Significance
A simple strategy is described to discover cyclin dependency of general cell cycle regulatory kinase cyclin-dependent kinase 1 for substrates in vivo. γ-Tubulin is discovered to be a specific B cyclin Clb3–cyclin-dependent kinase 1 substrate. The strategy can link proteins to specific phases of the cell cycle, and it can be used to determine subunit dependencies for other posttranslational modifying enzymes.
Keywords: in vivo enzyme complexes screen, cyclin specificity
Abstract
Cyclin-dependent kinases (Cdks) are regulatory enzymes with temporal and spatial selectivity for their protein substrates that are governed by cell cycle-regulated cyclin subunits. Specific cyclin–Cdk complexes bind to and phosphorylate target proteins, coupling their activity to cell cycle states. The identification of specific cyclin–Cdk substrates is challenging and so far, has largely been achieved through indirect correlation or use of in vitro techniques. Here, we use a protein-fragment complementation assay based on the optimized yeast cytosine deaminase to systematically identify candidate substrates of budding yeast Saccharomyces cerevisiae Cdk1 and show dependency on one or more regulatory cyclins. We identified known and candidate cyclin dependencies for many predicted protein kinase Cdk1 targets and showed elusory Clb3–Cdk1-specific phosphorylation of γ-tubulin, thus establishing the timing of this event in controlling assembly of the mitotic spindle. Our strategy can be generally applied to identify substrates and accessory subunits of multisubunit protein complexes.
A central problem in biology is determining the functions of individual enzyme subunits and the synergistic relationships among them. For example, enzymes that perform posttranslational modifications on proteins, such as the ubiquitin ligases, protein kinases and protein phosphatases, require different subunits to perform multiple transfer steps, assure specific subcellular localization, or provide additional specificity to substrate recognition (1–4). Cyclin-dependent kinases (Cdks) are a case in point, and the budding yeast Saccharomyces cerevisiae Cdk1 (also called Cdc28) is a very well-studied example of an enzyme of this category (5). Cdk1 requires the association of one of nine available cyclin partner proteins to recognize and phosphorylate its substrates (6, 7). The different Cdk1–cyclin complexes play critical roles in orchestrating the temporal and spatial ordering of events from initiation of the G1 transcriptional program (Cln1, -2, and -3) to DNA replication (Clb5 and -6), spindle assembly (Clb3 and -4), and mitosis (Clb1 and -2) (8).
The crucial role of Cdk1 in cell cycle regulation has prompted several extensive or proteome-wide studies to identify Cdk1 substrates or cyclin targets (9–12). To date, no experimental approach has captured interactions between Cdk1 and its substrates and the dependency of this interaction on one or more cyclins in the context of a living cell. In this study, we describe an approach that captures direct interactions between Cdk1 and its substrates and reveals the dependency of this interaction on one or more cyclins in living cells.
We devised a simple in vivo screening strategy to both identify potential Cdk1 substrates and establish dependencies of the Cdk1 interactions with these substrates on specific cyclins using the optimized yeast S. cerevisiae prodrug-converting enzyme cytosine deaminase protein-fragment complementation assay (OyCD PCA) (Fig. 1) (13). The OyCD PCA consists of two complementary N- and C-terminal fragments (OyCD-F[1] or F[2]) of the yCD gene (FCY1) fused individually to two genes of interest and performed in an FCY1Δ deletion strain (13). If the proteins of the two protein–OyCD fusion fragments interact, OyCD refolds from its complementary fragments, reconstituting its enzymatic activity. The assay provides two potential outputs: positive growth under cytosine-limited conditions or no growth when cells are treated with a yCD-specific prodrug called 5-fluorocytosine (5-FC) (13). Positive selection assays depend on careful control of colony density, number of cells plated, and composition of media (14). Here, we exploited the 5-FC negative selection assay of the OyCD PCA to provide sensitive detection of Cdk1 interactions as well as their cyclin dependence. First, we performed the OyCD screen with Cdk1 as bait and potential substrates as prey in the negative selection assay. Second, we retested the observed Cdk1–prey interactions in strains in which individual cyclin genes were deleted.
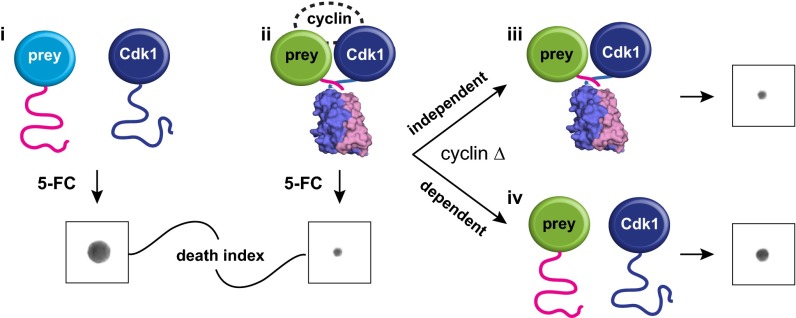
Fig. 1.
Dissecting Cdk1 complexes using the OyCD PCA. Detecting the interaction between Cdk1 and a protein of interest using the OyCD PCA. Cdk1 and proteins of interest (prey proteins) are fused to OyCD fragments. In the death selection OyCD PCA, (i) the absence of an interaction fails to allow the OyCD reporter enzyme to fold and restore the activity of the native enzyme. Cells expressing these fusion proteins are resistant to 5-FC. (ii) If prey protein interacts with Cdk1, cells are sensitive to 5-FC. When a cyclin gene is deleted (cyclin Δ), (iii) a prey protein can still interact with Cdk1, allowing the reporter fragments to fold; consequently, cells are sensitive to 5-FC. The prey protein–Cdk1 interaction is independent of any cyclin. (iv) If the interaction is cyclin-dependent, the absence of a specific cyclin results in partial or total resistance to 5-FC.
We could predict four potential outcomes for the screens (Fig. 1). In a primary screen, (Fig. 1, i) if Cdk1 does not interact with a prey protein in the WT strain (Fig. 1, i), cells are insensitive to 5-FC (colony growth). (Fig. 1, ii) However, if there is an interaction, OyCD PCA activity is reconstituted, and cells become sensitive to 5-FC (no colony growth). In the second screen of Cdk1–prey interactions in individual cyclin deletion strains, (Fig. 1, iii) if none of the cyclins were essential to the interaction, we would expect identical results to those observed in the WT strain (no growth in cells treated with 5-FC). (Fig. 1, iv) In cases where the Cdk1–prey interaction depends on one or more than one cyclin, we would expect to see restoration of total or partial growth when a certain cyclin knockout (KO) strain is grown on 5-FC.
