Significance
Although the vitamin D endocrine system has been well defined and the enzyme responsible for converting 25-hydroxyvitamin D to the final hormone, 1α,25-dihydroxyvitamin D3, is well understood, the enzyme responsible for the conversion of vitamin D to the blood form, 25-hydroxyvitamin D, has not been clearly identified. A case has been made for vitamin D 25-hydroxylase CYP2R1 as the responsible enzyme, but proof is lacking. We have produced a null mutant mouse lacking CYP2R1. With this model, we have shown that CYP2R1 is the major but not exclusive 25-hydroxylase and that there remains another significant enzyme responsible for this step in vitamin D activation that has yet to be identified.
Abstract
Bioactivation of vitamin D consists of two sequential hydroxylation steps to produce 1α,25-dihydroxyvitamin D3. It is clear that the second or 1α-hydroxylation step is carried out by a single enzyme, 25-hydroxyvitamin D 1α-hydroxylase CYP27B1. However, it is not certain what enzyme or enzymes are responsible for the initial 25-hydroxylation. An excellent case has been made for vitamin D 25-hydroxylase CYP2R1, but this hypothesis has not yet been tested. We have now produced Cyp2r1−/− mice. These mice had greater than 50% reduction in serum 25-hydroxyvitamin D3. Curiously, the 1α,25-dihydroxyvitamin D3 level in the serum remained unchanged. These mice presented no health issues. A double knockout of Cyp2r1 and Cyp27a1 maintained a similar circulating level of 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3. Our results support the idea that the CYP2R1 is the major enzyme responsible for 25-hydroxylation of vitamin D, but clearly a second, as-yet unknown, enzyme is another contributor to this important step in vitamin D activation.
The active vitamin D hormone, 1α,25-dihydroxyvitamin D, plays a pivotal role in calcium homeostasis and phosphate metabolism, and is likely involved in other biological actions such as the immune system (1-3). 1α,25-Dihydroxyvitamin D3 [1,25(OH)2D3] is synthesized from vitamin D3 in two sequential steps in vivo. The production of 25-hydroxyvitamin D3 [25(OH)D3], the major circulating form of vitamin D3, occurs predominantly in the liver (4). The final activation step occurs largely in the proximal convoluted tubule of the kidney to produce 1,25(OH)2D3 (5).
25-Hydroxyvitamin D 1a-hydroxylase CYP27B1 (CYP27B1) has long been identified as the sole 25(OH)D3 1α-hydroxylase in a number of species, including human (5), whereas the specific vitamin D3 25-hydroxylase has yet to be elucidated. Many candidates have been proposed, but in vivo proof has yet to appear. Most of the potential 25-hydroxylases are primarily expressed in the liver, and all are members of the cytochrome P450 family (CYP2C11, CYP2D25, CYP27A1, CYP3A4, CYP2R1, and CYP2J2/3) (4). Among them, CYP27A1 and CYP2R1 are considered the most promising candidates for vitamin D 25-hydroxylation. CYP27A1, also known as the sterol 27-hydroxylase, is a key enzyme in bile acid formation (6, 7). Recombinant CYP27A1 is able to catalyze multiple oxidation reactions with broad substrate specificity in vitro (8–14). However, it is a low-affinity, high-capacity vitamin D 25-hydroxylase and is certainly involved in steroidogenesis. Ablation of Cyp27a1 in mouse disrupted cholesterol metabolism and bile acid synthesis severely but did not alter vitamin D metabolism (15, 16). Indeed, these animals had supranormal serum 25(OH)D3 levels (15). Patients with cerebrotendinous xanthomatosis caused by mutations in the Cyp27a1 gene show dysfunctions that result from reduced bile acid production, but generally do not present vitamin D-related pathology (17, 18). These findings suggest that CYP27A1 is a minor factor, if it plays a role at all, in 25(OH)D3 synthesis in vivo. CYP2R1 was recently identified as vitamin D 25-hydroxylase in mouse and human through the screening of a liver cDNA library from Cyp27a1-null mice (19). Heterologous expression of CYP2R1 in HEK cells, yeast, and Escherichia coli revealed that CYP2R1 catalyzes 25-hydroxylation of both vitamin D3 and vitamin D2 at similar rate, and much more efficiently than CYP27A1 (19-21). The clinical relevance of CYP2R1 as vitamin D 25-hydroxylase is from a handful of patients of Nigerian and Saudi Arabian decent having 25(OH)D3 deficiency (22–26). These patients exhibited symptoms of vitamin D-dependent rickets. This condition appeared to mostly result from an amino acid mutation (Leu99Pro) that altered the CYP2R1 structure and abolished 25-hydroxylase activity (21), although other mutations have been reported (26).
We have created Cyp2r1−/− and Cyp2r1/Cyp27a1 double-knockout mouse models and evaluated the physiological role of CYP2R1 in the vitamin D activation pathway. Our results strongly support the concept that CYP2R1 is a major contributor to 25-hydroxylation of vitamin D in vivo, but show there is another unidentified enzyme that participates in this important activation.
Results
Generation of Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− Mice.
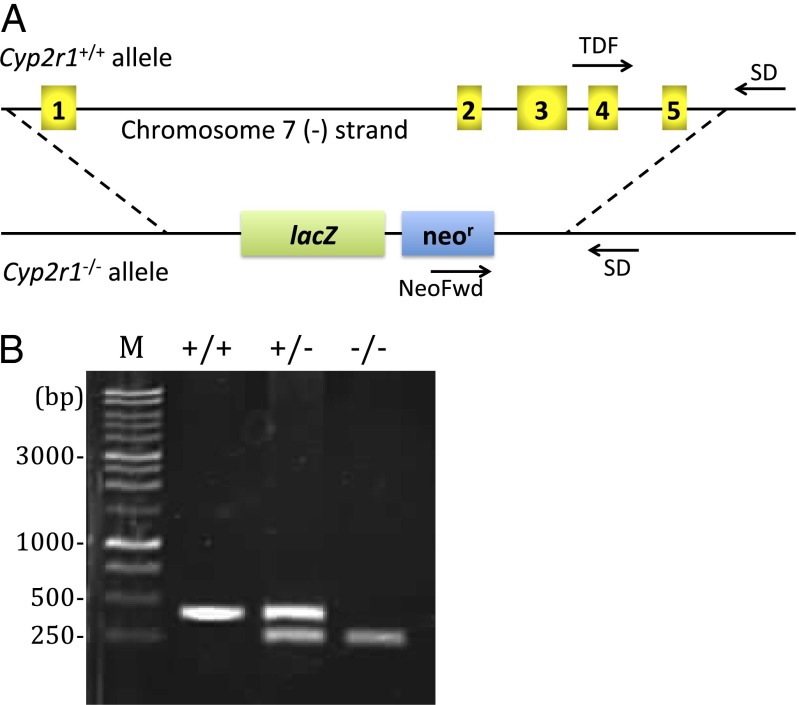
A mouse embryonic stem cell clone carrying Cyp2r1-null allele, in which the entire Cyp2r1 gene spanning a 12.9-kb region on chromosome 7 was deleted and replaced with a lacZ reporter and selection cassette, was used to generate chimeras. Crossbreeding with C57BL/6 eventually established Cyp2r1 knockout mice (Fig. 1). The offspring from heterozygous breeding pairs had no sex bias and followed Mendelian genetic distribution with 26% wild-type, 50% heterozygotes, and 24% knockout in 199 mice. The genotypes of stillborn pups and those dead before weaning were analyzed to be 22% wild-type, 54% heterozygotes, and 24% knockout among 45 bodies collected, confirming that Cyp2r1 ablation did not cause prenatal death. Double-knockout mice were generated by crossing Cyp2r1−/− mice with the Cyp27a1−/− strain.
