterone levels (29–31). Thus, an inverse correlation between testes size and parenting effort would reflect a trade-off between spermatogenesis, a form of mating effort, and parental care. Here we investigate whether testes size explains variance in parenting effort over and above testosterone alone.
If human fathers vary in their allocation of resources to parenting and mating, brain function should mediate this variability. Animal models point to the importance of the mesolimbic dopamine (DA) system in the appetitive drive to nurture offspring, with DA-producing cell bodies in the ventral tegmental area (VTA) projecting to the nucleus accumbens to motivate proactive care of infants (32). Activity in the VTA is consistently observed in parents exposed to child auditory (cry) and visual (face) stimuli (reviewed in ref. 33), and gray matter in the VTA increases in new mothers during the early postpartum period (34).
These data suggest the following hypotheses: (i) testosterone and testes size will be negatively correlated with paternal investment; (ii) neural activity in the VTA in response to viewing pictures of one’s own child will predict paternal investment; and (iii) testosterone and testes size will be negatively correlated with neural activity in the VTA. To test these hypotheses, we recruited biological fathers of children age 1 or 2 y, and assessed mother- and father-reported paternal caregiving and desired levels of caregiving, as well as testes volume, plasma testosterone levels, and brain activity while fathers viewed pictures of their child.
Results
Reproductive Biology and Parenting Behavior.
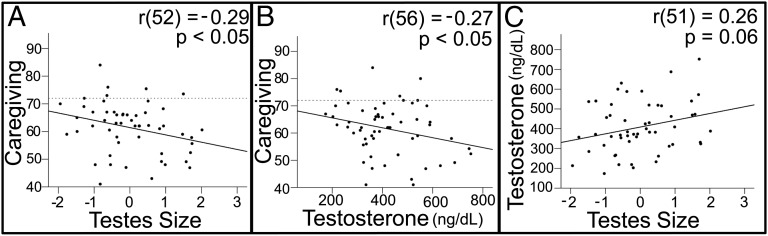
Although testes volume was not related to body mass, there was a significant linear correlation between testes volume and height [r(53) = 0.27, P < 0.05]. Therefore, residual testes volume, controlling for height, was used in subsequent analyses. Residual testes volume was negatively related to paternal caregiving [r(52) = −0.29, P < 0.05] (Fig. 1A). Testosterone levels were also negatively correlated with caregiving [r(56) = −0.27, P < 0.05] (Fig. 1B).
Fig. 1.
Relationship between reproductive biology and paternal investment. (A) Caregiving vs. testes volume residuals after testes volume was regressed against height. The dotted line indicates the score (72) at which mothers and fathers are equally responsible for their child’s daily care. Scores below 72 imply that the mother does more than the father and scores above 72 imply the opposite. (B) Caregiving vs. plasma testosterone levels. (C) Plasma testosterone levels vs. testes volume residuals (regressed against height).
Although these data support our first hypothesis, it could be the case that extraneous factors, such as stress or socioeconomic status, were related to testosterone and also interfered with fathers’ ability to take a more active parental role, despite a desire to be active in their child’s life. However, testosterone was also negatively correlated with fathers’ desired level of caregiving [r(64) = −0.26, P < 0.05] and there was a strong trend in the same direction for testes volume [r(53) = −0.25 P = 0.06], limiting this possibility and further supporting the Life History Theory interpretation. It could also be the case that fathers who provide less instrumental support for their children are investing in them in other ways. For example, they might work longer hours to provide greater financial investment. However, neither testes size nor testosterone were correlated with the number of hours fathers worked per week [residual testes: r(43) = −0.05, P = 0.75; testosterone: r(60) = 0.04, P = 0.79], nor with fathers’ earnings [residual testes: r(44) = −0.02, P = 0.911; testosterone: r(61) = 0.15, P = 0.24].
Testes volume showed a moderate positive correlation with testosterone levels [r(51) = 0.26, P = 0.06] (Fig. 1C). To determine the relative contributions of testosterone, testicular volume, and other independent variables related to paternal caregiving, we conducted linear regression analyses with caregiving as the dependent measure. We began with a model that included all independent variables that were related to caregiving in simple bivariate correlation analyses: testicular volume, testosterone, father’s earnings [r(63) = −0.33, P < 0.01], and father’s hours worked per week [r(61) = −0.28, P < 0.05]. The only independent variable that accounted for a significant amount of the variance in caregiving was testosterone [β = −0.42, t(46) = −3.07, P < 0.005] (Table 1). Thus, upon removal of the insignificant variables, our initial reduced model had only a single predictor variable. However, given our a priori hypothesis regarding the relationship between caregiving and testicular volume, we then added testes volume to this reduced model and found that both testosterone and testicular volume account for unique variance in caregiving, with testosterone accounting for slightly more of the variance (Table 1). In summary and in support of the first hypothesis, testicular volume and testosterone were inversely related to paternal caregiving and desired paternal caregiving and unrelated to occupational or financial investment, and both testicular volume and testosterone account for unique variance in caregiving.
