Significance
SDS/PAGE is a protein analysis technique universally used in biochemistry, cell biology, immunology, and virology, where proteins are separated by size on a gel matrix of polyacrylamide. However, most helical membrane proteins, which are biomolecules that comprise 20–30% of genomes and the majority of drug targets, migrate to positions on SDS/PAGE that have for decades been unpredictably larger or smaller than their actual size. We have found that the magnitude and direction of migration among membrane protein mimetics are controlled by the acrylamide concentration in the gel. Our results facilitate straightforward SDS/PAGE analysis of these important biomolecules.
Keywords: gel mobility, protein migration, protein identification, apparent size, immunoblotting
Abstract
SDS/PAGE is universally used in biochemistry, cell biology, and immunology to resolve minute protein amounts readily from tissue and cell extracts. Although molecular weights of water-soluble proteins are reliably determined from their SDS/PAGE mobility, most helical membrane proteins, which comprise 20–30% of the human genome and the majority of drug targets, migrate to positions that have for decades been unpredictably slower or faster than their actual formula weight, often confounding their identification. Using de novo designed transmembrane-mimetic polypeptides that match the composition of helical membrane-spanning sequences, we quantitate anomalous SDS/PAGE fractionation of helical membrane proteins by comparing the relative mobilities of these polypeptides with typical water-soluble reference proteins on Laemmli gels. We find that both the net charge and effective molecular size of the migrating particles of transmembrane-mimetic species exceed those of the corresponding reference proteins and that gel acrylamide concentration dictates the impact of these two factors on the direction and magnitude of anomalous migration. Algorithms we derived from these data compensate for this differential effect of acrylamide concentration on the SDS/PAGE mobility of a variety of natural membrane proteins. Our results provide a unique means to predict anomalous migration of membrane proteins, thereby facilitating straightforward determination of their molecular weights via SDS/PAGE.
Laemmli’s system for polyacrylamide gel protein electrophoresis in the presence of the detergent SDS (SDS/PAGE) is one of the most cited methodological papers in life sciences (1). The facility with which SDS/PAGE resolves minute amounts of proteins revolutionized the analysis of tissue and cell extracts, resulting in “overnight” adoption of the technique in biochemistry, cell biology, immunology, and virology (2). Considered “the single most useful analytical tool to study protein molecules” (3), SDS/PAGE is routinely used for simultaneous determination of protein heterogeneity and molecular weight in applications ranging from diagnosis of hereditary red cell membrane disorders to evaluation of recombinant protein expression and purification procedures. Protein analysis by SDS/PAGE is relatively simple, affordable, and rapid (4): A buffer containing a tracking dye and SDS is added to the sample of interest, the mixture is applied to a polyacrylamide gel, and a potential difference is used to drive the dye and the resulting anionic particle composed of protein and dodecyl sulfate (DS) through the gel. The distance traveled by the protein/DS particle from the top of the gel is then divided by that of the dye to obtain relative migration (Rf), and molecular weight [as relative molecular mass (Mr)] determined by comparison of this value with a logarithmic plot derived from the Rfs and Mrs of reference proteins.
Fractionation on SDS/PAGE is controlled by the molecular size and shape of the protein/DS particle, its net charge, and the accessible spaces among the acrylamide fibers that comprise the gel matrix as determined by the total concentration of acrylamide and bis-acrylamide cross-linker [T; Materials and Methods (5)]. Larger particles become trapped within the gel meshwork and migrate slower than smaller species. Low-percentage gels are therefore typically used to resolve larger proteins, and vice versa. Acrylamide concentrations compatible with routine use are usually from 4–20% T due to practical considerations, because gels outside of this range are too fragile or too brittle, respectively, to withstand the physical manipulation(s) required for protein visualization and/or immunoblotting.
Most globular, water-soluble proteins are reliably identified by their SDS/PAGE mobility relative to corresponding reference proteins typically used to estimate molecular weight. However, this group of well-behaved polypeptides does not include helical transmembrane (TM) proteins, macromolecules that comprise 20–30% of the human genome (6), comprise the majority of drug targets (7), and are the focus of major pharmaceutical discovery efforts (8). For example, the first true G protein-coupled receptor to be determined to high resolution, 39-kDa bovine rhodopsin (9), migrates on SDS/PAGE to positions consistent with sizes as low as 30 kDa (10). In fact, we have previously shown that the gel mobility of helical TM proteins seldom corresponds to formula molecular weight (11). This phenomenon of “anomalous migration” can arise as a consequence of the high hydrophobicity and concomitant binding of DS by TM proteins at levels that exceed those of water-soluble polypeptides (12). However, quantitation of DS binding stoichiometry is not routine and consumes milligram amounts of purified samples. Thus, the impact of enhanced DS binding on the direction and magnitude of anomalous migration has remained unpredictable for decades, with helical TM proteins variously exhibiting gel mobility reduced, equivalent, or increased relative to reference proteins (11–13). Such differences are generally disregarded when protein identity is known or can be confirmed in orthogonal molecular weight determination procedures but, in many instances, raise questions of protein folding, oligomeric organization, proteolytic processing, posttranslational modification(s), alternative splicing, antibody cross-reaction, and/or degradation. These issues become acute in SDS/PAGE analyses of tissue or cell extracts, where reasonable molecular weight estimates remain crucial for protein identification.
