Significance
Using a combination of approaches, a unique organization of the synaptic architecture of the dorsal raphe nucleus is revealed. That is, presynaptic GABAergic and glutamatergic boutons often pair with each other and converge on a single postsynaptic target, forming synaptic triads.
Keywords: axoaxonic, glutamate decarboxylase 65, vesicular glutamate transporter (VGLUT), ultrastructure
Abstract
The dorsal raphe nucleus (DR) controls forebrain serotonin neurotransmission to influence emotional states. GABA neurotransmission in the DR has been implicated in regulating sleep/wake states and influencing anxiety and aggression. To gain insight into how GABA regulates DR activity, we analyzed the organization of both GABA and glutamate axons in the rat DR using a high-resolution immunofluorescence technique, array tomography, as well as EM. This analysis revealed that a third or more of GABA-containing axons are organized in synaptic triads with a glutamatergic axon and a common postsynaptic target. Electrophysiological recordings showed that GABA has the capacity to presynaptically gate glutamate release in the DR through a combination of GABA-A and GABA-B receptor-mediated effects. Thus, GABA–glutamate synaptic triads are a common feature of the network architecture of the DR with the potential to regulate excitation of the nucleus.
Serotonin released from the dorsal raphe nucleus (DR) modulates forebrain circuits involved in emotional states, sleep, motivation, and aggression (1–5). Moreover, dysregulation of the DR has been implicated in the pathophysiology of affective disorders including anxiety and depression (5–7). The DR is an important area for many behaviors and neuropathologies, and its network architecture is complex. The DR contains several types of neurons that use neurotransmitters including serotonin, dopamine, GABA, and glutamate, potentially in combination with a variety of neuropeptides (8–10). In addition, afferent innervation of the DR, both inhibitory and excitatory, arises from multiple sources (11–15).
Is this complex circuitry organized at the synaptic level in the DR? In highly laminated structures such as the cortex, hippocampus, or cerebellum, the relationships between axons and dendrites are known to be highly precise (16). There are stereotypic patterns of glutamatergic innervation onto dendritic spines, and GABAergic synapses are spatially segregated. However, the DR is not a laminated structure, and DR neurons are only sparsely spiny, leaving the organizing principles of the DR at the synaptic level unknown.
To understand whether and how populations of glutamate and GABA axons may be organized within the DR, we used a recently developed high-resolution immunofluorescence imaging technique called array tomography (AT) (17, 18). With this approach, we could analyze and quantify the relationship of six different pre- and postsynaptic markers with respect to each other in the same volume of tissue. Relationships revealed using AT were further interrogated with ultrastructural and electrophysiological analysis to yield unique insight into the synaptic organization and function of DR microcircuits.
Results
The array tomographic visualization of the DR showed a high abundance of serotonin neurons densely surrounded by synaptic elements (Fig. 1A). Synapsin-labeled puncta could be individually resolved and characterized by double immunolabeling for markers of specific subtypes of axons (e.g., glutamate decarboxylase 65; GAD2) (Fig. 1 B–D).
Fig. 1.
High-resolution array tomographic images rendered from 16 ultrathin (70-nm) sections. (A) A field of view showing the high density of TPOH-labeled dendrites and soma (white), abundantly surrounded by synapsin (blue) and GAD2 (red) puncta. (B) Closer view of the boxed region in A showing individually resolved puncta of synapsin (blue), some of which are double immunolabeled for GAD2 (red; arrowheads). (C and D) Synapsin and GAD2 labeling individually. Notice the absence of out-of-focus light resulting in a very discrete pattern of immunolabeling suitable to quantitative analysis.
Abundance and Specificity of Immunolabeling.
Axonal boutons were defined as objects that were double immunolabeled for synapsin and either vesicular glutamate transporter (VGLUT)1–3 or GAD2 (Fig. 2 A–D). Using the contingency of colocalization with synapsin increases the confidence that boutons are specifically identified (19). However, this is a conservative approach, and underdetection may be caused by incomplete detection of antigens. In addition, if two boutons with the same neurotransmitter transporter are adjacent and their labeling is contiguous, they would be detected as one bouton. Analysis of the absolute density of axonal boutons in three dimensions using image stacks showed that both VGLUT2- and GAD2-containing boutons were more prevalent than those containing other VGLUT types (F3,20 = 156.08, P < 0.001; P < 0.001 vs. either VGLUT1 or VGLUT3 for both individually) (Fig. 2E). For this analysis, the sampled volumes contained a total of 7,468 axon boutons with VGLUT1 immunolabeling, 24,252 with VGLUT2, 4,407 with VGLUT3, and 24,476 with GAD2.
