Abstract
The continuous emergence of resistant bacteria has become a major worldwide health threat. The current development of new antibacterials has lagged far behind. To discover reagents to fight against resistant bacteria, we initiated a chemical approach by synthesizing and screening a small molecule library, reminiscent of the polycyclic indole alkaloids. Indole alkaloids are a class of structurally diverse natural products, many of which were isolated from plants that have been used as traditional medicine for millennia. Specifically, we adapted an evolutionarily conserved biosynthetic strategy and developed a concise and unified diversity synthesis pathway. Using this pathway, we synthesized 120 polycyclic indolines that contain 26 distinct skeletons and a wide variety of functional groups. A tricyclic indoline, Of1, was discovered to selectively potentiate the activity of β-lactam antibiotics in multidrug-resistant methicillin-resistant Staphylococcus aureus (MRSA), but not in methicillin-sensitive S. aureus. In addition, we found that Of1 itself does not have antiproliferative activity but can resensitize several MRSA strains to the β-lactam antibiotics that are widely used in the clinic, such as an extended-spectrum β-lactam antibiotic amoxicillin/clavulanic acid and a first-generation cephalosporin cefazolin. These data suggest that Of1 is a unique selective resistance-modifying agent for β-lactam antibiotics, and it may be further developed to fight against resistant bacteria in the clinic.
Antibiotics are one of the most important and widely used medicines. However, the emergence of resistance to the antibiotics has become a global public health threat. Serious infection of multidrug-resistant microorganisms has caused considerable patient mortality and modality (1). For example, more people died from methicillin-resistant Staphylococci aureus (MRSA) infection than those from HIV/AIDS, Parkinson disease, and homicide combined (2). S. aureus is the most common Gram-positive bacteria pathogen that can cause skin infection, respiratory disease, and food poisoning. There are two predominant resistance mechanisms in MRSA (3). Foremost, the mecA gene (4) encodes penicillin-binding protein 2a (PBP2a) that has low affinity to β-lactam antibiotics such as methicillin, allowing sufficient peptidoglycan cross-linking in the presence of β-lactam antibiotics. The second is the blaZ gene (5), which encodes β-lactamases that chemically deactivate β-lactam antibiotics. The pharmaceutical industry has been developing structural analogs of β-lactam antibiotics that have higher affinity to PBP2a and lower activity to β-lactamases. This strategy has kept up with the emergence of new resistant MRSA strains until recently. However, there are not enough analogs in the current antibiotic pipeline to combat current and future resistance emergence (6, 7).
Another successful strategy is to use resistance-modifying agents (RMAs) in combination with antibiotics. RMAs are highly favorable because they can increase the life span of the antibiotics that are currently used in the clinics, which have already been optimized for toxicity and large-scale production. For example, clavulanic acid is a serine-dependent β-lactamase inhibitor from Streptomyces clavuligerus (8). Its use in combination with amoxicillin restores the efficacy of amoxicillin against bacteria producing β-lactamases, and this combination has become one of the most prescribed antibiotics in the United States. Encouraged by the discovery of clavulanic acid, numerous efforts have attempted to discover novel RMAs from natural sources, such as membrane permeablizing agents and inhibitors of efflux pumps (9). However, the only RMAs that have been proven clinically useful are β-lactamase inhibitors. Recently, there are a number of reports that showed plant extracts from a variety of different species can potentiate the activity of β-lactam antibiotics (10). However, the discovery of the active compounds has been very difficult (11). This challenge is due to the chemical complexity of plant extracts, the lack of standardization, difficulties in access and supply, and the inherent slowness and costs of working with natural products. Only a few plant natural products with RMA activity have been characterized, such as epigallocatechin gallate (i.e., EGCG, a flavonoid from green tea) (12) and reserpine (i.e., a polycyclic indole alkaloid from the root of an Indian medical plant; Fig. 1A) (13). Herein, we describe our recent discovery of a highly selective RMA for β-lactam antibiotics against MRSA from a polycyclic indole alkaloid library.
Fig. 1.
