Significance
Outside of permafrost, no contiguous DNA sequences have been generated from material older than ∼120,000 y. By improving our ability to sequence very short DNA fragments, we have recovered the mitochondrial genome sequence of a >300,000-y-old cave bear from Sima de los Huesos, a Spanish cave site that is famous for its rich collection of Middle Pleistocene human fossils. This finding demonstrates that DNA can survive for hundreds of thousands of years outside of permafrost and opens the prospect of making more samples from this time period accessible to genetic studies.
Abstract
Although an inverse relationship is expected in ancient DNA samples between the number of surviving DNA fragments and their length, ancient DNA sequencing libraries are strikingly deficient in molecules shorter than 40 bp. We find that a loss of short molecules can occur during DNA extraction and present an improved silica-based extraction protocol that enables their efficient retrieval. In combination with single-stranded DNA library preparation, this method enabled us to reconstruct the mitochondrial genome sequence from a Middle Pleistocene cave bear (Ursus deningeri) bone excavated at Sima de los Huesos in the Sierra de Atapuerca, Spain. Phylogenetic reconstructions indicate that the U. deningeri sequence forms an early diverging sister lineage to all Western European Late Pleistocene cave bears. Our results prove that authentic ancient DNA can be preserved for hundreds of thousand years outside of permafrost. Moreover, the techniques presented enable the retrieval of phylogenetically informative sequences from samples in which virtually all DNA is diminished to fragments shorter than 50 bp.
Trace amounts of DNA can occasionally survive the decomposition of organic matter for long periods of time after the death of an organism. However, the retrieval of these ancient DNA molecules is severely impeded by their small size. DNA fragmentation is at least partly driven by depurination (1, 2), a continually occurring process. It is thus predicted that the degree of DNA fragmentation increases with sample age. This correlation has, in fact, been established in a recent study that analyzed samples of different ages from the same archeological sites (3), but the correlation vanishes in comparisons across different sites (4). The important role of environmental conditions, especially temperature, in DNA preservation is well recognized and reflected—for example, in the concept of thermal age (5). Unsurprisingly, permafrost environments have yielded the oldest credible records of DNA survival, including short stretches of plant and invertebrate DNA with an estimated age of up to 800,000 y that were amplified by PCR from Artic ice cores (6, 7) and the genome sequence of a 700,000-y-old horse published recently (8). More temperate environments have yielded many DNA sequences from the Holocene and the Late Pleistocene, some as old as ∼100,000 (9) or ∼120,000 y (10), but only a single study has convincingly raised the possibility of DNA survival extending far into the Middle Pleistocene outside of permafrost (11). In this study, short PCR products of ∼50 bp were retrieved from several bone samples of Middle Pleistocene cave bears from European caves, the oldest coming from the site of Sima de los Huesos (Atapuerca, Spain) and estimated to be >300,000 y old.
It is important to note that direct PCR amplification provides limited power to reconstruct sequences from short DNA fragments, because only fragments that are long enough to allow for the hybridization of two PCR primers around a stretch of informative sequence are amenable to direct amplification and sequencing. If, as in the study above (11), amplicon size is decreased to ∼50 bp, only ∼10 bp of informative sequence remain between the priming sites, which compromises the security of sequence identification while at best allowing genotyping of single nucleotide polymorphisms. In contrast, with current library-based techniques, even shorter DNA fragments can, in principle, be sequenced in their entirety because the priming sites required for amplification and sequencing are added externally by attaching artificial adaptor sequences to the molecule ends. As an additional benefit, this approach allows the determination of damage patterns unique to ancient molecules, thus providing a framework for verifying the authenticity of ancient sequences (4, 12).
The preparation of DNA libraries and high-throughput sequencing have, without doubt, greatly advanced the scope of sequence retrieval from ancient DNA in recent years, as is documented by the generation of entire genome sequences (e.g., refs. 8 and 13–17). However, the possibility remains that not all sequence information residing in ancient specimens is optimally recovered with these methods. This possibility becomes apparent when inspecting the size distributions of sequences reported from ancient DNA (e.g., refs. 3, 8, and 15), which consistently show a mode of ∼40 bp or larger. It is unclear whether the deficit of shorter molecules is due to poor preservation in ancient biological specimens or their exclusion during sample preparation. This question is of importance because it is expected that the number of DNA fragments in an ancient sample increases exponentially as length decreases and, hence, that most information resides in very short molecules (3, 8). An obvious step in which loss of short molecules is expected to occur is in library preparation, because commonly used techniques require size-selective DNA purification to remove excess adaptor molecules after ligation. However, such purification steps are absent in a single-stranded library preparation method that was recently developed to sequence the genome of an archaic Denisovan individual to high coverage (16). A direct comparison of fragment size distributions obtained from sequencing the same DNA extracts following single- and double-stranded library preparation indeed revealed an improved recovery of short sequences with the single-stranded method, but Denisovan sequences of <40 bp still remained underrepresented (16).