Table 1.
Results of linear regression analyses of variance in paternal caregiving
| Actual caregiving | R2 | Intercept | Variable | Standard β | t |
| Full model | 0.35 | 83.7 | Testosterone | −0.42 | −3.07*** |
| Testes volume | −0.11 | −0.83 | |||
| Father’s earnings | −0.13 | −0.83 | |||
| Father’s hours worked | −0.24 | −1.53 | |||
| Reduced model | 0.21 | 77.9 | Testosterone | −0.34 | −2.61** |
| Testes volume | −0.23 | −1.75* |
Results of linear regression analyses showing the variance in paternal caregiving explained by the full model, which includes all variables correlated with caregiving in a simple correlation analysis, and the reduced model, which includes only measures of reproductive biology. *P < 0.10, **P < 0.05, and ***P < 0.005. P values are two-tailed.
Main Effect of the Child Task.
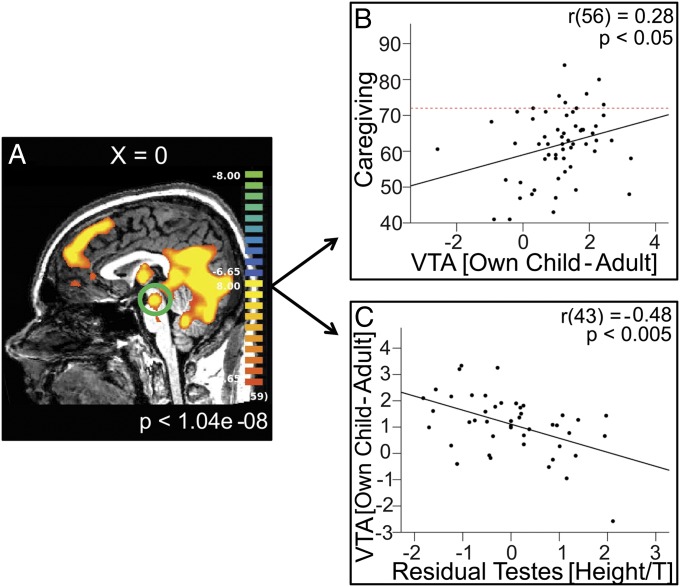
The child picture stimuli robustly activated regions of the brain known to be important for face processing (fusiform gyrus), a region involved in thinking about others’ mental states (dorsal medial prefrontal cortex) (35), and areas known to be crucial to the onset of maternal behavior and consistently activated when mothers and fathers view pictures of their children (thalamocingulate and mesolimbic DA systems) (Fig. 2A). Table 2 lists significant activations for the contrasts: “Own–Adult” and “Unknown–Adult.”
Fig. 2.
Relationship between brain function, paternal behavior, and reproductive biology. (A) Main effect of the contrast Own Child–Adult for all emotions combined, Bonferroni-corrected P < 0.001, uncorrected P < 1.04 × 10−8. (B) Plot of caregiving vs. β-contrast values Own Child–Adult from functionally derived VTA ROI. The dotted red line indicates the score (72) at which mothers and fathers are equally responsible for their child’s daily care. (C) Plot of β-contrast values Own Child–Adult from VTA ROI vs. residual testes volume (regressed against height and testosterone).
Table 2.
Main effect of the child task
| Category | Brodmann's area | x | y | z | Peak t | voxels |
| Own Child–Adult | ||||||
| Right fusiform gyrus, occipital gyrus, extending into posterior cingulate gyrus and thalamus | 37, 19 | 11 | −68 | −12 | 11.83 | 5,581 |
| Ventral tegmental area/substantia nigra* | −4 | −20 | −15 | 8.75 | 34 | |
| Right precentral gyrus | 6 | 44 | 4 | 33 | 8.49 | 166 |
| Superior frontal gyrus (medial part) | 8 | −1 | 46 | 39 | 9.16 | 632 |
| Medial frontal gyrus | 10 | −1 | 52 | 0 | 7.00 | 15 |
| Left middle frontal gyrus | 9 | −43 | 16 | 39 | 8.71 | 87 |
| Left superior temporal sulcus, extending into middle and superior temporal gyrus | 39 | −46 | −59 | 24 | 10.11 | 248 |
| Unknown Child–Adult | ||||||
| Right fusiform gyrus, extending into precuneus, occipital gyrus | 37, 31, 19 | 26 | −50 | −9 | 11.46 | 712 |
| Right medial frontal gyrus | 10 | 8 | 58 | 6 | 8.71 | 70 |
| Precuneus | 7 | −4 | −62 | 30 | 7.96 | 92 |
| Left fusiform gyrus | 37 | −25 | −47 | −9 | 9.45 | 246 |
| Left middle occipital gyrus | 19 | −28 | −80 | 18 | 7.15 | 20 |
The results are thresholdeded using Bonferroni-corrected P < 0.001, uncorrected P < 1.04 e-08.