Here, we quantitate anomalous SDS/PAGE fractionation of helical membrane proteins by comparing the relative mobilities of de novo designed TM-mimetic peptide polymers with typical water-soluble reference proteins on Laemmli gels ranging from 11–18% T. We find that net charge and effective molecular size among the migrating TM-mimetic species exceed those of the corresponding reference proteins and that gel acrylamide concentration dictates the impact of these two factors on the direction and magnitude of anomalous migration. Algorithms derived from these data compensate for the differential effect of acrylamide concentration on the SDS/PAGE mobility of a variety of natural membrane proteins. Our results provide a straightforward means to predict anomalous migration of membrane proteins relative to reference polypeptides, facilitating their identification by molecular weight in SDS/PAGE applications.
Results
A variety of buffer systems for SDS/PAGE have been described (e.g., refs. 1, 14, 15). From these, Laemmli buffers (also known as the Tris-glycine system) were selected for the present work because they are used in the majority of determinations of protein complexity and molecular weight (16), and are used most often among reported systems in our database of helical TM protein SDS/PAGE mobilities (11) (SI Materials and Methods). Gels using these buffers at >10% T resolve reference proteins in the range of ≥14–200 kDa, whereas those with ≥14% T fractionate species ≥3.5 kDa (Mark12 Unstained Standard on Life Technologies Novex Tris-Glycine Gel SDS/PAGE Migration Charts, www.invitrogen.com). Given that our TM-mimetics encompass the range from 3.5–41 kDa (13), we accordingly chose to analyze SDS/PAGE migration behavior on gels of 11–18% T, in 1% T intervals.
Gel Mobility of TM-Mimetics Relative to Reference Proteins Changes with Acrylamide Concentration.
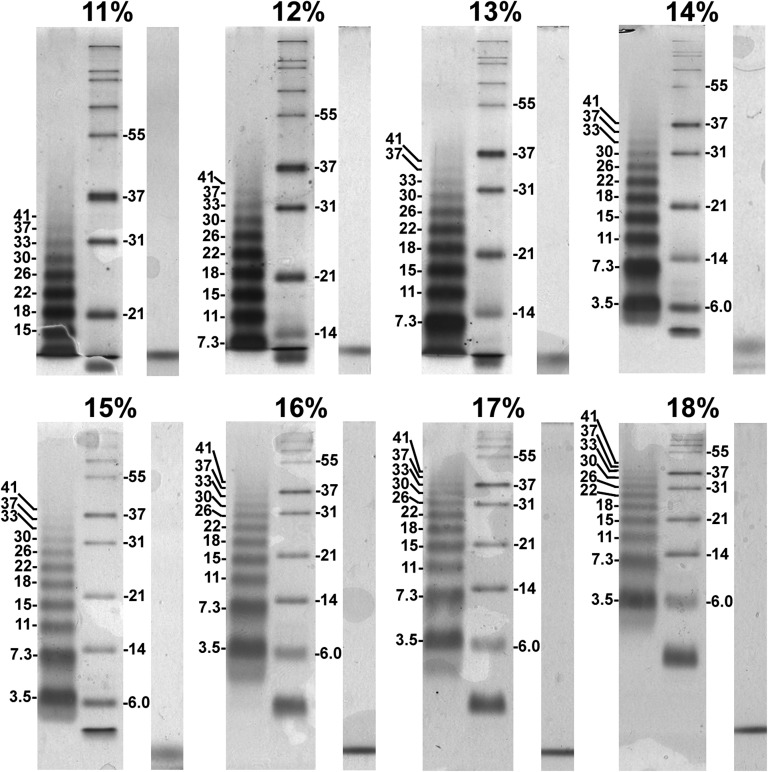
The set of TM protein mimetics we designed and prepared are polymers of a peptide with the core sequence NH2-SKSKS-Leu20-SKSKS-NH2, termed “pL20” (13). The average length, high hydrophobicity, and abundance of Leu in natural membrane-spanning regions are recapitulated in the 20-Leu segment of pL20, whereas its basic Lys and hydrophilic Ser residues resemble the native termini of such sequences (17). Sequence patterns that mediate oligomerization (e.g., refs. 18–20) were specifically excluded from the design. CD spectra of pL20 and its derivatives were characteristic of stable helical secondary structure (Figs. S1 A and B and S2), whereas Förster resonance energy transfer between dansyl- and dabsyl-derivatized pL20 could not be detected in SDS (Fig. S1 C–E), confirming its monomeric state. Nevertheless, the 3.5-kDa TM-mimetic corresponding to pL20 migrated as an apparent dimer of ∼7 kDa at ≥14% T (Fig. 1), whereas TM-mimetics greater than or equal to ∼30 kDa had enhanced mobility on 11–13% gels (Fig. 1). Indeed, each TM-mimetic less than or equal to ∼18 kDa migrated slower than reference proteins of comparable size (Fig. 1), whereas those greater than or equal to ∼30 kDa changed gel shift direction from migration faster to slower than the reference proteins as T was raised (Fig. 1). Plots relating gel mobility (Rf) to the logarithm of molecular weight (Mr) confirmed that the SDS/PAGE mobility of TM-mimetics relative to reference proteins was reduced at smaller Mrs, equivalent at an Mr corresponding to the intersection point of the two curves, and increased as Mrs exceeded the intersection point (Fig. S3 and Table S1).