Here we present improvements to a widely used silica-based DNA extraction technique (18) that, in combination with single-stranded library preparation, allows ancient DNA molecules as short as 30 bp to be efficiently recovered and sequenced. We describe the results from applying these methods to a bone sample of a Middle Pleistocene cave bear (Ursus deningeri) from Sima de los Huesos, representing the same type of material for which the longest DNA survival outside of permafrost has been proposed based on genotyping 17 mitochondrial positions where Late Pleistocene cave bears differ from brown bears (Ursus arctos) and American black bears (Ursus americanus) (11). These data support the existence of a monophyletic cave bear clade, in congruence with morphological analyses, but the exact genetic relationships within this clade remain to be determined.
Results and Discussion
An Improved Ancient DNA Extraction Technique.
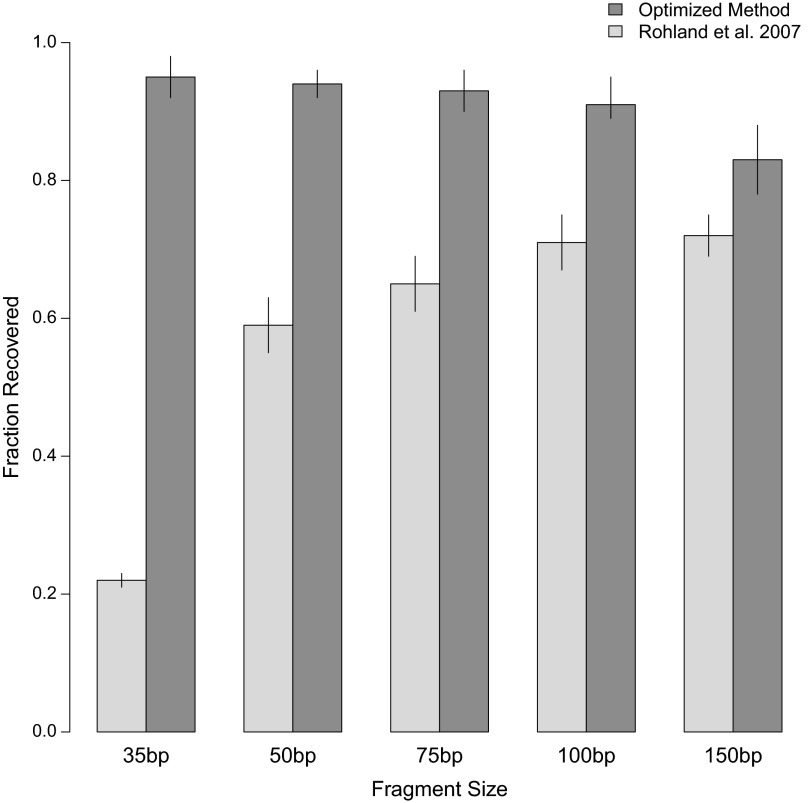
To evaluate the efficiency at which short DNA fragments are isolated from ancient samples, we first devised a simple test assay, in which we subjected a pool of synthetic DNA fragments ranging from 35 to 150 bp to DNA extraction and measured their recovery by capillary gel electrophoresis (SI Text, section 2). In this experiment we focused on a single silica-based DNA extraction method, which was optimized for ancient bones and teeth by Rohland and Hofreiter (18, 19) and has been widely applied in ancient DNA studies, including, for example, the generation of the Neandertal and Denisovan genome sequences (15, 20). In brief, this method involves the following steps. First, an extraction buffer consisting of only two reagents, EDTA and proteinase K, is used to release DNA from powdered samples. DNA is then bound to silica, which is added as a suspension together with a binding buffer containing sodium acetate, sodium chloride, and guanidine thiocyanate. Finally, the silica particles with the conjugated DNA are desalted by using an ethanol wash buffer, and the DNA is eluted into a low-salt buffer. Using the test assay, we determined that the recovery of DNA fragments is indeed length-dependent, decreasing from 72% at 150 bp to only 22% at 35 bp (Fig. 1). To ameliorate this bias, we optimized the DNA extraction process with the aim of equivalently recovering DNA fragments of all sizes. This goal was achieved by the following modifications to the original protocol: (i) the use of a binding buffer containing guanidine hydrochloride, sodium acetate, and isopropanol; (ii) an increase in the volume of binding buffer relative to that of extraction buffer; and (iii) the replacement of silica suspension with commercially available silica spin columns with a custom-adapted extension reservoir to enable large loading volumes (SI Text, section 3).