The VTA/substantia nigra fell within the larger activation listed above, and was defined by the local maxima.
Brain Activity, Reproductive Biology, and Parenting Behavior.
Region of interest analysis.
Averaged β-values across all subjects from the functionally derived regions of interest (ROI) in the VTA as a function of stimulus type are plotted in Fig. S1. Contrast values were calculated for Own–Adult and Unknown–Adult and entered into bivariate correlation analyses with caregiving, testosterone, and testes volume. The second hypothesis, that activity of the mesolimbic DA system, and specifically the VTA, would predict paternal caregiving, was confirmed: β-contrast values for Own–Adult were positively correlated with caregiving [r(56) = 0.28, P < 0.05] (Fig. 2B). Similarly, β-contrast values for Unknown–Adult were significantly correlated with caregiving [r(56) = 0.29, P < 0.05]. The final hypothesis, that testosterone and testes volume would be inversely correlated with VTA activity, was partially confirmed. Residual testes size was negatively correlated with activity in the VTA for the contrast Own–Adult [r(45) = −0.48, P < 0.005], and the correlation did not weaken when controlling for the portion of testes volume associated with testosterone [r(43) = −0.48, P < 0.005] (Fig. 2C). There were significant correlations in the same direction for the contrast Unknown–Adult [with caregiving: r(56) = 0.29, P < 0.05; with residual testes volume: r(43) = −0.31, P < 0.05]. Testosterone levels were not significantly related to VTA activity for either contrast.
Whole-brain exploratory analysis.
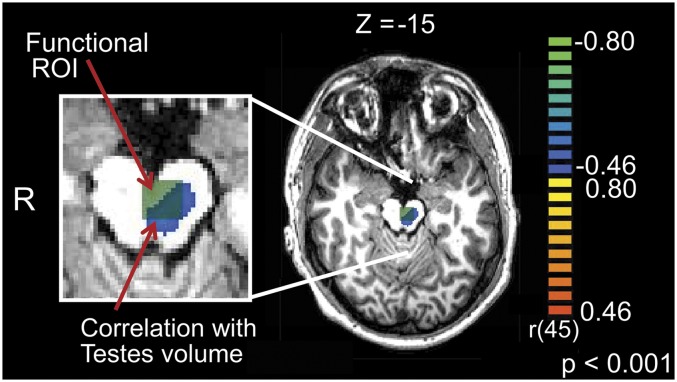
The results of a whole-brain exploratory analysis using testosterone and testes volume as covariates for the contrast Own–Adult are listed in Table S1. These analyses revealed a very specific correlation between testes volume and activation in the midbrain, in a region that overlapped the VTA functional ROI used in the above bivariate correlation analyses (Fig. 3).
Fig. 3.
Functionally derived ROI in the VTA/substantia nigra (green) overlaid on the results of a whole-brain exploratory analysis using testes volume as a covariate (blue). Results are thresholded at P < 0.001, uncorrected.
Discussion
Collectively, these data provide the most direct support to date that the biology of human males reflects a trade-off between mating and parenting effort. Fathers’ testicular volume and testosterone levels were inversely related to parental investment and testes volume was inversely correlated with nurturing-related brain activity when viewing pictures of their own child. The fact that both testosterone and testicular volume predicted unique variance in paternal caregiving suggests that these two measures may exert independent influence on paternal behavior. Given the role of testosterone in the development of secondary sexual characteristics, such as muscle and bone growth, as well as its role in status-related competition (21), testosterone levels may be more related to precopulatory, intrasexual competition. If this is the case, the data presented here suggest that fathers with greater investment in precopulatory competition invest less in day-to-day care of their children. On the other hand, testicular volume is most directly related to spermatogenesis and sperm quality (30), and thus testicular volume may reflect postcopulatory mating investment. Taken together, these data suggest that men who invest more in both pre- and postcopulatory mating competition participate less in caregiving. Related to this, it is important to note that testicular volume is likely a more stable measure than is testosterone. The fact that testicular volume, but not testosterone, was correlated with nurturing-related brain activity may indicate that testicular volume is a constitutional feature that, when coupled with brain function, represents a broad phenotype that is associated with particular life-history strategies among human males. In further support of this interpretation, VTA activity in response to unknown children was also correlated with testicular volume, suggesting that testicular volume is related to specific responsiveness to children, rather than to familiar individuals more generally.
