Abstract
Circulating platelets are constantly exposed to nitric oxide (NO) released from the vascular endothelium. This NO acts to reduce platelet reactivity, and in so doing blunts platelet aggregation and thrombus formation. For successful hemostasis, platelet activation and aggregation must occur at sites of vascular injury despite the constant presence of NO. As platelets aggregate, they release secondary mediators that drive further aggregation. Particularly significant among these secondary mediators is ADP, which, acting through platelet P2Y12 receptors, strongly amplifies aggregation. Platelet P2Y12 receptors are the targets of very widely used antithrombotic drugs such as clopidogrel, prasugrel, and ticagrelor. Here we show that blockade of platelet P2Y12 receptors dramatically enhances the antiplatelet potency of NO, causing a 1,000- to 100,000-fold increase in inhibitory activity against platelet aggregation and release reactions in response to activation of receptors for either thrombin or collagen. This powerful synergism is explained by blockade of a P2Y12 receptor-dependent, NO/cGMP-insensitive phosphatidylinositol 3-kinase pathway of platelet activation. These studies demonstrate that activation of the platelet ADP receptor, P2Y12, severely blunts the inhibitory effects of NO. The powerful antithrombotic effects of P2Y12 receptor blockers may, in part, be mediated by profound potentiation of the effects of endogenous NO.
Keywords: anti-platelet therapy, atherothrombosis, prostacyclin
Activation of platelets in a damaged blood vessel leads to a well-characterized sequence of events, including the important release of secondary mediators of aggregation, notably ADP and thromboxane A2 (1–3). These mediators are the targets of antithrombotic drugs taken by many millions of patients as prophylactic protection against heart attacks and strokes. In particular, inhibition of thromboxane A2 production by aspirin explains the antithrombotic utility of this drug, whereas blockade of the ADP receptor, P2Y12, by drugs such as clopidogrel, prasugrel, and ticagrelor provides a second, and possibly even stronger, antithrombotic protection (4). Within the circulation, platelets are constantly exposed to antithrombotic mediators, notably prostaglandin I2 (PGI2), which is derived from the vascular endothelium, and nitric oxide (NO), which can be produced by the vascular endothelium (5) and platelets themselves (6, 7). This leads to the understanding that there is an important interplay between endothelial-derived antiaggregatory mediators and platelet-derived proaggregatory mediators. For example, it has been demonstrated that activation of platelet P2Y12 receptors by ADP causes inhibition of platelet adenylyl cyclase, which reduces the ability of PGI2 acting through prostaglandin IP receptors to elevate cAMP, and thus reduces platelet aggregation. Hence, blockade of platelet P2Y12 receptors increases the sensitivity of platelets to the antiaggregatory and antisecretory effects of PGI2 (8, 9). This interaction is readily understandable, as both P2Y12 receptors and PGI2 have actions on a common signaling pathway, cAMP.
Compared with PGI2, NO is less potent as an inhibitor of platelet activation (10–17). NO acts by directly activating platelet guanylyl cyclase, causing an increase in intraplatelet cGMP (13). Although this cGMP pathway is distinct from the cAMP pathway that is modulated by PGI2 and P2Y12, earlier studies have shown that activation of these two inhibitory pathways produces a synergistic antiplatelet effect (18). Further, it has been shown that a NO donor drug can boost the effectiveness of P2Y12 inhibitors against thrombus formation ex vivo (19, 20). Here we report that treatment of platelets with P2Y12 receptor blockers dramatically increases the antiplatelet effects of NO, an increase that is very much greater than that already established for PGI2. This reveals that activation of platelet P2Y12 receptors by ADP could provide a common switching mechanism by which platelet sensitivity to the antithrombotic effects of NO and PGI2 is rapidly reduced to allow platelet activation, as required for hemostasis. It also raises the important possibility that for individual patients, the effectiveness of P2Y12 receptor blockers may vary on the basis of their vascular production of NO and PGI2. This provides a rationale for the commonly reported disconnect between the results of ex vivo testing of platelet responses and thrombotic outcomes.
Results and Discussion
P2Y12 Receptor Blockade Greatly Increases the Inhibition of Platelet Aggregation by NO.
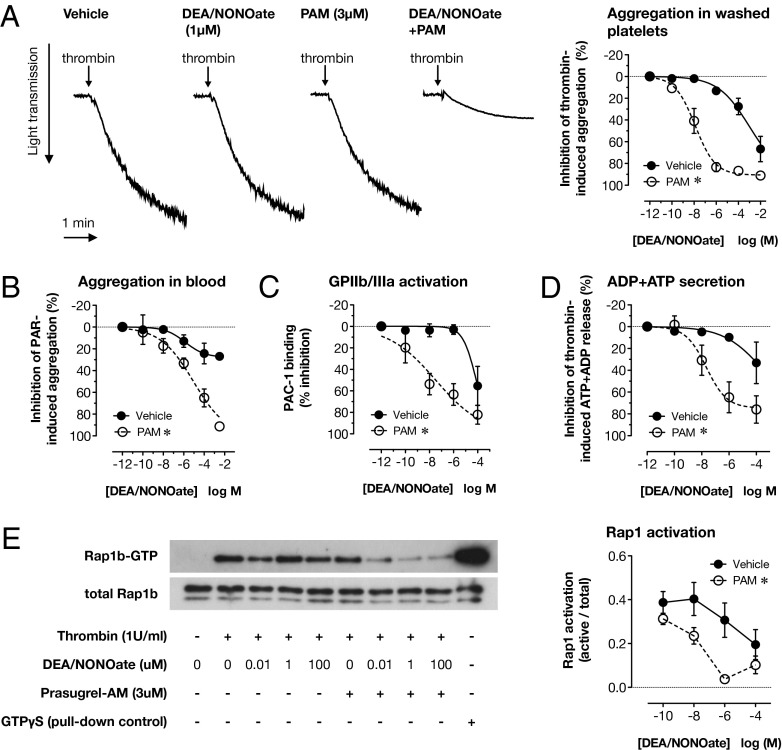
To study interactions between P2Y12 receptor activation and the inhibitory effect of NO on platelet aggregation, we used thrombin as the stimulus for platelet activation. Thrombin is a physiologically relevant agonist that provides a strong signal for ADP release. Similar to previous studies of this type (8), we found 1 U/mL thrombin to provide a maximal aggregation and secretion response that was relatively insensitive to P2Y12 receptor blockade (Fig. S1). By using 1 U/mL thrombin as an agonist, we could therefore study the effects of P2Y12 receptor blockade on responses to NO without the confounding effects of a differing baseline. In control conditions, the NO donor diethlyammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA/NONOate), which spontaneously releases NO at physiological pH (half-life ∼2 min), produced only weak inhibition of thrombin (1 U/mL)-induced washed platelet aggregation, even at supraphysiological concentrations (Fig. 1A). However, in the presence of the P2Y12 receptor blocker prasugrel active metabolite (PAM), both the potency and the maximal effect of DEA/NONOate were dramatically increased (Fig. 1A; logIC50 DEA/NONOate, −3.0 ± 0.4; DEA/NONOate +PAM, −7.9 ± 0.2; P < 0.0001; n = 8). This clearly suggests a powerful interaction between NO and P2Y12 blockade.