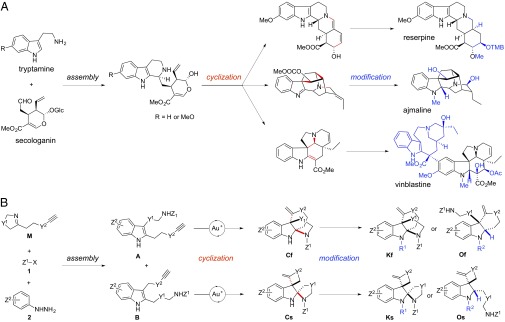
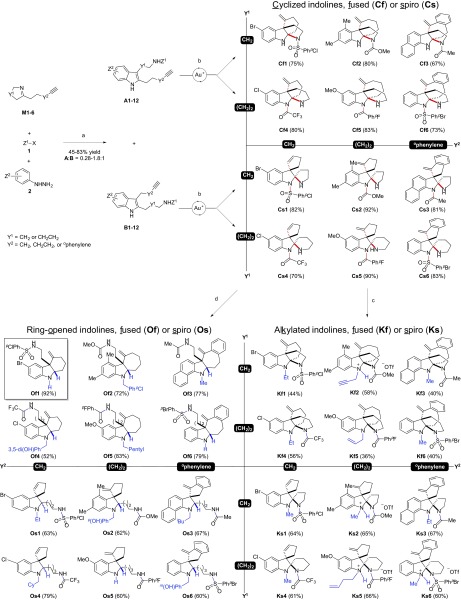
Synthetic strategy for representative polycyclic indole alkaloids and our synthetic library. (A) Biosynthetic strategy for representative indole alkaloids. (B) Synthetic plan for a polycyclic indoline alkaloid library. Bonds in red are formed in the cyclization phase, and functional groups in blue are installed in the modification phase.
Natural indole alkaloids represent a large and highly diverse family of nitrogen-containing compounds (14). Most of these are secondary metabolites of medical plants that have been used as traditional medicine for millennia. Many have been used to treat diseases, such as hypertension (reserpine and ajmaline; Fig. 1A) (15, 16) and cancer (vinblastine; Fig. 1A) (17). The broad structural and pharmaceutical properties of indole alkaloids make this family of natural products ideal targets for synthetic efforts. Several synthetic pathways have also been developed for the synthesis of indole alkaloid libraries (18–24). However, systematic and efficient approaches toward highly diverse polycyclic indoline-containing skeletons still remain a main challenge in diversity-oriented synthesis (25, 26).
Inspired by their bioactivity and structural features, we designed a diversity synthesis pathway of a polycyclic indoline alkaloid library, based on a common biosynthetic strategy of natural products (27), such as indole alkaloids (Fig. 1A), polyketides (e.g., erythromycin A) (28), terpenoids (e.g., taxol) (29), and nonribosomal peptides (e.g., vancomycin) (30). Specifically, as shown in Fig. 1B, we planned to assemble an alkynyl imine M, an activating agent Z1X (1), and an aryl hydrazine (2) using a one-pot three-component reaction that we recently developed (31). (Bold terms represent the whole classes of molecules; terms not in bold font are individual compounds.) Based on our previous studies on gold catalysis (32, 33), we envisioned that the resulting two regioisomeric indoles (A and B) could undergo gold(I)-catalyzed tandem cyclization reactions to form a fused (Cf) or spiro- (Cs) tetracyclic indoline, respectively (34). These indoline products are poised for further modifications, such as alkylations of the aniline nitrogen to afford Kf and Ks, or tandem ring-opening reduction-reductive aminations to produce Of and Os. We envisioned that a highly diverse collection of polycyclic indolines may be accessed by varying the linkers Y1 and Y2 of alkynyl imines M.
Results and Discussion
Preparation of Building Blocks M.
The alkynyl imine building blocks M were readily synthesized by alkylations of cyclic imine 3 with iodides 4 (Fig. 2) under basic conditions followed by desilylation. Only a single purification using basic alumina is required for each alkynyl imine M. Using this general procedure, we obtained six alkynyl imines (M1–6) in good yields (60–91%), each on multigram scale.
Fig. 2.
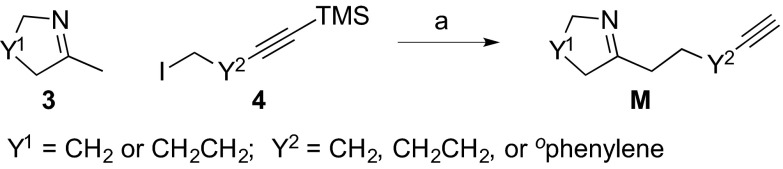
Preparation of alkynyl imine building blocks. Conditions and reagents: (a) (i) LDA, 4, THF, −78 °C to 23 °C, 12 h; (ii) TBAF, THF, 23 °C, 10 min, 60–91%. LDA, lithium diisopropylamine; THF, tetrahydrofuran; TBAF, tetrabutylammonium fluoride.