Fig. 2.
Glutamatergic and GABAergic axons in the DR. (A–D) Immunolabeling for VGLUT1–3–containing (green, magenta, and light blue, respectively) or GAD2-containing (red) can be found in the same location in serial sections, and these puncta are often coincident with synapsin (blue). In these examples, axons are also adjacent to serotonin neuron dendrites (white), identified by the presence of TPOH labeling and the lack of synapsin labeling. (E) The total density of puncta containing synapsin in combination with either VGLUT1–3 or GAD2 in the neuropil. VGLUT2- and GAD2-containing axons are most abundant. Bars represent SEM (n = 3 rats; *P < 0.001 vs. either VGLUT1 or VGLUT3). (F) Cross-correlation analysis between VGLUT1–3 or GAD2 and synapsin (SYN). When imaging channels are aligned (x = 0-μm displacement), VGLUT2 and GAD2 show the highest absolute correlation with synapsin, and this is likely driven by their higher relative abundance (shown in E). When imaging channels are displaced with respect to each other, all of the correlations decrease. (G) The normalized cross-correlation analysis shows the relative decrease in correlations with image displacement; that is, all of the curves are divided by the initial correlation value at no shift. This analysis provides a measure of how specific the correlations are, unbiased by the abundance of antigens. All curves drop similarly, indicating that VGLUT1–3 and GAD2 each have a fairly equal likelihood of coexisting with synapsin, supporting the specificity of immunolabeling.
Although colocalization of synapsin with a neurotransmitter transporter increases the confidence that the identified puncta are truly axon boutons, the absolute density of objects with double immunolabeling could include both specific and random events, depending on the abundance and specificity of labeling. To examine the specificity of the relationship between synapsin and the neurotransmitter transporters, cross-correlation analysis was pursued. The cross-correlation function is the plot of the Pearson’s correlation coefficient between two channels (on the y axis) and the alignment/misalignment of the images on the x axis. If two antigens specifically colocalize, their correlation will be maximal when the images are aligned, or when x = 0 μm. When one channel is gradually misaligned, or shifted with respect to the other as designated by increasing x values, the correlation should decrease to a nadir reflecting only random interactions. The cross-correlation graphs show that VGLUT2- and GAD2-labeled puncta had a stronger initial correlation with synapsin compared with those labeled for VGLUT1 or VGLUT3 (Fig. 2F). This is likely driven by the higher abundance of VGLUT2- and GAD2-containing puncta. However, all of the correlations decreased with image displacement, suggesting each correlation reflected a specific relationship between the antigens.
Because the initial and final correlations are influenced by how abundant the given antigens are, we also plotted the normalized cross-correlation, where the cross-correlation at every point is divided by the initial correlation value at no shift. These plots provide a measure of how specific an interaction is, unbiased by the abundance of immunolabeling. The normalized cross-correlation showed that all curves decrease to a similar extent with displacement. Taken together, these results show that VGLUT1–3 and GAD2 puncta have a similar propensity to overlap with synapsin (Fig. 2G), indicating each antigen identifies a population of axon boutons, validating the specificity of the approach. Further, VGLUT2 and GAD2 boutons are more abundant in the DR than those containing VGLUT1 or VGLUT3.
Associations Between GABA and Glutamate Boutons.
To determine whether there are any specific relationships between different types of axon boutons in the DR, we used a variation of the same approach to query the dataset for instances of adjacencies between VGLUT1–3– and GAD2-containing boutons (Fig. 3). Because two objects that are adjacent may not have overlapping immunolabeling, the “dilate” function was used to increase the size of the first bouton by less than 0.25 μm, and subsequently instances of overlap within another neurotransmitter marker were measured. We found that all three types of glutamatergic boutons (VGLUT1–3) are closely apposed to GABA puncta (Fig. 3 A–C). The density analysis showed that adjacencies between GABA- and VGLUT2-containing boutons were more common than adjacencies between GABA and other VGLUT types (F2,12 = 44.95, P < 0.001; P < 0.001 vs. either VGLUT1 or VGLUT3) (Fig. 3D). This involved about ∼36% (2,712/7,468) of total VGLUT1-, ∼24% (5,738/24,252) of VGLUT2-, and ∼57% (2,528/4,407) of VGLUT3-immunolabeled boutons. From the perspective of GABA boutons, about 11% of GABA boutons (2,712/24,476) were found adjacent to VGLUT1-containing boutons, whereas ∼23% (5,738/24,476) and ∼10% (2,528/24,476) were close to VGLUT2 and VGLUT3 boutons, respectively. On the other hand, when we analyzed possible interactions between all three types of glutamatergic boutons with each other, we found that an average of 8% of any type of glutamate bouton was adjacent to another glutamate bouton (Fig. 3D).
