Significance
The role of herbivore-associated microbes in modifying plant defenses has received scant attention. The Colorado potato beetle secretes symbiotic bacteria to wounds to manipulate plant defenses. The bacteria elicit salicylic acid (SA)-regulated defenses, and because SA signaling often negatively cross-talks with jasmonate signaling, plants are unable to fully activate their jasmonate-mediated resistance against the herbivore. From the plants’ perspective, they recognize herbivores not as such, but as microbial threats. We identified the specific bacteria from the beetle secretions and also characterized one of the bacterial effectors responsible for defense suppression. This clever, deceptive strategy for suppressing defenses has not been previously documented. Our results add a significant, unique concept to plant–insect interactions and how herbivores hijack plant defense signaling.
Abstract
Induced plant defenses in response to herbivore attack are modulated by cross-talk between jasmonic acid (JA)- and salicylic acid (SA)-signaling pathways. Oral secretions from some insect herbivores contain effectors that overcome these antiherbivore defenses. Herbivores possess diverse microbes in their digestive systems and these microbial symbionts can modify plant–insect interactions; however, the specific role of herbivore-associated microbes in manipulating plant defenses remains unclear. Here, we demonstrate that Colorado potato beetle (Leptinotarsa decemlineata) larvae exploit bacteria in their oral secretions to suppress antiherbivore defenses in tomato (Solanum lycopersicum). We found that antibiotic-untreated larvae decreased production of JA and JA-responsive antiherbivore defenses, but increased SA accumulation and SA-responsive gene expression. Beetles benefit from down-regulating plant defenses by exhibiting enhanced larval growth. In SA-deficient plants, suppression was not observed, indicating that suppression of JA-regulated defenses depends on the SA-signaling pathway. Applying bacteria isolated from larval oral secretions to wounded plants confirmed that three microbial symbionts belonging to the genera Stenotrophomonas, Pseudomonas, and Enterobacter are responsible for defense suppression. Additionally, reinoculation of these bacteria to antibiotic-treated larvae restored their ability to suppress defenses. Flagellin isolated from Pseudomonas sp. was associated with defense suppression. Our findings show that the herbivore exploits symbiotic bacteria as a decoy to deceive plants into incorrectly perceiving the threat as microbial. By interfering with the normal perception of herbivory, beetles can evade antiherbivore defenses of its host.
Plants defend themselves against herbivores via the induction of defense metabolites or proteins that are triggered when plants recognize insect-derived cues such as touch, tissue disruption, oviposition, and oral secretions (OSs; saliva and/or regurgitant) (1, 2). Phytohormones mediate specific defense responses depending on biotic attackers (3). Microbial pathogens that feed on dead tissues or cells and most herbivorous insects are susceptible to jasmonic acid (JA)/ethylene (ET)-regulated defenses, whereas microbial pathogens that require living hosts are susceptible to salicylic acid (SA)-regulated defense (3). It is well documented that cross-talk between JA/ET and SA plays an important role in fine tuning induced defenses to enhance plant fitness (3, 4).
As plants have evolved defense strategies in response to biotic attack, some herbivores produce effector molecules that suppress induced defenses of plants (5). Herbivore effectors are found in OSs and eggs but only a few of the effectors have been identified (7–9). Herbivore suppression of JA-regulated defenses can be mediated by cross-talk with the SA-signaling pathway or through other SA-independent means (6, 8, 10).
Many herbivorous insects harbor microbial symbionts that provide essential nutrients or vitamins and/or are associated with insect defenses against predators or parasites (11). In addition, symbionts can influence plant–insect interactions (12, 13). Symbiotic bacteria may allow some herbivores to expand the range of available host plants. When endosymbiotic bacteria of stinkbugs (Megacopta punctatissima), which feed on both leguminous crops and wild legumes, were transferred to Megacopta cribaria, whose host plants are restricted to wild legumes, M. cribaria performed better on soybean crops (14). Moreover, insect-associated microbes can change host plant physiology to benefit their insect host. Wolbachia-infected leaf miners (Phyllonorycter blancardella) elicit a green-island phenotype in apple, preserving photosynthetically active tissues in senescent leaves, and in turn, increase leaf miner performance (12). In maize roots, Wolbachia in western corn rootworms (Diabrotica virgifera virgifera) may suppress defense gene expression (13), although this is disputed (15). Despite a few examples, the role of insect symbionts as elicitors or effectors in modifying plant–insect interactions has received scant attention.
Previous research has shown that application of OSs from Colorado potato beetle (CPB; Leptinotarsa decemlineata) larvae to mechanically wounded plants suppressed induced defenses in tomato and potato compared with wounded and water-treated plants (16–18). To investigate potential mechanisms of defense suppression by CPB larvae, we asked if microbes in OSs from CPBs manipulate induced defenses. We found that larvae that fed on antibiotic-treated leaves failed to suppress JA-regulated defenses, whereas larvae not treated with antibiotics were able to suppress these defenses. The suppression of antiherbivore defenses was dependent on a functional SA-signaling pathway. Moreover, we identified symbiotic bacteria in OSs that suppressed plant defenses and this suppression increased larval performance. Thus, these data show that CPB larvae use orally secreted bacteria to manipulate plant defenses by triggering an ineffective defense pathway.
Results
Symbiotic Microbes in OSs Are Deposited During Larval Feeding.