Assembly of Building Blocks.
With these six alkynyl imines in hand, we next subjected them to the one-pot three-component assembly reactions. Because the resulting two regioisomeric indole products were expected to provide distinct ring skeletons on cyclizations, we slightly modified the reaction conditions and used dimethyl formamide (DMF) as the solvent to obtain moderate selectivity of regioisomers A and B. Under these modified conditions, all six alkynyl imines were converted to the corresponding indoles in moderate to good yields (45–83%; Table 1). The two regioisomers A and B from each assembly reaction were separable by silica gel chromatography. This assembly reaction showed excellent compatibility with a wide range of activating reagents Z1X, such as sulfonyl chlorides, acyl chlorides, anhydrides, and chloroformates. In addition, a variety of aryl hydrazines substituted with either electron-donating groups (e.g., methoxy and methyl) or electron-withdrawing groups (e.g., bromo and chloro) were well tolerated in the reaction conditions. For each alkynyl imine, we chose two different combinations of activating groups and hydrazines in the assembly reactions. As such, we ran 12 reactions and prepared 24 highly functionalized alkynyl indoles A1–12 and B1–12 (Table 1).
Table 1.
One-pot synthesis of alkynyl indoles A and B
| Entry | M | Y1 | Y2 | Z1X | Z2 | Yield (%) | A:B |
| 1 | M1 | CH2 | CH2 | pClPhSO2Cl | 4-Br | 49 | 0.56:1 |
| 2 | M2 | CH2 | CH2CH2 | MeOCOCl | 3,5-diMe | 55 | 1.8:1 |
| 3 | M3 | CH2 | ophenylene | AcCl | ophenylene | 60 | 1.1:1 |
| 4 | M4 | CH2CH2 | CH2 | (CF3CO)2O | 4-Cl | 45 | 0.77:1 |
| 5 | M5 | CH2CH2 | CH2CH2 | pFPhCOCl | 4-OMe | 55 | 1.3:1 |
| 6 | M6 | CH2CH2 | ophenylene | pBrPhSO2Cl | H | 54 | 0.5:1 |
| 7 | M1 | CH2 | CH2 | pClPhCOCl | 2-OMe | 60 | 1:1 |
| 8 | M2 | CH2 | CH2CH2 | pClPhSO2Cl | H | 83 | 0.4:1 |
| 9 | M3 | CH2 | ophenylene | MeOCOCl | 4-OMe | 55 | 1.8:1 |
| 10 | M4 | CH2CH2 | CH2 | (CF3CO)2O | 4-Me | 70 | 1:1 |
| 11 | M5 | CH2CH2 | CH2CH2 | pFPhCOCl | ophenylene | 60 | 1.3:1 |
| 12 | M6 | CH2CH2 | ophenylene | pBrPhSO2Cl | 3,5-diMe | 65 | 0.28:1 |
Gold(I)-Catalyzed Tandem Cyclizations of Alkynyl Indoles to Afford Polycyclic Indolines.
We used a commercially available gold(I) catalyst, Ph3PAuNTf2, for the cyclization step to simplify our library production. When subjected to the standard gold catalysis conditions (5 mol% Ph3PAuNTf2, toluene, 50 °C), each of the 24 alkynyl indoles underwent the expected tandem cyclization to produce the corresponding indolines Cf1–12 or Cs1–12 as a single regio- and diastereomer in good to excellent yields (see representative examples in Fig. 3). Nearly all reactions were initiated by the exo-dig cyclizations followed by the intramolecular nucleophilic attack of the iminium ion by the nitrogen nucleophiles. The only exceptions were Cs1 and Cs4, which reacted in an endo-dig fashion due to the severe ring strain of an exo-dig cyclization if they cyclized in the exo mode (i.e., 5-endo-dig cyclization is favored over 4-exo-dig cyclization) (35). Hence, over a dozen distinct polycyclic indoline skeletons were constructed under the same reaction condition in this step. In most cases, the Z1 groups appeared to migrate to the aniline nitrogens spontaneously after cyclizations, presumably to avoid the severe steric interactions.
Fig. 3.