Fig. 3.
Associations between glutamate and GABA boutons. (A–C) Serial section images showing that GAD2-labeled puncta (yellow) were often found adjacent to VGLUT1–3–containing puncta (green, magenta, and light blue, respectively). Synapsin is depicted in blue. (D) Total density of objects where markers of two types of axon terminals and synapsin overlapped. The most common overlap was seen between VGLUT2 and GAD2 (*P < 0.001 vs. either VGLUT1 or VGLUT3). Lower densities of associations between different glutamatergic axons (e.g., VGLUT1/SYN/VGLUT2, VGLUT1/SYN/VGLUT3, and VGLUT2/SYN/VGLUT3) were also detected. Bars represent SEM (n = 3 rats). (E) Cross-correlation analysis of pixels between different types of boutons shows the highest degree of colocalization for pairs containing VGLUT2 and GAD2, consistent with their higher overall abundance. (F) Normalized cross-correlation analysis to evaluate the specificity of correlations, unbiased by abundance. All curves of VGLUT1–3/SYN/GAD2 associations drop sharply, suggesting a similar spatial proximity of all three VGLUT–SYN pairs with GAD2 puncta. Association curves between different types of glutamate axons drop, but to a higher plateau, indicating a greater fraction of stochastic associations.
To further analyze the specificity of the relationship between GABA and glutamate axon boutons, we performed a cross-correlation analysis (Fig. 3E). The results showed that all VGLUT1–3–synapsin pairs associate with GAD2-labeled puncta to a greater extent than VGLUT1–3 boutons associate with each other. Further, the glutamate–GABA interactions in particular decreased with image displacement. In the normalized cross-correlation, all three glutamate–GABA pairs had similar, sharply dropping curves (Fig. 3F), suggesting that GAD2 axons equally interact with all three types of glutamatergic boutons, relative to their proportionate abundance. That is, the association between GAD2 and VGLUT2 boutons is the most common because of the relative abundance of VGLUT2 boutons. In contrast, when we analyzed the interactions between glutamate–glutamate pairs, the curves dropped to a higher plateau value at maximal displacement (Fig. 3F). Therefore, glutamate boutons interact with each other with a lower absolute frequency (Fig. 3 D and E), and these interactions are more nonspecific (Fig. 3F).
Ultrastructure of GABA Axons.
The relationships found between glutamate and GABA boutons using AT raised the possibility of selective axoaxonic associations. This possibility was further examined using immuno-EM for GAD2 in the same region of the DR. Immunolabeling for GAD2 was primarily localized to presynaptic boutons defined by the presence of synaptic vesicles. GAD2-immunolabeled boutons often formed symmetric, or inhibitory-type, synapses on dendrites. Of all GAD2 axon boutons sampled, many were adjacent to unlabeled axons (∼34%, 62/183) or to other GAD2-immunolabeled boutons (∼8%, 15/183) without an intervening glial process (Fig. 4 and Fig. S1). Unlabeled axons adjacent to GAD2-immunolabeled boutons were detected forming asymmetric, excitatory-type synapses consistent with the morphology of glutamate boutons (Fig. 4 A–C). Except in cases involving the axon initial segment, the morphological criteria for axoaxonic synapses remains poorly defined (20), and therefore these were not quantified. However, instances of close apposition of membranes between axons associated with an accumulation of synaptic vesicles were detected, consistent with potential neurotransmitter release sites (Fig. 4 D and E). Taken together, the AT and ultrastructural analysis indicate the existence of synaptic “triads” consisting of presynaptic GABAergic and glutamatergic boutons and a single postsynaptic dendrite.
Fig. 4.