However, the etiology of the relationship between reproductive biology and nurturing-related brain activity is unclear from this cross-sectional study. Although seasonal fluctuations in testes size are well documented in species with breeding seasons, surprisingly little is known about the relative stability of testes size in humans (23) or about the environmental, social, or nutritional factors that may influence it, and thus, it remains unclear whether greater testes volume is a cause or a consequence of male life-history strategies. If testicular volume is causal, individual differences in testes may be governed by evolved genetic influences, or they may be shaped by environmental factors analogous to the role of father absence in the timing of female menarche (36). Similar effects of early life experience on reproductive strategies have been found for males, with stress and unpredictability correlated with relatively earlier and more promiscuous sexual activity (37). It is possible, for example, that fathers in the present study who invest more in reproductive biology and less in parenting had experienced a more stressful and unpredictable childhood, or one without a father present.
Although the data presented herein link the reproductive biology related to mating effort with reduced parenting effort and related brain function, an important next step will be to more thoroughly examine the link between reproductive biology and sexual behavior in human males. Previous studies have found an inverse correlation between mating effort and parenting effort in humans (38), and future studies should investigate whether male biology and physiology are linked with a more promiscuous mating strategy, in addition to the decreased investment in parenting shown here. Because accurately assessing sexual behavior is notoriously difficult and may be particularly difficult in men who are married and have children (39, 40), future studies will require creative and well-validated methods (e.g., ref. 41).
The finding that the VTA response to one’s child was correlated with paternal care is consistent with studies of parental care in several species of animals, including humans (32, 42–44), and extends what is known about the neural systems supporting human paternal caregiving. Numan (45) argues that adult female rats have separate systems motivating approach and avoidance of offspring, and that maternal behavior emerges when the former exceeds the latter. The mesolimbic DA reward and motivation system is crucial for motivating approach and for the appetitive drive to nurture offspring. For example, this system is more active in rat mothers that lick and groom their offspring more frequently (46), and selective destruction of the DA neurons in the VTA disrupts pup retrieval (32). The finding that activity in the VTA in response to viewing their child predicts paternal caregiving suggests that this model of the neurobiology of rat maternal care is relevant to paternal care in humans. Given that oxytocin (OT) facilitates maternal behavior in rats by acting at the VTA to facilitate DA release in the nucleus accumbens (32), an important next step will be to image fathers following pretreatment with intranasal OT (47) to determine if OT augments activity in the reward system pathways when viewing pictures of children.
One prior study has attempted to relate paternal functional MRI (fMRI) activity to paternal behavior outside the scanner, and showed that fathers who exhibited more sensitive caregiving had less activity in the medial orbitofrontal cortex in response to their own child (48), a finding that is difficult to interpret within existing models of the neurobiology of parental care. We interpret the present results to suggest that fathers who are more rewarded by their child’s face are more involved in their care, perhaps because the child’s appearance positively reinforces caregiving.
An alternative interpretation of these data is that fathers who spend more time with their children may come to find the child more rewarding by virtue of the stronger bond they develop. These findings highlight the need to conduct longitudinal studies that would more definitively establish the direction of causality linking VTA activity and paternal caregiving. Such studies would determine whether more invested caregivers have more activity in the VTA in response to their child from the outset, which in turn motivates caregiving, or whether interacting with their child entrains a father’s reward system to be more responsive to that child. Or perhaps the causal arrow runs both directions, implicating a positive feedback loop in which fathers with a greater neural response in the VTA are more motivated to care for their child, leading to increased caregiving that further augments the reward response.
Materials and Methods
Subjects.
We recruited 70 biological fathers of children aged 1 or 2 y who were currently cohabitating with the child’s mother, using flyers posted around the Emory University campus, at local parks, daycare centers, and with an electronic advertisement on Facebook. Enrollment in the study required participation by the father, mother, and child. The study was approved by the Emory Institutional Review Board, and all participants gave written informed consent (mothers signed on behalf of children). Fathers had normal or corrected-to-normal (with contact lenses) vision and were screened and excluded for self-reported history of head trauma, seizures, or other neurological disorders, psychiatric illness, alcoholism, or any other substance abuse, serious medical illness, claustrophobia, and for ferrous metal in any part of body.
Fathers were between the age of 21 and 55 y (mean = 33.0, SD = 5.80) and had between one and four children, with two as the modal number (mean = 1.86, SD = 0.82) (see Table S2 for descriptive statistics). Sixty-five of the fathers were married to their partner. Two married fathers did not indicate how long they had been married, but for those that provided that information (n = 63), the average amount of time married fathers had been married was 5.87 y (SD = 3.41). For the five fathers who were not married to their partner, the average amount of time they had lived with their partner was 2.3 y (SD = 0.45).