Fig. 1.
Blockade of the P2Y12 receptor sensitizes platelets to the inhibitory effect of NO against thrombin-induced aggregation, secretion, and Rap1 activation. Thrombin (1 U/mL)-induced aggregation of washed platelets, determined by changes in light transmission, was only weakly inhibited by the NO donor, DEA/NONOate (A) (n = 8), but this effect was greatly increased in platelets pretreated with the P2Y12 receptor blocker PAM (3 μM). Traces show the effects of 1 μM DEA/NONOate in the absence and presence of PAM. Similarly, inhibition by DEA/NONOate of platelet aggregation in hirudin-anticoagulated whole blood stimulated with SFLLRN-amide (10 μM) + AYPGKF-amide (30 μM) and measured by impedance aggregometry (B), thrombin (1 U/mL)-induced GPIIb/IIIa activation by flow cytometry (C) and ADP+ATP secretion, measured by a modified luminometric method (D), were also strongly potentiated by P2Y12 receptor blockade (n = 4 for each). Thrombin (1 U/mL) also stimulated rapid activation of Rap1, as determined by affinity purification of Rap1-GTP and Western blotting of lysates of platelets stimulated under stirring conditions for 15 s (E). Rap1 activation was little altered by DEA/NONOate or P2Y12 receptor blockade alone but was strongly inhibited by the combination. Platelet lysates incubated with GTPγS were included on blots as a positive control for affinity isolation of Rap1-GTP (n = 4 for each). *P < 0.05 by two-way ANOVA vs. vehicle.
We observed a similar potentiation of the inhibitory effect of DEA/NONOate when we examined either aggregation of washed platelets induced by the glycoprotein VI (GPVI) agonist, collagen-related peptide (CRP; 1 μg/mL; Fig. S1), or aggregation in whole blood in response to activating peptides for the thrombin receptors, protease-activated receptor 1 (PAR1) and protease-activated receptor 4 (PAR4) (Fig. 1B). Importantly, this demonstrated that the powerful inhibitory interaction between P2Y12 receptor blockade and NO exists for another strong platelet agonist and is not an artifact of the method of platelet preparation used (i.e., is not unique for washed platelets).
Platelets themselves have been previously described to release NO to regulate aggregation responses (6, 7). To study the interaction between P2Y12 and platelet-derived NO, we measured thrombin- and collagen-induced platelet aggregation when platelet NO synthase activity was stimulated with l-arginine or inhibited with N5-[imino(nitroamino)methyl]-L- ornithine, methyl ester, monohydrochloride (L-NAME). In our experimental system, however, we were unable to observe any role for platelet-derived NO, either in the presence or absence of P2Y12 blockade (Fig. S2).
Inhibition of Thrombin-Induced Glycoprotein IIb/IIIa Activation, ADP+ATP Secretion, and Activation of the GTPase Rab1b by NO Is Increased by P2Y12 Receptor Blockade.
In parallel experiments, we confirmed that potentiation of the inhibitory effects of NO on platelet aggregation was reflected at the biochemical level of platelet activation. To do this, we studied glycoprotein IIb/IIIa (GPIIb/IIIa) activation (Fig. 1C and Fig. S3) and alpha granule section by flow cytometry (Fig. S3), dense granule secretion by ADP+ATP release (Fig. 1D), and Rap1 activation by using Ral guanine nucleotide dissociation stimulator (RalGDS) affinity pull-down for GTP-bound Rap1 (Fig. 1E). Thrombin stimulated a robust response in each activation assay, which was only modestly inhibited by DEA/NONOate. However, in each case, the inhibitory potency of DEA/NONOate was greatly increased by P2Y12 receptor blockade. For example, 10 nM DEA/NONOate in the presence of PAM caused the same inhibition (31% ± 14%) of thrombin-induced ADP+ATP secretion as 100 μM DEA/NONOate in the absence of PAM (34% ± 19% inhibition; n = 4; Fig. 1D). This represents a 10,000-fold increase in the inhibitory potency of DEA/NONOate.
Blockade of P2Y12 Receptors Acts Synergistically with NO to Inhibit Platelet Aggregation Induced by Thrombin or CRP.
To establish whether the interactions observed between NO and P2Y12 blockade for inhibition of thrombin- and CRP-induced platelet aggregation were additive or synergistic, we performed an isobolographic analysis (21). To do this, we performed multiple matched aggregation studies in 96-well microtiter plates, using both thrombin (0.5 and 1 U/mL) and CRP (0.5 and 1 μg/mL) as agonists. Aggregation was measured in the presence of increasing concentrations of two structurally distinct P2Y12 receptor blockers, PAM and ticagrelor. From these data, isobolograms were constructed (described in Fig. S4) showing the concentrations of PAM and DEA/NONOate required, in combination, to produce 50% (Fig. S5) or 25% and 75% (Fig. S6) inhibition of platelet aggregation. For a simple additive relationship between two inhibitors, an isobologram displays a straight line in which reducing concentration of one inhibitor requires a linear increase in the concentration of the other to maintain the same effect. However, data displayed here for the interaction between DEA/NONOate and PAM or ticagrelor show a very different pattern (Figs. S5 and S6), with the isoboles curving strongly toward the axes, indicating a powerful synergy between NO and blockade of P2Y12 receptors.