Bio-inspired synthesis of polycyclic indolines. Numbers in parentheses are isolated yields. Conditions and reagents: (a) 1, DMAP, 23 °C, 0.5 h, DMF; M, 2–12 h; TsOH⋅H2O, 23–80 °C, 24 h, 45–83%; (b) Ph3PAuNTf2, 50 °C, toluene, 1–12 h; 67–95%; (c) R1OTf, DCM, 23 °C, 2–12 h, 30–92%; (d) AcOH, NaBH3CN, MeOH, 0 °C, 0.5 h, and then aldehyde, 0–23 °C, 2–12 h, 35–99%. DMAP, 4-dimethylaminopyridine; DMF, N,N-dimethylformamide; TsOH·H2O, p-toluenesulfonic acid monohydrate; Ph3PAuNTf2, [bis(trifluoromethanesulfonyl)imidate]-(triphenylphosphine)gold(I); OTf, trifluoromethanesulfonate; DCM, dichloromethane; AcOH, acetic acid, NaBH3CN, sodium cyanoborohydride; MeOH, methanol; TMS, trimethylsilyl; Ph, phenyl; iBu, isobutyl; Cy, cyclohexanyl; Me, methyl; Et, ethyl. Full details are in SI Appendix, SI Methods.
Modifications of the Cyclized Indolines.
To further modify the cyclized indolines, we developed two general methods. When treated with alkyl triflates, the Z1 groups migrated back to the amine nitrogens and the aniline nitrogens were alkylated to provide Kf and Ks (Fig. 3). This phenomenon is likely due to the poor accessibility of the amine nitrogens to the alkylating agents. In these transformations, the ring skeletons of the indolines were maintained. However, the migrations of the Z1 groups caused significant changes of their pharmacological properties (e.g., pKa values). The alkyl triflates are either commercially available or can be prepared from their corresponding alcohols easily. Alternatively, treatment of the cyclized products Cf and Cs with acetic acid and NaBH3CN at 0 °C afforded 24 ring-opened indoline products Of and Os, respectively. The hydride was typically added to the iminium ion intermediates from the opposite face of the exo-methylene group (e.g., Of1 and Os5). For the endo-cyclization products (e.g., Cs1 and Cs4), the hydride was added nonselectively. Furthermore, subsequent addition of an aldehyde to the above reaction mixture produced the reductive amination product with a ring-opened indoline bearing an additional alkyl group on the aniline nitrogen. We chose two different aldehydes for each cyclized indoline, which provided another 48 ring-opened indoline products.
To summarize, we developed a concise and systematic synthetic pathway that enables rapid access (two to three steps) to highly functionalized and diverse polycyclic indolines. Using this pathway, we synthesized a pilot library of 120 indolines that contain 26 distinct skeletons each bearing three to six rings (SI Appendix, Fig. S1). The reactions used in this pathway showed high tolerance of functional groups, which allowed us to choose a wide range of commercially available building blocks, including the activating reagents Z1X (e.g., acyl chlorides, sulfonyl chlorides, chloroformates, and anhydrides), aryl hydrazines with both electron-rich (e.g., methyl and methoxy), and electron-deficient (e.g., chloro and bromo) substitutions, and a variety of different aldehydes, both aliphatic and aromatic. The gold(I)-catalyzed tandem cyclizations are particularly interesting, because these reactions, under essentially the same reaction conditions, underwent the expected tandem cyclizations to afford over a dozen different ring skeletons with high yields and diastereoselectivities.
Screening of Polycyclic Indolines That Potentiate the Activity of Methicillin in MRSA.
With this highly diverse and functionalized indoline library in hand, we next screened their ability to potentiate the activity of methicillin in MRSA. A multidrug-resistant MRSA strain [American Type Culture Collection (ATCC): BAA-44] was selected for the initial screening. In addition to methicillin, this MRSA strain is also resistant to many other β-lactam antibiotics, as well as antibiotics from other structural classes, such as erythromycin, tetracycline, and ciprofloxacin. We first determined the minimum inhibitory concentration (MIC) of methicillin for this MRSA strain as 128 µg/mL using the standard Clinical Laboratory Standards Institute (CLSI) broth microdilution method (36). Our initial screen was adapted from this standard microdilution assay; however, the Mueller Hinton Broth (MHB) was supplemented with methicillin at one-quarter of its MIC value (i.e., 32 µg/mL). To each well, 20 µM of each individual indoline alkaloid was added, and the plates were incubated at 37 °C for 18 h. Nine indoline alkaloids (8% hit rate) were identified that reduced the MIC of methicillin to at least 32 µg/mL. We further evaluated the ability of these nine compounds to potentiate methicillin by assessing the MIC of methicillin for MRSA using the standard microdilution method in media supplemented with 20 µM of each alkaloid. Of1 (Fig. 3) was found to be the most active compound and reduced the MIC of methicillin from 128 to 8 µg/mL. Hence, Of1 resensitizes this multidrug-resistant MRSA strain to methicillin, because S. aureus with an MIC ≤8 µg/mL is defined as methicillin sensitive.