Ultrastructural identification of synaptic triads. (A–D) Examples of GABA axons (white font A) adjacent to (arrows) unlabeled axons (black font A). The unlabeled axon often formed an excitatory-type (asymmetric) synapse (S, arrowheads) with a common postsynaptic dendrite (D). (E) Zoomed-in view from D, showing close apposition of membranes of the GABAergic and unlabeled axon without intervening glial processes. (Scale bars, 0.5 μm.)
Relationships to Serotonin Neurons.
To determine whether GABA and glutamate boutons individually or as pairs may have any preferential relationship to serotonin neurons, we analyzed the proportion that are within close proximity (<0.25 μm) to tryptophan hydroxylase (TPOH)-immunolabeled objects. To do this, we used the dilate function for TPOH immunolabeling and analyzed boutons with overlapping voxels of immunolabeling. We found that about 29% of VGLUT1 boutons (2,159/7,468), 30% of VGLUT2 boutons (7,266/24,252), 25% of VGLUT3 boutons (1,114/4,407), and 25% of GAD2 boutons (6,194/24,476) were associated with TPOH-immunolabeled processes (Fig. 5A). This is in agreement with the idea that about one-third of the neurons within the DR contain serotonin (21). The analysis of associations between glutamate and GABA boutons showed that about 20% of VGLUT1–GAD2 pairs (546/2,712), 21% of VGLUT2–GAD2 pairs (1,193/5,738), and 21% of VGLUT3–GAD2 pairs (528/2,528) overlapped with serotonin neurons (Fig. 5B), percentages that roughly parallel overall abundance of boutons. That is, synaptic triads do not have a selective relationship to serotonin neurons; rather, they appear to involve nonserotonin neurons in the DR as well.
Fig. 5.
Synaptic triads are associated with both serotonin and nonserotonin neurons. (A) Quantitative analysis of VGLUT1–3 and GAD2 axons (paired with synapsin) related to TPOH-labeled processes. Both VGLUT2- and GAD2-containing axons are the most common types of axons in association with serotonin neurons, consistent with their overall abundance in the neuropil (n = 3 rats; *P < 0.001 vs. either VGLUT1 or VGLUT3). (B) The rate that VGLUT1–3–GAD2 pairs are adjacent to serotonin neurons is proportional to their overall abundance in the neuropil (*P < 0.001 vs. VGLUT1 or VGLUT3), and therefore triads appear to involve both serotonin and nonserotonin neurons. Bars represent SEM.
Presynaptic GABAergic Modulation of Glutamate Release.
The adjacency between GABA and glutamate boutons detected by both AT and EM could provide the basis for axoaxonic cross-talk. Therefore, we explored the possible functional aspects of these axoaxonic arrangements. For this, we performed electrophysiological recordings from putative DR serotonin neurons based upon their size and action potential characteristics (22). In the presence of tetrodotoxin (TTX) (300 nM) and strychnine (3 µM), miniature inhibitory and excitatory postsynaptic currents (IPSCs and EPSCs, respectively) were readily distinguished as outward and inward synaptic currents, respectively, in DR neurons voltage-clamped at −55 mV (Fig. 6C). These miniature IPSCs and EPSCs were abolished by the addition of the GABA-A receptor antagonist gabazine (10 µM) and the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 µM), respectively (Fig. 6C).
Fig. 6.
GABA-B receptor activation presynaptically inhibits glutamate release in DR neurons. (A) Time plot of miniature EPSC rate during application of baclofen (10 µM), CGP55845 (CGP; 1 µM), gabazine (GBZ; 10 µM), and CNQX (10 µM) in the presence of TTX (300 nM) and strychnine (3 µM). (B) Average traces of miniature EPSCs before (Pre) and during application of baclofen (264 and 115 miniature EPSCs during each 2-min epoch, respectively). (C) Raw traces of miniature synaptic currents before (Pre), then during the addition of baclofen, then CGP55845, then gabazine and CNQX. Miniature EPSCs are inward, whereas miniature IPSCs are outward (the latter are indicated by asterisks). Cumulative probability distributions of miniature EPSC (D) interevent interval and (E) amplitude for the epochs during which traces were averaged in B.