Fig. 1.
(A) Schematic of mouse Cyp2r1 genomic sequence depicting the null allele design and genotyping strategy. All 5 exons of Cyp2r1 gene on chromosome 7, indicated by numbered boxes, were replaced with β-galactosidase coding sequence (lacZ) and neomycin selection cassette (neor) to create a Cyp2r1−/− allele. Genotyping primers are indicated by arrows (Materials and Methods). (B) Agarose electrophoresis visualized by ethidium bromide staining showing Cyp2r1+/+, Cyp2r1+/−, and Cyp2r1−/− genotypes. Tissues from ear punch were used as DNA sources. PCR product sizes are 399 bp for wild-type and 245 bp for Cyp2r1−/−. M, DNA marker.
General Assessment.
All Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice were weaned at 3 wk of age and maintained on a chow diet. They gained weight normally and displayed no obvious sign of developmental or behavioral problems. The major organs including liver, kidney, heart, lung, thymus, spleen, small intestine, testis, and brain were weighed and compared with those isolated from age- and sex-matched wild-type animals. No anomalies were observed via visual examination, except for the enlarged livers in double-knockout mice, which were ∼35% larger, likely the result of Cyp27a1 ablation, as reported previously (16). In our experience, Cyp27a1−/− mice housed under the same conditions had enlarged livers (∼45%) as well. All animals had a stable serum calcium level of 9.9 mg/dL since weaning. The phosphorus level in serum decreased slightly from 12.3 mg/dL at 3–5 wk of age to 10.2 mg/dL on maturity and remained stable from then on in all mice. The serum concentration of parathyroid hormone was low (22 pg/mL), and there was no noticeable difference between wild-type and Cyp2r1−/− mice. Both Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice were fertile and were able to produce as many normal-sized litters as wild-type mice.
Circulating 25(OH)D3 Level.
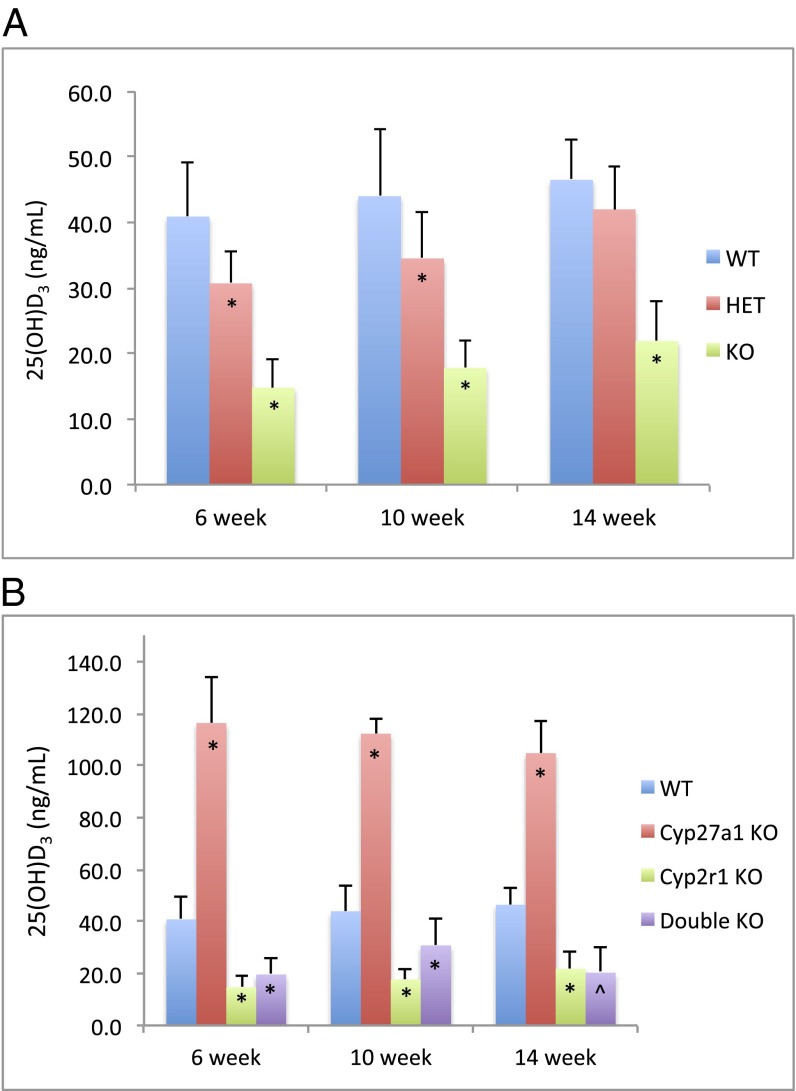
The serum 25(OH)D3 concentration of mice at 6–14 wk of age was measured using an RIA. A clear decrease in serum 25(OH)D3 level was observed in Cyp2r1−/− mice compared with that of the wild-type, with a reduction of more than 50% (Fig. 2 and Table 1). The serum level of 25(OH)D3 in heterozygotes was somewhere in between but was close to that of the wild type; it seemed to catch up to the wild type at 14 wk. A marked two- to threefold increase in 25(OH)D3 concentration was detected in Cyp27a1−/− mice, which is consistent with an earlier report (15). However, the Cyp2r1−/−/Cyp27a1−/− mice presented a 25(OH)D3 level similar to that of Cyp2r1−/− mice (Fig. 2 and Table 1). Possibly, it is CYP2R1 that is responsible for the supranormal 25(OH)D3 level in Cyp27a1−/− mice. Selected serum samples were reanalyzed by HPLC and LC-MS/MS. Both analyses revealed that the 25(OH)D3 levels were much lower than those measured by the RIA. The values from HPLC analysis were 19.4 ng/mL for wild-type, 5.2 ng/mL for Cyp2r1−/−, 52.2 ng/mL for Cyp27a1−/−, and 5.9 ng/mL for Cyp2r1−/−/Cyp27a1−/−. The LC-MS/MS analysis was in good agreement with the HPLC analysis (Table 1). Overall, it is clear that the circulating 25(OH)D3 level fell significantly, but was still detectable, in Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice. In addition, vitamin D 25-hydroxylase activities were compared in liver homogenates from these mice. All of the liver homogenates were able to convert vitamin D3 to 25(OH)D3 when supplemented with NADPH, with a relative conversion rate of 4:1.5:15:2 for wild-type, Cyp2r1−/−, Cyp27a1−/−, and Cyp2r1−/−/Cyp27a1−/− mice, respectively.
