Significance
We document the behavioral interactions among three ant species: a fungus-growing host ant, a permanently associated parasitic guest ant, and a raiding agro-predator ant. We show that the presence of guest ants becomes advantageous when host ants are attacked by raider ants, because guest ants use alkaloid venom to defend their host ant colony. Furthermore, detection of the guest ant odors is sufficient to discourage raider scouts from recruiting nestmates to host colonies. Guest ants likely have evolved this protective behavior because they also perish when their host colony dies.
Keywords: evolutionary transition, symbiosis, Attini, Solenopsidini
Abstract
The ants are extraordinary in having evolved many lineages that exploit closely related ant societies as social parasites, but social parasitism by distantly related ants is rare. Here we document the interaction dynamics among a Sericomyrmex fungus-growing ant host, a permanently associated parasitic guest ant of the genus Megalomyrmex, and a raiding agro-predator of the genus Gnamptogenys. We show experimentally that the guest ants protect their host colonies against agro-predator raids using alkaloid venom that is much more potent than the biting defenses of the host ants. Relatively few guest ants are sufficient to kill raiders that invariably exterminate host nests without a cohabiting guest ant colony. We also show that the odor of guest ants discourages raider scouts from recruiting nestmates to host colonies. Our results imply that Sericomyrmex fungus-growers obtain a net benefit from their costly guest ants behaving as a functional soldier caste to meet lethal threats from agro-predator raiders. The fundamentally different life histories of the agro-predators and guest ants appear to facilitate their coexistence in a negative frequency-dependent manner. Because a guest ant colony is committed for life to a single host colony, the guests would harm their own interests by not defending the host that they continue to exploit. This conditional mutualism is analogous to chronic sickle cell anemia enhancing the resistance to malaria and to episodes in human history when mercenary city defenders offered either net benefits or imposed net costs, depending on the level of threat from invading armies.
Ant societies retain much of their coherence through chemical nestmate recognition (1, 2), which allows resident workers to differentiate between friend and foe by colony-specific chemical signatures (3). This recognition system is not infallible, however; numerous species have evolved ways to evade detection so they can exploit ant colonies through invasion, usurpation, or thievery (2). One common route to social parasitism is shown convergently by many ant genera in which social parasites are their host’s closest relatives, a scenario that might have arisen through sympatric speciation (4, 5). At the other end of the spectrum are interactions with different insect orders, such as parasitic beetles and butterfly caterpillars, that drain host ant colonies of resources while remaining protected by crypsis or chemical mimicry (6).
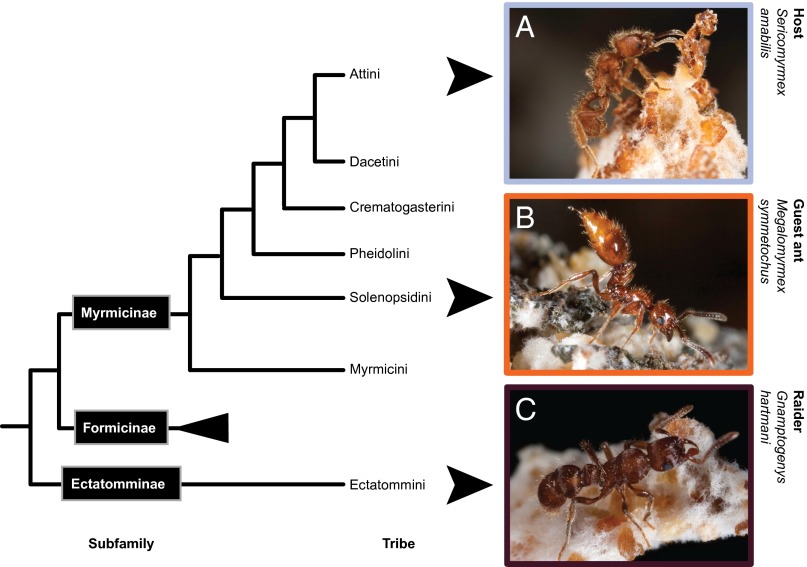
Intermediate types of parasitic interactions involving ants that exploit other, distantly related ants are rare (7). Several species of Megalomyrmex (Solenopsidini) belong to this category, associating in varying capacities with fungus-growing ant hosts (Attini), a clade of the same ant subfamily (8–12) (Fig. 1). Most details about the diversity and life history adaptations of these ants have been clarified only recently (11), and much of their biology remains to be discovered (SI Text: Study System and Tables S1 and S2). Free-living predatory Megalomyrmex are known to biosynthesize venom alkaloids that are used in defense (13, 14) whereas the fungus-growing ant associates seem to aggressively dispense these venoms when they attack host colonies (15). Some of these parasites are obligate or facultative thief ants consuming brood and fungus gardens (15), whereas others are specialized agro-predators that move from one host colony to the other after usurping fungus gardens and killing or chasing away the resident ants (10). Despite the often high densities of their attine hosts, these Megalomyrmex social parasites remain rare, with parasitism rates of ∼1.5–14% (10, 15), similar to the rates of many other social parasites (5).
Fig. 1.
Subfamily and tribe-level tree after Brady et al. (8) indicating the different phylogenetic positions of the interacting ant species. (A) The fungus-growing ant host S. amabilis. (B) The guest ant social parasite M. symmetochus (in a stilted stance, emitting volatile alkaloids from its protruding sting). (C) The G. hartmani raiding agro-predator. M. symmetochus and G. hartmani have independently specialized on using S. amabilis fungus gardens and brood as food.