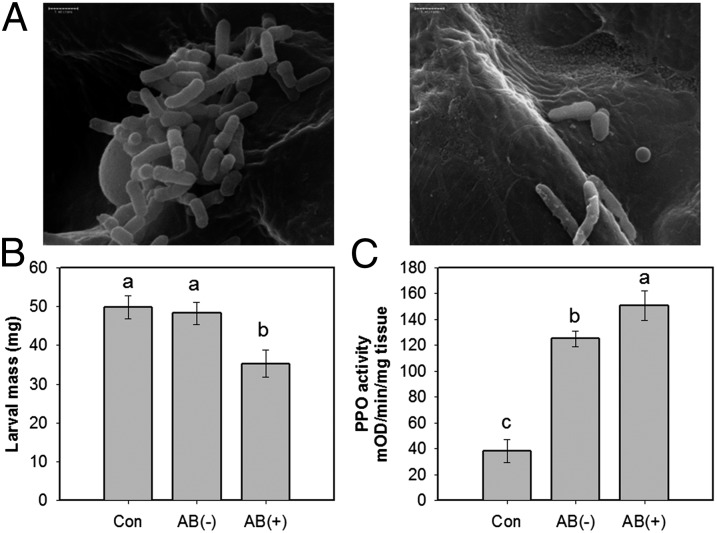
CPB larvae produce oral secretions when they feed on tomato leaves (18) (Movie S1). To determine if microbes in OSs are deposited on leaves during herbivory, we performed scanning electron microscopy (SEM) of leaves after 1 h of larval feeding, during which frass was not excreted. We found abundant bacteria on the wounded area damaged by larvae that fed on artificial diet without the addition of antibiotic (AB) compared with leaves damaged by larvae fed with AB (Fig. 1A). We also confirmed that larvae that fed on leaves treated or untreated with AB, hereafter referred to as AB-treated or untreated larvae, secreted similar amounts of OSs on leaves (SI Appendix, Figs. S1 and S2). These results indicate that CPB larvae deposited symbiotic microbes in their OSs.
Fig. 1.
(A) Scanning electron microscopy images of bacteria secreted onto leaves during feeding by CPB larvae after first feeding on an artificial diet with antibiotic (Right) or without antibiotic (Left). Damaged leaf tissues were prepared for SEM images 1 h after larval feeding. (Scale bar, 1 μm.) (B) Larval growth and (C) polyphenol oxidase (PPO) activities in plants damaged by larvae that fed on AB-treated or untreated leaves. Neonates were allowed to feed on excised leaflets from each treatment for 5 d and larval mass was determined. Values are means ± SEM. Different letters represent significant differences [ANOVA, P < 0.05; followed by LSD test; F(2,71) = 6.63, P = 0.0023, n = 23–26]. PPO activities were measured on subsamples from each treatment 48 h after insect feeding [F(2,9) = 27.13, P = 0.0002, n = 4]. To collect subsamples, two leaf discs from each of two leaves were pooled as one replicate. AB(−), plants damaged by untreated larvae; AB(+), plants damaged by AB-treated larvae; Con, undamaged plants.
Presence of Symbiotic Microbes Suppresses Plant Defense and Enhances Larval Growth.
To investigate whether the presence of symbiotic microbes benefits CPB larvae, we measured growth of neonate larvae on detached leaves damaged by AB-treated or untreated larvae and polyphenol oxidase (PPO) activity in plants damaged by these larvae. Measurement of PPO activity serves as a rapid, sensitive, and quantitative assay to study JA-responsive defenses. The neonate larvae that fed on leaves damaged by untreated larvae gained more weight than those that fed on leaves damaged by AB-treated larvae (Fig. 1B). Plants damaged by untreated larvae showed lower PPO activities than those damaged by AB-treated larvae (Fig. 1C). Interestingly, the neonate larvae that fed on leaves damaged by untreated larvae grew as well as those on undamaged leaves (Fig. 1B), although PPO activities in undamaged leaves were lower than leaves damaged by untreated larvae (Fig. 1C). Three of four independent experiments showed similar results (SI Appendix, Fig. S3). These results indicate that the suppression of induced defenses by symbionts was sufficient to enhance larval performance.
Plant Defenses Are Suppressed by Symbiotic Microbes in OSs.
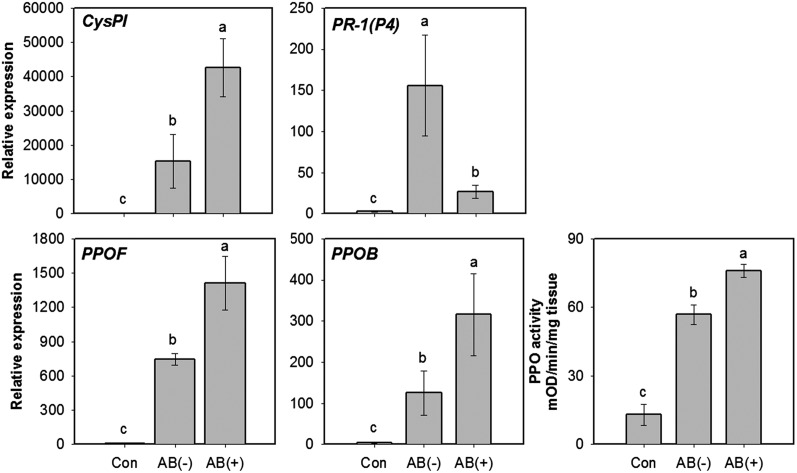
To investigate if microbial symbionts present in OSs affect induced defenses, we measured the expression of selected defense genes and enzymatic activities in plants damaged by AB-treated or untreated larvae. The cysteine proteinase inhibitor (CysPI) and polyphenol oxidase F/B (PPOF/B) were selected as JA-marker genes because they are well-established JA-inducible proteins that function against CPBs (19–21). For an SA-marker gene, pathogenesis-related 1 (PR-1(P4)) was selected (22). Feeding by untreated larvae decreased expression levels of the JA-responsive CysPI and PPOF/B compared with feeding by AB-treated larvae, whereas feeding by untreated larvae increased SA-responsive PR-1(P4) expression (Fig. 2). PPO activities in plants damaged by AB-treated larvae were higher than plants damaged by untreated larvae (Fig. 2). To further support our hypothesis, we applied OSs from AB-treated or untreated larvae to mechanically wounded plants. Plants wounded and treated with OSs from untreated larvae had lower PPO activity than those wounded and treated with water or OSs from AB-treated larvae (SI Appendix, Fig. S4). However, PPO activities in plants treated with OSs from AB-treated larvae were similar to plants treated with wounding and water (SI Appendix, Fig. S4). Application of AB to wounded plants had no effect on gene expression or PPO activity (SI Appendix, Fig. S5). These results demonstrate that the microbes in OSs from CPB larvae suppressed antiherbivore defenses.