Fig. 2.
Serum 25(OH)D3 concentration in Cyp2r1 wild-type (WT), heterozygote (HET), and knockout (KO) mice (A) and in wild-type, Cyp27a1−/−, Cyp2r1−/−, and Cyp2r1−/−/Cyp27a1−/− mice (B) at 6, 10, and 14 wk of age measured by RIA. *P < 0.01 and ^P < 0.05, compared with wild-type groups of the same age.
Table 1.
Serum 25(OH)D3 concentration (ng/mL) measured by RIA, HPLC, and LC-MS/MS analyses
| RIA |
HPLC |
LC-MS/MS |
||||
| Cyp2r1/Cyp27a1 | 6 wk | 10 wk | 14 wk | 10 wk | 10 wk | 10 wk* |
| ++/++ | 40.9 ± 8.3 | 44.0 ± 10.1 | 46.5 ± 6.2 | 19.4 ± 4.5 | 16.8 ± 1.6 | 34.7 ± 3.1 |
| +−/++ | 30.7 ± 4.9 | 34.5 ± 7.2 | 42.0 ± 6.4 | — | 13.9 ± 1.6 | 28.5 ± 2.2 |
| −−/++ | 14.8 ± 4.5 | 17.8 ± 4.3 | 21.9 ± 6.1 | 5.2 | 6.8 ± 1.2 | 14.0 ± 2.1 |
| ++/−− | 116.4 ± 17.5 | 112.3 ± 6.0 | 104.8 ± 12.5 | 52.2 | 70.5 ± 13.1 | 133.4 ± 17.0 |
| −−/−− | 19.7 ± 6.0 | 30.9 ± 10.2 | 20.4 ± 9.7 | 5.9 | 10.9 ± 1.7 | 24.2 ± 4.1 |
25(OH)D3, 24,25(OH)2D3, and 25(OH)D3-26,23-lactone combined.
Serum 1,25(OH)2D3 Level.
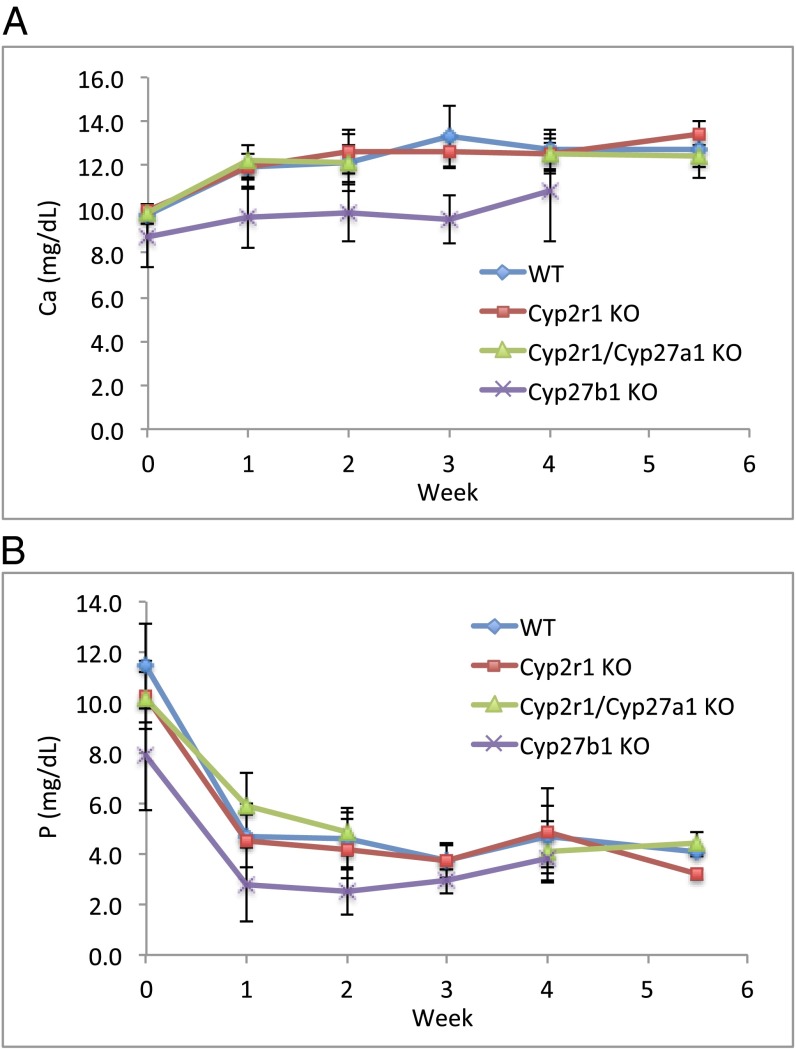
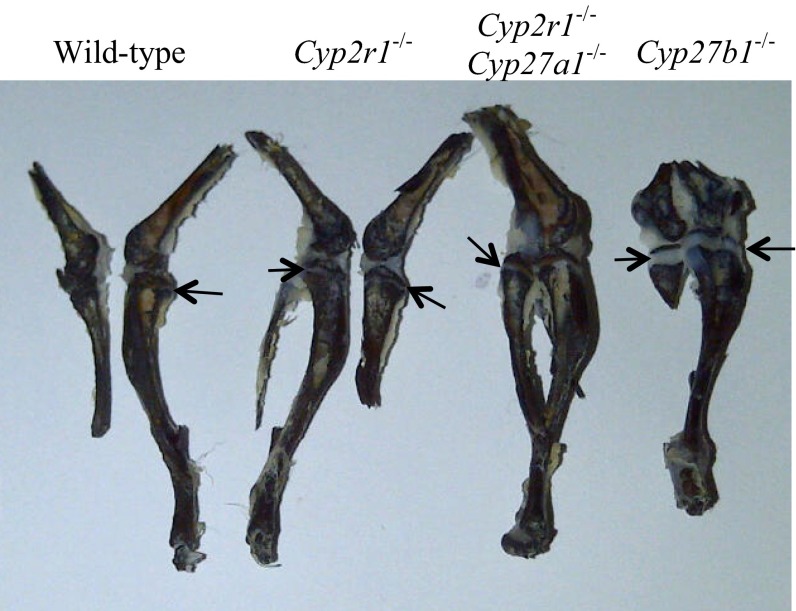
Unlike the concentrations of 25(OH)D3, the serum level of 1,25(OH)2D3, determined using a luciferase reporter assay (27), remained comparable in all age groups and genotypes at 51–68 pg/mL (Table 2). The normal serum concentration of 1,25(OH)2D3 in Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice was further supported by the fact that the mice did not develop rickets in response to a rachitogenic high-calcium and low-phosphorus diet. At 4–6 wk of age, mice were switched to a purified diet containing 1.2% Ca/0.02% P. The 1.2% Ca/0.02% P diet is expected to produce rickets in the absence of vitamin D (28). The serum calcium level of the mice on the diet steadily increased, whereas the phosphorus level quickly dropped, rendering the mice hypophosphatemic (Fig. 3). However, the epiphyseal plates in the tibia from Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice were indistinguishable from those in wild-type mice, whereas the Cyp27b1−/− mice used as controls had very wide growth plates, which is characteristic of rickets (Fig. 4). The Cyp27b1−/− mice receiving a 1.2% Ca/0.02% P diet remained hypocalcemic and hypophosphatemic (Fig. 3), whereas the Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice had serum calcium levels identical with those of the wild-type mice, confirming an adequacy of vitamin D in all mice except the Cyp27b1−/− mice. The normal growth plates in Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice compared with those in Cyp27b1−/− mice strongly suggest that the circulating level of 1,25(OH)2D3 was adequate in the Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− groups.
Table 2.
Serum 1,25(OH)2D3 concentration (pg/mL) determined by luciferase reporter assay
|
Cyp2r1/Cyp27a1 |
||||
| Age, wk | ++++ | ––++ | ++–– | −−−− |
| 5 | 56.5 | 60.7 | 66.2 | 51.2 |
| 10 | 65.6 | 54.8 | 61.5 | 63.1 |
| 16 | 67.6 | 62.3 | 64.4 | 66.7 |
Fig. 3.
Serum calcium (A) and serum phosphorus (B) in mice receiving a rachitogenic diet (1.2% Ca/0.02% P) over the course of 5–6 wk. Cyp27b1−/− mice were included as positive controls that illustrate the dependency of serum calcium and serum phosphorus on vitamin D activation.
Fig. 4.
Epiphyseal plates in wild-type, Cyp2r1−/−, and Cyp2r1−/−/Cyp27a1−/− mice compared with those in Cyp27b1−/− mice. The epiphyseal plates are indicated by arrows. Epiphyseal plates are almost absent in wild-type, Cyp2r1−/−, and Cyp2r1−/−/Cyp27a1−/− mice but are very wide in Cyp27b1−/− mice.
CYP2R1 Transcript Level.
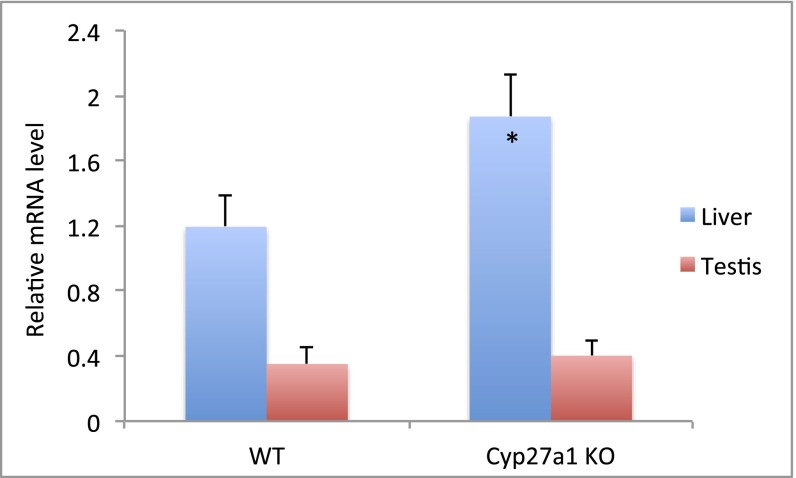
CYP2R1 transcript level was examined in tissues from liver, kidney, testis, and ovaries. Real-time PCR analysis revealed that CYP2R1 transcripts were abundant in liver and testis, with an approximate 3:1 ratio in wild-type mice (Fig. 5). Note that the relative abundance of CYP2R1 transcripts is dependent on the reference gene used as normalizing standard, which may explain the different ratios seen in earlier reports (19, 29). The CYP2R1 mRNA level was slightly higher than CYP27A1 in the liver, although the opposite trend has been reported (19), probably because of variations in the strains. A 1.5-fold increase in CYP2R1 transcripts was observed in Cyp27a1−/− mice in the liver, with less in the testis.
Fig. 5.
Real-time PCR analysis of CYP2R1 mRNA in the liver and testis from wild-type and Cyp27a1−/− mice. The relative mRNA levels were normalized against β-actin. *P < 0.01 compared with wild-type mice.