Results
Identifying Cdk1 Complexes in Vivo.
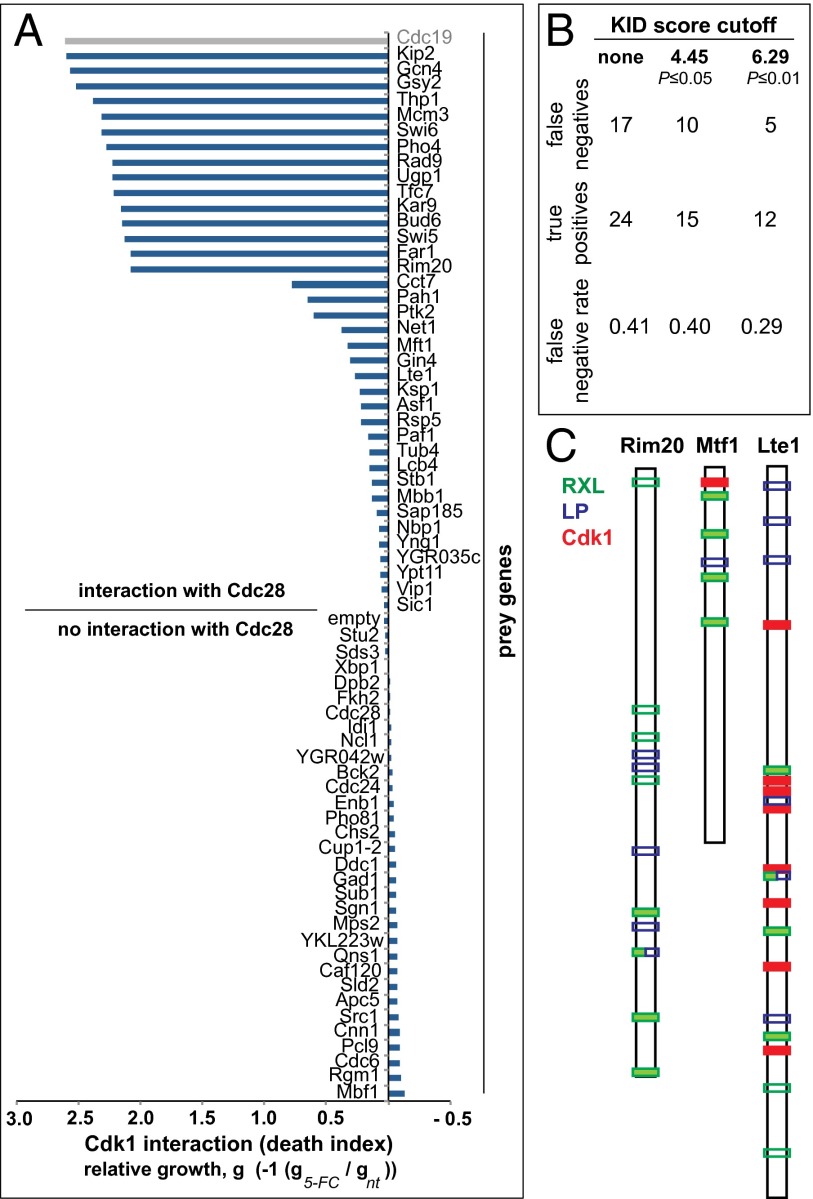
We selected 94 known or potential Cdk1 substrates from the literature or randomly chosen proteins as controls (9, 15). These proteins included those involved in transcription, cell cycle regulation, and mitotic spindle assembly. Of 94 candidates, we successfully generated OyCD PCA expression vectors of 68 candidate ORFs fused to OyCD-F[1]. Expression plasmids were cotransformed into the FCY1Δ deletion strain with Cdk1 fused to the complementary OyCD F[2] or a control plasmid that only expresses the OyCD-F[2]. We performed the OyCD PCA by inoculating two colonies from each transformation and growing them on medium with or without 5-FC at a concentration of 1 mg/mL; 38 of 68 preys showed a positive interaction with Cdk1–OyCD-F[2] (Fig. 2A and Dataset S1). One prey-expressing strain (Cdc19) among thirty-eight strains gave a false-positive signal when expressed with the fragment OyCD-F[2] alone (indicated in gray in Fig. 2A) and was not considered further.
Fig. 2.
Identification of interaction partners of Cdk1. (A) Quantification of Cdk1–prey protein interactions. Death index is calculated as the log10 of the ratio of pixel mean intensities of 5-FC–treated divided by untreated colonies (5-FC/not treated). The Cdk1–Cdc19 interaction was found to be a technical false positive (see text). (B) Estimation of the FNR for 37 proteins identified to interact with Cdk1 by OyCD PCA (details of calculation in SI Text). (C) Examples of Cdk1 interacting partners with cyclin binding motifs and with (Lte1 and Mft1) or without (Rim20) consensus Cdk1 phosphorylation sites. Filled bars are high-quality sites (P ≤ 0.01, open bars are low-quality sites).
We used the Kinase Interaction Database (KID) (16) as a gold standard to calculate false negatives (FNs) and true positives (TPs) in 37 prey proteins that interacted with Cdk1 in the OyCD PCA, and in this way, we estimated the false-negative rate [FNR; FNR = FN/(TP + FN)] to be 29% for the most significant KID scores (KID score for P ≤ 0.01) (Fig. 2B and SI Text). Twenty-two of thirty-seven preys were previously identified as Cdk1 substrates, and the remaining fifteen preys have not been linked to a specific cyclin–Cdk1 complex (9, 10, 17, 18). Of 15 unique interactions observed, 13 of the prey had full (S/T-P-X-K/R) or minimal (S/T-P) Cdk1 consensus sites, two criteria previously used to define likely Cdk1 substrates (Fig. 2B and Table S1) (9, 10). One of these candidates, Rim20, does not have a full or minimal Cdk1 consensus site but has four high-quality cyclin binding motifs [RXL; P ≤ 0.01, Eukaryotic Linear Motif (ELM) database] and five LP motifs that have been previously implicated in G1 cyclin–substrate binding in budding yeast (19). Rim20 is a regulator of Ime2, a protein kinase involved in activating meiosis (20). Rim20 resembles cyclin–Cdk inhibitors, such as Sic1 and p27Kip1, and has one or more RXL cyclin binding motifs (19, 21, 22). More typically, proteins contained various numbers of G1 and B-type cyclin binding motifs and minimal Cdk1 phosphorylation motifs (Table S1). For example, Mft1, a protein involved in mitotic recombination (23), has five cyclin binding motifs (four RXL and one LP) and one minimal Cdk1 site. Lte1, a spindle-positioning checkpoint protein that regulates the Ras-like small GTPase Tem1 (24), has many sites, including 6 RXL, 5 LP, and 8 full and 20 minimal Cdk1 sites. Phosphorylation of Lte1 by Cdk1 regulates the transition from apical to isotropic growth (25).