Fig. 2.
Expression levels of JA- and SA-regulated genes in plants damaged by larvae that fed on AB-treated or untreated leaves. Gene expression was measured 24 h after initiation of insect feeding. Values are untransformed means ± SEM (n = 4–5). Different letters represent significant differences [ANOVA, P < 0.05; followed by LSD test; CysPI, F(2,11) = 214.7, P < 0.0001; PPOF, F(2,11) = 185.5, P < 0.0001; PPOB, F(2,12) = 36.7, P < 0.0001; PR-1(P4), F(2,11) = 19.7, P = 0.0002]. AB(−), plants damaged by untreated larvae; AB(+), plants damaged by AB-treated larvae; Con, undamaged plants. CysPI, cysteine proteinase inhibitor; PPOF/B, polyphenol oxidase F/B; PR-1(P4), pathogenesis-related protein 1 (P4).
Defense Suppression by Symbiotic Microbes in OSs Is Dependent on SA-Signaling Pathway.
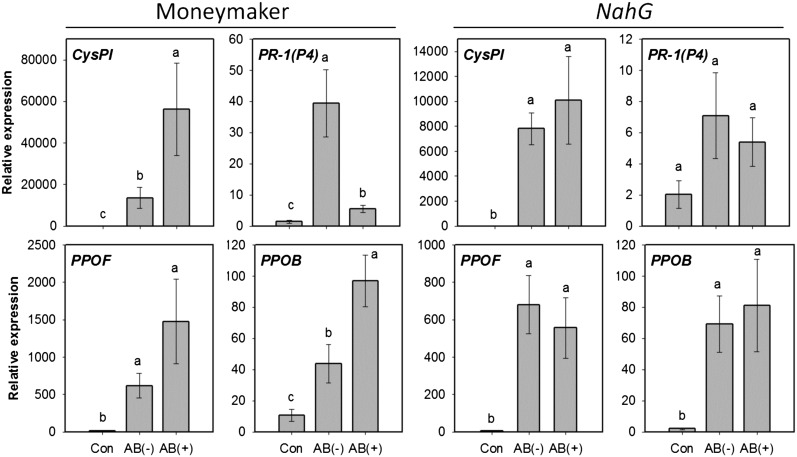
Negative cross-talk between the JA- and SA-signaling pathways plays a major role in suppression of induced defenses mediated by herbivore effectors (10). To determine if an antagonistic interaction between the JA- and SA-signaling pathways is mediated by symbionts, we measured cis-JA and SA levels in plants damaged by AB-treated or untreated larvae. Compared with feeding by AB-treated larvae, feeding by untreated larvae decreased cis-JA accumulation, but increased SA production at 2 h and 4 h after insect infestation (SI Appendix, Fig. S6). We also measured defense gene expression in Moneymaker (wild type) and SA-deficient NahG tomato plants damaged by AB-treated or untreated larvae. In Moneymaker, feeding by untreated larvae decreased expression of JA-responsive genes but increased SA-responsive gene expression (Fig. 3). In contrast, suppression of defense gene expression did not occur in NahG plants attacked by AB-untreated larvae (Fig. 3). Moreover, feeding by AB-untreated larvae decreased PPO activities in Moneymaker, but not in NahG plants (SI Appendix, Fig. S7). These results indicate that negative cross-talk between the JA- and SA-dependent pathways was involved in defense suppression.
Fig. 3.
Expression levels of JA- and SA-regulated genes in wild-type Moneymaker and SA-deficient NahG plants damaged by larvae that fed on AB-treated or untreated leaves. Values are untransformed means ± SEM (n = 4–5). Gene expression was measured 24 h after initiation of insect feeding. Different letters represent significant differences [ANOVA, P < 0.05; followed by LSD test; for Moneymaker, CysPI, F(2,12) = 176, P < 0.0001; PPOF, F(2,12) = 49.7, P < 0.0001; PPOB, F(2,1) = 14.7, P = 0.0006; PR-1(P4), F(2,11) = 17.5, P = 0.0004; for NahG, CysPI, F(2,12) = 233, P < 0.0001; PPOF, F(2,12) = 64.6, P < 0.0001; PPOB, F(2,12) = 33.97, P < 0.0001; PR-1(P4), F(2,11) = 2.83, P > 0.05]. AB(−), plants damaged by untreated larvae; AB(+), plants damaged by AB-treated larvae; Con, undamaged plants. CysPI, cysteine proteinase inhibitor; PPOF/B, polyphenol oxidase F/B; PR-1(P4), pathogenesis-related protein 1 (P4).
Symbiotic Bacteria Isolated in OSs Suppress Plant Defenses.
To investigate whether fungi and/or bacteria in OSs from CPB larvae suppress plant defenses, we fed larvae with leaves containing antibacterial (anti-B) agents, antifungal agents (anti-F), or both (anti-B/F). Feeding by untreated larvae or anti-F–treated larvae decreased PPO activities compared with feeding by anti-B or anti-B/F–treated larvae (SI Appendix, Fig. S8), indicating that bacteria, but not fungi, are involved in suppressing this JA-responsive defense. To characterize the symbiotic bacteria responsible for defense suppression, we isolated 22 bacterial colonies in OSs from untreated larvae. We cultured each isolate in liquid media (2xYT, 2x yeast extract and trypton) and then applied a specific amount of each isolate to mechanically wounded plants. Application of culture media to wounds (W + 2xYT) did not affect PPO activities compared with wounding followed by application of water (SI Appendix, Fig. S9A). Of the 22 bacterial isolates, three isolates significantly decreased PPO activities compared with W + 2xYT treatment (SI Appendix, Fig. S9). Based on the 16S rRNA gene sequence, we classified the isolates that suppressed PPO activity as members of the genera Stenotrophomonas, Pseudomonas, and Enterobacter. Of the remaining isolates that did not suppress PPO activities, we sequenced some of the nonsuppressing bacteria and classified them as Raoultella sp., Pseudomonas sp., and Enterobacteriaceae.