Discussion
We report the creation and characterization of Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice. The most striking feature of both knockout mice was the more than 50% reduction in serum 25(OH)D3 level, whereas the circulating 1,25(OH)2D3 concentration remained unchanged. The RIA afforded high-throughput analysis of hundreds of serum samples, but apparently also detected substances other than 25(OH)D3. The HPLC analysis was able to separate 25(OH)D3 from potential interfering compounds, and the determined 25(OH)D3 concentration was two- to fourfold lower than that from the RIA. The third method, using LC-MS/MS, confirmed the values measured by HPLC analysis and offered insight regarding the other metabolites that were detected in the RIA. Because the RIA relies on an antibody raised against a side chain truncated vitamin D derivative, it cannot distinguish 25(OH)D3, 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], and 25(OH)D3-26,23-lactone and seemed to have measured all three metabolites combined (Table 1). Not surprisingly, Cyp2r1 ablation also caused reduction in 24,25(OH)2D3 and 25(OH)D3-26,23-lactone levels. In conclusion, the serum 25(OH)D3 level in Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− mice may be as low as one third that in wild-type mice. The difference in serum 25(OH)D3 levels between wild-type and knockout mice, however, was not manifested until mice reached sexual maturity, probably because 25(OH)D3 is in greater demand at early stage of development, which makes its circulating levels relatively low. The serum 1,25(OH)2D3 level, in contrast, is under tight control through the transcriptional regulation of 1α-hydroxylase via a vast array of cellular agents, including autoregulatory feedback (5), and remained unchanged throughout the life span, despite the significantly low 25(OH)D3 level. This coincides with the observation that patients carrying CYP2R1 mutation had an abnormally low 25(OH)D3 level (4–5 ng/mL) but normal 1,25(OH)2D3 concentration (22, 26), suggesting that such a low level of 25(OH)D3 is sufficient for production of adequate 1,25(OH)2D3 and that the minimal threshold of 25(OH)D3 might be lower than currently defined. Interestingly, a substantial increase in 25(OH)D3 concentration was observed in Cyp27a1−/− mice. Although 24- and 26(27)-hydroxylase and other hydroxylation activities of CYP27A1 toward 25(OH)D3 were detected in vitro (11, 13, 14), they were trivial and were probably not able to carry out meaningful degradation of 25(OH)D3 in vivo. Therefore, the high level of 25(OH)D3 in Cyp27a1−/− mice is unlikely a result of hindered catabolism. A more plausible explanation has been that Cyp27a1 ablation causes increased expression of the Cyp2r1 gene. Because Cyp2r1−/−/Cyp27a1−/− mice had dramatically reduced 25(OH)D3 levels, similar to that of Cyp2r1−/− mice, and the real-time PCR analysis indicated that the CYP2R1 transcript level in the liver was increased in Cyp27a1−/− mice, it seems clear that CYP2R1 is the major, albeit not the only, vitamin D 25-hydroxylase.
CYP2R1 is highly conserved across vertebrate species (30) and shows no sexual dimorphism (19, 31). An in vitro reconstituted P450 system has been used to demonstrate that CYP2R1 acts equally well on vitamin D3 and vitamin D2 (19–21). The only known pathological condition associated with CYP2R1 disruption is the rare mutations identified in patients with 25(OH)D3 deficiency and vitamin D-dependent rickets (22–26). CYP2R1 is primarily expressed in liver and testis (19, 29, 32–34). The former is consistent with liver being the major site of 25-hydroxylation, but its abundance in testis awaits further investigation to decipher the physiological relevance, although it has been linked to male reproduction, testicular cancer, and other testiculopathic conditions (33–35). Cyp2r1 polymorphisms have been implicated in the variation of circulating 25(OH)D3 concentrations (36–38) and as risk factors in type 1 diabetes and multiple sclerosis in humans (39–42).
Despite the significantly reduced 25(OH)D3 level, Cyp2r1 ablation did not abolish the synthesis of 25(OH)D3, suggesting the presence of other vitamin D 25-hydroxylases. Some of the candidates hypothesized earlier have been deemed less likely to be the physiological vitamin D 25-hydroxylase, at least in humans. For example, CYP2C11, the first isolated enzyme that contains vitamin D 25-hydroxylation activity, is only found in rat and is only present in males, with its major function being testosterone hydroxylase (4). Similarly, porcine CYP2D25 does not have a functionally related human ortholog. CYP2D6, the only P450 isoform of human CYP2D subfamily, exhibits no detectable vitamin D 25-hydroxylation activity despite 77% sequence identity (4). The species specificity and sexual dimorphism makes them less relevant as potential 25-hyroxylases. In contrast, CYP27A1, the well-studied sterol 27-hydroxylase and the only mitochondrial enzyme with vitamin D 25-hydroxylase activity, has been cloned in rabbit, rat, and human (8, 9, 43–45) and was considered a strong candidate for some time. However, Cyp27a1-null mice showed a normal phenotype in the vitamin D activation pathway (15). Over the years, the identification of the nuclear hormone response elements in the Cyp3a4 gene promoter has drawn attention to the most abundant P450 in human liver and intestine as a possible vitamin D 25-hydroxylase (46–50), although the vitamin D receptor is undetectable in the liver (51). CYP3A4 is a well-known xenobiotic-metabolizing enzyme and can act on approximately half of the drugs in use. Although CYP3A4 can hydroxylate vitamin D compounds in vitro (52, 53), it is unclear whether it functions as a physiological 25-hydroxylase for vitamin D in vivo. Finally, rat CYP2J3, better known as the arachidonic acid epoxygenase (54), has shown very high 25-hydroxylase activities toward vitamin D3 and is mostly expressed in the liver (31). CYP2J2, the human counterpart, is mainly involved in the cardiovascular system and intestinal metabolism of antihistamine drugs (55, 56). Its 25-hydroxylase activity was much lower and seemed to favor vitamin D2 over vitamin D3 (57). The potential contribution of CYP2J2 in vitamin D 25-hydroxylation in vivo is not known. With Cyp2r1−/−/Cyp27a1−/− mice available, a screening of their liver cDNA library, similar to the one that was used to discover CYP2R1 (19), may provide a way to identify the remaining vitamin D 25-hydroxylases.
Materials and Methods
Generation of Cyp2r1−/− and Cyp2r1−/−/Cyp27a1−/− Mice.
Two embryonic stem cell clones from Velocigene at Regeneron, which replaced a 12.9-kb span covering all 5 exons of Cyp2r1 gene on chromosome 7 with β-galactosidase coding sequence (lacZ) and neomycin selection cassette, were purchased through knockout mouse project repository (University of California Davis-CHORI), with 10% and 75% euploidy, respectively. The clone with 75% euploidy was used for blastocyst injection into pseudopregnant C57BL/6 albino females at University of Michigan Transgenic Animal Model Core for chimera production. The chimeric founders were transferred to the University of Wisconsin–Madison and crossbred with C57BL/6 females (Harlan). The Cyp2r1−/− mice were generated by crossing F1 heterozygotes. Offspring from generations F3 through F5 were used in the study. The Cyp2r1−/−/Cyp27a1−/− mice were created by crossing Cyp2r1−/− and Cyp27a1−/− strains. The Cyp27a1−/− breeder pair (B6.129-Cyp27a1tm1Elt/J) was purchased from Jackson Laboratory. All mice were maintained on C57BL/6 background and fed a commercial chow diet [LabDiet 5015 (0.80% Ca/0.50% P) for breeders and LabDiet 5008 (1.00% Ca/0.65% P) for others] unless otherwise stated. Vitamin D3 intake was 0.25–0.4 μg (10–16 IU) per day per adult mouse based on food consumption. Genotyping was carried out according to Velocigene genotyping strategy or by Transnetyx genotyping service (Cordova, TN). Primers used in Velocigene genotyping strategy were as follows: SD, 5′-GTATGTGTGGGGGAATACAC-3′; TDF, 5′- CCCAGGTTGGGCATGACATTAC-3′; and NeoFwd, 5′- TCATTCTCAGTATTGTTTTGCC-3′. CYP2R1 mRNA in Cyp2r1−/− mice was measured to be negative (vide infra). All mice were managed in compliance with protocols approved by the University of Wisconsin Research Animal Resources Center.