We next examined whether GABA ligands presynaptically modulate spontaneous miniature EPSCs in these neurons. Application of the GABA-B receptor agonist baclofen (10 µM) produced a gradual reduction in the frequency of occurrence of spontaneous miniature EPSCs in all DR neurons tested, which was reversed by addition of the GABA-B antagonist 3-[-1-(S)-(3,4-dichlorphenyl)-ethyl]amino-2(S)-hydroxypropyl-(P-benzyl)-phosphinic acid (CGP55845) (1 µM) (Fig. 6 A and C). The reduction in frequency was observed as a rightward shift in the cumulative probability distribution of miniature EPSC interevent intervals (Fig. 6D). By contrast, baclofen had no effect on the amplitude of miniature EPSCs, or on the cumulative probability distribution of spontaneous miniature EPSC amplitudes (Fig. 6 B and E). On average, the rate and amplitude of spontaneous miniature EPSCs was 32 ± 5% and 99 ± 4% in the presence of baclofen compared with preapplication levels (P < 0.001 and P = 0.8, respectively; n = 7) (Fig. 7E).
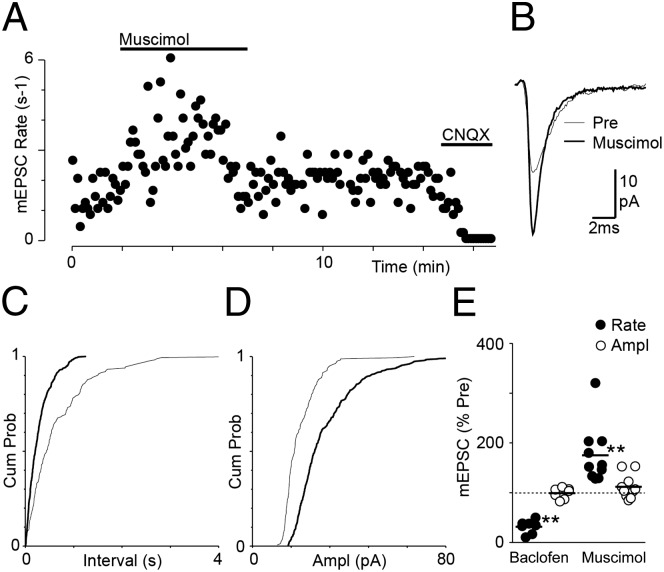
Fig. 7.
GABA-A receptor activation presynaptically enhances glutamate release in DR neurons. (A) Time plot of miniature EPSC rate during application of muscimol (2 µM) and CNQX (10 µM). All recordings are in the presence of TTX (300 nM) and strychnine (3 µM). (B) Average traces of miniature EPSCs before (Pre) and during application of muscimol (163 and 382 miniature EPSCs during each 2-min epoch, respectively). Cumulative probability distributions of miniature EPSC (C) interevent interval and (D) amplitude for the epochs during which traces were averaged in B. (E) Scatter plots of the effect of baclofen and muscimol on miniature EPSC rate and amplitude, expressed as a percentage of the preagonist level. Bars represent mean values (**P < 0.001).
Application of the GABA-A receptor agonist muscimol (2 µM) produced a rapid increase in the frequency of occurrence of spontaneous miniature EPSCs in all DR neurons tested, which often desensitized during its application (Fig. 7A). The increase in frequency was observed as a leftward shift in the cumulative probability distribution of miniature EPSC interevent intervals (Fig. 7C). In 3/10 neurons, muscimol produced an increase in the amplitude of spontaneous miniature EPSCs, which was observed as a rightward shift in the cumulative probability distribution of miniature EPSC amplitudes (Fig. 7 B and D). On average, the rate and amplitude of spontaneous miniature EPSCs was 175 ± 18% and 112 ± 8% in the presence of muscimol compared with preapplication levels (P < 0.004 and P = 0.15, respectively; n = 10) (Fig. 7E). It was noted that there was no correlation between the increase in rate produced by muscimol and the age of animals from which slices were obtained (Pearson’s r2 = 0.02, P = 0.68, animal age 21–35 d). In addition, both baclofen and muscimol produced outward currents in these neurons.