We also determined if the concentration of symbiotic bacteria applied is critical for defense suppression. We applied serial dilutions of the suppressing Stenotrophomonas sp., Pseudomonas sp., and Enterobacter sp. and the nonsuppressing bacterial isolates Raoultella sp., Pseudomonas sp., and Enterobacteriaceae to wounded plants. Defense suppression by the three suppressing bacteria was dose dependent, with a threshold of 105 CFU/mL (SI Appendix, Fig. S10). When we applied the same concentration of each isolate (109 CFU/mL) to wounded plants, the suppressing Pseudomonas sp. decreased PPO activities compared with W + 2xYT treatment. Application of other nonsuppressing bacteria did not decrease PPO activity (SI Appendix, Fig. S11).
In addition to suppression of PPO activity, we measured expression levels of JA/SA-regulated genes in plants that were wounded and treated with the suppressing Pseudomonas sp. culture. We found that this isolate decreased JA-responsive CysPI and PPOF expression, but increased SA-regulated PR-1(P4) expression compared with W + 2xYT (SI Appendix, Fig. S12).
To investigate the effects of a mixture of the suppressing bacterial isolates on plant defenses, we applied all possible combinations by applying two or three isolates at a time to wounded plants. We did not find synergistic, antagonistic, or additive effects of the mixture on suppression of PPO activities, but all combinations decreased PPO activities compared with W + 2xYT treatment (SI Appendix, Fig. S13). These results indicate that the presence of at least one of the three bacteria, Stenotrophomonas sp., Pseudomonas sp., or Enterobacter sp. in OSs was sufficient to suppress induced defenses.
Reintroduction of the Suppressing Bacteria to AB-Treated Larvae Suppresses Plant Defenses.
To confirm that the AB treatment used in our studies was indeed active on the bacteria of interest, we performed zone of inhibition assays with the three isolates of cultured bacteria described above. We found that the AB mixture inhibited growth of the three bacterial spp. that suppress plant defenses (SI Appendix, Table S1). In addition, we quantified the amount of Pseudomonas sp. on leaves after larval feeding by measuring rpoD (sigma factor subunit of RNA polymerase) abundance. Relative abundance of rpoD on plants damaged by untreated larvae was much higher than plants fed on by AB-treated larvae (SI Appendix, Fig. S14). We could not detect rpoD on undamaged plants. These data indicated that Pseudomonas sp. was deposited on leaves by untreated larvae and that AB treatment effectively inhibits Pseudomonas sp. in AB-treated larvae.
We further tested whether bacteria in OSs from untreated larvae were responsible for manipulation of induced defenses by reinoculation of the suppressing bacteria individually into AB-treated larvae. AB-treated or untreated larvae fed on leaves that received cultured bacteria or control buffer for 2 d. Addition of cultured-suppressing bacteria to untreated larvae did not affect PPO activities compared with the addition of control buffer to untreated larvae. Feeding by untreated larvae decreased PPO activities compared with feeding by AB-treated larvae that fed on leaves with control buffer. In contrast, reinoculating suppressing bacteria into AB-treated larvae restored defense suppression so that PPO activities in plants damaged by AB-treated larvae that received Stenotrophomonas sp., Pseudomonas sp., or Enterobacter sp. were similar to plants damaged by untreated larvae with control buffer or the suppressing bacteria (Fig. 4). These data demonstrate that the suppressing bacteria were delivered to wounded plants through OSs and that they significantly down-regulate induced defenses.
Fig. 4.
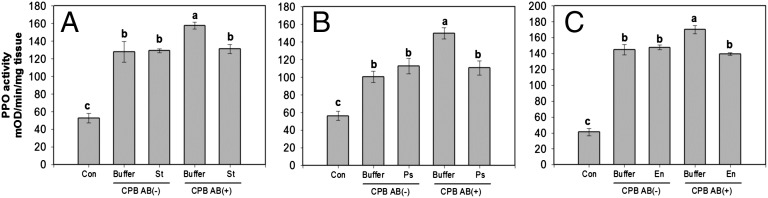
PPO activities in plants damaged by AB-treated or untreated larvae following reinoculation of the larvae with three bacterial isolates (Stenotrophomonas sp., Pseudomonas sp., and Enterobacter sp.) cultured from CPB larvae and found to suppress JA-mediated plant defenses. Larvae were allowed to feed on AB-treated or untreated leaves for 2 d and then fed on leaves that were reinoculated with suspension buffer (10 mM MgCl2) or the bacterial isolates in suspension buffer for 2 d. PPO activities were measured 48 h after initiation of insect feeding. Values are means ± SEM (n = 6). Different letters represent significant differences [ANOVA, P < 0.05; followed by LSD test; (A) Stenotrophomonas sp., F(4,25) = 33.8, P < 0.0001; (B) Pseudomonas sp., F(4,25) = 22.6, P < 0.0001; (C) Enterobacter sp., F(4,25) = 128, P < 0.0001]. Buffer, 10 mM MgCl2; En, Enterobacter sp.; Ps, Pseudomonas sp.; St, Stenotrophomonas sp. AB(−), plants damaged by untreated larvae; AB(+), plants damaged by AB-treated larvae; Con, undamaged plants.
Flagellin Suppresses Plant Defenses.