Testosterone values were acquired from 66 of the fathers. Blood was drawn between 0730 hours and 1455 hours. There was no significant correlation between testosterone and time of blood draw (Fig. S2). Fathers were told that the MRI scan of the testes was an optional portion of the study. Therefore, total testes volume was obtained from 55 men.
Photograph Stimuli.
Unknown adult photographs were acquired from male and female trained actors who were asked to generate happy, sad, and neutral facial expressions. Unknown child photographs were obtained from male and female age-matched children. We captured eight pictures of unknown and own children making each facial expression during a play session. If the child did not make one of the facial expressions naturally, sad faces were elicited by the mother leaving the room or taking a favorite toy or cell phone from the child, and happy faces were elicited with singing, dancing, or tickling. In addition to their own children, fathers viewed pictures of an unknown child and unknown adult matched on sex and ethnicity with their own child. See Fig. S3 for examples of each condition.
Design.
During the first session, the mother and child came to the laboratory and mothers completed self-report parenting questionnaires while experimenters photographed children as described above (Fig. S4). In a separate session, fathers began by completing self-report parenting questionnaires (described in more detail below). After completing the questionnaires, fathers’ height and weight were measured. Next, the fathers were fitted with an indwelling intravenous catheter and 16 mL of blood was drawn immediately upon catheterization. Subjects were next positioned in the MRI scanner, where they received structural and fMRI scans of their brain (n = 65) followed by a structural MRI of their testes. Five fathers completed the behavioral and testes imaging portion of the study but not the fMRI scan.
Parenting Questionnaires.
To measure caregiving, we used Sherryl Goodman’s Parental Responsibility Scale that combines two scales, the McBride and Mills Parental Responsibility Scale and the Montague and Walker-Andrews Child Care Activity Questionnaire (49, 50). The measure asks the parent to designate who has primary responsibility for 24 tasks along a five-point scale, ranging from 1 (mother almost always) to 5 (father almost always). Responsibility was defined for the parent as remembering, planning, and scheduling the task. Example items include: “Take the baby to preventative health care clinic,” “Bathe baby,” and “Attend to baby during night waking.” The measure also allows parents to report “N/A” if the item is not applicable to their family (for example, “Pick up baby at day care/sitter”). If “N/A” was chosen for an item, we performed a mean replace using that participant’s mean score. Answers were summed to create an actual caregiving score, such that a higher relative score reflected greater paternal caregiving. If mothers and fathers were equally responsible for all items, the total score would be 72. For each item, the parent was also asked “How would you like it to be?” These items were summed to acquire a desired caregiving score, for which a higher relative score reflected greater desired paternal caregiving.
Completed actual caregiving scores were acquired from 66 fathers and 67 mothers. There was very high agreement between father’s and mother’s ratings of actual caregiving: r(61) = 0.70, P < 0.001 (Fig. S5). We used mother’s ratings of actual caregiving in all analyses for two reasons. First, it was determined a priori that mothers’ ratings would likely be less subject to social desirability biases. Second, post hoc reliability analyses revealed that although both measures had good internal reliability, the Cronbach’s α for the mother’s actual caregiving was higher (mother: α = 0.83; father: α = 0.82). Complete scores on the desired caregiving scale were acquired from 65 fathers, and this measure had acceptable reliability (α = 0.74).
Testosterone.
Blood samples were centrifuged at 4 °C within 20 min of blood draw. Plasma was collected and frozen at −80 °C until assayed. Assays were analyzed in duplicate by the Biomarkers Core Laboratory of the Yerkes National Primate Research Center at Emory University using a coated-tube RIA kit (Coat-A-Count Total Testosterone, Cat No. TKTT1, Siemens). On the day of the assay, frozen plasma samples were thawed, centrifuged for 30 min at 3,000 revolutions per minute with a Thermo Scientific, Sorvall ST 16R rotor and centrifuge, and assayed according to the protocol provided by the manufacturer. The interassay CV% ranged from 4.05–4.37%, intra-assay CV% ranged from 2.07–2.28%, and the sensitivity of the assay was 6.0–1,667.00 ng/dL.
Anatomical Image Acquisition.
Subjects were positioned in the Siemens Trio 3T MRI scanner. Subjects lay motionless in a supine position in the scanner with padded head restraint to minimize head movement during scanning. Each scanning session began with a 15-s scout, followed by a 5-min T1-weighted MPRAGE scan [repetition time (TR) = 2,600 ms, echo time (TE) = 3.02 ms, matrix = 256 × 256, field of view (FOV) = 256 mm, slice thickness = 1.00 mm, gap = 0 mm].
fMRI Image Acquisition.