Behaviorally derived lineages of Megalomyrmex have become guest ant parasites of the higher attine genera Trachymyrmex and Sericomyrmex (11, 16, 17) (Fig. 1A). The Trachymyrmex specialist, Megalomyrmex adamsae, appears to have remained as equally rare as the agro-predators and thief ants (11), but the Sericomyrmex specialist Megalomyrmex symmetochus (Fig. 1B) is surprisingly common, with a prevalence of >80% in some host populations (16). Newly mated guest ant queens of both species likely enter host colonies by stealth and establish themselves in the fungus garden, where their developing colony will consume host brood and fungus garden for years (11). Their presence slows host colony growth and also prevents or reduces host reproduction, because both guest ant species clip the wings of host gynes (virgin queens), but not males (11). Such mutilation reduces host reproduction and dispersal via mating flights, but likely increases guest ant fitness when these mutilated females adopt worker tasks (11, 18). Like all guest ant social parasites, M. symmetochus retains a fully functional worker caste (16, 19), in contrast to many social parasites that exploit the services of their phylogenetically similar host colony without the need to produce workers (5).
The maintenance of a large worker caste despite permanent cohabitation with a host colony may have several purposes, all based on some specialized role for the parasite workers. First, it may be that the distantly related host workers are unable to feed the social parasite larvae (5) even though adult hosts and parasites are adapted to the same highly specific fungal diet. Another possibility is that the guest ant colony remains at risk of occasional attack by the host workers; such antagonism has been observed between M. symmetochus workers and host workers in older colonies (SI Text, Study System). But Sericomyrmex ants have only vestigial stings (20) and often feign death when attacked, and so this does not explain the large number of parasite workers. Thus, it seems reasonable to hypothesize that a large number of Megalomyrmex guest ant workers continue to remain essential for the survival and reproductive success of their own mother queen in ways other than merely reinforcing their dominance over Sericomyrmex host workers and caring for their own brood.
Neither founding queens nor mature colonies of M. symmetochus guest ants are known to move to other host colonies later in life, implying that guest ant reproductive success is completely dependent on the continued well being (albeit not the reproduction) of host colonies (11). Thus, we conjectured that M. symmetochus guest ants might act as defenders when host colonies are attacked by natural enemies that are sufficiently effective to pose a significant threat. Such a specialized enemy, the unrelated agro-predator ant Gnamptogenys hartmani (Ectatomminae: Ectatommini) (Fig. 1C and Fig. S1A), was recently seen to raid colonies of Sericomyrmex in Panama and to usurp their gardens and nest structures with remarkable efficiency (21). This finding suggests that the enhanced mortality risk emanating from these raids might have produced an unusual secondary mutualism between the socially parasitic guest ants and their hosts. Rather than merely reducing worker production as ant social parasites normally do, the M. symmetochus guest ants produce a seemingly excess number of workers (Table S2) that constantly patrol the host nest. These guest ants’ potent alkaloid weaponry, which originally secured their establishment at a chronic cost to the host colony, potentially could also serve to protect the host from greater harm in the direct interest of the guest ant parasite.
We tested this idea in a series of controlled laboratory experiments aimed at quantifying the damage by G. hartmani agro-predator parasites and the defense efficiency of Sericomyrmex hosts with and without guest ants (SI Text: Study System). We found that hosting even a moderate number of Megalomyrmex guest ants provides almost complete protection against G. hartmani raids, because (i) guest ants are much more efficient than host ants in killing intruding G. hartmani workers; (ii) guest ants reduce host ant mortality inflicted by the raiding agro-predators; and (iii) scouts of G. hartmani preferentially recruit nestmates to Sericomyrmex host colonies whose odor indicates an absence of guest ants.
Results and Discussion
After a number of staged encounters with guest ant-infected subcolonies of S. amabilis in the laboratory, it became clear that G. hartmani scouts were immediately attacked by the M. symmetochus guest ants, and that raids were often deterred (Movies S1 and S2). Not only were G. hartmani workers killed during the altercations with the M. symmetochus defenders, but some were also attacked later by members of their own raiding party (Fig. S1B), suggesting that M. symmetochus venom is both toxic and causes confusion in G. hartmani ants. In addition, experimentally stung G. hartmani ants more often avoided contact with a naïve sister compared with controls (2.88 ± 0.72% of time spent together vs. 33.27 ± 7.01%; Welch’s t = 4.31, df = 1, P = 0.0073) (SI Text: Methods and Results and Fig. S1 C and D), but when contact was made, stung ants were often attacked (four of six replicates) and sometimes killed, indicating that M. symmetochus venom disrupts nestmate recognition abilities of G. hartmani, much like other antagonistic chemicals used by ant exploiters (2, 22).