Fig. 1.
TM-mimetics switch migration positions relative to reference proteins on SDS/PAGE at various acrylamide concentrations. Representative gels indicating the migration position and Mr × 10−3 of the apolar peptide polymers comprising our TM-mimetics (Left lanes), the globular water-soluble polypeptides comprising a commercial blend of reference proteins (Right lanes), and the migration of the bromophenol blue tracking dye (far right of each set of gels) are shown. T is given at the top of each gel image. The sharpness of bromophenol blue may reflect the moving boundaries known to occur in SDS/PAGE (36, 37).
Molecular Size and Net Charge Are Larger Among TM-Mimetics than Reference Proteins.
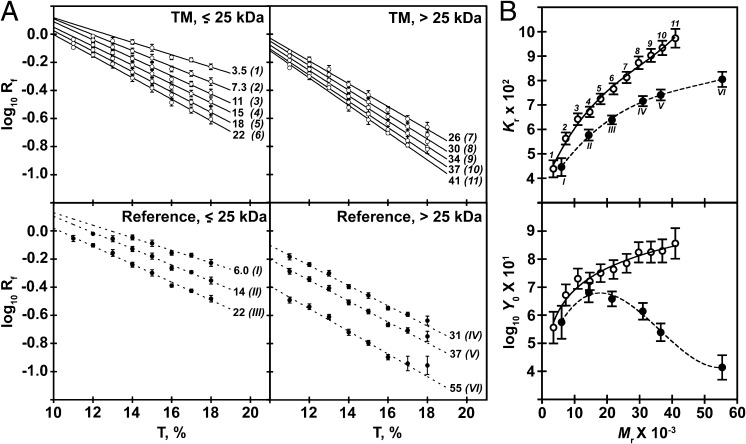
Having excluded the possibility of self-assembly as the origin of these phenomena, we used the procedure first proposed by Ferguson (21) and summarized by Chrambach and Rodbard (5) to measure two parameters of protein/DS complexes that together contribute to migration on SDS/PAGE: retardation coefficient (Kr), a measure of effective molecular size that is determined by the total mass and shape of the migrating particle, and free electrophoretic mobility (as log10 Y0; Materials and Methods), a descriptor of net charge. Both quantities are measured by determining mobility on gels of various acrylamide concentrations and generating a Ferguson plot of log10 Rf vs. T that is linear with slope −Kr and y-intercept log10 Y0 (Eq. 1). Ferguson plots (Fig. 2A) derived from the Rf values determined at 11–18% T (Fig. 1) were linear on gels <18%, although some concavity was observed as T and Mr increased (10, 22–25) (Fig. 2A, Right). The linear regression analysis used to obtain Kr and log10 Y0 therefore eliminated Rf of ≥30-kDa polypeptides at 18% T. Kr and log10 Y0 among the TM-mimetics were larger than those of the reference proteins (Fig. 2B), an outcome consistent with the enhanced DS loading capacity expected for sequences of enhanced hydrophobicity (12) (Table S2). To quantify these increases, nonlinear regression was applied to define the relationships of Kr to Mr and of log10 Y0 to Mr. Kr was found to be related to Mr by a third-order polynomial in each case (25) (Fig. 2B, Upper), whereas the function relating log10 Y0 to Mr differed for the TM-mimetic and reference proteins (logarithmic vs. polynomial; Fig. 2B, Lower). The latter finding presumably arises from a change in the balance of the charge and frictional forces known to underlie Y0 as Mr is increased (22). In certain systems, the relationship of Y0 to Mr among typical reference proteins may be approximated by monotonic or polynomial functions (23); in others, Y0 values among globular, water-soluble polypeptides are nearly independent of Mr (22, 25–27).