Evaluation of the Ability Of1 to Potentiate Other Antibiotics.
To evaluate Of1’s ability to potentiate other antibiotics in MRSA, we determined the MIC values of these antibiotics in the presence and absence of 20 µM of Of1. In addition to methicillin, we tested other β-lactam antibiotics, such as oxacillin (i.e., the replacement of methicillin, a narrow spectrum antibiotic), amoxicillin/clavulanic acid (amox/clav, an extended spectrum antibiotic), cephalexin and cefazolin (first-generation cephalosporins), and meropenem and imipenem (carbapenems). Antibiotics from other structural classes tested include streptomycin (aminoglycoside), rifampicin (ansamycin), tetracycline (tetracycline), ciprofloxacin (quinolone), erythromycin and azithromycin (macrolides), clindamycin (lincosamide), vancomycin (glycopeptide), and linezolid (oxazolidinone). As summarized in Table 2, the results showed that Of1 potentiates all β-lactam antibiotics with variable fold of potentiation (8- to 128-fold). Although this MRSA strain is not resistant to carbapenems such as imipenem and meropenem, Of1 still showed 8- and 16-fold potentiation of these, respectively. In addition, this MRSA strain showed intermediate resistance to rifampicin with an MIC of 2 µg/mL. Of1 showed weak potentiation effect for rifampicin (twofold), but not any other classes of antibiotics tested, such as ciprofloxacin, tetracyclin, vancomycin, and linezolid. It should be noted that this MRSA strain is highly resistant to azithromycin, erythromycin, streptomycin, and clindamycin. Of1 (20 µM) was unable to lower their MICs to 256 µg/mL, the highest concentration tested.
Table 2.
Of1 selectively potentiates β-lactam antibiotics in multidrug-resistant MRSA
| Compound | MIC (µg/mL) | MIC (+Of1)* (µg/mL) | Fold of potentiation | Sensitive range† (µg/mL) |
| Of1 | >128 | — | — | — |
| Methicillin | 128 | 8 | 16 | ≤8 |
| Oxacillin | 64 | 0.5 | 128 | ≤2 |
| Amox/clav | 32/16 | 4/2 | 8 | ≤4/2 |
| Meropenem | 4 | 0.25 | 16 | ≤4 |
| Imipenem | 8 | 1 | 8 | ≤4 |
| Cephalexin | 256 | 16 | 16 | ≤8 |
| Cefazolin | 128 | 4 | 32 | ≤8 |
| Rifampicin | 2 | 1 | 2 | ≤1 |
| Tetracycline | 64 | 64 | 1 | ≤4 |
| Ciprofloxacin | 8 | 8 | 1 | ≤1 |
| Azithromycin | >256 | >256 | — | ≤2 |
| Erythromycin | >256 | >256 | — | ≤0.5 |
| Clindamycin | >256 | >256 | — | ≤0.5 |
| Streptomycin | >256 | >256 | — | — |
| Vancomycin | 1 | 1 | 1 | ≤2 |
| Linezolid | 2 | 2 | 1 | ≤4 |
MIC value in the presence of 20 µM Of1.
Values obtained from Clinical Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Testing; 17th informational supplement (36).
Furthermore, we evaluated Of1’s potentiation effect for β-lactam antibiotics in a methicillin-sensitive S. aureus (MSSA) strain (ATCC: 25923). Intriguingly, we found Of1 does not potentiate any β-lactams tested, such as methicillin, amox/clav, and cefazolin (SI Appendix, Table S1). The highly tuned specificity of Of1 for β-lactam antibiotics in MRSA largely rules out the general potentiation mechanisms, such as efflux pump inhibition and enhancement of membrane permeability (37). Instead, Of1 may selectively target β-lactam–specific resistance mechanism, such as β-lactam–induced expression of the mecA and/or blaZ genes, which encode PBP2a and β-lactamase, respectively (3,38–41). Because Of1 can potentiate the combination of amoxicillin and the β-lactamase inhibitor clavulanic acid, and Of1 does not have the reactive β-lactam functional group, it is highly likely that Of1 modifies the resistance of MRSA via an unknown mechanism.