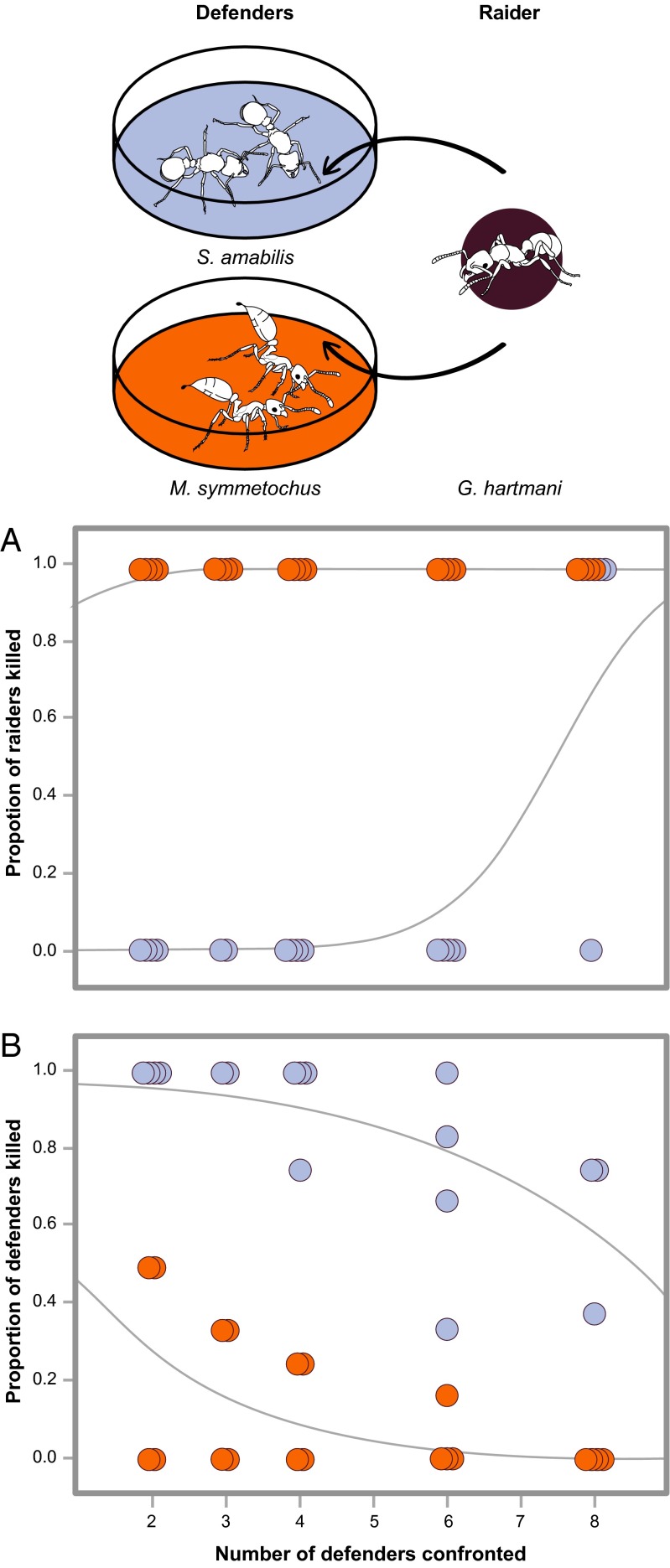
To formally investigate the efficiency of M. symmetochus and S. amabilis defenses against a single G. hartmani ant, we conducted a series of experiments with a varying number of opponents (two, three, four, six, or eight workers of S. amabilis or M. symmetochus) (Fig. 2). We found that M. symmetochus workers were much more effective than S. amabilis host workers at killing G. hartmani raiders [binomial generalized linear model (GLZ), likelihood ratio (LR) χ2 = 42.3, P < 0.0001] (Fig. 2A). Surviving S. amabilis hosts sometimes fled to the roof of the nest box to escape their predators, as reported previously by Dijkstra and Boomsma (21), and never killed the intruding G. hartmani worker in the first hour of the trial (n = 17). However, M. symmetochus workers pursued, attacked, and killed the G. hartmani raiders in 45% of 20 trials in the first hour and in 100% of 20 trials within 24 h (Fig. 2A). Furthermore, the guest ant workers were significantly less likely than the S. amabilis host workers to be killed by the G. hartmani worker (binomial GLZ, LR χ2 = 7.29, P = 0.007) (Fig. 2B). A higher number of defenders of either species was generally associated with a reduced average casualty rate (LR χ2 = 18.85, P < 0.0001), and this reduction was similar for both species (species × number interaction, LR χ2 = 0.0996, P = 0.752).
Fig. 2.
Defense efficiencies of host and guest ants. (A) The resulting mortality after a single G. hartmani agro-predator interacted with groups of two to eight S. amabilis host ants (blue Petri dish) or M. symmetochus guest ants (orange Petri dish) after 24 h. Defender category significantly affected G. hartmani worker mortality (binomial GLZ, LR χ2= 42.34, P < 0.0001), with S. amabilis effective in killing only when greatly outnumbering G. hartmani (blue dots) and M. symmetochus significantly more effective in killing regardless of their number (orange dots). (B) The overall mortality inflicted by the G. hartmani worker on host or guest ant defenders differed significantly (binomial GLZ, LR χ2 = 18.84, P < 0.0001), with S. amabilis defenders (blue dots) taking proportionally much higher casualties. The proportional mortality of both defenders decreased significantly with an increasing number of defenders (binomial GLZ, LR χ2 = 7.29, P = 0.0069), but this decrease did not differ between the defending species (interaction between number and species of defenders, binomial GLZ, LR χ2 = 0.0996, P = 0.752). Ant drawings courtesy of Rozlyn Haley.