Fig. 2.
Molecular size and net charge of TM-mimetics are larger than those of reference proteins. (A) Ferguson plots of TM-mimetics (n = 5–8, ○) and reference proteins (n = 5–8, ●), grouped by molecular weight for clarity of presentation. Points represent the average of at least three independent Rf measurements and error bars ± SD. The best-fit line to each TM-mimetic (solid lines) and reference protein (dashed lines) is shown (R2 > 0.9, P < 0.0001; Tables S3 and S4). Mr × 10−3 is indicated at the right of the best-fit line; bracketed Arabic or Roman numerals indicate the degree of polymerization of the TM-mimetic (13) or the identity of the reference protein (Table S2), respectively. (B) Values of Kr (Upper) and log10 Y0 (Lower), obtained from the best-fit lines shown in A, are plotted vs. Mr. Points corresponding to the TM-mimetics (n = 11, ○) and reference proteins (n = 6, ●) are shown ± fit errors, labeled according to the scheme in A. Lines connecting Kr and log10 Y0 values of TM-mimetics (solid lines) and reference proteins (dashed lines) were obtained by nonlinear regression (R2 > 0.9).
Predicting Differential Gel Mobility from Gel Acrylamide Concentration, Molecular Size, and Net Charge.
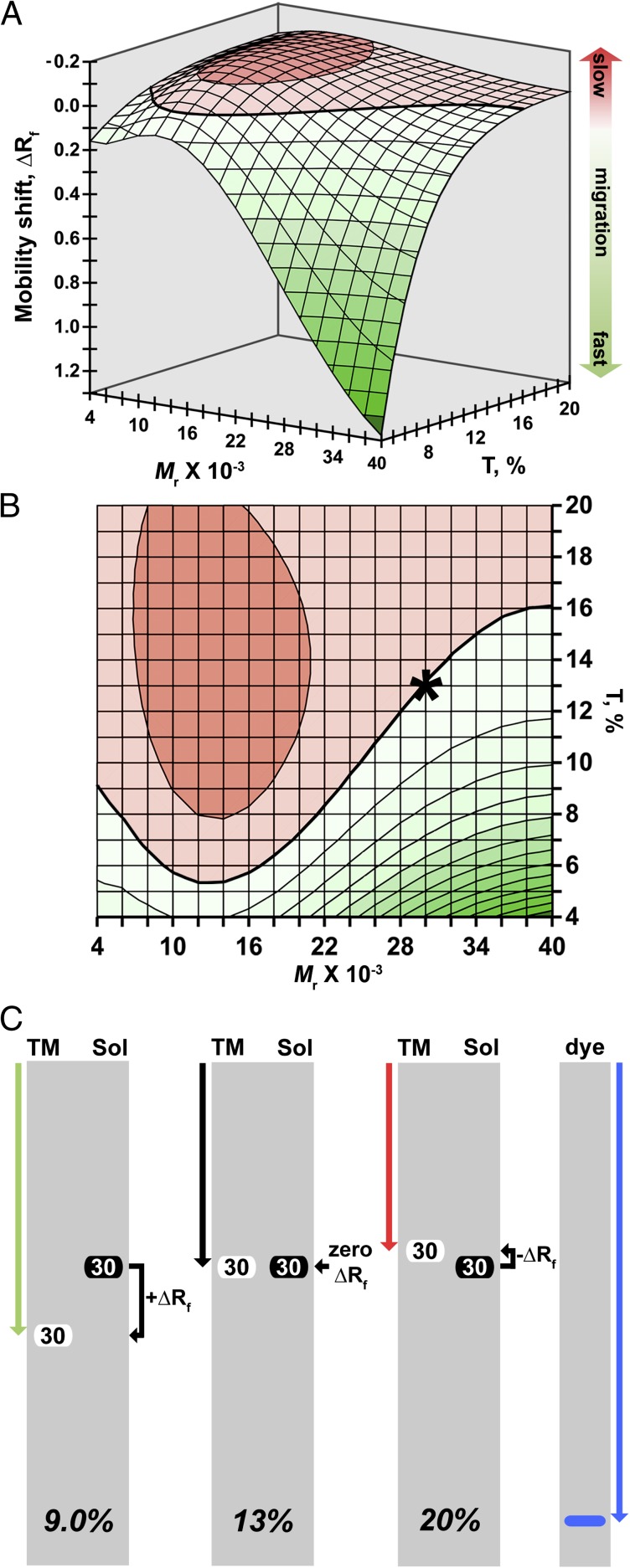
Relationships of Kr and of log10 Y0 to Mr were used to derive equations that predict anomalous migration in terms of the difference in gel mobility of a TM-mimetic(s) vs. reference protein(s), expressed as a fraction of the distance traveled by the tracking dye (Fig. 3 A and B). These equations show that gel mobility among the TM-mimetics is variously equal, reduced, or increased relative to reference proteins as a function of Mr and T (Fig. 3 A and B). As illustrated schematically in Fig. 3C, a 30-kDa TM-mimetic will migrate equivalently to a water-soluble reference protein of the same size on SDS/PAGE at 13% acrylamide. However, the same TM-mimetic will migrate anomalously slow on a 20% gel, with mobility reduced by 3.3% of the distance traveled by the tracking dye. Conversely, at 9.0% acrylamide, the 30-kDa TM-mimetic will migrate faster than a 30-kDa reference protein, with mobility increased by 15% of the distance traveled by the tracking dye.