Functional scans used an echo-planar imaging sequence with the following parameters: TR = 2,000 ms, TE = 28 ms, matrix =64 × 64, FOV = 224 mm, slice thickness = 2.5 mm, gap thickness = 1.05 mm, 34 axial slices. TE was minimally decreased from the typical value (32 ms) to reduce magnetic susceptibility artifact in the orbitofrontal region. Subjects were imaged while viewing pictures of happy, sad, and neutral facial expressions in three different people: (i) their own child, (ii) an unknown child, and (iii) an unknown adult. Participants were instructed to “please observe each picture and try to share the emotions of the person in the picture.” For each expression, fathers viewed eight different pictures of the person making that expression over the course of four blocks, and each picture was viewed twice (Fig. S6). During a single block, four photographs of the same type were shown, each for 3 s. There was a 0.5-s fixation between each photograph. Thus, the duration of each block was 14 s. After every six blocks, subjects viewed a fixation block of equal duration. The total duration of the task was 9 min 48 s (36 face blocks + 6 fixation blocks × 14 s per block). Photographs were presented in pseudorandom order, and fathers always viewed own children at the end so that their responses to unknown children could be compared with the responses from nonfathers in a related study.
Functional Image Analysis.
Image preprocessing was conducted with Brain Voyager QX (v2.0.8) software (Brain Innovation). The first eight volumes of each run were discarded to allow the tissue magnetization to equilibrate. Preprocessing involved image realignment by six-parameter 3D motion correction, slice scan time correction using linear interpolation, spatial smoothing with a 8-mm full-width at half-maximum Gaussian kernel, and temporal smoothing with voxel-wise linear detrending and high-pass filtering of frequencies below three cycles per run length. Five subjects were excluded because of movement greater than 2 mm in the x, y, or z direction, so the final dataset contained 60 participants. Images were subsequently normalized into Talairach space (Talairach and Tournoux 1988).
A separate general linear model (GLM) was defined for each subject that examined the neural response to the following nine regressors: own infant’s happy face, own infant’s sad face, own infant’s neutral face, unknown infant’s happy face, unknown infant’s sad face, unknown infant’s neutral face, unknown adult’s happy face, unknown adult’s sad face, and unknown adult’s neutral face. Because there was substantial attenuation of the blood oxygen level-dependent response upon viewing each block repetition, only the first block of each condition was included in the GLM. Each regressor was convolved with a standardized model of the hemodynamic response. The resulting GLM was corrected for temporal autocorrelation using a first-order autoregressive model. For each subject, contrasts of parameter estimates for various predictors were computed at every voxel of the brain.
To evaluate our hypotheses of interest, we focused on Unknown–Adult and Own–Adult to assess fathers’ responses to children in general and to their own children, more specifically. The large sample size allowed us to threshold results at a Bonferroni-corrected P value of < 0.001, uncorrected P = 1.04 × 10−8. A functional ROI was defined in the VTA/substantia nigra by identifying the peak voxel for the contrast Own–Adult. All contiguously activated voxels within 10 voxels in the x, y, and z direction from the peak voxel were included in the ROI. Individual subject contrast values from this ROI for the contrasts Own–Adult and Unknown–Adult were explored in bivariate correlation analyses with testosterone, testes volume, caregiving, and desired caregiving.
We also conducted a whole-brain exploratory analysis using testosterone and testes volume as a covariate for the contrast Own–Adult. Results were thresholded at P < 0.001, uncorrected for multiple comparisons. Results for these analyses are listed in Table S1.
Testes MRI.
Upon completion of the fMRI, body array and spinal coils were placed on the subjects and they were moved further into the MRI to receive a testes scan. Each scanning session began with a 16-s scout, followed by a 5-min T2-weighted scan, during which two averages were acquired axially (TR = 3,500 ms, TE = 90 ms, matrix = 180 × 180, FOV = 180 mm, slice thickness = 2.00 mm, gap = 0 mm).
Testes images were analyzed using tools from the Oxford Center for Functional Magnetic Resonance Imaging of the Brain’s software library (FMRIB, Oxford; www.fmrib.ox.ac.uk/fsl). First, masks were drawn on the left and right testes separately, determined based on the axial slice with 0.6 × 0.6 × 2.0-mm voxels. Experimenters were blind to subject identity and characteristics. Masks were drawn by three experimenters. After extensive training and assessment standardization, the interrater reliability for each experimenter was assessed by measurement of four testes that had been measured by another experimenter, blinded to the other’s segmentation. Reliability was calculated using the absolute value of the difference between the two measures divided by the first expressed as a percentage (100 × |x − y|/x). All raters had interrater reliability exceeding 95%. Finally, the volume of the masks was calculated and summed to acquire total testes volume. One participant’s testes volume measurement was excluded because his value was 2.8 SDs above the mean (mean = 38,064; SD = 11,183) and was more than 13,000 mm3 larger than any recorded value found in the literature (30, 51–53). Of the more than 1,500 healthy, age-matched men in these studies, the largest reported value was 56,000 mm3, and this participant’s measurement was 69,736 mm3.