Because complete genome sequences and proteome databases are available for several Pseudomonas sp., we focused on Pseudomonas sp. among the suppressing bacteria to investigate molecular mechanisms underlying suppression of plant defenses. To identify potential effectors in the suppressing bacteria, we purified flagellin from Pseudomonas sp. and applied it to wounded plants. Purified flagellin was analyzed by SDS/PAGE and the band with ∼67 kDa was excised and subjected to MALDI-TOF/TOF mass spectrum analysis (SI Appendix, Fig. S15A). The mass spectra of the purified flagellin were identified as Pseudomonas flagellin (SI Appendix, Table S2). Application of varying flagellin concentrations decreased PPO activities compared with wounding and buffer treatment (SI Appendix, Fig. S15B), showing that flagellin was an effector protein involved in suppressing plant defenses.
Discussion
Microbial symbionts provide important roles in the survival of their hosts including providing nutrition, detoxifying toxins, and priming of host immunity, among others. In the case of certain parasitic insects, symbiotic viruses suppress the defensive responses of their hosts (23). However, in the case of herbivores, very little is known about how their symbionts may mediate host plant defenses. Here, we demonstrate that CPB larvae secrete bacteria in their OSs to evade antiherbivore defenses in tomato. These responses did not occur with plants, which have a defective SA-signaling pathway (Fig. 3 and SI Appendix, Fig. S7); the plant responded as if it was being attacked by a pathogen rather than an herbivore. Thus, the beetle, by presenting bacteria in its oral secretions, is hijacking the defensive machinery of the host plant for its own benefit. In essence, the beetle is diverting resources away from the appropriate immune response of its host plant. Conversely, a common strategy used by plant pathogens (e.g., Pseudomonas syringae pathovar tomato) is to hijack the JA pathway at the expense of resources to the SA immune response (24). Our findings indicate that the beetles, by harboring bacteria in their oral secretions, are recognized, in part, by plants as a microbial threat. We do not know the extent of this exploitive strategy, but in a few other examples that we have tested, it appears that beetles routinely release copious amounts of oral secretions during feeding. The role of microbial symbionts in the coevolution of plants and herbivores has no doubt received inadequate attention.
When plants are attacked by herbivores, plants perceive elicitors or herbivore-associated molecular patterns in OSs and trigger biosynthesis of phytohormones and antiherbivore defense responses (2). As microbial pathogens secrete effector proteins into their host to suppress immune defenses that are triggered by microbial-associated molecular patterns (MAMPs) (25), insect herbivores often evolve strategies to circumvent plant defenses. Although there is an emerging literature indicating that some chewing/piercing-sucking herbivores use effectors to manipulate plant defenses, there is scant evidence that symbiotic microbes of herbivores manipulate plant defenses. Among herbivore effectors identified so far, glucose oxidase in saliva of Helicoverpa zea caterpillar was the first effector identified reducing JA-regulated nicotine production in Nicotiana tabacum (6). In aphids (Myzus persicae), the salivary protein MpCOO2 has been shown to be an effector and its overexpression in Nicotiana benthamiana promoted aphid fecundity (7). It also has been reported that nonproteinaceous components of the OSs of Pieris brassicae and Spodoptera littoralis caterpillars reduced induction of antiherbivore defense genes in Arabidopsis in a manner independent of JA/SA-signaling pathways (8). Saliva of Spodoptera exigua suppressed JA-regulated defense responses in Arabidopsis (10). Spider mites (Tetranychus evansi) suppressed direct defenses so that mites had higher oviposition and survival rates on plants that they attacked (26). Because spider mites inject saliva into host plants (27), it is possible that unknown effectors present in T. evansi saliva manipulate plant defenses. Silverleaf whiteflies (Bemisia tabaci) decreased JA-responsive gene transcripts and increased SA-regulated gene transcripts, which allowed faster nymph development (28). It is presumed that silverleaf whiteflies secrete saliva to suppress plant defenses (28). However, none of the above studies indicated an involvement of microbial symbionts in modifying plant defenses.
To our knowledge, there has been only one previous report of defense suppression by herbivore-harbored microbes. In maize roots, larvae of the western corn rootworm (D. virgifera virgifera) inhibited the induction of defense gene expression, which was not observed when insects were treated with the antibiotic tetracycline (13). The authors reported that larvae with Wolbachia infection suppressed plant-defense–related gene transcripts, although the effect of suppression on insect performance was not examined. Additionally, it is unclear whether Wolbachia or Wolbachia-derived compounds are secreted into plants when western corn rootworm larvae feed on root tissues. More recently, it was shown that Wolbachia of the rootworm did not suppress maize defense responses (15). In contrast to herbivore symbionts, which are not pathogenic to plants, phytopathogens associated with insects can modify plant responses (29). The plant pathogenic bacterium, Candidatus Liberibacter psyllaurous (Lps), vectored by tomato psyllids (Bactericerca cockerelli), suppressed JA/SA-regulated defense transcripts (29), although it was not determined whether the modified responses were beneficial to psyllids or Lps and how effectors from psyllids or Lps change defense responses. In this study, we clearly demonstrated that CPB larvae released symbiotic bacteria, which was confirmed by confocal microscopy (SI Appendix, Fig. S1 and Movie S1), electron microscopy (Fig. 1A), and quantitative PCR (qPCR) (SI Appendix, Fig. S14). Further we demonstrated that specific bacteria in OSs suppressed the induced defenses in tomato (SI Appendix, Figs. S9–S13), which promotes CPB larval growth (Fig. 2).