When attacking, G. hartmani workers lock on to their S. amabilis opponents and sting repeatedly, releasing their potent nonvolatile venom (SI Text: Methods and Results). These one-to-one engagements can last from approximately 20 seconds to up to 10 min, during which time the S. amabilis defenders may use their relatively powerful mandibles to bite off legs or antennae of the G. hartmani attackers (Fig. S1A) but then feign death, prompting the G. hartmani workers to release them. This cycle can repeat itself several times before the S. amabilis workers are eventually killed. In contrast, the Megalomyrmex defensive strategy is aggressive and uses an approximate 1:1 mix of two isomers of butylhexylpyrrolizidine alkaloids (SI Text: Methods and Results) dispensed from their specialized sting as an aerosol or contact venom (Fig. 1B, SI Text: Methods and Results, and Movie S2). To evaluate the complex interactions between all three ant species, we conducted a second experiment using larger and more natural (i.e., with fungus garden fragments) subcolonies of S. amabilis with and without a variable number of guest ants in which we introduced two G. hartmani raiders.
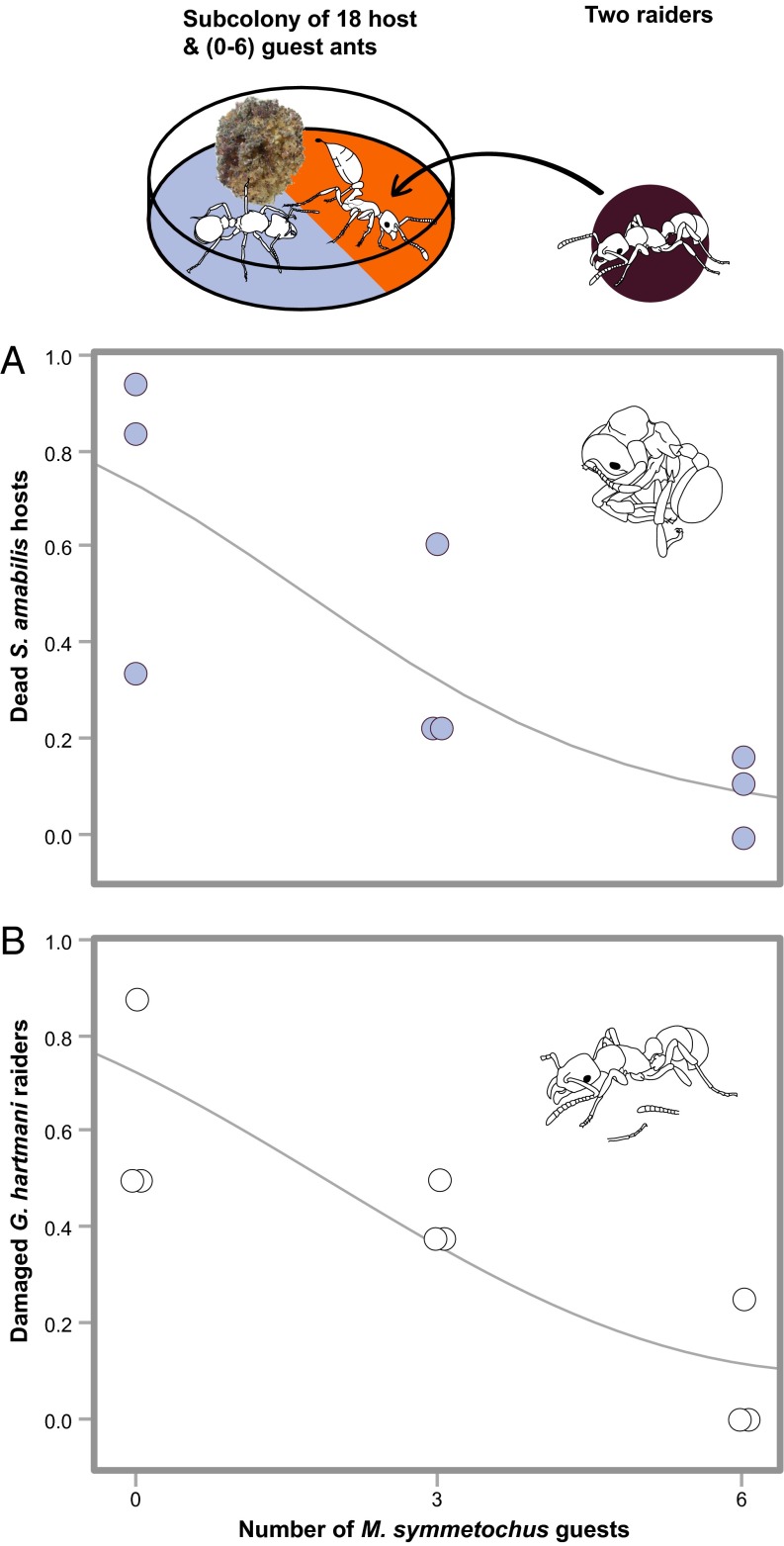
In this more complex interaction experiment, both introduced G. hartmani workers were killed in six of the nine S. amabilis subcolonies across the range of zero, three, or six M. symmetochus defenders (Fig. 3). When mortal damage was also considered (i.e., at least six of eight possible G. hartmani extremities lost), all trials left at least one G. hartmani dead or doomed after 24 h independent of the number of guest ants (binomial GLZ, LR χ2 = 0.286, P = 0.593). As in the first experiment (Fig. 2B), the G. hartmani raiders were very effective at killing a high proportion (70% on average) of the S. amabilis host workers in the absence of M. symmetochus, but as the number of M. symmetochus guest ants increased, host casualties decreased to rather low values (LR χ2 = 10.93, P = 0.0009) (Fig. 3A). This finding indicates that the numerical ratios of interacting ants used in this experiment were well balanced, such that adding defenders of each category had a noticeable effect. The external damage to G. hartmani workers (i.e., the proportion of the maximum 2 × 8 raider legs and antennae that were missing) decreased with increasing numbers of defending guest ants (LR χ2 = 14.17, P = 0.0002) (Fig. 3B), supporting behavioral observations that G. hartmani workers were indeed killed by the guest ants and not by the host ants. As in the first experiment (Fig. 2B), there was little difference in guest ant mortality between treatments (LR χ2 = 0.232, P = 0.630), indicating that as few as three M. symmetochus workers offer adequate protection to S. amabilis colonies when there are two G. hartmani intruders. This protection was even greater when the ratio of M. symmetochus to S. amabilis was increased to 1:3 (6 to 18; Fig. 3A), a ratio close to that seen in field colonies (Table S2).