Discussion
In this study, we used the high-resolution immunofluorescence technique AT to quantitatively examine the organization of axonal boutons within the DR. We analyzed several immunohistochemical antigens with respect to each other in the same tissue volumes, including glutamatergic (VGLUT1–3) and GABAergic (GAD2) presynaptic markers as well as synapsin as a general marker for synaptic boutons. These five markers were combined with immunolabeling for TPOH, to study the relationship of boutons to serotonin neurons. This analysis revealed preferential interactions between glutamate and GABAergic presynaptic boutons, providing a basis for axoaxonic communication between these neurotransmitters. This relationship appeared to be driven by GABA, because GABA boutons were associated with every subtype of glutamatergic bouton, and glutamatergic boutons did not commonly interact with each other. Confirming the relationship between GABA and glutamate boutons, GAD2-labeled boutons directly apposed unlabeled boutons at the ultrastructural level. Finally, we found that GABA-A and GABA-B receptors presynaptically enhance and inhibit glutamate release, respectively, and thus this axoaxonic communication likely modulates the net glutamate neurotransmission into the nucleus.
Synaptic triads are easily detected by EM. However, using EM, it is extremely difficult to determine whether the relationship between two axons merely reflects a stochastic event, driven by the coincidental arrival of two axons at a common postsynaptic target. This is particularly true because frank synapses are not commonly present between axonal boutons, even when functional relationships are known to exist (20, 23). Furthermore, multiple rounds of double immuno-EM would be needed to identify the neurotransmitter in the adjacent axons. AT is unique in the ability to immunolabel the same sections for multiple antigens and then query the data in different ways, for example using cross-correlation analysis to analyze the specificity of relationships. This analysis revealed that axoaxonic relationships occur at greater than random rates for GABAergic axons and involve all types of glutamate axons. This relationship is brain region-specific; that is, previous studies using AT analysis have shown a lack of correlation between GABAergic and glutamatergic axon markers in the cortex (18). In the cortex, as in several other brain regions, excitatory axons tend to innervate dendritic spines that are isolated from other axon terminals. Neurons in the DR are typically aspiny or sparsely spiny, indicating a basic difference in the way synaptic information is received by DR neurons. Our results suggest that one of these differences could be a higher prevalence of axoaxonic cross-talk.
Estimates of the percent of GABAergic axons involved in a presynaptic relationship with glutamate are on the order of 45% with AT and 34% with EM. Although both approaches are subject to sampling errors, these percentages would suggest that axoaxonic interactions represent a fairly common feature of GABAergic boutons. The DR receives GABAergic innervation from multiple sources. Local GABAergic neurons reside both in the DR and laterally within the adjacent periaqueductal gray (24–26). The DR also receives GABAergic projections from the hypothalamus, preoptic areas, substantia nigra, ventral tegmental area, and possibly the rostromedial tegmental nucleus (13–15). Because a subpopulation of GABAergic boutons was involved in presynaptic relationships with glutamatergic boutons, an intriguing question is whether this corresponds to a particular subgroup of GABAergic neurons, as might be suspected given the propensity of GABAergic interneurons in areas such as the cortex to exhibit highly stereotypic patterns of innervation.
GABAergic synaptic inputs directly inhibit DR neurons via GABA-A ligand-gated ion channels and G protein-coupled GABA-B receptors (27–29). In addition, the present results also show that GABA indirectly regulates neuronal activity within the DR by modulating glutamatergic synaptic transmission. Interestingly, this presynaptic GABAergic modulation of glutamate release had two components: GABA-A–mediated facilitation and GABA-B–mediated inhibition, demonstrated by an increase and a decrease in the rate of miniature EPSCs, respectively. The GABA-B–mediated inhibition of glutamatergic synaptic transmission is similar to that previously observed in the DR (30). The GABA-A–induced presynaptic facilitation suggests that GABA-A activation depolarizes glutamate nerve terminals within the DR, as observed previously in the hypothalamus, hippocampus, and cerebellum (31–34). This GABA-A–induced depolarization was not an age-dependent phenomenon but rather was likely due to the relatively high chloride concentration in axon terminals resulting from enhanced presynaptic Na–K–Cl cotransporter activity (31, 35). The GABA-A–induced enhancement of transmitter release was TTX-resistant, unlike the cerebellum, suggesting that it was independent of voltage-gated Na+ channels and might be due to direct control of the resting potential of presynaptic glutamatergic synaptic terminals (36). The concomitant presynaptic excitation and inhibition displayed a similar time course of action as that observed in the cerebellum (34), where rapid/transient GABA-A–mediated facilitation and delayed but more sustained GABA-B–mediated inhibition have the capacity to temporally gate glutamatergic synaptic transmission.