Fig. 1.
Fragment size recovery in DNA extraction. A constructed DNA ladder of sizes relevant to ancient DNA was run through a previously published extraction method (n = 2), as well as the current, optimized method (n = 4). Recovered DNA was then quantified against a control ladder. Error bars represent one standard deviation.
DNA Sequence Generation.
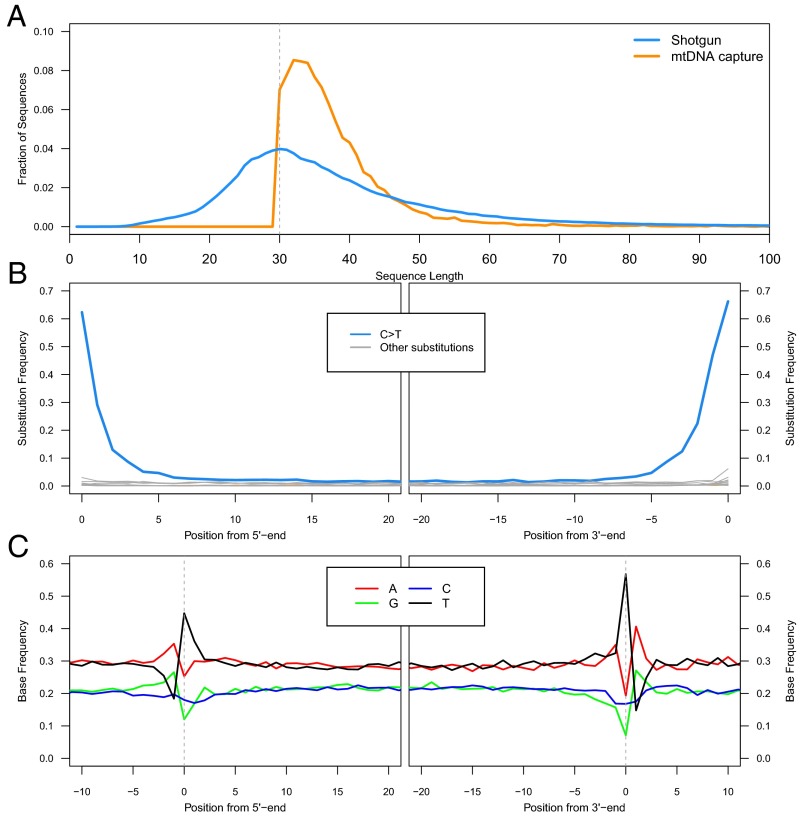
Using the optimized DNA extraction protocol, we generated DNA extracts from the U. deningeri sample (SI Text, section 1) and converted them into Illumina sequencing libraries using the single-stranded library preparation method (21). To determine the size distribution of the extracted DNA fragments, we first performed shallow shotgun sequencing for a subset of the libraries. The size distribution indeed indicates highly efficient recovery of DNA fragments ≥30 bp (Fig. 2A). Because none of the sequences aligned to the mitochondrial genome of bear, we enriched the sequencing libraries for mitochondrial sequences using hybridization capture (22, 23). Temperatures of the hybridization reaction and posthybridization wash steps were lowered to facilitate the annealing of short library molecules to the probes (SI Text, section 4). Of the sequences obtained from the enriched libraries, only a relatively small proportion (∼4%) aligned to the published mitochondrial genome sequence of a Late Pleistocene cave bear (24) (SI Text, section 5, and Dataset S1), presumably due to the lowered stringency of hybridization enrichment. Nonetheless, after duplicate removal, 19,576 uniquely mapped sequences of a length ≥30 bp were retained and analyzed further (Dataset S1). Strikingly, despite a bias toward hybridizing longer molecules (Fig. S1), 94% of the sequences are no longer than 50 bp and 76% are no longer than 40 bp, respectively (Fig. 2A). The vast majority of sequenced DNA fragments are thus in a size range that was not efficiently recovered with previous methods.