Serum Analysis.
Serum calcium was determined by atomic absorption at 422.7 nm on a Perkin-Elmer AA3110 spectrometer after diluting serum in 0.1% LaCl3 solution. Serum phosphorus was determined using a colorimetric assay, in which serum was mixed with ammonium molybdate and malachite green to form a chromogenic complex measured at 660 nm (58). Parathyroid hormone was determined using mouse PTH 1–84 ELISA kit (Immutopics).
25(OH)D3 concentration was measured using a 25(OH)D3 RIA kit (DiaSorin) and by HPLC and LC-MS/MS analyses. No extraction was performed before the RIA. For HPLC analysis, pooled serum (500 μL) was extracted with ethyl acetate. The organic layer was evaporated under a stream of N2 and redissolved in HPLC mobile phase (60% CH3CN/40% H2O). The mixture was applied onto a reverse phase column (Symmetry C18, 3.9 × 150 mm, Waters) run in the same solvent monitored at 265 nm. 26,27-[3H]-25(OH)D3 (Perkin-Elmer) was spiked into the serum to evaluate extraction efficiency. 25(OH)D3 concentration was calculated on the basis of the area under the curve and injection of known amounts of standard. The limit of quantitation for 25(OH)D3 by HPLC analysis is 1.5 ng. For LC-MS/MS analysis, 100 μL aliquots of serum were diluted with 200 μL of H2O and supplemented with 80 ng/mL [2H3]-25(OH)D3 (Isosciences), 65 ng/mL [2H3]-25(OH)D2 (Isosciences), and 6 ng/mL [2H6]-24,25(OH)2D3 (courtesy of A. Mouriño, Universidad de Santiago de Compostela, Spain, and M. Maestro, Universidad de A Coruna, Spain). One hundred microliters of 0.1 M HCl was added, and protein precipitation was carried out by adding 0.2 M ZnSO4 and 450 μL of CH3OH, with vortexing after the addition of each component. The supernatant was extracted with hexane and methyl t-butyl ether. The extracts were derivatized with 4-[2-(6,7-dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalyl)ethyl]-1,2,4-triazoline-3,5-dione (DMEQ-TAD) (Key Synthesis) in ethyl acetate, as described previously (59), and redissolved in 60 μL of 60% CH3OH/40% H2O. Triplicate 10 μl-aliquots of each serum extract were subjected to analysis, using an ACQUITY ultraperformance LC coupled to a TQ-S triple-quadrupole mass spectrometer in electrospray-positive mode (Waters). Chromatography was achieved using a 65–90% (vol/vol) CH3OH/H2O exponential gradient (curve 8) at a flow rate of 0.4 mL/min over the course of 5 min on a UPLC BEH-Phenyl column (1.7 μm, 2.1 × 50 mm, Waters). The UPLC solvents were supplemented with 0.1% formic acid and 2 mM ammonium acetate. Quantitation was performed using calibration curves generated with internal standards.
1,25(OH)2D3 concentration was measured using a luciferase reporter assay with modifications (27). Serum (100 μL) was extracted with Oasis MCX extraction cartridge (Waters), following the manufacturer’s protocol. The CH3OH eluent was dried in a SpeedVac, and the residues were resuspended in CH3OH:C2H5OH (1:1) and filtered through a GHP Acrodisc filter (Pall). The filtrate was dried in the SpeedVac, and the residues were redissolved in the same media used in the luciferase assay by continuous vortexing. 1,25(OH)2D3 concentration was calculated from a standard curve generated using 1,25(OH)2D3-spiked Cyp27b1−/− mouse serum and analyzed on the same plate. Cyp27b1−/− mice were produced as previously reported (60) and maintained on a rescue diet (61).
25-Hydroxylase Activity.
Livers, freshly isolated from mice killed by CO2 asphyxiation, were quickly minced on ice-cold metal plates using razor blades and homogenized in 0.25 M sucrose solution on ice. To 2.5 mL of 25% liver homogenate were added 0.015 μCi of 1,2-[3H]-D3 (62), 2.5 mL PBS, and 10 mM NADPH (final concentration). NADPH was omitted in the control reactions. The mixture was incubated at 37 °C for 2.5 h on a shaker at 220 rpm and subjected to modified Bligh-Dyer extraction (63), in which CHCl3 was replaced with CH2Cl2. The CH2Cl2 layer was dried down in a SpeedVac to less than 50 μL of oil and diluted in HPLC mobile phase of 97% hexane/3% isopropanol. The lipid extract was applied onto a straight phase column (ZORBAX SIL, 4.6 × 250 mm, Agilent) run in the same solvent. Fractions were collected, and the conversion rate of vitamin D3 to 25(OH)D3 was calculated from tritium count. The retention time of tritiated vitamin D3 and 25(OH)D3 was based on comigration with authentic compounds under the same condition monitored at 265 nm.
Analysis of Epiphyseal Plate.
Mice at 4–6 wk of age were switched to a rachitogenic diet that contains essential vitamins and minerals in addition to the basal composition (64) but has imbalanced calcium and phosphorus content (1.2% Ca/0.02% P). The mice were maintained on the diet for 5–6 wk. Blood was collected weekly or every 2 wk for serum analysis. Mice were killed by CO2 asphyxiation, and the tibia-fibula joint sections were isolated. The bones were split longitudinally, revealing epiphyseal plates, and stained briefly in 1% AgNO3. Cyp27b1−/− mice were included in the study as positive controls, and most were terminated within 4 wk because of severe rickets.
Real-Time PCR Analysis.