GABA is thought to provide a state-dependent modulation of serotonin activity. Extracellular GABA levels in the DR increase with slow-wave sleep and more dramatically with paradoxical sleep (13, 37). On the other hand, glutamatergic neurotransmission in the DR is thought to be responsible for phasic responses to sensory stimulation (38). The interactions of GABAergic boutons extended to all different types of glutamatergic boutons in the DR, which probably arise from different sources. VGLUT1 axons likely derive from cortical sources, VGLUT2 from subcortical, and VGLUT3 from local neurons (12, 39). The common position of GABAergic boutons with respect to glutamate boutons in the DR seems ideal to contribute to the role of GABA in regulating sleep/wake states. In addition, the axoaxonic relationship between glutamate and GABA could underlie, at least in part, previous pharmacological evidence that these two neurotransmitters interact in their control of serotonin release (40).
Conclusions
The understanding of molecular and fine structural features of synaptic circuits will contribute to elucidating their role in normal and pathological neurotransmission, and whether altered circuit structure could participate in the pathophysiology of mental illnesses. Indeed, GABAergic transmission has been implicated in the pathophysiology and therapeutics of anxiety and depression both associated with sleep disturbances (41–44). Even though the DR receives innervation from multiple brain areas, AT analysis revealed that these axon terminals converge with other axon terminals on the dendritic tree in an ordered manner. Ultrastructure and electrophysiology confirmed a relationship between pairs of axonal boutons, yielding insight into how GABA may regulate the activity within the DR, with an ultimate impact on serotonin neurotransmission in the forebrain.
Materials and Methods
Array Tomography.
Serial ultrathin sections were immunolabeled as described (17, 19) using primary antisera raised against TPOH (sheep, 1:200; Millipore; AB1541) and VGLUT1, VGLUT2, and VGLUT3 (all three guinea pig, 1:1,000; Millipore; AB5905, AB2251, and AB5421, respectively) and monoclonal antibodies for synapsin 1 and GAD2 (rabbit, 1:200; Cell Signaling Technologies; 5297 and 5843, respectively). Antibodies from different hosts were applied together in a random order and imaged, followed by elution and reimmunolabeling (17, 19). Images from serial sections were converted into stacks and aligned using Fiji software and StackReg and MultiStackReg plug-ins (45) using TPOH immunolabeling as the fiduciary (19). The “enhance contrast” and “subtract background” functions of Fiji were also used on images before the analysis. The image’s contrast was defined at 0.1% of saturated pixels, whereas spurious background was removed using a rolling ball radius of 20 pixels. Volumetric images were rendered using the Fiji 3D view function with a resampling factor of 1. Two blocks were sectioned from each of three rats. Two regions were sampled from each block, yielding a total of 12 stacks containing at least 25 images each. For each stack, 90 × 90 μm areas were arbitrarily selected within the midline DR. Image stacks were converted to binary images using the threshold function initially selected using the JACoP plug-in (46) and in some cases manually adjusted (Fig. S2). Overlapping voxels were identified using the “multiply” operation of the “image calculator” function and counted using the Object Counter 3D plug-in. When searching for adjacencies where voxels may not overlap, we applied the dilate function to capture nearby associations. Cross-correlation analysis involved the JACoP plug-in (46, 47).
EM and Electrophysiology.
Standard protocols were followed (48, 49). For immuno-EM, the primary antibody used was against GAD2 (rabbit, 1:1,000; Cell Signaling Technologies; 5843).
Further experimental details are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Dr. Sun Jung Bang for tissue processing in EM experiments. This work was supported by National Institutes of Health Grant DA021801.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304505110/-/DCSupplemental.