Cyclin Dependency of Cdk1 Complexes.
We next tested whether the interactions between Cdk1 and prey were contingent on a particular cyclin. We performed the OyCD PCA in nine cyclin deletion strains (cln1-3 and clb1-6) for 21 of 37 proteins that interact with Cdk1 (Fig. 3A, Fig. S1, and Table S1). As a positive control, to assure that none of the cyclin deletion strains affected the performance of the OyCD PCA, we tested a constitutive homomeric GCN4 leucine zipper-forming peptide (Zip) interaction with the OyCD PCA in all the different strains. As a negative control, we tested for the activity of OyCD PCA in the different strains expressing Cdk1–OyCD-F[2] alone. All results were evaluated by taking the ratio of integrated colony intensity of each sample grown on selection medium with 1 mg/mL 5-FC divided by the intensity of colonies grown on selection medium without 5-FC.
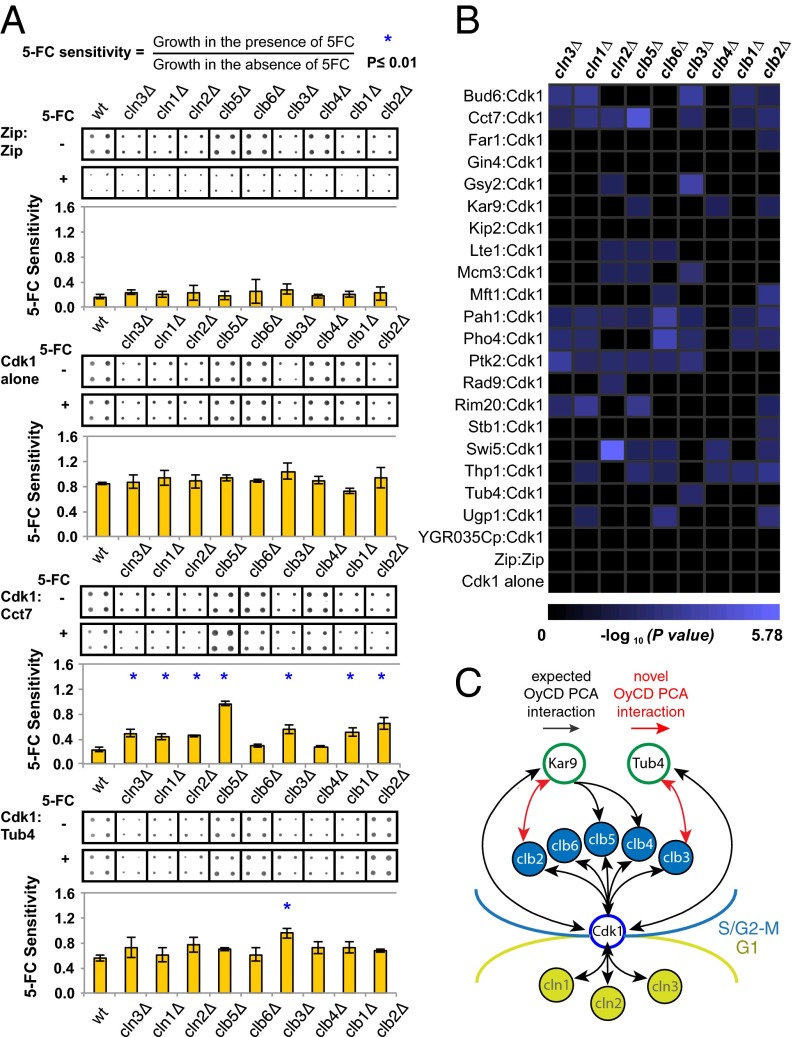
Fig. 3.
Cyclin dependence of Cdk1–prey protein interactions. (A) Quantification of cyclin dependence. The overexpression of some fusion genes slightly affects the fitness of the yeast strain compared with yeast expressing only Cdk1 or Zip:Zip fused to OyCD fragments. The deletion of some cyclin genes affects the ability of the strain to grow compared with the WT strain. The effect of the OyCD PCA activity on growth is relatively constant in the different cyclin deletion strains (mean ± SD). Statistical significance was assessed using Student t test. P = 0.04 was obtained for WT and cln3Δ strains expressing Zip:Zip interaction. P = 0.02 was obtained for WT and clb1Δ deletion strains expressing Cdk1 alone (contingency of the Cdk1 complexes). The gene of interest and Cdk1 fused to OyCD fragments were transformed into the FCY1Δ deletion strain, which is referred to as the WT, as well as in nine different cyclins and FCY1Δ double deletion strains, which are represented by their gene name in italic followed by a Δ (e.g., cln1Δ). Four colonies of each transformation were assayed for OyCD PCA activity in the presence of 1 mg/mL 5-FC in three different experiments. The growth of each sample was quantified using ImageJ. Only the results of one set of a quadruple experiment are represented. All strains expressing only Cdk1–OyCD-F[2] (Cdk1 alone) are resistant to the 5-FC death selection assay with P < 0.02. All strains expressing the GCN4 leucine zipper domains (Zip:Zip) fused to the OyCD fragments are sensitive to 5-FC in the death selection assay with a P < 0.04. A loss of interaction detected in the different cyclin deletion strains is depicted with corresponding P value. P ≤ 0.01 was used as a cutoff for this experiment (indicated with blue asterisk). (B) Results for P ≤ 0.01 are represented on the matrix (44). (C) Summary of previously described (black arrows) and discovered (red arrows) interactions between Cdk1–cylin complexes, Kar9, and Tub4 and timing of their functions in the mitotic cell cycle.
We observed that the overexpression of Cdk1 combined with some of the proteins (Thp1, Bud6, and Gin4) did affect the growth of the yeast strains compared with control strains overexpressing Cdk1 alone or the Zip:Zip complex (Fig. S1 and Dataset S2). We also found that some cyclin deletion strains grew poorly compared with the WT strain (e.g., CLN1-3 and CLB1-3) (Fig. 3A). However, the effect of Cdk1 overexpression affected the WT and cyclin deletion strains uniformly and did not affect the OyCD PCA activity for all of the different yeast strains (Fig. 3A and Fig. S1). Overall, the effect of the OyCD PCA activity was dominant over the effect of strain variability as well as over the effect of overexpression of the two genes of interest.