Fig. 2.
Length distributions and damage patterns. (A) Fragment length distributions of shotgun sequences (blue) and captured mitochondrial sequences (orange) as the fraction of sequences in each size bin. (B) Substitution patterns at the 5′ and 3′ ends of the aligned sequences. (C) DNA fragmentation patterns inferred from the reference base composition around alignment start and end points.
Damage Patterns.
Given the extraordinary age of the fossil (>300,000 ka), cytosine deamination is expected to have converted a large proportion of terminal cytosines to uracils (4, 8). The frequency of this conversion can be approximated as the fraction of sequences that carry a T at positions where the reference sequence carries a C. In fact, we find that 62% of terminal cytosines are deaminated to uracils at the 5′ end and 66% at the 3′ end, respectively (Fig. 2B). These numbers are higher than those reported from other cave samples (e.g., refs. 4 and 16), and even exceed the theoretical maximum of 50% that is predicted by a model where cytosine deamination occurs predominantly in single-stranded overhangs (2). However, this excess may be explained by a bias in the sampling of sequences. DNA strands with overhangs on both ends, which are on average longer, are more likely to be recovered in hybridization capture and to pass the length threshold of 30 bp. Another observation arising from this analysis is a noticeable asymmetry in the frequency of C > T substitutions between 5′ and 3′ ends. Because this pattern extends from the terminal positions into the interior of the sequence, it is unlikely to be the result of biases in library preparation but may indicate that 3′ overhangs are on average longer in ancient DNA. Upon reexamination, the same asymmetry, albeit less pronounced, is also observed with Denisovan (16) and Neandertal (21) sequences that were generated with the single-stranded library preparation method. Last, an analysis of DNA fragmentation patterns, which are inferred from the reference base composition around alignment start and end points, confirms previous observations (Fig. 2C). These include an elevation of G and A bases immediately adjacent to the aligned sequenced, a pattern thought to reflect strand breaks caused by depurination (2, 4, 25), as well as an excess of T bases at the first and last positions of the sequences, a pattern for which no satisfying explanation exists (2, 16).
Mitochondrial Sequence Assembly.
Based on the raw sequence alignments, average coverage of the mitochondrial genome is 45 (Fig. S2). However, upon inspection of the alignments, we found that some regions are covered by more than one sequence variant. BLAST searches (26) of these variants revealed the presence of contaminant sequences from human, bat, corynebacteria, and, in smaller numbers, pig and fox. We therefore eliminated all sequences showing a better alignment to any of these contaminant genomes than to cave bear (8.1% of the data). After masking damage-derived Ts from the first and last three bases of each sequence (SI Text, section 6), a consensus was called for all positions where ≥80% of the sequences agreed and coverage was at least two. For some positions with lower consensus support, manual consensus calls were made where justifiable (Table S1). The final consensus sequence covers 16,305 bp of the cave bear mitochondrial genome. Most of the missing sequence information (∼480 bp) is in the D loop, including a ∼320-bp stretch of repetitive sequence that cannot be resolved with short sequences. Outside the D loop, only five positions remain undetermined.
Phylogenetic Position of U. deningeri.
The earliest fossil evidence of cave bear-derived morphological features is found ∼1.2 Ma in Ursus dolinensis, a species that was defined in Atapuerca Gran Dolina (TD4) (27) but is also recorded at Atapuerca Trinchera Elefante (TE9) (28) and Untermassfeld (29, 30). An abundant fossil record in Europe and parts of Asia indicates that subsequent cave bear evolution proceeded through the Middle Pleistocene form U. deningeri, which transitioned into the Late Pleistocene form Ursus spelaeus sensu lato (31), before cave bears went extinct 28 ka (32). Genetic and morphological analyses support a further differentiation of three types of Late Pleistocene cave bears. The first two, U. spelaeus sensu stricto and Ursus ingressus, are predominantly found in Europe and are thought to have become reproductively isolated (33). The third type has been found only in the Caucasus and the Yana river region in Eastern Siberia and was designated U. deningeri kudarensis (34) based on its more ancestral dental morphology. It also shows a divergent mitochondrial haplotype (35, 36).