It is important to note that neonate larvae gained more weight on leaves that were damaged by untreated larvae than on those that were damaged by AB-treated larvae (Fig. 1 and SI Appendix, Fig. S3). Also, increased larval growth corresponded with decreased expression of JA-responsive antiherbivore genes (CysPI and PPOF/B) and reduced PPO activity, when plants were damaged by AB-untreated larvae (Fig. 2). Thus, suppression of antiherbivore defenses is highly beneficial to the performance of CPB larvae. Earlier reports have shown that defense suppression mediated by herbivore effectors enhance herbivore performance (6, 7, 26, 28). For example, S. littoralis larvae grew better on Arabidopsis treated with OSs of S. littoralis than on wounded plants (8). The growth of neonate larvae on plants damaged by untreated larvae was similar to that on undamaged plants. It may be attributed to the fact that untreated larvae induced insufficient production of defensive proteins such as CysPI and PPO to retard neonatal growth. Alternatively, it is possible that CPB larvae adapted to lower levels of defenses induced by untreated larvae. It was shown that CPB larvae can overcome induced defenses by changing the profile of their digestive proteases (30).
Among the bacterial colonies isolated from OSs of untreated larvae, three isolates, genera Stenotrophomonas, Pseudomonas, and Enterobacter, suppressed PPO activities when applied to wounded plants (SI Appendix, Figs. S9 and S10). Similar bacteria have been found in other geographic locations. CPB larvae from potato fields in Maryland and Virginia contained these genera (31) and CPB populations in Turkey had Pseudmononas sp. (32), but the role(s) these bacteria may play in association with the insect was not investigated in these cases. These bacteria from field populations showed high sequence similarities (>97%) to the 16S rRNA gene sequences from the isolates we cultured from CPB in our laboratory colony. Furthermore, CPB larvae collected from potato fields in Pennsylvania have symbiotic bacteria that suppress plant defenses. Plants damaged by untreated larvae from this field population showed lower PPO activities than those damaged by AB-treated larvae (SI Appendix, Fig. S16). We also detected the same Pseudomonas sp. from the field population (12 larvae and four adults; n = 16) as the suppressing Pseudomonas sp. in our colony (9 larvae and two adults; n = 11) (SI Appendix, Fig. S17). The sequence similarity of the rpoD gene between these samples was 100%. Thus, it is noteworthy to investigate whether symbiotic bacteria isolated from other field populations of CPB suppress plant defenses.
We found that antiherbivore defenses were suppressed by symbiotic microbes in OSs in an SA-dependent manner (Figs. 2 and 3 and SI Appendix, Fig. S6). When NahG plants were damaged by AB-treated or untreated larvae, suppression of JA-responsive genes and PPO activity was not observed (Fig. 3 and SI Appendix, Fig. S7). Application of suppressing bacteria isolated from OSs to wounded plants reduced both JA-responsive defense gene expression and PPO activity compared with plants wounded and treated with culture medium (SI Appendix, Figs. S9–S12). Flagellin isolated from the culture medium of Pseudomonas sp., one of the suppressing bacteria, decreased PPO activities (SI Appendix, Fig. S15). In Arabidopsis, MAMPs, such as flagellin, induced SA biosynthesis and triggered SA-regulated responses (33). Tomato can recognize flagellin and its conserved domain (flg15) through FLS2 (Flagellin Sensing 2), a flagellin receptor (34). Flagellin from Pseudomonas sp. in OSs thus acts as an effector of CPB larvae by eliciting a SA-signaling pathway to down-regulate JA-regulated defenses triggered by feeding. In addition to flagellin, other components may be produced by CPB symbiotic bacteria, which suppress JA-regulated defense responses.
Other studies have shown that phytopathogens suppress host plant defenses to enhance the performance of their insect vectors (35). For example, Tomato spotted wilt virus vectored by western flower thrips (Frankliniella occidentalis) decreased JA-regulated defenses through induction of the SA-signaling pathway, benefitting the insect vector (36). Phytoplasmas secreted the protein SAP11 to down-regulate lipoxygenase and inhibit JA biosynthesis, which increases survival and fecundity of the leafhopper vector and phytoplasma transmission (37). It remains to be determined if other metabolites produced by CPB symbiotic bacteria suppress plant defenses or if other MAMPs from these bacteria activate the SA-signaling pathway to interfere with JA-regulated defenses. We cannot rule out the possibility that unculturable, AB-sensitive microbes in OSs also could be involved in modifying defenses.
In conclusion, we provide direct evidence that orally secreted microbial symbionts function as effectors and mediate plant–insect interactions. In other words, microbial symbionts in OSs from CPB larvae are secreted onto plant tissues during herbivory and manipulate induced defenses through negative cross-talk between JA- and SA-regulated signaling pathways. Thus, CPB larvae hijack these signaling pathways, which benefits the larvae by improving their growth. Our results provide important clues to how these unrecognized and underappreciated players (i.e., insect-associated microbes) shape the complex interaction between plants and insect herbivores. This adds an exciting unique dimension to our understanding of how herbivores deploy counterdefense strategies in response to plant defenses.
Materials and Methods
Plant and Insect Materials.
Tomato (Solanum lycopersicum cv. Betterboy) and transgenic NahG and its wild-type cv. Moneymaker plants were grown in Promix potting soil (Premier Horticulture) in a greenhouse. The laboratory and field colonies of CPBs (L. decemlineata) were maintained separately. Details are provided in SI Appendix, Materials and Methods.
Fluorescent Pictures and Movie of Regurgitation by CPB Larvae.
To demonstrate that CBP larvae deposit OSs during herbivory, the fluorescent dye Alexa Fluor 488 (Invitrogen) (38) was fed to the larvae, which were then transferred to fresh leaf tissues. Details are provided in SI Appendix, Materials and Methods.
Antibiotics Treatment.
To reduce microbes present in OSs from larvae, larvae were fed antibacterial and/or antifungal agents (SI Appendix, Materials and Methods).
SEM Images.
Leaves were damaged by larvae that fed on AB-treated or untreated artificial diets for 2–3 d. The larvae were then transferred to fresh leaves. After 1 h of herbivory, the damaged sections of leaves were prepared for SEM (details are provided in SI Appendix, Materials and Methods).
Herbivore Treatment and Application of OSs to Wounded Plants.