P2Y12 Activation Limits the Inhibitory Activity of NO Through a Mechanism Requiring Activation of Guanine Nucleotide Binding Protein αi/z but Not Cyclooxygenase.
We next wanted to confirm the specific role of P2Y12-dependent Gαi/z signaling in regulating the responses of platelets to DEA/NONOate. To do this, we determined the effect of epinephrine (10 μM), which restores Gαi/z activation in the presence of P2Y12 blockade (22), on the sensitivity of platelets to DEA/NONOate. As described earlier, DEA/NONOate produced only weak inhibition of platelet aggregation, but this was not altered by addition of epinephrine (Fig. S7). Similarly, as described earlier, pretreatment of platelets with PAM enhanced the inhibitory potency of DEA/NONOate, but this effect was completely reversed by the addition of epinephrine, indicating that this phenomenon is mediated by Gαi/z signaling.
To determine whether the interactions between P2Y12 receptor blockade and DEA/NONOate occurred at the level of thromboxane A2, we repeated experiments in the presence of aspirin (100 μM). Aspirin, however, had no effect on the inhibitory potency of DEA/NONOate or the potentiating effects of P2Y12 receptor blockade (Fig. S7).
NO-Induced cGMP Formation and Activity Is Not Affected by P2Y12 Receptor Blockade.
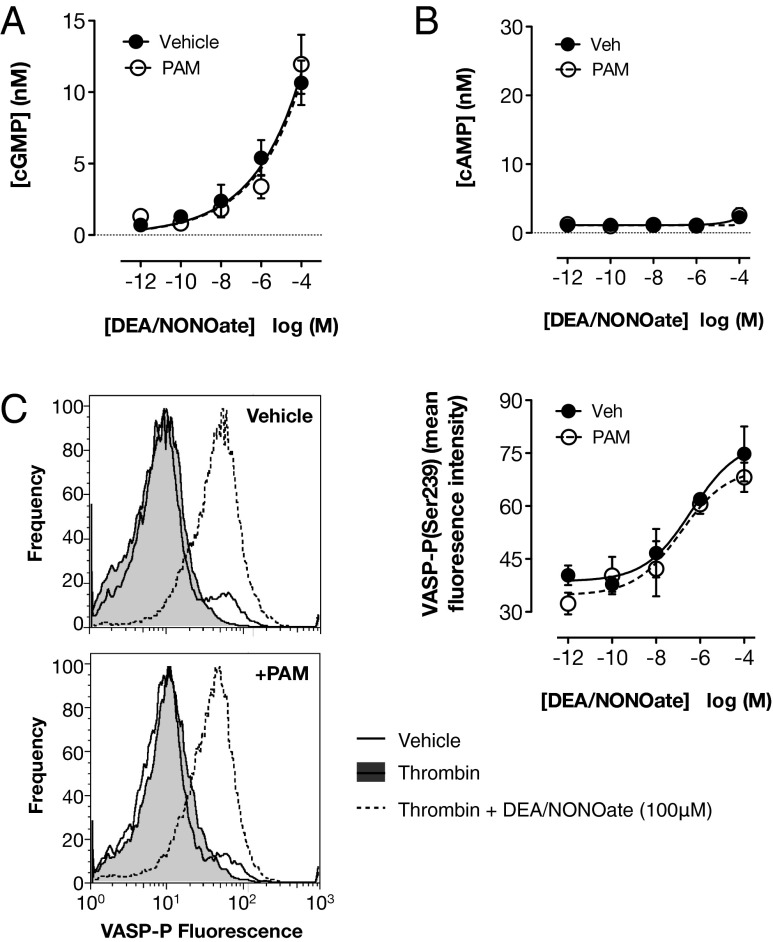
It has been recently demonstrated that physiologically relevant concentrations of DEA/NONOate and other NO donors act in platelets exclusively through activation of soluble guanylyl cyclase, driving the production of cGMP (13). cGMP is an important signaling molecule with its own downstream effectors but may also act in part by influencing cAMP signaling. For example, it has been previously suggested that cGMP can elevate cAMP levels, possibly by competitive inhibition of phosphodiesterase (PDE) 3 (23, 24). To determine whether platelet cGMP and/or cAMP was affected by the interaction between NO and P2Y12 receptor activation, we measured platelet cGMP and cAMP levels, as well as vasodilator-stimulated phosphoprotein (VASP) phosphorylation at serine 239, a composite cGMP and cAMP response (25). P2Y12 blockade alone had no detectable effect on platelet cGMP or cAMP content or on VASP phosphorylation (Fig. 2). DEA/NONOate caused a concentration-dependent increase in the platelet content of cGMP (Fig. 2A) without affecting cAMP (Fig. 2B), but with a concomitant increase in VASP phosphorylation (Fig. 2 C and D). These actions were unaffected by blockade of P2Y12 receptors, suggesting that the interaction between DEA/NONOate and P2Y12 is downstream of the formation of cAMP and cGMP.
Fig. 2.
P2Y12 receptor blockade does not alter the ability of NO to stimulate cAMP or cGMP formation or VASP phosphorylation. cGMP content, determined by immunoassay in lysates of washed platelets stimulated with thrombin (1 U/mL) under stirring conditions, was increased by DEA/NONOate (A). This response was not different in platelets pretreated with the P2Y12 receptor blocker PAM (3 μM; n = 9). cAMP content, measured in the same way, was not altered by either DEA/NONOate or P2Y12 receptor blockade (B) (n = 9). The level of phospho(Ser239)-VASP, measured by flow cytometry in washed platelets stimulated with thrombin under nonstirring conditions, was also increased by DEA/NONOate, and this response was not altered by P2Y12 receptor blockade (C). Representative histograms show the effect of 100 μM DEA/NONOate in the presence and absence of PAM (n = 4).
Blockade of P2Y12 Receptors Does Not Interact with Inhibitors of PDE2, PDE3, or PDE4 in Affecting Platelet Aggregation.