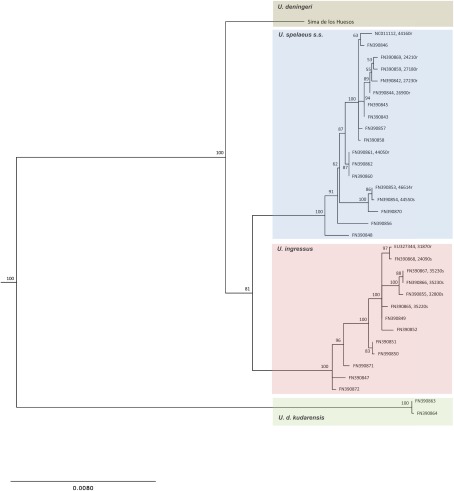
After aligning the sequence of the Sima de los Huesos specimen, which is considered a typical representative of Middle Pleistocene U. deningeri based on skeletal morphology (31), to published mitochondrial genome sequences of Late Pleistocene cave bears (24, 36, 37), we used a maximum-likelihood (ML) approach to reconstruct the phylogenetic relationships among cave bears (SI Text, section 7). The U. deningeri lineage branches off basal to the common ancestor of U. spelaeus s.s. and U. ingressus with good statistical support (Fig. 3), a result that is in line with morphological analyses. It is noteworthy, however, that the Sima de los Huesos sequence is located on a branch of substantial length. Sequences from additional specimens will therefore be needed to determine how closely the Sima de los Huesos population is related to the U. deningeri population that gave rise to Late Pleistocene cave bears. Interestingly, Late Pleistocene U. deningeri kudarensis from the Caucasus remain the most divergent cave bear lineage, further supporting the hypothesis that they may represent a separate branch of cave bear evolution.
Fig. 3.
Phylogenetic position of U. deningeri within Late Pleistocene cave bears. ML reconstruction of cave bear relationships is shown. Numbers on branches represent bootstrap support. The tree is rooted with five brown bear sequences as outgroup (branch not depicted). Age of the samples is provided in brackets where known (r and s denote radiocarbon and stratigraphic dates, respectively).
As expected due to its Middle Pleistocene origin, the cave bear sequence from Sima de los Huesos exhibits a shorter branch than any of the Late Pleistocene sequences in the tree. Combined ESR and U-series dating of two sets of cave bear bones from Sima de los Huesos have previously suggested minimum ages of 200 ka for one set and 300 ka for the other (38), but based on the macro- and microfaunal associations, an age >300 ka seems very likely for all bears in the site (39). We attempted molecular dating of the fossil via tip calibration (40) using the radio-carbon and stratigraphic dates associated with 14 of the Late Pleistocene cave bear sequences (SI Text, section 8), yielding an age estimate of 409 ka, but with poor resolution (95% confidence interval: 179–680 ka). It should also be noted that the bear sample analyzed here was found in a layer that contained hominin remains. For these, an age >530 ka has been suggested based on geological analyses (41). However, the age of the Sima de los Huesos fossils is currently being re-evaluated using additional geological data, work that will be important especially for interpreting the human fossil record.
Conclusion
We demonstrate that very short ancient DNA fragments can be efficiently extracted and sequenced and can be used for the reconstruction of contiguous, phylogenetically informative DNA sequences. The strong signal of cytosine deamination, the short size of the DNA fragments, and the unique positioning of the sample in the phylogenetic tree provide multiple lines of evidence for the authenticity of the mitochondrial genome sequence from the Sima de los Huesos cave bear, confirming that DNA can indeed survive for hundreds of thousands of years outside of permafrost (11), albeit in an extremely fragmentary state.
We note that, although the vast majority of cave bear sequences are only 30–50 bp in length, we have not yet systematically explored the lower size limit of DNA fragments surviving in ancient bone. It is therefore possible that even shorter molecules can be made available for sequencing in the future, by using library-based techniques as described here or directly via single-molecule sequencing (8, 25). However, in addition to further optimizations of the DNA extraction method, such attempts will have to include improvements to hybridization enrichment of very short molecules and the development of new sequence analysis strategies that allow for confidently aligning very short sequences to a reference genome while discriminating endogenous sequences from contaminating environmental DNA. We hope that the methodology presented here will help to retrieve ancient DNA sequences from additional organisms of the Middle Pleistocene period. The fossil remains from Sima de los Huesos will undoubtedly remain in the focus of such efforts, because they include the largest assembly of Middle Pleistocene hominin fossils in the world (42).
Materials and Methods
Sampling, DNA Extraction, and Library Preparation.