To evaluate the impact of larval feeding on induced plant responses, one AB-treated or untreated larva was placed on the terminal leaflet of each four-leaf stage plant using clip cages. Undamaged control plants received the clip cage without insects. After 100% of the area confined by the cage was consumed, leaf tissues were harvested for gene expression and defense enzymatic activity (polyphenol oxidase activity). To verify that observed plant responses required the presence of microbes in the OSs from the larvae and not just wounding, OSs from AB-treated or untreated larvae were applied to mechanically wounded plants. Details are provided in SI Appendix, Materials and Methods.
Impact of JA-Mediated Defense Suppression on CPB Performance.
To investigate whether the suppression of JA-dependent plant defenses enhances larval performance, we measured larval growth. Plants were damaged by AB-treated or untreated larvae that were confined to a single leaflet as described above and the damaged leaflets were detached after 48 h. One neonate was then placed on an excised leaflet in a 1-oz cup containing 1% agar to maintain leaf moisture. Control larvae received the leaflets from undamaged plants that received an empty clip cage. Larval weight was measured after 5 d. PPO activities were measured in subsamples from each treatment at 48 h after treatment. To collect subsamples for PPO activity, one leaf disc (diameter, 1.8 cm) was removed surrounding the leaf tissue that had been consumed by the larvae and two leaf discs from each of two leaves were pooled as one replicate. This feeding experiment was repeated four times.
PPO Activity.
PPO activities for all experiments were measured 48 h after treatment using caffeic acid as the substrate as described previously (39).
Quantification of JA and SA.
Plants were damaged by AB-treated or untreated larvae that were confined to a single leaflet as described above. Leaf tissues were harvested 2, 4, 24, and 48 h after placing insects. JA and SA were extracted and measured using GC/MS as described previously (40).
DNA and RNA Extractions and Quantitative Real-Time PCR.
Leaf tissues were ground in liquid nitrogen. RNA was extracted with an RNeasy Plus Mini kit (Qiagen) following the manufacturer's protocol and quantified using NanoDrop (Thermo Scientific). Total genomic DNA from plants was extracted using DNeasy Plant Mini kit (Qiagen) following the manufacturer's protocol and quantified using NanoDrop (Thermo Scientific). Details are provided in SI Appendix, Materials and Methods.
Isolation of Bacteria in OSs and Application of the Bacteria to Wounded Plants.
To isolate bacteria in OSs from CPB larvae, OSs was collected from AB-untreated larvae and cultured on 2xYT agar plates. Cultured bacterial isolates were applied to wounded plants. Details are provided in SI Appendix, Materials and Methods.
Reinoculation of Symbiotic Bacteria to Larvae and Zone of Inhibition Assays.
To verify that the cultured bacterial isolates found to suppress JA-dependent responses are indeed secreted by larvae onto leaves, we reintroduced the bacterial isolates to AB-treated larvae and again evaluated plant responses. Details are provided in SI Appendix, Materials and Methods.
DNA Extraction, PCR, 16S rRNA, and rpoD Gene Sequencing to Taxonomically Classify Bacterial Isolates.
Direct colony PCR followed by Sanger sequencing was performed to identify bacterial isolates using universal 16S rRNA primers. Details are provided in SI Appendix, Materials and Methods.
Flagellin Purification and Identification.
Flagellin was purified as described previously (41). Details are provided in SI Appendix, Materials and Methods.
Statistical Analysis.
All data were analyzed using analysis of variance or Student's t test. Details are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. Kim for helpful discussion, J. H. Tumlinson and N. McCartney for phytohormone extraction, and P. Schmitt for use of ultracentrifuge. This work was supported by US Department of Agriculture–National Institute of Food and Agriculture Grant 2011-67013-30352 (to G.W.F. and D.S.L.) and National Science Foundation Grant IOS-1256326 (to G.W.F., C.R., and K.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JX296529, JX296530, JX296531, and KC977253–KC977257).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308867110/-/DCSupplemental.
References
- 1.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 2.Felton GW, Tumlinson JH. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr Opin Plant Biol. 2008;11(4):457–463. doi: 10.1016/j.pbi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5(5):308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 4.Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17(5):260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Walling LL. In: Advances in Botanical Research. Van Loon LC, editor. London: Academic; 2009. pp. 551–612. [Google Scholar]
- 6.Musser RO, et al. Herbivory: Caterpillar saliva beats plant defences. Nature. 2002;416(6881):599–600. doi: 10.1038/416599a. [DOI] [PubMed] [Google Scholar]
- 7.Bos JIB, et al. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid) PLoS Genet. 2010;6(11):e1001216. doi: 10.1371/journal.pgen.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consales F, et al. Insect oral secretions suppress wound-induced responses in Arabidopsis. J Exp Bot. 2012;63(2):727–737. doi: 10.1093/jxb/err308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atamian HS, et al. In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol Plant Microbe Interact. 2013;26(1):67–74. doi: 10.1094/MPMI-06-12-0144-FI. [DOI] [PubMed] [Google Scholar]