To further exclude any role for regulation of phosphodiesterase activity as underlying the interactions between NO and P2Y12 receptors, we performed experiments using selective inhibitors of the 3 major platelet PDE isoforms: PDE2, PDE3, and PDE5 (26). Inhibition of PDE2 by erythro-9-2-hydroxy-3-nonyladenine, or PDE3 by milrinone, produced no change in the effects of DEA/NONOate on platelet aggregation, cAMP formation, or cGMP formation (Fig. S8). In contrast, inhibition of PDE5 by sildenafil enhanced the inhibitory activity of DEA/NONOate against thrombin (1 U/mL)-induced platelet aggregation, which was associated with an increased platelet content of cGMP but not cAMP (Fig. S8). The addition of PAM increased the inhibitory effects of DEA/NONOate; this increase was not altered by either milrinone or erythro-9-2-hydroxy-3-nonyladenine but was further increased by sildenafil. The results of these experiments are consistent with the well-established role for PDE5, but not PDE2 or PDE3, as a limiter of cGMP accumulation in platelets. More relevantly, these results indicate that neither PDE inhibition nor cAMP play a specific role in the interaction between P2Y12 receptor blockade and NO. Indeed, there were no detectable changes in platelet cAMP content even when platelet cGMP content was elevated more than 150-fold by the combination of DEA/NONOate and sildenafil.
P2Y12 Blockade Increases the Ability of cGMP-Analogs to Inhibit Platelet Aggregation, but Not VASP Phosphorylation.
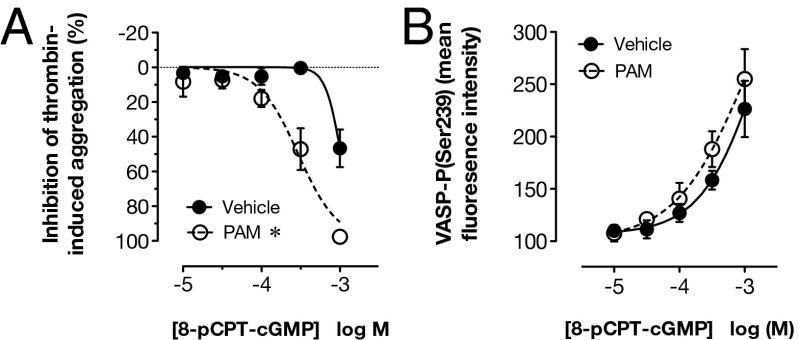
Having shown that P2Y12 receptor blockade was not influencing the formation or metabolism of cAMP and cGMP, we next sought to determine whether P2Y12 receptor blockade alters the sensitivity of platelets to exogenous cGMP. We found that PAM enhanced the ability of a cell-permeable analog of cGMP, 8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate (8-pCPT-cGMP), to inhibit aggregation (Fig. 3A), but it did not alter the ability of cGMP to stimulate VASP phosphorylation (Fig. 3B). This confirmed that the interaction between P2Y12 receptor blockade and NO occurs downstream of cGMP.
Fig. 3.
P2Y12 receptor blockade potentiates inhibition of platelet aggregation to exogenous cGMP but does not potentiate phosphorylation of VASP. Thrombin (1 U/mL)-induced aggregation of washed platelets, determined by changes in light transmission, was inhibited by the cell-permeable cGMP analog, 8-pCPT-cGMP (A), and this action was enhanced in platelets pretreated with the P2Y12 receptor blocker PAM (3 μM). An increase in platelet phospho(Ser239)-VASP levels also was produced by 8-pCPT-cGMP, as determined by flow cytometry, but this was not altered by P2Y12 receptor blockade (B). *P < 0.05 by two-way ANOVA vs. vehicle (n = 3–4).
To further explore the role for cGMP, we performed experiments using two structurally distinct inhibitors of cGMP regulated protein kinase (cGK), which is a major downstream effector of cGMP. We found that neither 8-(4-chlorophenylthio)guanosine-3′,5′cyclic monophosphorothioate, Rp-isomer (Rp-8-pCPT-cGMPS) (200 μM) nor KT5823 (5 μM) affected the inhibition of platelet aggregation following the combination of DEA/NONOate and PAM (Fig. S8). This may indicate that the inhibition of platelet aggregation occurs through a cGK-independent mechanism. Alternatively, and possibly more likely, this may reflect the limitations of these experimental compounds (27–29).
Inhibition of Thrombin-Induced Intracellular Calcium Mobilization by NO Is Not Limited by P2Y12 Receptor Activation.