Fig. 3.
SDS/PAGE mobility shifts of TM-mimetics relative to reference proteins. (A and B) Mobility shift is expressed as a fraction of the distance traveled by the tracking dye (∆Rf = Rf, TM − Rf, Ref; SI Materials and Methods). Positive and negative values of ∆Rf (green and red, respectively) indicate increased or reduced mobility of TM-mimetics relative to reference proteins. A ∆Rf of zero (bold contour) indicates equivalent mobility. Graphs are shaded according to mobility shift, with darker colors corresponding to regions of larger magnitude migration differences. (B) Contour map of the data presented in A, viewed along the ∆Rf axis (a 90° rotation toward the reader). We caution that mobility shifts calculated for 4–10% T represent extrapolations of present data; in practice, this acrylamide concentration range is not typically applied to low-Mr proteins. (C) Quantitative cartoon of anomalous SDS/PAGE migration calculated for a 30-kDa TM-mimetic (TM, white) vs. a 30-kDa water-soluble reference protein (Sol, black). Gels (gray rectangles) are arranged in order of increasing total acrylamide concentration, with the mobility of the tracking dye (dye, blue arrow) shown at the far right. At 9.0% T, mobility of the TM-mimetic (green arrow) is increased by 15% of the distance traveled by the dye (∆Rf of +0.15). At 13% T, the TM-mimetic migrates equivalently to the water-soluble protein (black arrow, ∆Rf of zero; asterisk in B). At 20% T, mobility of the same TM-mimetic (red arrow) is reduced by 3.3% of the distance traveled by the dye (∆Rf of −0.033).
When we tested predictions made by our equations with a database of helical TM protein SDS/PAGE mobilities (11), we found that gel acrylamide concentration was the predominant factor dictating the direction and magnitude of anomalous migration among 16 (73%) of 22 of these proteins (Eq. S9, Dataset S1, and Fig. S4). This level of accuracy is remarkable, given that the SDS/PAGE experiments in the database were performed by various groups over 20 y and were not filtered for bis-acrylamide concentration, an important determinant of Kr (5). Given that each TM-mimetic has a fixed number of 10 flanking polar residues per TM sequence, whereas the extramembrane regions of natural helical TM proteins may encompass a few to hundreds of residues, it seemed reasonable that the six exceptions might be distinguished by the proportion of extramembrane vs. TM sequence. The formula Mr per TM domain among the outliers was nevertheless statistically indistinguishable from those with correctly predicted gel mobility (Dataset S1). However, we note that proteins with large extramembrane regions are generally excluded from the Mr range of the present data. It thus remains possible that the SDS/PAGE mobility of the TM-mimetics may more closely resemble natural membrane proteins with short loops than that of proteins with large extramembrane domains.
Discussion
Anomalous SDS/PAGE mobility of helical membrane proteins thus arises as a direct consequence of enhanced net charge and effective molecular size among their protein/DS particles relative to the particles of water-soluble reference proteins. The outcome of this difference on gel mobility, however, is tied to the interplay between these two quantities, polypeptide molecular weight, and the space available within the acrylamide gel matrix [as determined by T (5)]. Thus, it may be surmised that on gels of acrylamide concentration lower than equivalent gel mobility (Teq), increased net charge dominates Rf, resulting in increased mobility. However, as acrylamide concentration increases, migration becomes increasingly impeded by larger particle size, resulting in a gradual reduction in gel mobility, and ultimately a “switch” to reduced mobility once Teq is exceeded. Trends of increased mobility among larger helical membrane proteins and of reduced mobility among smaller helical membrane proteins (11) may therefore arise from using low- or high-percentage acrylamide gels, respectively, for SDS/PAGE analysis.
Any factor(s) that alter the molecular size and shape, and/or net charge, of the polypeptide/DS particle are expected to affect SDS/PAGE migration in a complex manner that will depend on both acrylamide concentration and Mr. The smaller polypeptides that are typically analyzed on high-percentage gels may be particularly subject to these variations (25). As examples, alterations in DS loading and/or distribution of DS along the polypeptide chain might increase or decrease SDS/PAGE mobility of small polypeptides (e.g., refs. 12, 28, 29), depending on the acrylamide concentration of the gel and the buffer system used for analysis. Indeed, differential protein/DS contacts among folded vs. unfolded β-barrel membrane proteins may underlie variation in their gel shifts (30).