We noted that we did not observe complete loss of 5-FC sensitivity for a Cdk1–prey protein interaction in any of the cyclin deletion strains compared with a negative control strain expressing only Cdk1–OyCD-F[2]. Among the potential reasons for this difference is that a unique cyclin was not responsible for the Cdk1–prey protein interaction in any of the cases studied here. Other cyclins could, thus, compensate for the deleted one. Also, residual binding of Cdk1 to prey proteins may always occur, despite deletion of specific cyclins. Finally, other proteins may also contribute to observed Cdk1–prey protein binding.
To compare the activity of the OyCD PCA of each interaction in the 10 different yeast strains (WT or cyclin null), a Student t test was performed using the OyCD PCA activity obtained. For the Zip:Zip control, we observed minor differences in growth in the different strains compared with the WT strain, but the strain background did not significantly affect results compared with the assay in any case (Student t test, P < 0.04). We did not observe significant differences among strain results for a negative control Cdk1–OyCD-F[2] expressed alone (Student t test, P < 0.02). We considered results with a P ≤ 0.01 to be significant, because this cutoff is more stringent than the lowest P value for the PCA activity of cells expressing the Cdk1 alone control in the CLB1Δ deletion strain (Fig. 3 A and B and Fig. S1). Sensitivity and specificity of the test were calculated to be between 67% and 100% and 60% and 63%, respectively, depending on reference data used (SI Text).
Among 21 preys that interacted with Cdk1, three proteins (Gin4, Kip2, and YGR035C) remained unchanged in all of the cyclin deletion strains with respect to the WT strain, similar to the Zip:Zip interaction. The interaction between Cdk1 and the remaining 18 preys decreased in one or more cyclin deletion strains (decreased sensitivity to 5-FC). For example, growth of yeast expressing the γ-tubulin ortholog Tub4 and Cdk1 increased when the CLB3 gene was deleted alone. We observed the same decrease in sensitivity to 5-FC for Far1 and Stb1 in the CLB2Δ deletion strain and Rad9 in the CLN2Δ deletion strain. In contrast, the majority of interactions between Cdk1 and prey were dependent on more than one cyclin. In some cases, multiple cyclin interactions are consistent with previous findings; for example, the Kar9–Cdk1 interaction was dependent on CLB2, CLB4, and CLB5. Likewise, the interaction between Rim20 and Cdk1 was dependent on G1, S-phase, and mitotic cyclins: CLN3, CLN1, CLB5, and CLB2. Efficient targeting by G1 cyclin–Cdk1 protein complexes (Cln1, -2, and -3) is promoted by LP motifs, five of which are clustered among the four RXL motifs located in the carboxyl terminal of Rim20. Indeed, all of the proteins with interaction with Cdk1 that depend on G1 cyclins (Rim20, Lte1, Bud6, Swi5, Cct7, Ugp1, Gsy2, Pah1, Rad9, Mcm3, Thp1, Ptk2, and Pho4) contain both canonical RXL cyclin binding motifs as well as LP motifs (Table S1), the latter residing within a patch of hydrophobic residues as previously described (26). Finally, the distribution of cyclins associated with Lte1 is consistent with its phosphorylation and binding partners during the cell cycle. Phosphorylation of Lte1 during G2/M relocalizes the protein from the bud tip isotropically over the cortex of the bud, thereby inhibiting polarized growth as cells progress to mitosis. This inhibition is achieved through phosphorylation of Lte1 by the PAK kinase Cla4, which requires G1 cyclin activity and subsequent, hyperphosphorylation by Cdk1 in S/G2 (25). The association of Lte1 with both G1 and S/G2 cyclins is consistent with its multiple roles (control of polarization and spindle positioning) during the transition from S phase to mitosis.
γ-Tubulin Is an in Vivo Target of Cdk1–Clb3.
Our results provide specific molecular mechanistic insights into regulation of the function of the mitotic spindle, which has numerous component proteins that are Cdk1 targets (Fig. 3C) (10). Cdk1 promotes early spindle positioning to the bud neck by driving the asymmetric distribution of Kar9 to the old spindle pole and associated cytoplasmic microtubules (27). Interaction and phosphorylation of Kar9 is mediated by several cyclins, including Clb3, Clb4, and Clb5 (27, 28). Our finding that Kar9 binds to Clb2 could arise from the close association of Kar9, dynein, and dynactin at the positive ends of cytoplasmic microtubules associated with cortical actin (29). In addition, we found that the evolutionarily conserved microtubule nucleator γ-tubulin, Tub4 in budding yeast, is a target of Clb3–Cdk1 but not the other S-phase and mitotic cyclin–Cdk1 complexes Clb1 and -2 and Clb4, -5, and -6. This finding suggests that Tub4 regulation could be temporally coupled with but stay distinct from the Clb4–Cdk1 regulation that enforces Kar9 asymmetry during spindle assembly, which is concomitant with spindle orientation perpendicular to the future plane of cytokinesis at the bud neck (27) (Fig. 3C).
Tub4 S360, which is in a conserved Cdk1 recognition motif, was previously shown to be a mitotic target in a phosphopeptide analysis of highly purified spindle pole bodies (SPBs) (18). Phosphomimetic mutations in S360 (S360D/E) caused defects in spindle function. Keck et al. (18) showed that S360 is specifically phosphorylated in vitro by Clb2–Cdk1. Clb2–Cdk1, however, is the most promiscuous cyclin–Cdk1 complex, because it does not require a cyclin docking motif and thus, can phosphorylate any minimal (S/TP) Cdk1 recognition motif (26). Furthermore, the SPBs used for the phosphopeptide analysis were isolated under a metaphase arrest condition produced by depletion of the anaphase promoting complex (APC)-activating protein Cdc20, in which all B-type cyclins (Clb1 -2, -3, -4, -5, and -6) are expected to be active (30, 31). It was, thus, not possible in that study to unambiguously identify which cyclin–Cdk1 complex was specific for Tub4 S360 phosphorylation. Knowledge of the cell cycle timing and therefore, the one of six possible cyclin–Cdk1 complexes that phosphorylates S360 is critical to understanding when and how γ-tubulin phosphorylation contributes to spindle function. Our evidence suggests that it is Clb3, a B cyclin that accumulates during S phase and spindle assembly (Fig. 4A), which is an important candidate Cdk1 partner for recognizing and phosphorylating γ-tubulin. We, thus, decided to test whether Clb3–Cdk1 could specifically phosphorylate γ-tubulin in vitro.