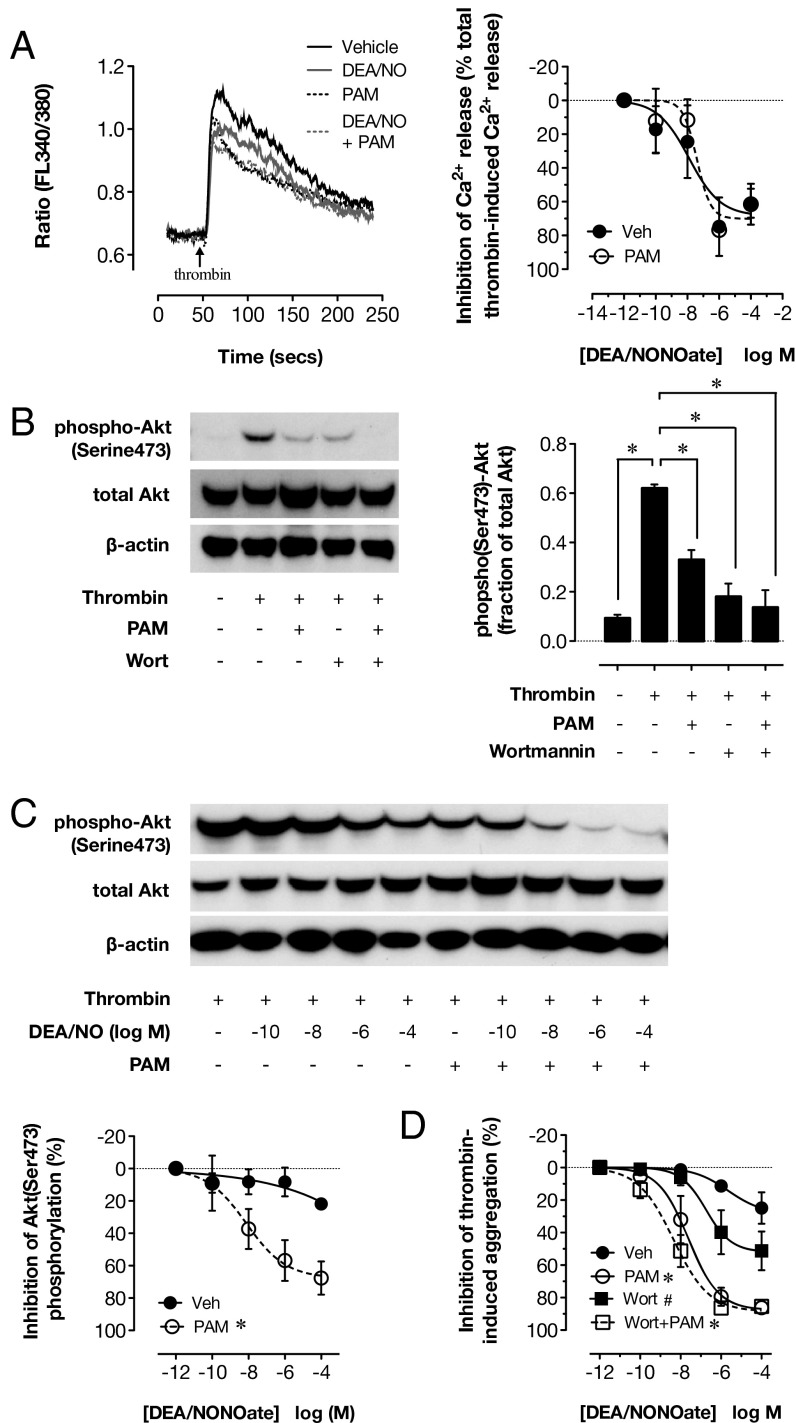
Current evidence indicates that the inositol triphosphate receptor associated cGK target (IRAG) is a primary mediator of the inhibitory effects of cGMP in platelets. IRAG exists in a complex with cGK-I and the type 1 InsP3 receptor in the membrane of the dense tubular system and regulates InsP3-mediated calcium release, a major component of aggregation and secretion responses to many agonists, including thrombin. IRAG deletion (14), or mutation to prevent its association with the InsP3 receptor, strongly blunts the inhibitory effects of NO donors on platelets (12). In comparison, deletion of other putative targets of platelet cGMP, such as VASP, causes more modest reductions in sensitivity to NO and cGMP (30). We therefore examined how P2Y12 blockade and NO interacted at this level. Stimulation of washed platelets with thrombin led to a rapid rise in free intracellular calcium concentration (Fig. 4A); DEA/NONOate produced concentration-dependent, but incomplete, inhibition of this calcium release (12). This inhibitory effect of DEA/NONOate was unaffected by PAM and occurred at concentrations of DEA/NONOate very much below those that inhibited platelet aggregation and secretion (Fig. 4A).
Fig. 4.
P2Y12 receptor blockade does not alter the ability of NO to inhibit intracellular Ca2+ mobilization but facilitates its ability to inhibit PI3K signaling. Thrombin (1 U/mL)-induced mobilization of intracellular calcium in Fura2-loaded washed platelets was inhibited by DEA/NONOate, and this inhibition was not altered by the P2Y12 receptor blocker PAM (3 μM) (A). Thrombin (1 U/mL) also stimulated phosphorylation of Akt(Ser473) under stirring conditions measured in washed platelets lysed 2 min after agonist addition (B). Akt(Ser473) phosphorylation was strongly inhibited by P2Y12 receptor blockade (PAM; 3 μM) or the PI3K inhibitor, wortmannin (Wort; 200 nM) (B). DEA/NONOate only weakly inhibited thrombin-induced Akt(Ser473) phosphorylation, but this effect was greatly potentiated in the presence of PAM (C). In parallel studies, specific inhibition of PI3K by wortmannin enhanced the ability of DEA/NONOate to inhibit thrombin (1 U/mL)-induced aggregation of washed platelets in the absence, but not the presence, of P2Y12 blockade (D). (A, C, and D) *P < 0.05 by two-way ANOVA vs. vehicle. (B) *P < 0.05 by one-way ANOVA vs. thrombin. (D) #P < 0.05 by two-way ANOVA vs. the same treatment in the absence of wortmannin (n = 4).
The Ability of P2Y12 Receptor Activation to Limit the Inhibitory Effect of NO Depends on P2Y12 Receptor-Dependent Phosphoinositide-3 Kinase Activation.
These data demonstrate that P2Y12 receptor activation drives a cGMP-insensitive pathway of platelet activation that supports platelet aggregation, even in the presence of NO. To investigate this hypothesis, we studied the effect of DEA/NONOate and P2Y12 receptor blockade on phosphoinositide-3-kinase (PI3K) activation, a principle signaling mechanism downstream of the P2Y12 receptor. To do this, we measured phosphorylation of the PI3K substrate, protein kinase B (Akt), at serine 473. Thrombin (1 U/mL)-induced phosphorylation of Akt (Fig. 4B) was partially inhibited by PAM and strongly inhibited by the specific PI3K inhibitor, wortmannin. This is consistent with the current understanding that Akt phosphorylation is primarily dependent on activation of P2Y12 receptors by secreted ADP (31). By itself, DEA/NONOate had little inhibitory effect on thrombin (1-U/mL)-induced Akt phosphorylation, but in the presence of PAM, it produced a much greater inhibitory effect (Fig. 4C). This suggests that P2Y12 receptor-dependent PI3K activation could constitute a cGMP-insensitive pathway that supports aggregation in the presence of NO.
To determine the functional consequences of P2Y12 receptor-mediated PI3K activation, we assessed how specific PI3K inhibition by wortmannin influenced the ability of DEA/NONOate to reduce platelet aggregation. PI3K inhibition increased the sensitivity and maximal inhibition of aggregation produced by DEA/NONOate (Fig. 4D), confirming that PI3K can limit the antiplatelet effect of NO. Further, addition of wortmannin to PAM produced no further increase in the sensitivity of platelets to DEA/NONOate, indicating that this NO-limiting PI3K pathway is driven by P2Y12 receptor activation.