Quantitation of the observed pivotal role of acrylamide concentration using the algorithm shown in Fig. 3 should nevertheless facilitate interpretation of any SDS/PAGE application where an accurate molecular weight is required (i.e., identification of helical membrane proteins can be accomplished with water-soluble reference proteins when gels as close as possible to Teq are used). However, the dependence of Kr on the electrophoresis buffer system (31, 32) and on cross-linker concentration (32, 33) means that the algorithms presented here will be most accurate when applied to Laemmli gels at the bis-acrylamide concentration used here (Materials and Methods). With this in mind, we note that anomalous SDS/PAGE migration among TM proteins should disappear if TM-mimetics are used as size standards (13); in applications where protein(s) are unknown, such reagents may prove to be essential.
Materials and Methods
Production of TM-Mimetics.
The TM-mimetics were produced from peptides with the sequence H2N-Cys-SKSKS-Leu20-SKSKS-Cys-NH2 as previously described (13). Briefly, purified peptide was self-polymerized at the sulfhydryl moiety of Cys in a “one-pot” reaction with a bis-maleimidoethane (BMOE) cross-linker in an aqueous 80% (vol/vol) 2,2,2-trifluoroethanol solution buffered at pH 7.5 with 20 mM Tris. BMOE was selected as a conjugating linkage because of the sulfhydryl specificity of its reaction at neutral pH, the stability of the resulting thioether linkage to changes in pH and to reducing agents, the trans-configuration of linkages, and the commonality of thioether conjugates in biomaterials.
SDS/PAGE.
Gels were cast in disposable minigel 8-cm × 8-cm, 1.0-mm-thick cassettes (Life Technologies) using mixtures containing appropriate volumes of a 40% T stock solution [29:1 acrylamide/N,N′-methylenebis(acrylamide), 3.3% C; BioRad], degassed ultrapure water, ultrapure SDS (BioUltra; Fluka), and stacking (0.375 M Tris⋅HCl, pH 6.8) or separating (1 M Tris, pH 8.8) buffers, where T [%, (wt/vol)] = [g acrylamide + g N,N′-methylenebis(acrylamide)] per 100 mL of solution and C [%, (wt/vol)] = 100 × [g N,N′-methylenebis(acrylamide)] per 100 mL of solution/T (34). The polymerization catalysts N,N,N′,N′-tetramethylethylenediamine (TEMED; BioRad) and freshly prepared 5% (wt/vol) ammonium persulfate (APS; BioRad) in ultrapure water were added immediately before casting. Separating gels were 6 cm in length and contained 11–18% T, 0.375 M Tris (pH 8.8), 0.1% (wt/vol) SDS, 0.025% (vol/vol) TEMED, and 0.125% (wt/vol) APS; stacking gels were 2 cm in length and contained 4% T, 0.125 M Tris (pH 6.8), 0.1% (wt/vol) SDS, 0.04% (vol/vol) TEMED, and 0.4% (wt/vol) APS. Separating and stacking gels were each allowed to polymerize for 1 h at room temperature, after which cassettes were sealed in packages containing a small volume of ultrapure water and stored at 4 °C overnight before use.
Aliquots of TM-mimetics were dissolved in 1× Tris-glycine SDS sample buffer (Life Technologies). Mark12 Unstained Standard (Life Technologies) was diluted 1/20 in 1× Tris-glycine SDS sample buffer before application to gels. Five microliters of the TM-mimetic blend and 5 microliters of the diluted Mark12 Standard were applied to each gel. Ten microliters of SeeBlue Pre-Stained Protein Standard (Life Technologies) was applied undiluted to the first and last lanes of each gel in order to facilitate alignment of images before and after staining. TM-mimetics and reference proteins were applied in parallel to at least three individual gels at each T. Electrophoresis was performed at 125 V in Tris-glycine SDS running buffer [25 mM Tris base, 192 mM glycine, 0.1% (wt/vol) SDS, pH 8.3] at room temperature for 100 min (11–14% T) or until the bromophenol blue tracking dye was near the gel end (15–18% T). Gels were removed from the cassettes and immediately imaged with visible light to record the positions of the tracking dye and the prestained marker proteins. After visible imaging, gels were silver-stained using the SilverXpress Silver Staining Kit (Life Technologies) according to the manufacturer’s directions. Gel images taken before and after staining were aligned based on the positions of the prestained marker proteins. The distances of each polypeptide band and of the bromophenol blue dye from the top of the separating gel were measured and divided to obtain relative mobility (Rf).
Ferguson Plots.