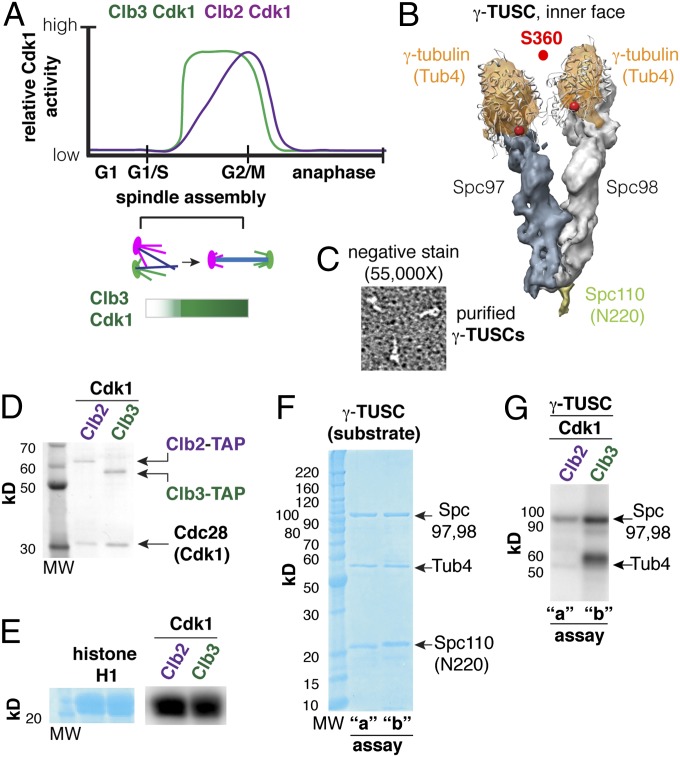
Fig. 4.
γ-Tubulin is a Clb3–Cdk1 substrate in vitro. (A) Early and late B-type cyclin–Cdk complexes (e.g., Clb3 and Clb2) are active during spindle assembly, with Clb3 activity peaking before Clb2 reaches its maximum activity. (B) The γ-tubulin small complex (γ-TUSC) is composed of Tub4, Spc97, and Spc98 (2:1:1 stoichiometry). γ-TUSCs are Y-shaped complexes that form spontaneously in coinfected Sf9 cells in the presence of a fragment of Spc110 (N-terminal 220). (C) Verification of the structure of purified γ-TUSCs using negative staining and transmission electron microscopy (TEM). (D) Clb–Cdk1 complexes purified from yeast. (E) Histone H1 was used as a control to verify Clb–Cdk1 activity. (F) γ-TUSC substrate (0.4 µM) reacted with Clb–Cdk1 in parallel kinase assays: a, 2 nM Clb2–Cdk1; b, 5 nM Clb3–Cdk1 for 30 min at 30 °C. (G) Tub4 was efficiently phosphorylated by Clb3–Cdk1 but not Clb2–Cdk1, whereas Spc97 and -98 are phosphorylated by both Clb2–Cdk1 and Clb3–Cdk1.
Clb3–Cdk1 Preferentially Targets γ-Tubulin in Vitro.
Active, functional Tub4 located at SPBs is not found as a monomer but rather, is found in a large complex with two other evolutionarily conserved proteins: Spc97 and Spc98. This complex, called the γ-tubulin ring complex (γ-TURC), is composed of seven Y-shaped smaller complexes called γ-TUSCs (Fig. 4B), each composed of two molecules of Tub4 and one molecule each of Spc97 and Spc98 (32). γ-TUSCs are anchored to the SPBs by receptor proteins, one of which is Spc110 (32). Using an Sf9 insect cell expression system, γ-TUSCs form spontaneously on coexpression and can be purified by affinity purification with GST-tev-Spc1101–220 fusion protein as the ligand (32, 33). We reasoned that purified γ-TUSCs would be appropriate as a substrate to test the specificity of cyclin–Cdk1 complexes for two reasons: first, this approach would test in vitro Cdk1 targeting of Tub4 in a state that is similar to that in which it exists in vivo, and second, Spc97 and Spc98 serve as internal controls for relative specificity. We purified γ-TUSCs as previously described (33) and verified their structure as Y-shaped particles using negative staining EM (Fig. 4C). We then performed parallel 32P (ATP) incorporation assays in the presence of purified Cdk1 (Cdc28) and cyclin (Fig. 4D) using either histone H1 (Fig. 4E) or γ-TUSCs (Fig. 4F) as substrates as described in Materials and Methods and ref. 18. Although Spc97 and/or Spc98 were targeted by both Clb2–Cdk1 and Clb3–Cdk1, we found that Clb3–Cdk1 selectively phosphorylated Tub4 with no detectable phosphorylation by histone H1-normalized Clb2–Cdk1 (Fig. 4F). These in vitro assays support our in vivo finding that Clb3–Cdk1 is the most likely Cdk1 complex targeting Tub4 in cells.
Discussion
It has proven extremely difficult to distinguish specific vs. redundant roles of a cyclin in cyclin–Cdk1 protein complexes using functional assays. We have established a systematic method to dissect the ternary interaction between a protein of interest and the Cdk1–cyclin complex using the OyCD PCA. We used the Gateway cloning strategy to generate expression plasmids for prey–OyCD PCA fragment fusions. There are sufficient ORFs in Gateway-ready vectors available to screen a substantial portion of the yeast proteome. Interactions can be further dissected through mutagenesis of potential substrates to determine whether cyclins that bind to a prey, bind to overlapping or unique sites. The OyCD PCA strategy will better allow us to place individual substrate phosphorylation events according to the timing of cyclin synthesis and degradation and therefore, when they occur during the mitotic cell cycle.
Our results provide insights into the mechanism of regulation of Cdk1 and its unique interaction partners. For instance, our results suggest that the Mft1–Cdk1 interaction depends on Clb6, consistent with its known function in mitotic recombination (23). Cdk1 could interact with Clb6 and phosphorylate Mft1 to regulate its activity during the S phase of the cell cycle. Equally, as noted above, Rim20 does not have any Cdk1 consensus site but interacts with Cdk1 in complexes with G1, G1-S, and S-phase cyclins (Cln3, Cln1, and Clb5), perhaps through the cyclin binding motifs (RXL and LP) that it possesses. Because Rim20 is a regulator of Ime2, a protein kinase involved in activating meiosis, it would be worth investigating whether Rim20 could inhibit Cdk1 activity to stop the mitotic cell cycle when diploid cells are nitrogen-starved, driving them into meiosis (similar to how Far1 inhibits Cdk1 activity in the presence of α-factor for haploid MATa yeast strain) (20, 34).