The Synergy Between P2Y12 Blockade and NO Is Very Much Greater than That Between P2Y12 Receptor Blockade and Prostacyclin.
It has been previously demonstrated that P2Y12 blockade can also potentiate the inhibitory effects of prostacyclin (8). To compare the magnitude of the interaction between P2Y12 receptor activation and prostacyclin with that described earlier for NO, we determined in paired experiments the effect of PAM on the inhibitory potency of prostacyclin and DEA/NONOate against thrombin (1 U/mL)-induced aggregation and secretion responses (Fig. S9). We found that although PAM potentiated the inhibitory effect of both agents, its effects on DEA/NONOate were very much greater than those on prostacyclin.
Summary and Conclusions.
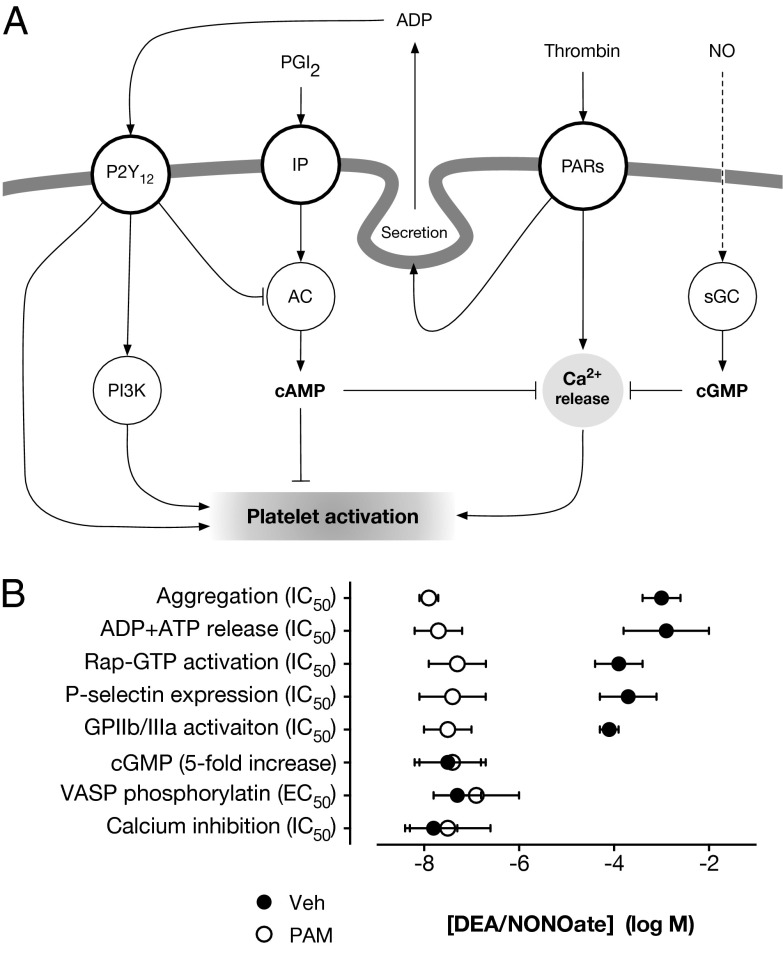
Here we report the unique observation that P2Y12 receptor activation strongly limits the antiplatelet effects of NO. The power of this limiting pathway is illustrated by the finding that blockade of P2Y12 receptors increases the inhibitory potency of the NO donor, DEA/NONOate, by many orders of magnitude. We observed this synergy between NO and two chemically unrelated blockers of P2Y12 receptors, PAM and ticagrelor, on multiple platelet responses after activation of thrombin or collagen receptors. The interplay between P2Y12 receptor blockade and NO was not related to altered synthesis or degradation of either cGMP or cAMP. Instead, it appears to reflect the ability of P2Y12 receptors to activate PI3K and other cGMP-insensitive signaling pathways that can sustain aggregation responses even in the presence of very high concentrations of NO (Fig. 5A). Thus, although platelets can sense nanomolar concentrations of NO, as determined by cGMP formation, VASP phosphorylation, or inhibition of Ca2+ mobilization, millimolar concentrations are required to inhibit platelet functional markers such as aggregation and secretion after strong activation. Only when P2Y12 receptors are blocked is the true inhibitory activity of NO revealed (Fig. 5B).
Fig. 5.
Schematic and summary for interaction between P2Y12 receptors and NO signaling in platelets. Schematic showing proposed mechanism by which P2Y12 receptor activation by secreted ADP limits the antiplatelet effect of NO by providing a cGMP-insensitive pathway for platelet aggregation, which depends partly on PI3K signaling (A). Relative potencies for DEA/NONOate on platelet responses (B) highlight that although DEA/NONOate is active on platelets at nanomolar concentrations (cGMP, VASP, Ca2+), inhibition of strong activation (aggregation, secretion, etc.) requires millimolar concentrations of DEA/NONOate. However, when P2Y12 receptors are blocked, the inhibitory potency of NO against activation markers closely correlates with its potency on cGMP production, VASP, and calcium.
In conclusion, previously published data (8) have shown the ability of P2Y12 receptor activation to limit the antiplatelet effect of prostacyclin (Fig. S9). Our observations on the interaction between NO and P2Y12 receptor signaling indicate that in the presence of a strong stimulus, P2Y12 receptor activation is a key mechanism by which platelets can overcome the effects of inhibitory autacoids released by the vascular endothelium (18). Overcoming the effects of these inhibitory autacoids allows the platelet activation essential for hemostasis. As such, potentiating the inhibitory actions of NO and prostacyclin in vivo may provide a key mechanism through which P2Y12 receptor blockers, such as clopidogrel, prasugrel, and ticagrelor, exert antithrombotic protection in man. The effectiveness of P2Y12 receptor blockers as antithrombotic agents in individual patients may, therefore, vary markedly as a function of endothelial activity. This raises many interesting questions as to how antithrombotic therapies can best be used relative to the vascular health of particular individuals or patient groups.
Materials and Methods
Additional methodological detail can be found in the SI Materials and Methods.
Preparation of Platelets.