Twelve wild-type and 12 Cyp27a1−/− mice including both sexes were killed by CO2 asphyxiation and perfused with cold PBS. Tissues from liver, kidney, testis, and ovaries were immediately collected, submerged in Tri reagent (Molecular Research Center), flash-frozen in liquid N2, and stored at −80 °C. RNA was isolated according to manufacturer’s protocol. cDNA synthesis was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR reactions were carried out in duplicate on a StepOnePlus real-time PCR system, using Taqman gene expression assays (Applied Biosystems). β-Actin mRNA was used as normalizing standard. The assay IDs were Mm01159413_m1 for Cyp2r1, Mm00470430_m1 for Cyp27a1, and Mm00607939_s1 for β-actin. Cyp2r1−/− mice were included as negative controls for CYP2R1 transcripts (CT > 40).
Statistical Analysis.
Data were expressed as mean ± SD. Statistical differences between experimental groups were quantified by two-tailed Student t test.
Acknowledgments
We thank Jean Prahl for assistance on the animal work and the luciferase assay; William Blaser, Heather Neils, and Steven Marling for conducting serum analysis of calcium and parathyroid hormone and 25(OH)D3 radioimmunoassay; and Chrystal Glidden for HPLC analysis of serum 25(OH)D3. This work is funded by the Wisconsin Alumni Research Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Holick MF. Vitamin D: Evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 2.Christakos S, DeLuca HF. Minireview: Vitamin D: Is there a role in extraskeletal health? Endocrinology. 2011;152(8):2930–2936. doi: 10.1210/en.2011-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen CJ, et al. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523(1):30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Henry HL. The 25-hydroxyvitamin D 1α-hydroxylase. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. 2nd Ed. Vol 1. San Diego, CA: Elsevier Academic Press; 2005. pp. 69–83. [Google Scholar]
- 6.Norlin M, Wikvall K. Enzymes in the conversion of cholesterol into bile acids. Curr Mol Med. 2007;7(2):199–218. doi: 10.2174/156652407780059168. [DOI] [PubMed] [Google Scholar]
- 7.Lorbek G, Lewinska M, Rozman D. Cytochrome P450s in the synthesis of cholesterol and bile acids—from mouse models to human diseases. FEBS J. 2012;279(9):1516–1533. doi: 10.1111/j.1742-4658.2011.08432.x. [DOI] [PubMed] [Google Scholar]
- 8.Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991;266(12):7774–7778. [PubMed] [Google Scholar]
- 9.Guo YD, Strugnell S, Back DW, Jones G. Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions. Proc Natl Acad Sci USA. 1993;90(18):8668–8672. doi: 10.1073/pnas.90.18.8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyoshi-Shibata M, et al. Expression of rat liver vitamin D3 25-hydroxylase cDNA in Saccharomyces cerevisiae. FEBS Lett. 1991;280(2):367–370. doi: 10.1016/0014-5793(91)80333-x. [DOI] [PubMed] [Google Scholar]
- 11.Axén E, Postlind H, Sjöberg H, Wikvall K. Liver mitochondrial cytochrome P450 CYP27 and recombinant-expressed human CYP27 catalyze 1 alpha-hydroxylation of 25-hydroxyvitamin D3. Proc Natl Acad Sci USA. 1994;91(21):10014–10018. doi: 10.1073/pnas.91.21.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pikuleva IA, Björkhem I, Waterman MR. Expression, purification, and enzymatic properties of recombinant human cytochrome P450c27 (CYP27) Arch Biochem Biophys. 1997;343(1):123–130. doi: 10.1006/abbi.1997.0142. [DOI] [PubMed] [Google Scholar]
- 13.Sawada N, Sakaki T, Ohta M, Inouye K. Metabolism of vitamin D(3) by human CYP27A1. Biochem Biophys Res Commun. 2000;273(3):977–984. doi: 10.1006/bbrc.2000.3050. [DOI] [PubMed] [Google Scholar]
- 14.Araya Z, Hosseinpour F, Bodin K, Wikvall K. Metabolism of 25-hydroxyvitamin D3 by microsomal and mitochondrial vitamin D3 25-hydroxylases (CYP2D25 and CYP27A1): A novel reaction by CYP27A1. Biochim Biophys Acta. 2003;1632(1-3):40–47. doi: 10.1016/s1388-1981(03)00062-3. [DOI] [PubMed] [Google Scholar]
- 15.Rosen H, et al. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J Biol Chem. 1998;273(24):14805–14812. doi: 10.1074/jbc.273.24.14805. [DOI] [PubMed] [Google Scholar]
- 16.Repa JJ, et al. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J Biol Chem. 2000;275(50):39685–39692. doi: 10.1074/jbc.M007653200. [DOI] [PubMed] [Google Scholar]
- 17.Moghadasian MH. Cerebrotendinous xanthomatosis: Clinical course, genotypes and metabolic backgrounds. Clin Invest Med. 2004;27(1):42–50. [PubMed] [Google Scholar]
- 18. Gallus GN, Dotti MT, Federico A (2006) Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci 27(2):143–149. [DOI] [PubMed]
- 19.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: A microsomal vitamin D 25-hydroxilase. J Biol Chem. 2003;278(39):38084–38093. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinkyo R, Sakaki T, Kamakura M, Ohta M, Inouye K. Metabolism of vitamin D by human microsomal CYP2R1. Biochem Biophys Res Commun. 2004;324(1):451–457. doi: 10.1016/j.bbrc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 21.Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW. Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol. 2008;380(1):95–106. doi: 10.1016/j.jmb.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 22.Casella SJ, Reiner BJ, Chen TC, Holick MF, Harrison HE. A possible genetic defect in 25-hydroxylation as a cause of rickets. J Pediatr. 1994;124(6):929–932. doi: 10.1016/s0022-3476(05)83184-1. [DOI] [PubMed] [Google Scholar]
- 23.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA. 2004;101(20):7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Q, Miller WL. Vitamin D 25-hydroxylase deficiency. Mol Genet Metab. 2004;83(1-2):197–198. doi: 10.1016/j.ymgme.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Levine MA, et al. Tropical rickets in Nigeria: Mutation of the CYP2R1 gene encoding vitamin D 25-hydroxylase as a cause of vitamin D dependent rickets. Bone. 2007;40:S60–S61. [Google Scholar]