Our identification of Cdk1–Clb3 as the specific form of Cdk1 interacting with γ-tubulin in vivo and in vitro provides critical temporal information and suggests that Tub4 phosphorylation occurs in S phase during the process of spindle assembly rather than during metaphase (Fig. 4A). Phosphorylation of S360 was detected in the pool of γ-tubulin associated with SPBs (18) but not soluble pools (35) from which the SPB-bound fraction is recruited. Although accessible to solvent, S360 is positioned in the cleft between the two molecules of Tub4 of the γ-TUSC and projects from the interior surface of the γ-TURC (36). Given its position inside the γ-TURC, it is unlikely that S360 would be accessible to Clb3–Cdk1 if the γ-TURC is occupied by a microtubule. Phosphorylation of S360 is, therefore, likely to occur in unoccupied γ-TURCs that result from the catastrophe of spindle microtubules, which is expected to occur as microtubules search for partners (e.g., bind to kinetochores or pair with microtubules projecting from the opposite pole) during early spindle assembly, at which time Clb3–Cdk1 activity is increasing.
The detection of a specific Clb3–Cdk1 interaction with Tub4 in vivo without any cell cycle arrest or perturbation reveals the exquisite sensitivity of the OyCD PCA for detecting cyclin–Cdk1 substrate complexes in vivo. The number of γ-tubulin molecules in a yeast cell is estimated to be ∼7,000 (37); however, the number associated with the spindle poles (where S360 is phosphorylated) is expected to be ∼600 [14 per γ-TURC and therefore, per microtubule (32) and 40 spindle microtubules and 2–3 astral microtubules per cell (38)]. However, the majority of the spindle microtubules are stabilized by their attachment to kinetochores or as a result of pairing to form polar microtubules (38, 39). As a result, the conversion of an occupied γ-TURC to an open γ-TURC with S360 residues that can be phosphorylated is expected to be a rare event.
Finally, the OyCD PCA strategy can be generally applied to dissect other natural multisubunit enzyme complexes, including kinases, phosphates, and ubiquitin ligases in vivo, for which determining the basis of substrate specificity and localization is as difficult as for cyclin–Cdk1 substrates.
Materials and Methods
Yeast Strains.
We used the MATa BY4741 yeast strain along with FCY1 (encoding yeast cytosine deaminase) and all nine single cyclin deletion strains (fcy1∆, cln1∆, cln2∆, cln3∆, clb1∆, clb2∆, clb3∆, clb4∆, clb5∆, and clb6∆) (40). The FCY1 gene was replaced by the nourceothricin resistance gene in all nine cyclin deletion strains by homologous recombination.
The Yeast ORF Collection of over 4,900 plasmid-based yeast genes in Gateway expression clones was purchased from Open Biosystems (41).
Plasmid Construction.
The pAG413GAL1-ccdB-EGFP (HIS3 marker) Gateway destination vector (42) was purchased from Addgene. The pAG413GAL1-ccdB-OyCD-F[1] Gateway destination vector was created by cloning an OyCD-F[1] sequence into the position of the EGFP gene in the pAG413GAL1-ccdB-EGFP destination vector using EcoRV and XhoI restriction sites. The p415Gal1-Linker-OyCD-F[2] was constructed by introducing Linker-OyCD-F[2] sequence in p415Gal1 (87330; ATCC) using BamHI and XhoI sites. The p415GAL1-Cdk1-OyCD-F[2] plasmid was obtained by cloning the CDK1 gene upstream of OyCD-F[2] using SpeI and BamHI. The negative control p415GAL1-Start-Linker-OyCD-F[2] was created by cloning a Linker-OyCD-F[2], which has an ATG codon before the linker sequence in the position of Cdk1-OyCD-F[2] in p415GAL1-Cdk1-OyCD-F[2] using SpeI and XhoI restriction sites.
Gateway Cloning.
The selected genes from the Yeast ORF Collection were transferred into a Gateway donor vector to obtain ENTRY clones by Gateway BP reactions according to the manufacturer’s protocol (Invitrogen), with the exception that the reaction were scaled down four times and the incubation time was prolonged to 16 h at 22 °C. We generated a Destination vector that carries the first fragment of OyCD (OyCD-F[1]) that we named pAG413GAL1-ccdB-OyCD-F[1]. This vector was used in an attL and attR LR reaction with an ENTRY clone that carries a gene encoding a protein of interest. The LR reactions were performed according to the manufacturer’s protocol (Invitrogen), with the exception that the reactions were scaled down four times and the incubation time was prolonged to 16 h at 22 °C. The product of the LR reaction is an Expression clone that contains the gene of interest fused to OyCD-F[1] (pAG413GAL1-GeneX-OyCD-F[1]) and a byproduct plasmid that is not recovered. This strategy enabled us to easily create a large number of Gateway expression clones using the selected genes from the Yeast ORF Collection with each fused to the OyCD-F[1] sequence.
OyCD PCA to Detect Protein–Protein Interactions with Cdk1.
The selected genes fused to the OyCD-F[1] sequence in Gateway expression clones were each separately transformed into BY4741 fcy1∆ yeast containing either p415GAL1-Cdk1-OyCD-F[2] or p415GAL1-Linker-OyCD-F[2]. After 3 d of growth, two colonies were picked from each transformation and grown in a 96-well plate in 400 μL synthetic complete medium without histidine and leucine and with 2% (wt/vol) raffinose for 16 h. Galactose was added to each culture at a final concentration of 2% (wt/vol) to induce the expression of the OyCD fusion proteins for 1 h at 30 °C before pinning the samples on selection plates with and without 1 mg/mL 5-FC (Sigma) with a manual pintool (1.58 mm, 1-mL slot pins, 45 mm, VP 408Sa; V&P Scientific Inc.). Raffinose, galactose, and glucose were purchase from Bioshop. Images of the plates were taken after 3 d of growth.
Detecting Protein–Protein Interactions in the Different Cyclin Deletion Strains.