Fig. 3.
Host survival rates and raider mutilations. When threatened, M. symmetochus guest ants use toxic venom, whereas Sericomyrmex hosts mutilate intruders by removing appendages. (A) When 18 host ants were confronted with 2 intruding Gnamptogenys workers without (0) or with (3 or 6) Megalomyrmex guest ants, the proportion of S. amabilis deaths was decreased (binomial GLZ, LR χ2 = 10.93, P = 0.0009). (B) The same increased number of guest ants was also associated with a reduced rate of extremity damage in G. hartmani workers (binomial GLZ, LR χ2 = 14.18, P = 0.0002), consistent with the fact that the agro-predator raiders were no longer attacked by S. amabilis defenders because they were killed by guest ants rather than by physical mutilation by the host ants. Ant drawings courtesy of Rozlyn Haley.
In the more realistic scenario of mixed subcolonies (Fig. 3), the guest ants remained more effective at killing G. hartmani raiders than their S. amabilis hosts, who needed much larger numbers to mount at least some resistance against one or two G. hartmani intruders. (Compare Fig. 2A, in which eight S. amabilis workers were able to kill a single G. hartmani raider in two out of three trials, but at the cost of 63% mortality.) However, under field conditions, scouts of G. hartmani normally return to their nest to recruit a column of nestmates before initiating a raid. This may easily involve 100 or more G. hartmani (Fig. S2), which can quickly overwhelm an S. amabilis colony (21). Thus, hosting and feeding an M. symmetochus guest ant colony likely would have substantial fitness payoffs for S. amabilis when the risk of a raid by G. hartmani is high, and these benefits would be even greater if G. hartmani colonies preferred to raid S. amabilis colonies without guest ants.
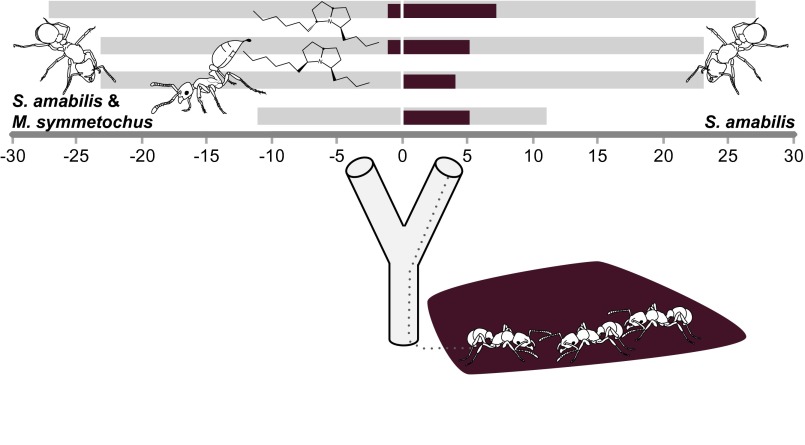
To test this possibility, we conducted a y-tube choice experiment, which showed that G. hartmani preferentially initiated raids on S. amabilis colonies without guest ants relative to control colonies with guest ants (binomial GLZ, LR χ2 = 18.12, P < 0.0001) (Fig. 4, SI Text: Methods and Results, Fig. S2, Movie S3, and Table S3). Experimental Gnamptogenys scouts were allowed minimal contact with resident S. amabilis workers through a mesh separating their colonies, and they were never observed to have physical contact with guest ants. Thus, it is likely that the volatile components of the M. symmetochus venom were the main factors deterring raids (Fig. 4). Fig. 1B depicts a worker projecting its sting to disperse two isomers of 3-butyl-5-hexylpyrrolizidine (Fig. S3); SI Text: Methods and Results provides information on chemical analysis. This finding suggests that direct contact with M. symmetochus defenders is not necessary for a G. hartmani scout to determine whether a S. amabilis colony is worth recruiting to. However, field colonies of M. symmetochus can have hundreds of workers that are spread out across all chambers of a host colony (Table S2). Furthermore, physical contact between a G. hartmani scout and an M. symmetochus defender is likely, given that M. symmetochus workers recruit nestmates from their deeper fungal cavity when an intruder is detected (Movie S2), further enhancing the prophylactic inhibition of G. hartmani raids.
Fig. 4.
A G. hartmani laboratory colony was given repeated choices between four pairs of S. amabilis host colonies with or without M. symmetochus guest ants (size-matched so that total number of ants, fungus garden volume, and nest box size were approximately equal). Gray bars represent the number of replicate trials for each pair, and dark-colored bars represent raids that were initiated after single G. hartmani scouts had inspected one or both of the maize separations with the experimental S. amabilis colonies. Recruited columns of raiding G. hartmani were preferentially directed toward S. amabilis colonies without guest ants (binomial GLZ, LR χ2 = 18.12, P < 0.0001). Chemical structures (Upper Left) represent the M. symmetochus venom compounds (5Z,8E)-3-butyl-5-hexylpyrrolizidine and (5E,8E)-3-butyl-5-hexylpyrrolizidine, detected from air samples. Ant drawings courtesy of Rozlyn Haley.