Supplementary Material
Acknowledgments
We thank Sherryl Goodman for guidance regarding the measurement of paternal behavior. Assay services were provided by the Biomarkers Core Laboratory at the Yerkes National Primate Research Center, which is supported by Yerkes National Primate Research Center Base Grant 2P51RR000165-51. This work was supported by a Positive Neuroscience Award from the John Templeton Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305579110/-/DCSupplemental.
References
- 1.Weitoft GR, Hjern A, Haglund B, Rosén M. Mortality, severe morbidity, and injury in children living with single parents in Sweden: A population-based study. Lancet. 2003;361(9354):289–295. doi: 10.1016/S0140-6736(03)12324-0. [DOI] [PubMed] [Google Scholar]
- 2.Gaudino JA, Jr, Jenkins B, Rochat RW. No fathers’ names: A risk factor for infant mortality in the State of Georgia, USA. Soc Sci Med. 1999;48(2):253–265. doi: 10.1016/s0277-9536(98)00342-6. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera NJ, Tamis-LeMonda CS, Bradley RH, Hofferth S, Lamb ME. Fatherhood in the twenty-first century. Child Dev. 2000;71(1):127–136. doi: 10.1111/1467-8624.00126. [DOI] [PubMed] [Google Scholar]
- 4.Sarkadi A, Kristiansson R, Oberklaid F, Bremberg S. Fathers’ involvement and children’s developmental outcomes: a systematic review of longitudinal studies. Acta Paediatr. 2008;97(2):153–158. doi: 10.1111/j.1651-2227.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 5.Hrdy SB. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Belknap Press; 2009. [Google Scholar]
- 6.Lack D. The Natural Regulation of Animal Numbers. Oxford: Clarendon; 1954. p. 343. [Google Scholar]
- 7.Trivers R. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man 1871–1971. Chicago, IL: Aldine; 1972. [Google Scholar]
- 8.Draper P, Harpending H. Father absence and reproductive strategy: An evolutionary perspective. J Anthropol Res. 1982;38(3):255–273. [Google Scholar]
- 9.Apicella CL, Marlowe FW. Men’s reproductive investment decisions—Mating, parenting, and self-perceived mate value. Hum Nat Interdiscip Biosoc Perspect. 2007;18(1):22–34. doi: 10.1007/BF02820844. [DOI] [PubMed] [Google Scholar]
- 10.Figueredo AJ, et al. The K-factor: Individual differences in life history strategy. Pers Individ Dif. 2005;39(8):1349–1360. [Google Scholar]
- 11.Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The “Challenge Hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136(6):829–846. [Google Scholar]
- 12.Wang C, et al. Testosterone Gel Study Group Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 13.Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc Natl Acad Sci USA. 2011;108(39):16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth A, Dabbs JM. Testosterone and men’s marriages. Soc Forces. 1993;72(2):463–477. [Google Scholar]
- 15.Alvergne A, Faurie C, Raymond M. Variation in testosterone levels and male reproductive effort: Insight from a polygynous human population. Horm Behav. 2009;56(5):491–497. doi: 10.1016/j.yhbeh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm Behav. 2001;40(2):139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- 17.Gray PB, Anderson KG. Fatherhood: Evolution and Human Paternal Behavior. Cambridge, MA: Harvard Univ Press; 2010. [Google Scholar]
- 18.Hunt KE, Hahn TP, Wingfield JC. Endocrine influences on parental care during a short breeding season: Testosterone and male parental care in Lapland Longspurs (Calcarius lapponicus) Behav Ecol Sociobiol. 1999;45(5):360–369. [Google Scholar]
- 19.Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42(4):399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- 20.Hermans EJ, Ramsey NF, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol Psychiatry. 2008;63(3):263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends Cogn Sci. 2011;15(6):263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 22.van Honk J, et al. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc Natl Acad Sci USA. 2011;108(8):3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin RD. The evolution of human reproduction: A primatological perspective. Am J Phys Anthropol. 2007;134(Suppl 45):59–84. doi: 10.1002/ajpa.20734. [DOI] [PubMed] [Google Scholar]
- 24.Harcourt AH, Harvey PH, Larson SG, Short RV. Testis weight, body weight and breeding system in primates. Nature. 1981;293(5827):55–57. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- 25.Harcourt A, Purvis A, Liles L. Sperm competition: Mating system, not breeding season, affects testes size of primates. Funct Ecol. 1995;9(3):468–476. [Google Scholar]
- 26.Schulte-Hostedde AI, Millar JS. Intraspecific variation of testis size and sperm length in the yellow-pine chipmunk (Tamias amoenus): Implications for sperm competition and reproductive success. Behav Ecol Sociobiol. 2004;55(3):272–277. [Google Scholar]