Blood was collected by venipuncture from healthy volunteers, who had provided written consent (St. Thomas’s Hospital, London, Research Ethics Committee, Reference 07/Q0702/24), into trisodium citrate (0.32% final). Washed platelets were prepared by centrifugation and resuspended in Tyrodes-Hepes buffer at pH 7.4 at 3 × 108 platelets/mL.
Light Transmission Aggregometry.
Washed platelet suspensions were incubated (30 min, room temperature) with the P2Y12 receptor blocker PAM (3 μM; AstraZeneca) or vehicle (0.5% DMSO). In some experiments, additional pretreatments were performed as detailed. Aggregation to thrombin (1 U/mL; Sigma) or CRP [1 μg/mL (a kind gift of Richard Farndale University of Cambridge, Cambridge, United Kingdom) in the presence of 1 mg/mL human fibrinogen (Sigma)] was measured in a Bio/Data PAP-8E turbidimetric aggregometer after preincubation (1 min, 37 °C) with the NO donor DEA/NONOate (Sigma) or vehicle.
96-Well Plate Aggregometry.
In some experiments, platelet aggregation was assessed in 96-well plates, as we have previously reported (32). Washed platelets were treated as described earlier, before aggregation was stimulated by the addition of thrombin (0.5 or 1 U/mL) or CRP (0.5 or 1 μg/mL in the presence of 1 mg/mL human fibrinogen) and shaking on a microplate shaker (BioShake IQ, QUANTIFOIL Instruments). Absorbance was then determined using a microplate reader and percentage platelet aggregation calculated with reference to the absorbance of Tyrodes-Hepes buffer, taken as a surrogate for 100% aggregation.
Isobolographic Analysis.
Using data from 96-well platelet aggregation experiments, IC25, IC50, and IC75 values for DEA/NONOate in the presence of increasing concentrations of PAM or ticagrelor were calculated. These were used to generate isobolograms, as detailed in Fig. S4.
Whole-Blood Aggregometry.
Whole blood, collected into lepirudin (25 μg/mL final; Celgene), was treated with P2Y12 receptor blockers and DEA/NONOate, as described earlier, before platelet aggregation to the thrombin receptor activating peptides SFLLRN amide (10 μM, Bachem) and AYPGKF-amide (30 μM; Bachem) was monitored by impedance aggregometry (Multiplate; Dynabyte).
ADP+ATP Secretion.
Washed platelets, treated with P2Y12 inhibitors and DEA/NONOate as described earlier, were stimulated with thrombin (1 U/mL), and ADP+ATP secretion was measured using ChronoLUME reagent (Chronolog).
P-Selectin Expression and GPIIb/IIIa Activation.
Washed platelets, treated with P2Y12 inhibitors and DEA/NONOate as described earlier, were activated with thrombin (1 U/mL) for 2 min before staining with anti–CD61-allophycocyanin (eBioscience), PAC-1-FITC (BD Bioscience), and anti–P-selectin-PE (eBioscience) for 15 min at 4 °C. PAC-1 and anti–P-selectin binding were determined by flow cytometry after gating of CD61+ events (Fig. S10).
Akt Phosphorylation.
Light transmission aggregometry was performed as described earlier, and platelets were lysed after 2 min. Platelet lysates were separated by Western blotting, using antiphospho(Ser473)-Akt (New England Biolabs) or anti–pan-Akt primary antibodies (New England Biolabs).
Rap1b Activation.
Light transmission aggregometry was performed as described earlier, and platelets were lysed after 15 s. Active Rap1-GTP was purified by RalGDS affinity pull-down, using a commercial available kit (Thermo Scientific). Rap1-GTP and total Rap1 were then separated and quantified by Western blotting as described earlier, using the supplied antibodies.
cAMP and cGMP Measurements.
Light transmission aggregometry was performed as described earlier, and platelets were lysed after 4 min. cAMP and cGMP concentrations were determined by homogenous time-resolved fluorescence-based competitive immunoassays (Cisbio).
VASP Phosphorylation.
Washed platelets, treated with P2Y12 inhibitors and DEA/NONOate as described earlier, were activated with thrombin (1 U/mL) for 4 min before staining with anti–VASP-P(Ser239) primary antibody (Enzo Lifesciences), Alexa647-conjugated secondary antibody (Invitrogen), and anti–CD42b-FITC (eBiosciences). VASP-P-associated fluorescence was quantified in CD42b+ events by flow cytometry (Fig. S10).
Calcium Mobilization.
Washed platelets, loaded with Fura2 (Sigma), were treated with P2Y12 receptor blockers and DEA/NONOate as described earlier. Thrombin (1 U/mL)-induced intracellular calcium mobilization was recorded using a Perkin-Elmer LS940B fluorometer.
Statistics and Data Analysis.
Unless otherwise stated, data were analyzed by two-way ANOVA, using GraphPad Prism 5 for Mac OS X, and differences were considered significant if P < 0.05. Summary data (IC50, EC50) were obtained by fitting of data to a logistic equation and tested by Student t test (2 groups) or one-way ANOVA (>2 groups).
Supplementary Material
Acknowledgments
This research was supported by the Wellcome Trust (085255/Z/08/Z), the British Heart Foundation (PG/11/75/29105), and the William Harvey Research Foundation. This work forms part of the research themes contributing to the translational research portfolio of Barts and the London Cardiovascular Biomedical Research Unit, which is supported and funded by the National Institute of Health Research.
Footnotes
Conflict of interest statement: T.D.W. has received consultancy fees from AstraZeneca and Daichii Sankyo/Eli Lilly relating to clinical development of P2Y12 inhibitors.
This article is a PNAS Direct Submission. M.W.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218880110/-/DCSupplemental.