By using a dentistry drill, 1.8 g of fine powder were obtained from an U. deningeri radius (SH-01-S16-38) from Sima de los Huesos. From this powder, 19 DNA extracts were generated with the optimized extraction protocol presented here (SI Text, section 3), aliquots of which were converted into 23 DNA libraries (21). In addition, three blank controls were added during both DNA extraction and library preparation and were carried alongside the sample libraries through all subsequent steps. Libraries were amplified by PCR using AccuPrime Pfx DNA polymerase (Life Technologies) (43) following a double-indexing scheme described elsewhere (44).
Enrichment, Sequencing, and Assembly of the Mitochondrial Genome.
Mitochondrial DNA was enriched in successive experiments by capture with three different probe sets (SI Text, section 4). Briefly, the first experiment was performed by using biotinylated PCR products as probes, which were derived from amplifying the brown bear mitochondrial genome in overlapping fragments of ∼2,000 bp. To minimize sequence divergence between sample and probes, we subsequently generated a second set of shorter probes (250-300 bp) using DNA from a Late Pleistocene cave bear specimen with very good DNA preservation (24). Last, we designed a set of biotinylated oligonucleotides to close gaps in the assembly remaining after enrichment with the brown and cave bear probes. Enriched libraries were sequenced on a total of 5 MiSeq lanes (Illumina) by using recipes for double-indexed paired-end sequencing (44). Full-length molecule sequences were generated from overlapping forward and reverse sequence reads (45). Unmerged reads and those that did not perfectly match one of the expected index combinations were discarded. The remaining sequences were aligned against a published mitochondrial genome sequence of the Late Pleistocene cave bear (U. spelaeus) by using BWA (46) with the seeding turned off and allowing up to five mismatches to the reference as well as one gap opening event. After generating basic summary statistics, the sequences of all three enrichment experiments from each library were pooled, and duplicates were removed by calling a consensus from sequences with identical alignment start and end coordinates (47) (see SI Text, section 5, for further details). The mitochondrial consensus sequence was reconstructed from these sequences as detailed in SI Text, section 6 and has been deposited in the GenBank database (accession no. KF437625). Sequences obtained from the extraction and library preparation blanks show no evidence of contamination with exogenous bear DNA (SI Text, section 5).
Phylogenetic Reconstructions and Molecular Dating.
A multiple sequence alignment was generated by using MAFFT (48). The alignment consisted of 33 published mitochondrial sequences of Late Pleistocene cave bears, the Sima de los Huesos U. deningeri sequence, and five brown bear mitochondrial genomes (HQ685901, HQ685919, HQ685926, HQ685945, and HQ685955) corresponding to five European brown bear haplotypes identified by Keis (49). Because the published data contains missing information, phylogenetic analyses were restricted to 9,592 bp where base calls have been made for all samples. Details of the phylogenetic reconstruction and molecular dating are available in SI Text, sections 7 and 8 (Table S2).
Supplementary Material
Acknowledgments
We thank Qiaomei Fu, Ignacio Martínez, and Bence Viola for helpful discussions; Ayinuer Aximu, Barbara Höber, and Barbara Höffner for performing the sequencing runs; Janet Kelso, Gabriel Renaud, and Udo Stenzel for bioinformatic support; Christoph Kantzke for help in the laboratory; and Torsten Blass for help with R. This work was supported by the Max Planck Society and Ministerio de Ciencia e Innovacion Grant CGL2009-12703-C03-03.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. KF437625).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314445110/-/DCSupplemental.