Evaluation of the Antiproliferative Activity of Of1 in MRSA and MSSA.
To evaluate the antiproliferative activity of Of1 alone, we performed the standard microdilution assay for Of1 using both MRSA (ATCC: BAA-44) and MSSA (ATCC: 25923) strains. The MICs of Of1 against both strains were found to be higher than 128 µg/mL, the highest concentration tested. Considering the effective concentration (20 µM or 10 µg/mL) required to resensitize MRSA to methicillin; this result suggests that Of1 has synergistic effect with methicillin, and it does not target any essential genes or gene products.
Determination of Of1’s Minimum Resensitizing Concentrations for MRSA.
Next we determined Of1’s minimum resensitizing concentration (MRC) for MRSA with three common β-lactam antibiotics: oxacillin, amox/clav, and cefazolin. A modified broth microdilution assay was used. The modified microdilution assay involves incubating MRSA with Of1 in twofold series dilution in the presence of each individual antibiotic at its highest sensitive concentration (i.e., 2, 4/2, and 8 µg/mL, respectively). As shown in Table 3, Of1 resensitizes BAA-44 to all three antibiotics, and the MRC of Of1 is dependent on the antibiotic used. We also determined the MRC values for three other MRSA strains. Strain ATCC 33592 behaves similarly to BAA-44, and the MRC for all three antibiotics is 4 µg/mL. BAA-1683 is not resistant to cefazolin, but resistant to both oxacillin and amox/clav. The MRC for oxacillin is found to be >32 µg/mL (the highest concentration tested), and the MRC for amox/clav is 4 µg/mL, similar to the other two strains. Strain ATCC 700789 is particularly interesting, because it is known as a vancomycin intermediate-resistant S. aureus (VISA). Our results showed that Of1 is able to resensitize this VISA strain to amox/clav at 4 µg/mL.
Table 3.
Of1’s minimum resensitizing concentrations (MRCs) for MRSAs
| β-Lactams | BAA-44 | 33592 | 700789 | BAA-1683 |
| Oxacillin | 2 | 4 | >32 | >32 |
| Amox/clav | 4 | 4 | 4 | 4 |
| Cefazolin | 4 | 4 | — | — |
All MRC values are in µg/mL; all strain names are ATCC numbers. —, this strain is not resistant to the antibiotic indicated.
Mammalian Cytotoxicity of Of1.
To evaluate the cytotoxicity of Of1 in mammalian cells, we treated human liver hepatocellular carcinoma HepG2 cells with Of1 at various concentrations for 24 h. The remaining viable cells were determined using the CellTiter Glo mammalian viability assay (Promega). Of1 showed weak inhibition (∼10%) of the growth of HepG2 cells at 4 µg/mL, which is the MRC value of Of1 required to resensitize all strains to amox/clav or cefazolin. The half growth inhibitory concentration (GI50) of Of1 in HepG2 cells is determined as 8.2 µg/mL. This was determined by fitting the data using KaleidaGraph (v4.1.1; Synergy Software) (SI Appendix, Fig. S2).
In summary, from the pilot library of 120 highly diverse and functionalized polycyclic indolines, we identified a tricyclic indoline Of1 that selectively potentiates the activity of all β-lactam antibiotics tested (e.g., methicillin, amox/clav, cefazolin, and imipenem) in multidrug-resistant MRSA strains but not in MSSA. In addition, Of1 alone does not have antiproliferative activity in either MRSA or MSSA, and it has low cytotoxicity at the concentration required to resensitize MRSA to amox/clav and cefazolin. These observations suggest that Of1 is a unique resistance-modifying agent that selectively resensitizes MRSA to β-lactam antibiotics through an unknown mechanism. Further structure–activity relationship studies of Of1 and investigation of its mechanism of action are ongoing and will be reported in due course.
Materials and Methods
Supplementary figures, table, detailed methods, and compound characterization data and NMR spectra can be found in the SI Appendix. S. aureus strains ATCC BAA-44 and ATCC 25923 were generously donated to us from Daniel Feldheim and Charles McHenry (Department of Chemistry and Biochemistry, University of Colorado, Boulder, CO), respectively. S. aureus strains 33592, 700789, BAA-1683, and HepG2 (ATCC HB-8065) cells were purchased from the ATCC.