Our results confirm that socially parasitic M. symmetochus guest ants can serve as protective symbionts of their S. amabilis hosts. This development is remarkable because, despite the parasitic (i.e., maintenance) costs, the relationship between the host and guest ants has shifted to a context-dependent mutualism in which the cost to the host is compensated for by a secondary protection benefit against a shared natural enemy. It has turned an interaction governed by negative antagonistic selection into one characterized by positive reinforcement, allowing the guest ants to become unusually common (in 73% of host nests on average; SI Text: Methods and Results and Table S2). This finding reinforces the concept that mutualistic interactions are actually driven by mutual exploitation (23), and that the outcomes can be mutualistic win-win situations under certain conditions and parasitic win-lose situations in other circumstances. This variation may be a key factor in the coevolution of such interactions, and is one of the cornerstones of the geographic mosaic theory of coevolution (24, 25).
Our results suggest that M. symmetochus guest ant prevalence should be positively correlated with Gnamptogenys agro-predator density across sites, as has been shown in other protective symbionts (26, 27). We would also expect the guest ant M. adamsae and its host T. zeteki to suffer much less from Gnamptogenys raids given the typically much lower host colony infection rates (∼1–6%; ref. 11), but we lack the comparative data needed to test this possibility. This dynamic coevolutionary scenario would seem to be conditional on each of the partners being largely or fully dependent on the others, with attine ants rearing a peculiar food source for which the two parasitic ants compete, one (Gnamptogenys) as a destructive and highly virulent agro-predator and the other (Megalomyrmex) as a milder and chronic disease. However, this tripartite interaction likely owes its evolutionary stability to the milder parasite’s alkaloid weaponry, which can control the more virulent raiders without major cost. Although pyrrolizidines are not unique to M. symmetochus and have convergently evolved in other ant genera (28), our study illustrates that such alkaloids are detectable and functional in interactions with ants from a distant subfamily, the Ectatomminae (Fig. 1). Previously, ant alkaloids were considered general repellents used during competitive intraspecific interactions and thief ant raids (ref. 29 and references therein). Such broad functionality remains compatible with specific effects on the Gnamptogenys raiders as long as they remain vulnerable to these venoms.
Examining the different strategies of the two exploiters may hold the key to understanding their stable coexistence. S. amabilis sites without G. hartmani raiders would be influenced by the maintenance costs of infection with M. symmetochus guest ant parasites, which would tend to reduce S. amabilis densities and impose selection on traits that would decrease host colony susceptibility to guest ant infiltration (e.g., queen aggression, detoxifying enzymes effective against Megalomyrmex alkaloids). This would make the mildly virulent chronic guest ant rare while at the same time creating ideal conditions for the more virulent G. hartmani raiders to invade. Such population invasion would shift selection on the host to allow more frequent guest ant infiltration. Colonies with protective guest ant symbionts would then increase in the population, providing fewer attractive host colonies for G. hartmani to raid, which in turn would reduce the fitness of G. hartmani, making the agro-predators rare once again. Renewed selection against guest ant infiltrations would then be expected.
Another major factor in maintaining some form of dynamic equilibrium between the two social parasites and their shared host appears to be the life-long association of M. symmetochus with a single host colony, similar to the association between M. adamsae guest ants and their Trachymyrmex host colonies (11). This form of obligate perennial colony-level association tends to select for low virulence or prudent exploitation (30), implying that the cost to host colonies of maintaining guest ants might quickly shift to a net benefit when more virulent and mobile alternative parasites appear. The characteristics of the association between Megalomyrmex guest ants and their hosts remains fundamentally antagonistic, however (11), explaining the aggression between S. amabilis host workers and their M. symmetochus guest ants seen in both the field and the laboratory. This effect is likely driven by a window of conflict over resource allocation, because the S. amabilis host can still realize some reproductive success by producing males and thus has no interest in allowing M. symmetochus guest ant colonies to grow much beyond the number of workers needed for optimal protection.
Overall, the dynamic interactions among the three ant species studied here resemble human military history. Many medieval cities maintained contingents of mercenary soldiers in times when mobile invasive armies posed a threat, despite their maintenance costs, which quickly became prohibitive after peace treaties were signed. Thus, both M. symmetochus guest ants and human mercenaries can be considered alien soldier castes that defend against larger evils as long as they are worth their keep. Another relevant analogy is the maintenance by heterozygote advantage of sickle cell anemia as a chronic human disease in areas where virulent malaria is endemic (31), that is, a chronic disease is maintained because it makes carriers resistant to a potentially lethal disease. Thus, we would expect Sericomyrmex populations without Gnamptogenys raiders to have a lower prevalence of M. symmetochus, because this situation would select for partial resistance against invasion by guest ants.
Materials and Methods
Biological Material.
Four parasitized and four nonparasitized S. amabilis colonies, along with a single G. hartmani colony, were collected in May 2009, 2010, and 2011 near Gamboa and El Llano in the Republic of Panama (Table S1). All colonies were transferred to Copenhagen, Denmark and kept in an environmentally controlled rearing room at a constant temperature of 25 °C and relative humidity of 60–70%. Ant vouchers from all colonies used in this study are deposited at the Museum of Natural History, Smithsonian Institute, Washington, DC and at the Smithsonian Tropical Research Institute, Balboa, Republic of Panama.