The logarithm of Rf values obtained by SDS/PAGE on at least three individual gels at each T for each TM-mimetic and reference protein in the Mr ranges of 3,500–41,000 and 6,000–55,400, respectively, was plotted as a function of T and fit with linear regression to the relationship (21):
To ensure that all data conformed to Eq. 1, Rfs of TM-mimetics and reference proteins were sequentially excluded from highest to lowest T until the probability of the linear model reached >0.5 in the runs test; Rf values of polypeptides ≥30 kDa obtained on gels of 18% T were omitted from regression analysis (25, 35). The resulting lines of best fit had R2 values ranging from 0.920–0.984, significantly nonzero slopes (P < 0.0001), and probabilities of linearity >0.5 (Tables S3 and S4). Kr and log10 Y0 were obtained from the absolute slope and y-intercept of these best-fit lines, and we have opted here to compare log10 Y0 values in lieu of transforming to Y0. This modification facilitates derivation of the equations that predict the SDS/PAGE mobility of TM-mimetics relative to reference proteins (Eqs. S3–S9).
Statistical Analysis.
Linear regression, nonlinear regression, runs tests, and statistical analyses were performed using Prism version 4.0a for Macintosh (GraphPad). P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Prof. Karen Fleming (Johns Hopkins University) for helpful suggestions regarding the analyses presented in this manuscript. This work was supported by grants to C.M.D. from the Medical and Related Sciences (MaRS) Innovation Proof of Principle Program (MaRS POP Grant MI-POP 2010-0088); the Natural Sciences and Engineering Research Council Idea-to-Innovation Program (NSERC I2I Grant 411522-10); and the Canadian Institutes of Health Research (Grant FRN-5810). The Hospital for Sick Children owns the intellectual property associated with the composition and preparation of the TM-mimetic polypeptide reagents.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311305110/-/DCSupplemental.
References
- 1.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 2.Pederson T. Turning a PAGE: The overnight sensation of SDS-polyacrylamide gel electrophoresis. FASEB J. 2008;22(4):949–953. doi: 10.1096/fj.08-0402ufm. [DOI] [PubMed] [Google Scholar]
- 3.Marchesi VT. The relevance of research on red cell membranes to the understanding of complex human disease: A personal perspective. Annu Rev Pathol. 2008;3:1–9. doi: 10.1146/annurev.pathmechdis.3.121806.154321. [DOI] [PubMed] [Google Scholar]
- 4.Goetz H, et al. Comparison of selected analytical techniques for protein sizing, quantitation and molecular weight determination. J Biochem Biophys Methods. 2004;60(3):281–293. doi: 10.1016/j.jbbm.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Chrambach A, Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971;172(3982):440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- 6.Wallin E, von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7(4):1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildirim MA, Goh KI, Cusick ME, Barabási AL, Vidal M. Drug-target network. Nat Biotechnol. 2007;25(10):1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- 8.Fleming KG. Riding the wave: Structural and energetic principles of helical membrane proteins. Curr Opin Biotechnol. 2000;11(1):67–71. doi: 10.1016/s0958-1669(99)00056-7. [DOI] [PubMed] [Google Scholar]
- 9.White SH. Biophysical dissection of membrane proteins. Nature. 2009;459(7245):344–346. doi: 10.1038/nature08142. [DOI] [PubMed] [Google Scholar]
- 10.Frank RN, Rodbard D. Precision of sodium dodecyl sulfate-polyacrylamide-gel electrophoresis for the molecular weight estimation of a membrane glycoprotein: Studies on bovine rhodopsin. Arch Biochem Biophys. 1975;171(1):1–13. doi: 10.1016/0003-9861(75)90001-6. [DOI] [PubMed] [Google Scholar]
- 11.Rath A, Deber CM. Correction factors for membrane protein molecular weight readouts on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 2013;434(1):67–72. doi: 10.1016/j.ab.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci USA. 2009;106(6):1760–1765. doi: 10.1073/pnas.0813167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rath A, Nadeau VG, Poulsen BE, Ng DP, Deber CM. Novel hydrophobic standards for membrane protein molecular weight determinations via sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 2010;49(50):10589–10591. doi: 10.1021/bi101840j. [DOI] [PubMed] [Google Scholar]
- 14.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 15. Engelhorn S, Updyke TV, inventors; Novex Experimental Technologies, assignee (1996) US Patent US5578180A.