References
- 1.Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res. 2007;100(9):1261–1275. doi: 10.1161/01.RES.0000264509.36234.51. [DOI] [PubMed] [Google Scholar]
- 2.Cattaneo M. Platelet P2 receptors: Old and new targets for antithrombotic drugs. Expert Rev Cardiovasc Ther. 2007;5(1):45–55. doi: 10.1586/14779072.5.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Warner TD, Nylander S, Whatling C. Anti-platelet therapy: Cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619–633. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattaneo M. The platelet P2Y₁₂ receptor for adenosine diphosphate: Congenital and drug-induced defects. Blood. 2011;117(7):2102–2112. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]
- 5.Vane JR. The Croonian Lecture, 1993. The endothelium: Maestro of the blood circulation. Philos Trans R Soc Lond B Biol Sci. 1994;343(1304):225–246. doi: 10.1098/rstb.1994.0023. [DOI] [PubMed] [Google Scholar]
- 6.Freedman JE, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999;84(12):1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 7.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA. 1990;87(13):5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo M, Lecchi A. Inhibition of the platelet P2Y12 receptor for adenosine diphosphate potentiates the antiplatelet effect of prostacyclin. J Thromb Haemost. 2007;5(3):577–582. doi: 10.1111/j.1538-7836.2007.02356.x. [DOI] [PubMed] [Google Scholar]
- 9.Iyú D, et al. P2Y₁₂ and EP3 antagonists promote the inhibitory effects of natural modulators of platelet aggregation that act via cAMP. Platelets. 2011;22(7):504–515. doi: 10.3109/09537104.2011.576284. [DOI] [PubMed] [Google Scholar]
- 10.Murata T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388(6643):678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 11.Ozüyaman B, et al. Endothelial nitric oxide synthase plays a minor role in inhibition of arterial thrombus formation. Thromb Haemost. 2005;93(6):1161–1167. doi: 10.1160/TH03-09-0588. [DOI] [PubMed] [Google Scholar]
- 12.Antl M, et al. IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood. 2007;109(2):552–559. doi: 10.1182/blood-2005-10-026294. [DOI] [PubMed] [Google Scholar]
- 13.Dangel O, et al. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J Thromb Haemost. 2010;8(6):1343–1352. doi: 10.1111/j.1538-7836.2010.03806.x. [DOI] [PubMed] [Google Scholar]
- 14.Schinner E, Salb K, Schlossmann J. Signaling via IRAG is essential for NO/cGMP-dependent inhibition of platelet activation. Platelets. 2011;22(3):217–227. doi: 10.3109/09537104.2010.544151. [DOI] [PubMed] [Google Scholar]
- 15.Moore C, Tymvios C, Emerson M. Functional regulation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb Haemost. 2010;104(2):342–349. doi: 10.1160/TH09-11-0764. [DOI] [PubMed] [Google Scholar]
- 16.Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, Egbrink MG. Endogenous nitric oxide and prostaglandins synergistically counteract thromboembolism in arterioles but not in venules. Arterioscler Thromb Vasc Biol. 2001;21(1):163–169. doi: 10.1161/01.atv.21.1.163. [DOI] [PubMed] [Google Scholar]
- 17.Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, oude Egbrink MG. Endogenous nitric oxide protects against thromboembolism in venules but not in arterioles. Arterioscler Thromb Vasc Biol. 1998;18(1):139–145. doi: 10.1161/01.atv.18.1.139. [DOI] [PubMed] [Google Scholar]
- 18.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilahur G, Pena E, Padró T, Badimon L. Protein disulphide isomerase-mediated LA419- NO release provides additional antithrombotic effects to the blockade of the ADP receptor. Thromb Haemost. 2007;97(4):650–657. [PubMed] [Google Scholar]
- 20.Vilahur G, Segalés E, Salas E, Badimon L. Effects of a novel platelet nitric oxide donor (LA816), aspirin, clopidogrel, and combined therapy in inhibiting flow- and lesion-dependent thrombosis in the porcine ex vivo model. Circulation. 2004;110(12):1686–1693. doi: 10.1161/01.CIR.0000142296.19558.99. [DOI] [PubMed] [Google Scholar]
- 21.Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther. 2006;319(1):1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 22.Fälker K, Lange D, Presek P. ADP secretion and subsequent P2Y12 receptor signalling play a crucial role in thrombin-induced ERK2 activation in human platelets. Thromb Haemost. 2004;92(1):114–123. doi: 10.1160/TH03-12-0729. [DOI] [PubMed] [Google Scholar]
- 23.Maurice DH, Haslam RJ. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: Inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990;37(5):671–681. [PubMed] [Google Scholar]
- 24.Dickinson NT, Jang EK, Haslam RJ. Activation of cGMP-stimulated phosphodiesterase by nitroprusside limits cAMP accumulation in human platelets: Effects on platelet aggregation. Biochem J. 1997;323(Pt 2):371–377. doi: 10.1042/bj3230371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butt E, et al. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269(20):14509–14517. [PubMed] [Google Scholar]
- 26.Hidaka H, Asano T. Human blood platelet 3′: 5′-cyclic nucleotide phosphodiesterase. Isolation of low-Km and high-Km phosphodiesterase. Biochim Biophys Acta. 1976;429(2):485–497. doi: 10.1016/0005-2744(76)90296-5. [DOI] [PubMed] [Google Scholar]
- 27.Burkhardt M, et al. KT5823 inhibits cGMP-dependent protein kinase activity in vitro but not in intact human platelets and rat mesangial cells. J Biol Chem. 2000;275(43):33536–33541. doi: 10.1074/jbc.M005670200. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MS, et al. Inhibition of cGMP-dependent protein kinase by the cell-permeable peptide DT-2 reveals a novel mechanism of vasoregulation. Mol Pharmacol. 2004;65(5):1111–1119. doi: 10.1124/mol.65.5.1111. [DOI] [PubMed] [Google Scholar]
- 29.Danielewski O, Schultess J, Smolenski A. The NO/cGMP pathway inhibits Rap 1 activation in human platelets via cGMP-dependent protein kinase I. Thromb Haemost. 2005;93(2):319–325. doi: 10.1160/TH04-09-0582. [DOI] [PubMed] [Google Scholar]
- 30.Aszódi A, et al. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18(1):37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, et al. Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling. Blood. 2002;99(10):3629–3636. doi: 10.1182/blood.v99.10.3629. [DOI] [PubMed] [Google Scholar]
- 32.Chan MV, Armstrong PC, Papalia F, Kirkby NS, Warner TD. Optical multichannel (optimul) platelet aggregometry in 96-well plates as an additional method of platelet reactivity testing. Platelets. 2011;22(7):485–494. doi: 10.3109/09537104.2011.592958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.