Proteins that interacted with Cdk1 were screened in yeast strains expressing all nine cyclin genes or lacking one of nine cyclin genes. The potential substrate genes fused to the OyCD-F[1] sequence in Gateway expression vectors were cotransformed with p415GAL1-Cdk1-OyCD-F[2] into the FCY1 deletion (fcy1∆) strain and the nine single cyclin and FCY1Δ double deletion strains. Each test necessitated serial transformation of each strain. This screen is a labor-intensive process, although consisting of no more steps than an alternative approach, such as transforming MATa and MATα haploid strains with the bait and prey plasmids followed by mating. We consider the FCY1∆ yeast strain as the control strain in this screen and referred to it as the WT strain, because it expresses all nine cyclins. The controls for protein–protein interaction detected in 10 yeast strains were the interactions between the homodimeric GCN4 leucine Zips fused to complementary OyCD fragments serving as positive controls and Cdk1 fused to OyCD-F[2] alone serving as negative controls. Four clones were picked and grown to saturation in synthetic complete medium lacking histidine and leucine with 2% (wt/vol) glucose and 200 μg/mL (wt/vol) G418 (Sigma). A glycerol stock was prepared with these cultures. All samples from the glycerol stock were printed on plates containing the same medium with 3% (wt/vol) agar and allowed to grow for 4 d. For evaluating the OyCD PCA activity, colonies were pinned on synthetic complete medium lacking histidine and leucine with 2% (wt/vol) raffinose, 2% (wt/vol) galactose, 200 μg/mL G418 (Sigma), and 3% (wt/vol) agar plates with and without 1 mg/mL 5-FC using a robotically manipulated 384 pintool (0.356-mm flat round-shaped pins, custom AFIX384FP8 BMP Multimek FP8N; V&P Scientific Inc.). Pictures were taken after 1, 2, 3, and 4 d of growth. This experiment was repeated three times.
Analysis of the Cyclin Deletion Strains.
Cell growth was quantified using ImageJ (43) by calculating the integrated intensity of each colony. This experiment was repeated three times. Results of one representative dataset, with images collected on day 4, are shown in Fig. 3A. The activity of the OyCD PCA was measured by taking the ratio of integrated intensity of each colony grown on 1 mg/mL 5-FC over the integrated intensity of colonies grown in the absence of 5-FC. A two-tailed type 1 Student t test was performed using the results of OyCD PCA activity obtained for the WT strain and each of nine single cyclin deletion strains. A minimum P = 0.04 was obtained for cells expressing the GCN4 leucine Zip:Zip in the Cln3Δ deletion strain. The minimum P value (P = 0.02) was obtained for cells expressing Cdk1 fused to OyCD-F[1] (Cdk1 alone) in the Clb1Δ deletion strain. Only P values ≤ 0.01 were considered as significant for the interaction between Cdk1 and test proteins in the different yeast strains. Results were represented in a matrix using iVici (44).
Purification of γ-TUSC Substrate and Particle Analysis.
Constructs used for γ-TUSC expression are described in refs. 32 and 33. An N-terminal fragment of Spc110 fused to TEV-GST (Spc110N220-tev-GST) was used for complex purification. For purification of γ-TUSCs, Sf9 cells were coinfected with four virus stocks prepared as described in ref. 45, and each virus encodes one of four components of the γ-TUSC (Tub4, Spc97, Spc98, and Spc110N220-GST). Infected Sf9 cells were incubated for 48 h, and γ-TUSC complexes formed spontaneously in the Sf9 cells. Cell lysis and all subsequent steps were performed in HB100 (40 mM K-hepes, pH 7.5, 100 mM KCL, 1 mM MgCl2, 1 mM EGTA, 0.1 mM GTP; 1 mM DTT) as described in ref. 33. Whole-cell extracts containing γ-TUSC complexes were incubated with glutathione resin, the resin was washed, and the bound γ-TUSCs were eluted in HB100 by TEV cleavage. Purified complexes, which can be in the form of full or partial γ-TURCs and γ-TUSCs (32), were diluted to 100 µg/mL and incubated on ice in HB100 buffer with KCL concentration raised to 500 mM to produce predominantly γ-TUSCs (Fig. 4C). Negative staining was performed as described in ref. 32 using an FEI Tecnai G2 F20 200 kV Cryo-S/TEM Microscope.
Cdk1 Kinase Assays.
Active Clb2–Cdk1 and Clb3–Cdk1 complexes were purified using the tandem affinity purification (TAP) method as described in ref. 26. Activity of the purified kinases was tested with histone H1 at a concentration of ∼10 µM using 2 nM Clb2 or 5 nM Clb3–Cdk1 kinase. The composition of the assay was as follows. A prereaction mixture of kinase, 100 µM cold ATP, and 10 µCi γ[32P]ATP was prepared in kinase buffer as described in ref. 26. The concentration of the purified γ-TUSC substrate was kept at 0.4 µM in Clb2–Cdk1 (2 nM; a reaction) and Clb3–Cdk1 (5 nM; b reaction) kinase assays. Parallel a and b reactions were started by adding the kinase mixture to the γ-TUSC substrate, with incubation for 30 min at 30 °C. Reactions were terminated by addition of 0.1 volumes β-mercaptoethanol and 0.25 volumes 4× SDS sample buffer. Parallel reactions were separated on a 10% SDS/PAGE gel, and proteins were visualized by Coomassie blue stain. Incorporation of P32 into the γ-TUSC components Spc98, Spc97, and Tub4 was measured using a TRIO Typhoon phosphoimager.
Note Added in Proof.
Prior to publication, Nazarova et al. described the significance of Clb3-Cdk1 phosphorylation of γ-tubulin (46).
Supplementary Material
Acknowledgments
We thank Emmanuel Levy for the ImageJ macro used to quantify the growth of the colonies (cyclin contingency experiments) and Gary Brouhard, Justin Kollman, Susanne Bechsted, and members of the laboratories of J.V. and S.W.M. for many helpful discussions. EM was performed at the Facility for Electron Microscopy Research at McGill University. The authors acknowledge support from Canadian Institutes of Health Research Grant MOP-123335 and Natural Sciences and Engineering Research Council (Canada) Grants RGPIN 262246 (to J.V.), MOP-GMX-152556 (to S.W.M.), and MOP-GMX-231013 (to S.W.M.). P.H.E. thanks the Faculté des Études Supérieures for scholarships. C.H. is supported by a Cellular Dynamics of Macromolecular Complexes-Collaborative Research and Training Experience Graduate Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305420110/-/DCSupplemental.