- 26.Al Mutair AN, Nasrat GH, Russell DW. Mutation of the CYP2R1 vitamin D 25-hydroxylase in a Saudi Arabian family with severe vitamin D deficiency. J Clin Endocrinol Metab. 2012;97(10):E2022–E2025. doi: 10.1210/jc.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbour NC, Ross TK, Zierold C, Prahl JM, DeLuca HF. A highly sensitive method for large-scale measurements of 1,25-dihydroxyvitamin D. Anal Biochem. 1998;255(1):148–154. doi: 10.1006/abio.1997.2439. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Deluca HF. Role of 1,25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc Natl Acad Sci USA. 1974;71(4):1040–1044. doi: 10.1073/pnas.71.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1-4) during development and in different adult tissues. Arch Biochem Biophys. 2005;436(1):50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Nelson DR. Comparison of P450s from human and fugu: 420 million years of vertebrate P450 evolution. Arch Biochem Biophys. 2003;409(1):18–24. doi: 10.1016/s0003-9861(02)00553-2. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki T, Izumi S, Ide H, Ohyama Y. Identification of a novel rat microsomal vitamin D3 25-hydroxylase. J Biol Chem. 2004;279(22):22848–22856. doi: 10.1074/jbc.M311346200. [DOI] [PubMed] [Google Scholar]
- 32.Bièche I, et al. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17(9):731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- 33.Foresta C, et al. Bone mineral density and testicular failure: Evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab. 2011;96(4):E646–E652. doi: 10.1210/jc.2010-1628. [DOI] [PubMed] [Google Scholar]
- 34.Blomberg Jensen M, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25(5):1303–1311. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 35.Foresta C, et al. Altered bone status in unilateral testicular cancer survivors: Role of CYP2R1 and its luteinizing hormone-dependency. J Endocrinol Invest. 2013;36(6):379–384. doi: 10.3275/8650. [DOI] [PubMed] [Google Scholar]
- 36.Ahn J, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang TJ, et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu H, et al. (2013) A genome-wide methylation study of severe vitamin D deficiency in African American adolescents. J Pediatr 162(5):1004–1009.e1. [DOI] [PMC free article] [PubMed]
- 39.Ramos-Lopez E, Brück P, Jansen T, Herwig J, Badenhoop K. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes Metab Res Rev. 2007;23(8):631–636. doi: 10.1002/dmrr.719. [DOI] [PubMed] [Google Scholar]
- 40.Cooper JD, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60(5):1624–1631. doi: 10.2337/db10-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussein AG, Mohamed RH, Alghobashy AA. Synergism of CYP2R1 and CYP27B1 polymorphisms and susceptibility to type 1 diabetes in Egyptian children. Cell Immunol. 2012;279(1):42–45. doi: 10.1016/j.cellimm.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Simon KC, et al. Genetic predictors of 25-hydroxyvitamin D levels and risk of multiple sclerosis. J Neurol. 2011;258(9):1676–1682. doi: 10.1007/s00415-011-6001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson S, Davis DL, Dahlbäck H, Jörnvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264(14):8222–8229. [PubMed] [Google Scholar]
- 44.Usui E, Noshiro M, Okuda K. Molecular cloning of cDNA for vitamin D3 25-hydroxylase from rat liver mitochondria. FEBS Lett. 1990;262(1):135–138. doi: 10.1016/0014-5793(90)80172-f. [DOI] [PubMed] [Google Scholar]
- 45.Su P, et al. A cDNA encoding a rat mitochondrial cytochrome P450 catalyzing both the 26-hydroxylation of cholesterol and 25-hydroxylation of vitamin D3: Gonadotropic regulation of the cognate mRNA in ovaries. DNA Cell Biol. 1990;9(9):657–667. doi: 10.1089/dna.1990.9.657. [DOI] [PubMed] [Google Scholar]
- 46.Thummel KE, et al. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60(6):1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- 47.Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277(28):25125–25132. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- 48.Thompson PD, et al. Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem Biophys Res Commun. 2002;299(5):730–738. doi: 10.1016/s0006-291x(02)02742-0. [DOI] [PubMed] [Google Scholar]
- 49.Jurutka PW, et al. Molecular and functional comparison of 1,25-dihydroxyvitamin D(3) and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. J Cell Biochem. 2005;94(5):917–943. doi: 10.1002/jcb.20359. [DOI] [PubMed] [Google Scholar]
- 50.Pavek P, et al. Intestinal cell-specific vitamin D receptor (VDR)-mediated transcriptional regulation of CYP3A4 gene. Biochem Pharmacol. 2010;79(2):277–287. doi: 10.1016/j.bcp.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523(1):123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 52. Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH (2004) CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res 19(4):680–688. [DOI] [PubMed]
- 53.Gupta RP, He YA, Patrick KS, Halpert JR, Bell NH. CYP3A4 is a vitamin D-24- and 25-hydroxylase: Analysis of structure function by site-directed mutagenesis. J Clin Endocrinol Metab. 2005;90(2):1210–1219. doi: 10.1210/jc.2004-0966. [DOI] [PubMed] [Google Scholar]
- 54.Wu S, et al. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem. 1997;272(19):12551–12559. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- 55.Spiecker M, Liao J. Cytochrome P450 epoxygenase CYP2J2 and the risk of coronary artery disease. Trends Cardiovasc Med. 2006;16(6):204–208. doi: 10.1016/j.tcm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Thelen K, Dressman JB. Cytochrome P450-mediated metabolism in the human gut wall. J Pharm Pharmacol. 2009;61(5):541–558. doi: 10.1211/jpp/61.05.0002. [DOI] [PubMed] [Google Scholar]
- 57.Aiba I, et al. Characterization of rat and human CYP2J enzymes as Vitamin D 25-hydroxylases. Steroids. 2006;71(10):849–856. doi: 10.1016/j.steroids.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 58. Itaya K, Ui M (1966) A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta 14(3):361–366. [DOI] [PubMed]
- 59.Higashi T, Awada D, Shimada K. Simultaneous determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma by liquid chromatography-tandem mass spectrometry employing derivatization with a Cookson-type reagent. Biol Pharm Bull. 2001;24(7):738–743. doi: 10.1248/bpb.24.738. [DOI] [PubMed] [Google Scholar]
- 60.Vanhooke JL, et al. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc Natl Acad Sci USA. 2006;103(1):75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amling M, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: Formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140(11):4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 62.Neville PF, DeLuca HF. The synthesis of [1,2-3H]vitamin D3 and the tissue localization of a 0.25-mu-g (10 IU) dose per rat. Biochemistry. 1966;5(7):2201–2207. doi: 10.1021/bi00871a007. [DOI] [PubMed] [Google Scholar]
- 63.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 64.Yang S, Smith C, Prahl JM, Luo X, DeLuca HF. Vitamin D deficiency suppresses cell-mediated immunity in vivo. Arch Biochem Biophys. 1993;303(1):98–106. doi: 10.1006/abbi.1993.1260. [DOI] [PubMed] [Google Scholar]