Supplementary Material
Acknowledgments
We are grateful to Dr. Richard Shoemaker and Mr. Patrick Barbour (Department of Chemistry and Biochemistry, University of Colorado, Boulder, CO) for NMR spectroscopic assistance. Funding for this project was provided by the University of Colorado Boulder (X.W.) and National Institutes of Health Institutional Training Grant GM008732 (to J.D.P).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310459110/-/DCSupplemental.
References
- 1.Payne DJ. Microbiology. Desperately seeking new antibiotics. Science. 2008;321(5896):1644–1645. doi: 10.1126/science.1164586. [DOI] [PubMed] [Google Scholar]
- 2.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis. 2007;13(12):1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: Mechanisms and modulation. Sci Prog. 2002;85(Pt 1):57–72. doi: 10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ubukata K, Nonoguchi R, Matsuhashi M, Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989;171(5):2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin JR, Murray CL, Rabinowitz JC. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981;256(21):11283–11291. [PubMed] [Google Scholar]
- 6.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6(1):29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 7.Coates AR, Halls G, Hu Y. Novel classes of antibiotics or more of the same? Br J Pharmacol. 2011;163(1):184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reading C, Cole M. Clavulanic acid: A beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stavri M, Piddock LJ, Gibbons S. Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemother. 2007;59(6):1247–1260. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- 10.Abreu AC, McBain AJ, Simões M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat Prod Rep. 2012;29(9):1007–1021. doi: 10.1039/c2np20035j. [DOI] [PubMed] [Google Scholar]
- 11.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15(8):639–652. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WH, Hu ZQ, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45(6):1737–1742. doi: 10.1128/AAC.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons S, Udo EE. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother Res. 2000;14(2):139–140. doi: 10.1002/(sici)1099-1573(200003)14:2<139::aid-ptr608>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Ban Y, Murakami Y, Iwasawa Y, Tsuchiya M, Takano N. Indole alkaloids in medicine. Med Res Rev. 1988;8(2):231–308. doi: 10.1002/med.2610080205. [DOI] [PubMed] [Google Scholar]
- 15.Arora RB, Madan BR. Antiarrhythmics. VI. Ajmaline and serpentine in experimental cardiac arrhythmias. J Pharmacol Exp Ther. 1956;117(1):62–67. [PubMed] [Google Scholar]
- 16.Shamon SD, Perez MI. Blood pressure lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst Rev. 2009;CD007655(4):CD007655. doi: 10.1002/14651858.CD007655.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Bensch KG, Marantz R, Wisniewski H, Shelanski M. Induction in vitro of microtubular crystals by vinca alkaloids. Science. 1969;165(3892):495–496. doi: 10.1126/science.165.3892.495. [DOI] [PubMed] [Google Scholar]
- 18.Benson SC, Lee L, Yang L, Snyder JK. Intramolecular inverse electron demand Diels-Alder reactions of tryptamine with tethered heteroaromatic azadienes. Tetrahedron. 2000;56(9):1165–1180. [Google Scholar]
- 19. Gan Z, Reddy PT, Quevillon S, Couve-Bonnaire S, Arya P (2005) Stereocontrolled solid-phase synthesis of a 90-membered library of indoline-alkaloid-like polycycles from an enantioenriched aminoindoline scaffold. Angewandte Chemie Int 44(9):1366–1368. [DOI] [PubMed]
- 20.Oguri H, Schreiber SL. Skeletal diversity via a folding pathway: Synthesis of indole alkaloid-like skeletons. Org Lett. 2005;7(1):47–50. doi: 10.1021/ol047945w. [DOI] [PubMed] [Google Scholar]
- 21.Prakesch M, et al. Building skeletally diverse architectures on the indoline scaffold: The discovery of a chemical probe of focal adhesion kinase signaling networks. Bioorg Med Chem. 2008;16(21):9596–9602. doi: 10.1016/j.bmc.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Lo MM, Neumann CS, Nagayama S, Perlstein EO, Schreiber SL. A library of spirooxindoles based on a stereoselective three-component coupling reaction. J Am Chem Soc. 2004;126(49):16077–16086. doi: 10.1021/ja045089d. [DOI] [PubMed] [Google Scholar]