- 16.Chiou SH, Wu SH. Evaluation of commonly used electrophoretic methods for the analysis of proteins and peptides and their application to biotechnology. Anal Chim Acta. 1999;383(1):47–60. [Google Scholar]
- 17.Ulmschneider MB, Sansom MS, Di Nola A. Properties of integral membrane protein structures: Derivation of an implicit membrane potential. Proteins. 2005;59(2):252–265. doi: 10.1002/prot.20334. [DOI] [PubMed] [Google Scholar]
- 18.Zhou FX, Merianos HJ, Brunger AT, Engelman DM. Polar residues drive association of polyleucine transmembrane helices. Proc Natl Acad Sci USA. 2001;98(5):2250–2255. doi: 10.1073/pnas.041593698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walters RF, DeGrado WF. Helix-packing motifs in membrane proteins. Proc Natl Acad Sci USA. 2006;103(37):13658–13663. doi: 10.1073/pnas.0605878103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li E, Wimley WC, Hristova K. Transmembrane helix dimerization: Beyond the search for sequence motifs. Biochim Biophys Acta. 2012;1818(2):183–193. doi: 10.1016/j.bbamem.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson KA. Starch-Gel Electrophoresis—Application to the Classification of Pituitary Proteins and Polypeptides. Metabolism. 1964;13(Suppl):985–1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- 22.Neville DM., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971;246(20):6328–6334. [PubMed] [Google Scholar]
- 23.Banker GA, Cotman CW. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972;247(18):5856–5861. [PubMed] [Google Scholar]
- 24.Repke H, Schmitt M. Electrophoretic characterization of muscarinic receptors under denaturating and nondenaturating conditions: Computer-assisted Ferguson plot analysis. Biochim Biophys Acta. 1987;929(1):62–73. doi: 10.1016/0167-4889(87)90241-2. [DOI] [PubMed] [Google Scholar]
- 25.Westerhuis WH, Sturgis JN, Niederman RA. Reevaluation of the electrophoretic migration behavior of soluble globular proteins in the native and detergent-denatured states in polyacrylamide gels. Anal Biochem. 2000;284(1):143–152. doi: 10.1006/abio.2000.4684. [DOI] [PubMed] [Google Scholar]
- 26.Shirahama K, Tsujii K, Takagi T. Free-boundary electrophoresis of sodium dodecyl sulfate-protein polypeptide complexes with special reference to SDS-polyacrylamide gel electrophoresis. J Biochem. 1974;75(2):309–319. doi: 10.1093/oxfordjournals.jbchem.a130398. [DOI] [PubMed] [Google Scholar]
- 27.Gentile F, et al. SDS-resistant active and thermostable dimers are obtained from the dissociation of homotetrameric beta-glycosidase from hyperthermophilic Sulfolobus solfataricus in SDS. Stabilizing role of the A-C intermonomeric interface. J Biol Chem. 2002;277(46):44050–44060. doi: 10.1074/jbc.M206761200. [DOI] [PubMed] [Google Scholar]
- 28.Walkenhorst WF, Merzlyakov M, Hristova K, Wimley WC. Polar residues in transmembrane helices can decrease electrophoretic mobility in polyacrylamide gels without causing helix dimerization. Biochim Biophys Acta. 2009;1788(6):1321–1331. doi: 10.1016/j.bbamem.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tulumello DV, Deber CM. Positions of polar amino acids alter interactions between transmembrane segments and detergents. Biochemistry. 2011;50(19):3928–3935. doi: 10.1021/bi200238g. [DOI] [PubMed] [Google Scholar]
- 30.Burgess NK, Dao TP, Stanley AM, Fleming KG. Beta-barrel proteins that reside in the Escherichia coli outer membrane in vivo demonstrate varied folding behavior in vitro. J Biol Chem. 2008;283(39):26748–26758. doi: 10.1074/jbc.M802754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Lith HA, Haller M, Van Zutphen LF, Beynen AC. The use of three ferguson-plot-based calculation methods to determine the molecular mass of proteins as illustrated by molecular mass assessment of rat-plasma carboxylesterases ES-1, ES-2, and ES-14. Anal Biochem. 1992;201(2):288–300. doi: 10.1016/0003-2697(92)90341-4. [DOI] [PubMed] [Google Scholar]
- 32.Rodbard D, Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacrylamide gel electrophoresis. Anal Biochem. 1971;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- 33.Rodbard D, Chrambach A. Unified theory for gel electrophoresis and gel filtration. Proc Natl Acad Sci USA. 1970;65(4):970–977. doi: 10.1073/pnas.65.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hjerten S. “Molecular sieve” chromatography on polyacrylamide gels, prepared according to a simplified method. Arch Biochem Biophys. 1962;1(Suppl 1):147–151. [PubMed] [Google Scholar]
- 35.Kozulić B. Models of gel electrophoresis. Anal Biochem. 1995;231(1):1–12. doi: 10.1006/abio.1995.1495. [DOI] [PubMed] [Google Scholar]
- 36.Wyckoff M, Rodbard D, Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: Properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]
- 37.Buzás Z, Chrambach A. Moving boundaries in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis. 2004;25(7-8):970–972. doi: 10.1002/elps.200305798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.