- 27.Preston BT, Stevenson IR, Pemberton JM, Coltman DW, Wilson K. Overt and covert competition in a promiscuous mammal: The importance of weaponry and testes size to male reproductive success. Proc Biol Sci. 2003;270(1515):633–640. doi: 10.1098/rspb.2002.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellis MA. Human Sperm Competition: Copulation, Masturbation and Infidelity. New York: Chapman & Hall; 1995. [Google Scholar]
- 29.Simmons LW, Firman RC, Rhodes G, Peters M. Human sperm competition: Testis size, sperm production and rates of extrapair copulations. Anim Behav. 2004;68(2):297–302. [Google Scholar]
- 30.Bahk JY, Jung JH, Jin LM, Min SK. Cut-off value of testes volume in young adults and correlation among testes volume, body mass index, hormonal level, and seminal profiles. Urology. 2010;75(6):1318–1323. doi: 10.1016/j.urology.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Møller AP. Ejaculate quality, testes size and sperm competition in primates. J Hum Evol. 1988;17(5):479–488. [Google Scholar]
- 32.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Rilling JK. The neural and hormonal bases of human parental care. Neuropsychologia. 2013;51(4):731–747. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Kim P, et al. The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 2010;124(5):695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieberman MD. Social cognitive neuroscience: A review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- 36.Tither JM, Ellis BJ. Impact of fathers on daughters’ age at menarche: A genetically and environmentally controlled sibling study. Dev Psychol. 2008;44(5):1409–1420. doi: 10.1037/a0013065. [DOI] [PubMed] [Google Scholar]
- 37.Belsky J, Schlomer GL, Ellis BJ. Beyond cumulative risk: Distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Dev Psychol. 2012;48(3):662–673. doi: 10.1037/a0024454. [DOI] [PubMed] [Google Scholar]
- 38.Apicella CL, Marlowe FW. Men’s reproductive investment decisions. Hum Nat. 2007;18(1):22–34. doi: 10.1007/BF02820844. [DOI] [PubMed] [Google Scholar]
- 39.Catania JA, Binson D, Canchola J, Pollack LM, Hauck W. Effects of interviewer gender, interviewer choice, and item wording on responses to questions concerning sexual behavior. Public Opin Q. 1996;60(3):345–375. [Google Scholar]
- 40.Fenton KA, Johnson AM, McManus S, Erens B. Measuring sexual behaviour: Methodological challenges in survey research. Sex Transm Infect. 2001;77(2):84–92. doi: 10.1136/sti.77.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehl MR, Pennebaker JW, Crow DM, Dabbs J, Price JH. The Electronically Activated Recorder (EAR): A device for sampling naturalistic daily activities and conversations. Behav Res Methods Instrum Comput. 2001;33(4):517–523. doi: 10.3758/bf03195410. [DOI] [PubMed] [Google Scholar]
- 42.Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: Brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36(13):2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49(1):12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 46.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wittfoth-Schardt D, et al. Oxytocin modulates neural reactivity to children's faces as a function of social salience. Neuropsychopharmacology. 2012;37(8):1799–1807. doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuo PX, Carp J, Light KC, Grewen KM. Neural responses to infants linked with behavioral interactions and testosterone in fathers. Biol Psychiatry. 2012;91(2):302–306. doi: 10.1016/j.biopsycho.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McBride BA, Mills G. A comparison of mother and father involvement with their preschool age children. Early Child Res Q. 1993;8(4):457–477. [Google Scholar]
- 50.Montague DR, Walker-Andrews AS. Mothers, fathers, and infants: The role of person familiarity and parental involvement in infants’ perception of emotion expressions. Child Dev. 2002;73(5):1339–1352. doi: 10.1111/1467-8624.00475. [DOI] [PubMed] [Google Scholar]
- 51.Aribarg A, Kenkeerati W, Vorapaiboonsak V, Leepipatpaiboon S, Farley TM. Testicular volume, semen profile and serum hormone levels in fertile Thai males. Int J Androl. 1986;9(3):170–180. doi: 10.1111/j.1365-2605.1986.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 52.Spyropoulos E, et al. Size of external genital organs and somatometric parameters among physically normal men younger than 40 years old. Urology. 2002;60(3):485–489, discussion 490–491. doi: 10.1016/s0090-4295(02)01869-1. [DOI] [PubMed] [Google Scholar]
- 53.Kothari LK, Gupta AS. Effect of ageing on the volume, structure and total Leydig cell content of the human testis. Int J Fertil. 1974;19(3):140–146. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.