- 10.Weech M-H, Chapleau M, Pan L, Ide C, Bede JC. Caterpillar saliva interferes with induced Arabidopsis thaliana defence responses via the systemic acquired resistance pathway. J Exp Bot. 2008;59(9):2437–2448. doi: 10.1093/jxb/ern108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser W, Huguet E, Casas J, Commin C, Giron D. Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc R Soc B Biol Sci. 2010;277(1692):2311–2319. doi: 10.1098/rspb.2010.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr KL, Hearne LB, Briesacher S, Clark TL, Davis GE. Microbial symbionts in insects influence down-regulation of defense genes in maize. PLoS ONE. 2010;5(6):e11339. doi: 10.1371/journal.pone.0011339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. Obligate symbiont involved in pest status of host insect. Proc R Soc B Biol Sci. 2007;274(1621):1979–1984. doi: 10.1098/rspb.2007.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert CAM, et al. Direct and indirect plant defenses are not suppressed by endosymbionts of a specialist root herbivore. J Chem Ecol. 2013;39(4):507–515. doi: 10.1007/s10886-013-0264-5. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence SD, Novak NG, Blackburn MB. Inhibition of proteinase inhibitor transcripts by Leptinotarsa decemlineata regurgitant in Solanum lycopersicum. J Chem Ecol. 2007;33(5):1041–1048. doi: 10.1007/s10886-007-9285-2. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence SD, Novak NG, Ju CJ, Cooke JE. Potato, Solanum tuberosum, defense against Colorado potato beetle, Leptinotarsa decemlineata (Say): Microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. J Chem Ecol. 2008;34(8):1013–1025. doi: 10.1007/s10886-008-9507-2. [DOI] [PubMed] [Google Scholar]
- 18.Chung SH, Felton GW. Specificity of induced resistance in tomato against specialist lepidopteran and coleopteran species. J Chem Ecol. 2011;37(4):378–386. doi: 10.1007/s10886-011-9937-0. [DOI] [PubMed] [Google Scholar]
- 19.Brunelle F, Girard C, Cloutier C, Michaud D. A hybrid, broad-spectrum inhibitor of Colorado potato beetle aspartate and cysteine digestive proteinases. Arch Insect Biochem Physiol. 2005;60(1):20–31. doi: 10.1002/arch.20078. [DOI] [PubMed] [Google Scholar]
- 20.Girard C, et al. A multicomponent, elicitor-inducible cystatin complex in tomato, Solanum lycopersicum. New Phytol. 2007;173(4):841–851. doi: 10.1111/j.1469-8137.2007.01968.x. [DOI] [PubMed] [Google Scholar]
- 21.Thipyapong P, Stout MJ, Attajarusit J. Functional analysis of polyphenol oxidases by antisense/sense technology. Molecules. 2007;12(8):1569–1595. doi: 10.3390/12081569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Kan JA, Joosten MH, Wagemakers CA, van den Berg-Velthuis GC, de Wit PJ. Differential accumulation of mRNAs encoding extracellular and intracellular PR proteins in tomato induced by virulent and avirulent races of Cladosporium fulvum. Plant Mol Biol. 1992;20(3):513–527. doi: 10.1007/BF00040610. [DOI] [PubMed] [Google Scholar]
- 23.Strand MR. In: Insect Virology. Asgari S, Johnson KN, editors. UK: Caister Academic Press, Wymondham; 2010. pp. 171–197. [Google Scholar]
- 24.Zhao Y, et al. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 2003;36(4):485–499. doi: 10.1046/j.1365-313x.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 26.Sarmento RA, et al. A herbivore that manipulates plant defence. Ecol Lett. 2011;14(3):229–236. doi: 10.1111/j.1461-0248.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takabayashi J, Shimoda T, Dicke M, Ashihara W, Takafuji A. Induced response of tomato plants to injury by green and red strains of Tetranychus urticae. Exp Appl Acarol. 2000;24(5-6):377–383. doi: 10.1023/a:1006497024175. [DOI] [PubMed] [Google Scholar]
- 28.Zarate SI, Kempema LA, Walling LL. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007;143(2):866–875. doi: 10.1104/pp.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casteel CL, Hansen AK, Walling LL, Paine TD. Manipulation of plant defense responses by the tomato psyllid (Bactericerca cockerelli) and its associated endosymbiont Candidatus Liberibacter psyllaurous. PLoS ONE. 2012;7(4):e35191. doi: 10.1371/journal.pone.0035191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruden K, et al. Molecular basis of Colorado potato beetle adaptation to potato plant defence at the level of digestive cysteine proteinases. Insect Biochem Mol Biol. 2004;34(4):365–375. doi: 10.1016/j.ibmb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Blackburn MB, Gundersen-Rindal DE, Weber DC, Martin PAW, Farrar RR., Jr Enteric bacteria of field-collected Colorado potato beetle larvae inhibit growth of the entomopathogens Photorhabdus temperata and Beauveria bassiana. Biol Control. 2008;46(3):434–441. [Google Scholar]
- 32.Muratoglu H, Demirbag Z, Sezen K. The first investigation of the diversity of bacteria associated with Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) Biologia (Bratisl) 2011;66(2):288–293. [Google Scholar]
- 33.Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F. Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 2008;53(5):763–775. doi: 10.1111/j.1365-313X.2007.03369.x. [DOI] [PubMed] [Google Scholar]
- 34.Robatzek S, et al. Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Mol Biol. 2007;64(5):539–547. doi: 10.1007/s11103-007-9173-8. [DOI] [PubMed] [Google Scholar]
- 35.Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. Herbivore arthropods benefit from vectoring plant viruses. Ecol Lett. 2005;8(1):70–79. [Google Scholar]
- 36.Abe H, et al. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 2012;53(1):204–212. doi: 10.1093/pcp/pcr173. [DOI] [PubMed] [Google Scholar]
- 37.Sugio A, Kingdom HN, MacLean AM, Grieve VM, Hogenhout SA. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc Natl Acad Sci USA. 2011;108(48):E1254–E1263. doi: 10.1073/pnas.1105664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peiffer M, Felton GW. Do caterpillars secrete “oral secretions”? J Chem Ecol. 2009;35(3):326–335. doi: 10.1007/s10886-009-9604-x. [DOI] [PubMed] [Google Scholar]
- 39.Felton GW, Donato K, Vecchio RJ, Duffey SS. Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J Chem Ecol. 1989;15(12):2667–2694. doi: 10.1007/BF01014725. [DOI] [PubMed] [Google Scholar]
- 40.Tooker JF, Rohr JR, Abrahamson WG, De Moraes CM. Gall insects can avoid and alter indirect plant defenses. New Phytol. 2008;178(3):657–671. doi: 10.1111/j.1469-8137.2008.02392.x. [DOI] [PubMed] [Google Scholar]
- 41.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18(3):265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.