- 23. Noren-Muller A, et al. Discovery of a new class of inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase B by biology-oriented synthesis. Angewandte Chemie Int 47(32):5973–5977. [DOI] [PubMed]
- 24. Meseguer B, Alonso-Diaz D, Griebenow N, Herget T, Waldmann H (1999) Natural product synthesis on polymeric supports: Synthesis and biological evaluation of an indolactam library. Angewandte Chemie Int 38(19):2902–2906. [DOI] [PubMed]
- 25.Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287(5460):1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 26. Nielsen TE, Schreiber SL (2008) Towards the optimal screening collection: A synthesis strategy. Angewandte Chemie Int 47(1):48–56. [DOI] [PMC free article] [PubMed]
- 27.Austin MB, O’Maille PE, Noel JP. Evolving biosynthetic tangos negotiate mechanistic landscapes. Nat Chem Biol. 2008;4(4):217–222. doi: 10.1038/nchembio0408-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staunton J, Wilkinson B. Biosynthesis of Erythromycin and Rapamycin. Chem Rev. 1997;97(7):2611–2630. doi: 10.1021/cr9600316. [DOI] [PubMed] [Google Scholar]
- 29.Chau M, Jennewein S, Walker K, Croteau R. Taxol biosynthesis: Molecular cloning and characterization of a cytochrome P450 taxoid 7 beta-hydroxylase. Chem Biol. 2004;11(5):663–672. doi: 10.1016/j.chembiol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Woithe K, et al. Oxidative phenol coupling reactions catalyzed by OxyB: A cytochrome P450 from the vancomycin producing organism. implications for vancomycin biosynthesis. J Am Chem Soc. 2007;129(21):6887–6895. doi: 10.1021/ja071038f. [DOI] [PubMed] [Google Scholar]
- 31.Yeo SJ, Liu Y, Wang X. A one-pot three-component reaction for the preparation of highly functionalized tryptamines. Tetrahedron. 2012;68(3):813–818. [Google Scholar]
- 32.Liu YX, Xu WQ, Wang X. Gold(I)-catalyzed tandem cyclization approach to tetracyclic indolines. Org Lett. 2010;12(7):1448–1451. doi: 10.1021/ol100153h. [DOI] [PubMed] [Google Scholar]
- 33.Noey EL, Wang X, Houk KN. Selective gold(I)-catalyzed formation of tetracyclic indolines: A single transition structure and bifurcations lead to multiple products. J Org Chem. 2011;76(9):3477–3483. doi: 10.1021/jo200556f. [DOI] [PubMed] [Google Scholar]
- 34.Bandini M. Gold-catalyzed decorations of arenes and heteroarenes with C-C multiple bonds. Chem Soc Rev. 2011;40(3):1358–1367. doi: 10.1039/c0cs00041h. [DOI] [PubMed] [Google Scholar]
- 35. Staben ST, Kennedy-Smith JJ, Toste FD (2004) Gold(I)-catalyzed 5-endo-dig carbocyclization of acetylenic dicarbonyl compounds. Angewandte Chemie Int 43(40):5350–5352. [DOI] [PubMed]
- 36. Clinical and Laboratory Standards Institute (2009) Methods for dilution antimicrobial susceptibility testing for bacteria that grew aerobically. (Clinical and Laboratory Standards Institute, Wayne, PA), Approved Standard M7-A10.
- 37.Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128(6):1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 38. Zhang HZ, Hackbarth CJ, Chansky KM, Chambers H. F. A proteolytic transmembrane signaling pathway and resistance to beta-lactams in staphylococci. Science 291(5510):1962–1965. [DOI] [PubMed]
- 39.Arede P, Ministro J, Oliveira DC. Redefining the role of beta-lactamase locus in MRSA: Beta-lactamase regulators disrupt the MecI-mediated strong repression on mecA and optimize the phenotypic expression of resistance in strains with constitutive mecA expression. Antimicrob Agents Chemother. 2013;57(7):3037–3045. doi: 10.1128/AAC.02621-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llarrull LI, Toth M, Champion MM, Mobashery S. Activation of BlaR1 protein of methicillin-resistant Staphylococcus aureus, its proteolytic processing, and recovery from induction of resistance. J Biol Chem. 2011;286(44):38148–38158. doi: 10.1074/jbc.M111.288985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambers HF. Methicillin resistance in staphylococci: Molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10(4):781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.