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Briggs AW, et al. Patterns of damage in genomic DNA sequences from a Neandertal. Proc Natl Acad Sci USA. 2007;104(37):14616–14621. doi: 10.1073/pnas.0704665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allentoft ME, et al. (2012) The half-life of DNA in bone: Measuring decay kinetics in 158 dated fossils. Proc Biol Sci 279(1748):4724-4733. [DOI] [PMC free article] [PubMed]
- 4.Sawyer S, Krause J, Guschanski K, Savolainen V, Pääbo S. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PLoS ONE. 2012;7(3):e34131. doi: 10.1371/journal.pone.0034131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith CI, Chamberlain AT, Riley MS, Stringer C, Collins MJ. The thermal history of human fossils and the likelihood of successful DNA amplification. J Hum Evol. 2003;45(3):203–217. doi: 10.1016/s0047-2484(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 6.Willerslev E, et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300(5620):791–795. doi: 10.1126/science.1084114. [DOI] [PubMed] [Google Scholar]
- 7.Willerslev E, et al. Ancient biomolecules from deep ice cores reveal a forested southern Greenland. Science. 2007;317(5834):111–114. doi: 10.1126/science.1141758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlando L, et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature. 2013;499(7456):74–78. doi: 10.1038/nature12323. [DOI] [PubMed] [Google Scholar]
- 9.Orlando L, et al. Revisiting Neandertal diversity with a 100,000 year old mtDNA sequence. Curr Biol. 2006;16(11):R400–R402. doi: 10.1016/j.cub.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Lindqvist C, et al. Complete mitochondrial genome of a Pleistocene jawbone unveils the origin of polar bear. Proc Natl Acad Sci USA. 2010;107(11):5053–5057. doi: 10.1073/pnas.0914266107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdiosera C, et al. Typing single polymorphic nucleotides in mitochondrial DNA as a way to access Middle Pleistocene DNA. Biol Lett. 2006;2(4):601–603. doi: 10.1098/rsbl.2006.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause J, et al. A complete mtDNA genome of an early modern human from Kostenki, Russia. Curr Biol. 2010;20(3):231–236. doi: 10.1016/j.cub.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 13.Miller W, et al. Sequencing the nuclear genome of the extinct woolly mammoth. Nature. 2008;456(7220):387–390. doi: 10.1038/nature07446. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen M, et al. Ancient human genome sequence of an extinct Palaeo-Eskimo. Nature. 2010;463(7282):757–762. doi: 10.1038/nature08835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green RE, et al. A draft sequence of the Neandertal genome. Science. 2010;328(5979):710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer M, et al. A high-coverage genome sequence from an archaic Denisovan individual. Science. 2012;338(6104):222–226. doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller A, et al. New insights into the Tyrolean Iceman’s origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698. doi: 10.1038/ncomms1701. [DOI] [PubMed] [Google Scholar]
- 18.Rohland N, Hofreiter M. Ancient DNA extraction from bones and teeth. Nat Protoc. 2007;2(7):1756–1762. doi: 10.1038/nprot.2007.247. [DOI] [PubMed] [Google Scholar]
- 19.Rohland N, Hofreiter M. Comparison and optimization of ancient DNA extraction. Biotechniques. 2007;42(3):343–352. doi: 10.2144/000112383. [DOI] [PubMed] [Google Scholar]
- 20.Reich D, et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature. 2010;468(7327):1053–1060. doi: 10.1038/nature09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gansauge MT, Meyer M. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat Protoc. 2013;8(4):737–748. doi: 10.1038/nprot.2013.038. [DOI] [PubMed] [Google Scholar]
- 22.Maricic T, Whitten M, Pääbo S. Multiplexed DNA sequence capture of mitochondrial genomes using PCR products. PLoS ONE. 2010;5(11):e14004. doi: 10.1371/journal.pone.0014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Q, et al. DNA analysis of an early modern human from Tianyuan Cave, China. Proc Natl Acad Sci USA. 2013;110(6):2223–2227. doi: 10.1073/pnas.1221359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause J, et al. Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary. BMC Evol Biol. 2008;8:220. doi: 10.1186/1471-2148-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlando L, et al. True single-molecule DNA sequencing of a pleistocene horse bone. Genome Res. 2011;21(10):1705–1719. doi: 10.1101/gr.122747.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia N, Arsuaga JL. Ursus dolinensis: A new species of early pleistocene ursid from Trinchera Dolina, Atapuerca (Spain) Cr Acad Sci Ii A. 2001;332(11):717–725. [Google Scholar]
- 28.Carbonell E, et al. The first hominin of Europe. Nature. 2008;452(7186):465–469. doi: 10.1038/nature06815. [DOI] [PubMed] [Google Scholar]
- 29. Garcia N (2004) New results on the remains of Ursidae from Untermassfeld: Comparisons with Ursus dolinensis from Atapuerca and other early and middle Pleistocene sites. Late Neogene and Quaternary Biodiversity and Evolution: Regional Developments and Interregional Correlations Conference, 18th International Senckenberg Conference, Weimar, Germany, eds Kahlke R-D, Maul LC, Mazza PP (Terra Nostra, Schriften der Alfred-Wegener-Stiftung, Berlin), pp 112–113.