Guest Ant Venom Function.
Pilot experiments were staged, introducing the G. hartmani colony into guest ant-infested S. amabilis subcolonies to establish whether guest ants exhibit defensive reactions. The observation of intracolony conflict of Gnamptogenys raiders prompted a more controlled study of pairwise interactions between two G. hartmani workers (SI Text: Methods and Results). The treatment consisted of a Megalomyrmex “stung” individual introduced to a naïve G. hartmani worker (n = 6; Fig. S1 C and D). Avoidance or attraction behavior was scored for 1 h. Control experiments were designed similarly, except that the introduced worker was rubbed with empty soft forceps rather than with a live Megalomyrmex worker stinger. The proportion of time that the workers spent in close proximity was compared using Welch’s t test (allowing for heterogeneous variances).
Defense Efficiency in Two-Species Interactions.
A single starved G. hartmani scout worker and two, three, four, six, or eight S. amabilis or M. symmetochus worker opponents (10 combinations in all) were placed in a small arena, and mortality was assessed after 24 h. The experiment was repeated using subcolonies derived from four source colonies: Mb, Mc, Md, and Me (SI Text: Methods and Results and Table S1). Mortality was analyzed using JMP version 9.02 (SAS Institute) to fit a generalized linear model with binomial errors. Opponent type and number of opponents were fitted as main effects, together with their interaction. Because the G. hartmani mortality data had quasi-complete separation, we performed Firth-adjusted maximum likelihood analysis.
Three-Species Interactions, Survival, and Mutilation.
Subcolonies consisting of 18 S. amabilis workers, a fungus garden fragment ca.1.5 cm in diameter, and zero, three, or six M. symmetochus workers were set up in medium-sized Petri dishes from three parasitized source colonies: Mb, Mc, and Md (SI Text: Methods and Results and Table S1). Two starved G. hartmani workers were introduced into each Petri dish, and ant mortality and damage to G. hartmani worker appendages were assessed after 1 h and 24 h. Mortality and damage were analyzed with JMP using generalized linear models with binomial errors, correcting for overdispersion of the data as necessary.
Raid Preference Choice: Parasitized vs. Nonparasitized.
With the use of a bifurcating olfactometer (i.e., y-tube), a laboratory colony of Gnamptogenys hartmani was given the choice of recruiting nestmates to size-matched pairs of parasitized or nonparasitized S. amabilis colonies (Fig. S2). Colony combinations (Mb+Sb, Mc+Sc, Md+Sd, and Me+Se; Table S1) were tested 11, 24, 29, and 23 times, respectively (SI Text: Methods and Results and Table S3). A mesh screen at the entrance of each S. amabilis colony allowed airflow and minimal (antennal) interactions between G. hartmani scouts and S. amabilis workers, and prohibited contact between G. hartmani scouts and M. symmetochus guest ants. Where raids occurred, their direction (accumulating G. hartmani workers on one side; Fig. S1 and Movie S3) was scored blindly from video recordings and analyzed using a generalized linear model with binomial errors to test for overall bias toward or away from parasitized nests, taking into account any differences between colony pairs. Firth-adjusted maximum likelihood estimates were used, because there were no raids on parasitized nests in two of the four pairs of colonies.
Volatile Chemical Analyses.
All three species were extracted in methanol and chemically analyzed for volatile compounds by GC-MS following established methods (32). To determine whether the venom alkaloids were dispensed in the air by M. symmetochus workers, headspace analysis using a solid-phase microextraction (SPME) fiber assembly carboxen/polydimethylsiloxane (57318 SUPELCO; Sigma-Aldrich) was conducted on a sample of eight M. symmetochus ants from colony Me (SI Text: Methods and Results). For comparison, 10 ants and a small amount of fungus garden from a nonparasitized colony (RMMA100611-03; Table S1) were analyzed as well.
Supplementary Material
Acknowledgments
We thank the staff and researchers at the Smithsonian Tropical Research Institute for help with logistics and facilities, the Autoridad Nacional del Ambiente y el Mar for permission to sample ants in Panama and export them to Denmark, Friluftsland A/S and John B. Anderson for equipment, and Rozlyn E. Haley for the ant drawings. We also thank two anonymous reviewers for comments and suggestions that improved this article. This work was funded by a Marie Curie International Incoming Fellowship [237266–evolutionAry traNsitions: Chemical Ecology of Parasitic Societies (ANCEPS), to R.M.M.A.], a Smithsonian Molecular Evolution Postdoctoral Fellowship (to R.M.M.A.), and a grant from the Danish National Research Foundation (DNRF57, to J.J.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311654110/-/DCSupplemental.