References
- 1.Thornton BR, Toczyski DP. Precise destruction: An emerging picture of the APC. Genes Dev. 2006;20(22):3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58(1989):453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 3.Morgan DO. Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13(1997):261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 4.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: In cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33(3):113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DO. Principles of CDK regulation. Nature. 1995;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall MD. An inhibitor of p34CDC28 protein kinase activity from Saccharomyces cerevisiae. Science. 1993;259(5092):216–219. doi: 10.1126/science.8421781. [DOI] [PubMed] [Google Scholar]
- 7.Mendenhall MD, Hodge AE. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62(4):1191–1243. doi: 10.1128/mmbr.62.4.1191-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8(2):149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 9.Ubersax JA, et al. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425(6960):859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- 10.Holt LJ, et al. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325(5948):1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archambault V, et al. Targeted proteomic study of the cyclin-Cdk module. Mol Cell. 2004;14(6):699–711. doi: 10.1016/j.molcel.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Loog M, Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434(7029):104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- 13.Ear PH, Michnick SW. A general life-death selection strategy for dissecting protein functions. Nat Methods. 2009;6(11):813–816. doi: 10.1038/nmeth.1389. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz JE, Exinger F, Erbs P, Jund R. New insights into the pyrimidine salvage pathway of Saccharomyces cerevisiae: Requirement of six genes for cytidine metabolism. Curr Genet. 1999;36(3):130–136. doi: 10.1007/s002940050482. [DOI] [PubMed] [Google Scholar]
- 15.de Lichtenberg U, Jensen LJ, Brunak S, Bork P. Dynamic complex formation during the yeast cell cycle. Science. 2005;307(5710):724–727. doi: 10.1126/science.1105103. [DOI] [PubMed] [Google Scholar]
- 16.Sharifpoor S, et al. A quantitative literature-curated gold standard for kinase-substrate pairs. Genome Biol. 2011;12(4):R39. doi: 10.1186/gb-2011-12-4-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ptacek J, et al. Global analysis of protein phosphorylation in yeast. Nature. 2005;438(7068):679–684. doi: 10.1038/nature04187. [DOI] [PubMed] [Google Scholar]
- 18.Keck JM, et al. A cell cycle phosphoproteome of the yeast centrosome. Science. 2011;332(6037):1557–1561. doi: 10.1126/science.1205193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 20.Su SS, Mitchell AP. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics. 1993;133(1):67–77. doi: 10.1093/genetics/133.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382(6589):325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 22.Schwob E, Bohm T, Mendenhall MD, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994;79(2):233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 23.Chavez S, et al. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. EMBO J. 2000;19(21):5824–5834. doi: 10.1093/emboj/19.21.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardin AJ, Visintin R, Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102(1):21–31. doi: 10.1016/s0092-8674(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 25.Geymonat M, Spanos A, Jensen S, Sedgwick SG. Phosphorylation of Lte1 by Cdk prevents polarized growth during mitotic arrest in S. cerevisiae. J Cell Biol. 2010;191(6):1097–1112. doi: 10.1083/jcb.201005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koivomagi M, et al. Dynamics of Cdk1 substrate specificity during the cell cycle. Mol Cell. 2011;42(5):610–623. doi: 10.1016/j.molcel.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liakopoulos D, Kusch J, Grava S, Vogel J, Barral Y. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell. 2003;112(4):561–574. doi: 10.1016/s0092-8674(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 28.Moore JK, Miller RK. The cyclin-dependent kinase Cdc28p regulates multiple aspects of Kar9p function in yeast. Mol Biol Cell. 2007;18(4):1187–1202. doi: 10.1091/mbc.E06-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grava S, Schaerer F, Faty M, Philippsen P, Barral Y. Asymmetric recruitment of dynein to spindle poles and microtubules promotes proper spindle orientation in yeast. Dev Cell. 2006;10(4):425–439. doi: 10.1016/j.devcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Tavormina PA, Burke DJ. Cell cycle arrest in cdc20 mutants of Saccharomyces cerevisiae is independent of Ndc10p and kinetochore function but requires a subset of spindle checkpoint genes. Genetics. 1998;148(4):1701–1713. doi: 10.1093/genetics/148.4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahal R, Amon A. Mitotic CDKs control the metaphase-anaphase transition and trigger spindle elongation. Genes Dev. 2008;22(11):1534–1548. doi: 10.1101/gad.1638308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kollman JM, Polka JK, Zelter A, Davis TN, Agard DA. Microtubule nucleating gamma-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature. 2010;466(7308):879–882. doi: 10.1038/nature09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinh DB, Kern JW, Hancock WO, Howard J, Davis TN. Reconstitution and characterization of budding yeast gamma-tubulin complex. Mol Biol Cell. 2002;13(4):1144–1157. doi: 10.1091/mbc.02-01-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyers M, Futcher B. Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes. Mol Cell Biol. 1993;13(9):5659–5669. doi: 10.1128/mcb.13.9.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin TC, et al. Phosphorylation of the yeast gamma-tubulin Tub4 regulates microtubule function. PLoS One. 2011;6(5):e19700. doi: 10.1371/journal.pone.0019700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by γ-tubulin complexes. Nat Rev Mol Cell Biol. 2011;12(11):709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 38.Winey M, Bloom K. Mitotic spindle form and function. Genetics. 2012;190(4):1197–1224. doi: 10.1534/genetics.111.128710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner MK, et al. Chromosome congression by Kinesin-5 motor-mediated disassembly of longer kinetochore microtubules. Cell. 2008;135(5):894–906. doi: 10.1016/j.cell.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giaever G, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418(6896):387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 41.Gelperin DM, et al. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 2005;19(23):2816–2826. doi: 10.1101/gad.1362105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alberti S, Gitler AD, Lindquist S. A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast. 2007;24(10):913–919. doi: 10.1002/yea.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 44.Tarassov K, Michnick SW. iVici: Interrelational visualization and correlation interface. Genome Biol. 2005;6(13):R115. doi: 10.1186/gb-2005-6-13-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wasilko DJ, et al. The titerless infected-cells preservation and scale-up (TIPS) method for large-scale production of NO-sensitive human soluble guanylate cyclase (sGC) from insect cells infected with recombinant baculovirus. Protein Expr Purif. 2009;65(2):122–132. doi: 10.1016/j.pep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Nazarova, et al. Distinct roles for anti-parallel microtubule pairing and overlap during early spindle assembly. Mol Biol Cell. 2013 doi: 10.1091/mbc.E13-05-0232. 10.1091/mbc.E13-05-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.