- 30. Kahlke R-D (2006) Untermassfeld: A Late Early Pleistocene (Epivillafranchian) Fossil Site Near Meiningen (Thuringia, Germany) and its Position in the Development of the European Mammal Fauna (Archaeopress, Oxford)
- 31.García N, Arsuaga JL, Torres T. The carnivore remains from the Sima de los Huesos Middle Pleistocene site (Sierra de Atapuerca, Spain) J Hum Evol. 1997;33(2-3):155–174. doi: 10.1006/jhev.1997.0154. [DOI] [PubMed] [Google Scholar]
- 32.Pacher M, Stuart AJ. Extinction chronology and palaeobiology of the cave bear (Ursus spelaeus) Boreas. 2009;38(2):189–206. [Google Scholar]
- 33.Hofreiter M, et al. Evidence for reproductive isolation between cave bear populations. Curr Biol. 2004;14(1):40–43. doi: 10.1016/j.cub.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 34. Baryshnikov G (1998) Cave bears from the Paleolithic of the Greater Caucasus. Quaternary Paleozoology in the Northern Hemisphere, eds Saunders JJ, Styles BW, Baryshnikov GF (Illinois State Museum, Springfield, IL), pp 69–118.
- 35.Knapp M, et al. First DNA sequences from Asian cave bear fossils reveal deep divergences and complex phylogeographic patterns. Mol Ecol. 2009;18(6):1225–1238. doi: 10.1111/j.1365-294X.2009.04088.x. [DOI] [PubMed] [Google Scholar]
- 36.Stiller M, Knapp M, Stenzel U, Hofreiter M, Meyer M. Direct multiplex sequencing (DMPS)—a novel method for targeted high-throughput sequencing of ancient and highly degraded DNA. Genome Res. 2009;19(10):1843–1848. doi: 10.1101/gr.095760.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bon C, et al. Deciphering the complete mitochondrial genome and phylogeny of the extinct cave bear in the Paleolithic painted cave of Chauvet. Proc Natl Acad Sci USA. 2008;105(45):17447–17452. doi: 10.1073/pnas.0806143105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bischoff JL, et al. Geology and preliminary dating of the hominid-bearing sedimentary fill of the Sima de los Huesos Chamber, Cueva Mayor of the Sierra de Atapuerca, Burgos, Spain. J Hum Evol. 1997;33(2-3):129–154. doi: 10.1006/jhev.1997.0130. [DOI] [PubMed] [Google Scholar]
- 39.Garcia N, Arsuaga JL. The Sima de los Huesos (Burgos, northern Spain): Palaeoenvironment and habitats of Homo heidelbergensis during the Middle Pleistocene. Quat Sci Rev. 2011;30(11-12):1413–1419. [Google Scholar]
- 40.Shapiro B, et al. A Bayesian phylogenetic method to estimate unknown sequence ages. Mol Biol Evol. 2011;28(2):879–887. doi: 10.1093/molbev/msq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bischoff JL, et al. High-resolution U-series dates from the Sima de los Huesos hominids yields: Implications for the evolution of the early Neanderthal lineage. J Archaeol Sci. 2007;34(5):763–770. [Google Scholar]
- 42.Arsuaga JL, et al. Sima de los Huesos (Sierra de Atapuerca, Spain). The site. J Hum Evol. 1997;33(2-3):109–127. doi: 10.1006/jhev.1997.0132. [DOI] [PubMed] [Google Scholar]
- 43.Dabney J, Meyer M. Length and GC-biases during sequencing library amplification: A comparison of various polymerase-buffer systems with ancient and modern DNA sequencing libraries. Biotechniques. 2012;52(2):87–94. doi: 10.2144/000113809. [DOI] [PubMed] [Google Scholar]
- 44.Kircher M, Sawyer S, Meyer M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 2012;40(1):e3. doi: 10.1093/nar/gkr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kircher M, Heyn P, Kelso J. Addressing challenges in the production and analysis of Illumina sequencing data. BMC Genomics. 2011;12:382. doi: 10.1186/1471-2164-12-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kircher M. Analysis of high-throughput ancient DNA sequencing data. Methods Mol Biol. 2012;840:197–228. doi: 10.1007/978-1-61779-516-9_23. [DOI] [PubMed] [Google Scholar]
- 48.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keis M (2013) Brown bear (Ursus arctos) phylogeography in northern Eurasia. Doctoral dissertation (Univ of Tartu, Tartu, Estonia)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.