References
- 1.Lenoir A, D’Ettorre P, Errard C, Hefetz A. Chemical ecology and social parasitism in ants. Annu Rev Entomol. 2001;46:573–599. doi: 10.1146/annurev.ento.46.1.573. [DOI] [PubMed] [Google Scholar]
- 2.Akino T. Chemical strategies to deal with ants: A review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol News. 2008;11:173–181. [Google Scholar]
- 3.Guerrieri FJ, et al. Ants recognize foes and not friends. Proc Biol Sci. 2009;276(1666):2461–2468. doi: 10.1098/rspb.2008.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buschinger A. Evolution of social parasitism in ants. Trends Ecol Evol. 1986;1(6):155–160. doi: 10.1016/0169-5347(86)90044-3. [DOI] [PubMed] [Google Scholar]
- 5.Buschinger A. Social parasitism among ants: A review (Hymenoptera: Formicidae) Myrmecol News. 2009;12:219–235. [Google Scholar]
- 6.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Belknap Press of Harvard Univ Press; 1990. pp. 471–529. [Google Scholar]
- 7.Huang MH, Dornhaus A. A meta-analysis of ant social parasitism: Host characteristics of different parasitism types and a test of Emery’s rule. Ecol Entomol. 2008;33:589–596. [Google Scholar]
- 8.Brady SG, Schultz TR, Fisher BL, Ward PS. Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc Natl Acad Sci USA. 2006;103(48):18172–18177. doi: 10.1073/pnas.0605858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longino JT. A taxonomic review of the ant genus Megalomyrmex Forel (Hymenoptera: Formicidae) in Central America. Zootaxa. 2010;2720:35–58. [Google Scholar]
- 10.Adams RMM, Mueller UG, Schultz TR, Norden B. Agro-predation: Usurpation of attine fungus gardens by Megalomyrmex ants. Naturwissenschaften. 2000;87(12):549–554. doi: 10.1007/s001140050777. [DOI] [PubMed] [Google Scholar]
- 11.Adams RMM, Shah K, Antonov LD, Mueller UG. Fitness consequences of nest infiltration by the mutualist-exploiter Megalomyrmex adamsae. Ecol Entomol. 2012;37:453–462. [Google Scholar]
- 12.Brandão CRF. Systematic revision of the Neotropical ant genus Megalomyrmex Forel (Hymenoptera: Formicidae: Myrmicinae), with the description of thirteen new species. Arquivos de Zoologia. 1990;31:411–481. [Google Scholar]
- 13.Jones TH, Blum MS, Fales HM, Brandão CRF, Lattke J. Chemistry of venom alkaloids in the ant genus Megalomyrmex. J Chem Ecol. 1991;17:1897–1908. doi: 10.1007/BF00993736. [DOI] [PubMed] [Google Scholar]
- 14.Jones T, et al. Dialkylpyrrolidines from the ants Megalomyrmex cyendyra Brandão and M. latreillei Emery. Caribb J Sci. 1999;35:310–311. [Google Scholar]
- 15.Adams RMM, Longino JT. Nesting biology of the arboreal fungus-growing ant Cyphomyrmex cornutus and behavioral interactions with the social-parasitic ant Megalomyrmex mondabora. Insectes Soc. 2007;54:136–143. [Google Scholar]
- 16.Wheeler WM. A new guest-ant and other new Formicidae from Barro Colorado Island, Panama. Biol Bull. 1925;49:150–181. [Google Scholar]
- 17.Schultz TR, Brady SG. Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA. 2008;105(14):5435–5440. doi: 10.1073/pnas.0711024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nehring V, Boomsma JJ, d’Ettorre P. Wingless virgin queens assume helper roles in Acromyrmex leaf-cutting ants. Curr Biol. 2012;22(17):R671–R673. doi: 10.1016/j.cub.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Martin SJ, Jenner EA, Drijfhout FP. Chemical deterrent enables a socially parasitic ant to invade multiple hosts. Proc Biol Sci. 2007;274(1626):2717–2721. doi: 10.1098/rspb.2007.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann H, Moser J, Hunt A. The hymenopterous poison apparatus, X: Morphological and behavioral changes in Atta texana (Hymenoptera: Formicidae) Ann Entomol Soc Am. 1970;63:1552–1558. [Google Scholar]
- 21.Dijkstra MB, Boomsma JJ. Gnamptogenys hartmani Wheeler (Ponerinae: Ectatommini): An agro-predator of Trachymyrmex and Sericomyrmex fungus-growing ants. Naturwissenschaften. 2003;90(12):568–571. doi: 10.1007/s00114-003-0478-4. [DOI] [PubMed] [Google Scholar]
- 22.Thomas JA, et al. Parasitoid secretions provoke ant warfare. Nature. 2002;417(6888):505–506. doi: 10.1038/417505a. [DOI] [PubMed] [Google Scholar]
- 23.Herre EA, Knowlton N, Mueller UG, Rehner SA. The evolution of mutualisms: Exploring the paths between conflict and cooperation. Trends Ecol Evol. 1999;14(2):49–53. doi: 10.1016/s0169-5347(98)01529-8. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 25.Forde SE, Thompson JN, Bohannan BJM. Adaptation varies through space and time in a coevolving host-parasitoid interaction. Nature. 2004;431(7010):841–844. doi: 10.1038/nature02906. [DOI] [PubMed] [Google Scholar]
- 26.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science. 2010;329(5988):212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 27.Kwiatkowski M, Vorburger C. Modeling the ecology of symbiont-mediated protection against parasites. Am Nat. 2012;179(5):595–605. doi: 10.1086/665003. [DOI] [PubMed] [Google Scholar]
- 28.Jones T, Blum M, Fales H, Thompson C. (5Z, 8E)-3-Heptyl-5-methylpyrrolizidine from a thief ant. J Org Chem. 1980;45:4778–4780. [Google Scholar]
- 29.Jones TH, Blum MS, Fales HM. Ant venom alkaloids from Solenopsis and Monomorium species. Tetrahedron. 1982;38:1949–1958. [Google Scholar]
- 30.Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71(1):37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- 31.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. BMJ. 1954;1(4857):290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams RMM, et al. A comparative study of exocrine gland chemistry in Trachymyrmex and Sericomyrmex fungus-growing ants. Biochem Syst Ecol. 2012;40:91–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.