References
- 1.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8(3):365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28(20):5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABA(B) receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J Neurosci. 2010;30(35):11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruchas MR, et al. Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71(3):498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockmeier CA. Neurobiology of serotonin in depression and suicide. Ann N Y Acad Sci. 1997;836:220–232. doi: 10.1111/j.1749-6632.1997.tb52362.x. [DOI] [PubMed] [Google Scholar]
- 7.Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- 8.Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755(2):229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- 9.Commons KG. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3) J Chem Neuroanat. 2009;38(4):273–281. doi: 10.1016/j.jchemneu.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hioki H, et al. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518(5):668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963(1-2):57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- 12.Soiza-Reilly M, Commons KG. Glutamatergic drive of the dorsal raphe nucleus. J Chem Neuroanat. 2011;41(4):247–255. doi: 10.1016/j.jchemneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gervasoni D, et al. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20(11):4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirouac GJ, Li S, Mabrouk G. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J Comp Neurol. 2004;469(2):170–184. doi: 10.1002/cne.11005. [DOI] [PubMed] [Google Scholar]
- 15.Lavezzi HN, Parsley KP, Zahm DS. Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: Psychostimulant-elicited Fos expression and collateralization. Brain Struct Funct. 2012;217(3):719–734. doi: 10.1007/s00429-011-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishchenko Y, et al. Ultrastructural analysis of hippocampal neuropil from the connectomics perspective. Neuron. 2010;67(6):1009–1020. doi: 10.1016/j.neuron.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micheva KD, Smith SJ. Array tomography: A new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55(1):25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-synapse analysis of a diverse synapse population: Proteomic imaging methods and markers. Neuron. 2010;68(4):639–653. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soiza-Reilly M, Commons KG. Quantitative analysis of glutamatergic innervation of the mouse dorsal raphe nucleus using array tomography. J Comp Neurol. 2011;519(18):3802–3814. doi: 10.1002/cne.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25(12):687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- 21.Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: A light and electron microscope radioautographic study. J Comp Neurol. 1982;207(3):239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- 22.Marinelli S, et al. Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J Neurophysiol. 2004;92(6):3532–3537. doi: 10.1152/jn.00437.2004. [DOI] [PubMed] [Google Scholar]
- 23.Isaacson JS, Solís JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10(2):165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 24.Belin MF, et al. GABA-accumulating neurons in the nucleus raphe dorsalis and periaqueductal gray in the rat: A biochemical and radioautographic study. Brain Res. 1979;170(2):279–297. doi: 10.1016/0006-8993(79)90107-0. [DOI] [PubMed] [Google Scholar]
- 25.Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122(1):193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- 26.Fu W, et al. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518(17):3464–3494. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- 27.Lemos JC, et al. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci. 2006;24(12):3415–3430. doi: 10.1111/j.1460-9568.2006.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abellán MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABA(B) receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36(1):21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Vaughan CW, Christie MJ. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol. 1996;117(8):1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colmers WF, Williams JT. Pertussis toxin pretreatment discriminates between pre- and postsynaptic actions of baclofen in rat dorsal raphe nucleus in vitro. Neurosci Lett. 1988;93(2-3):300–306. doi: 10.1016/0304-3940(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 31.Jang IS, Jeong HJ, Akaike N. Contribution of the Na-K-Cl cotransporter on GABA(A) receptor-mediated presynaptic depolarization in excitatory nerve terminals. J Neurosci. 2001;21(16):5962–5972. doi: 10.1523/JNEUROSCI.21-16-05962.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang IS, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience. 2006;138(1):25–35. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Stell BM, Rostaing P, Triller A, Marty A. Activation of presynaptic GABA(A) receptors induces glutamate release from parallel fiber synapses. J Neurosci. 2007;27(34):9022–9031. doi: 10.1523/JNEUROSCI.1954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugh JR, Jahr CE. Axonal GABAA receptors increase cerebellar granule cell excitability and synaptic activity. J Neurosci. 2011;31(2):565–574. doi: 10.1523/JNEUROSCI.4506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price GD, Trussell LO. Estimate of the chloride concentration in a central glutamatergic terminal: A gramicidin perforated-patch study on the calyx of Held. J Neurosci. 2006;26(44):11432–11436. doi: 10.1523/JNEUROSCI.1660-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48(1):109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: Role in the control of REM sleep. Am J Physiol. 1997;273(1 Pt 2):R451–R455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine ES, Jacobs BL. Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: Microiontophoretic studies in the awake cat. J Neurosci. 1992;12(10):4037–4044. doi: 10.1523/JNEUROSCI.12-10-04037.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herzog E, et al. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21(22):RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res. 2003;961(1):109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- 41.Krystal JH, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 42.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24(7):495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- 43.Liu GX, et al. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology. 2007;32(7):1531–1539. doi: 10.1038/sj.npp.1301281. [DOI] [PubMed] [Google Scholar]
- 44.Jindal RD, Thase ME. Treatment of insomnia associated with clinical depression. Sleep Med Rev. 2004;8(1):19–30. doi: 10.1016/S1087-0792(03)00025-X. [DOI] [PubMed] [Google Scholar]
- 45.Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7(1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 46.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 47.van Steensel B, et al. Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J Cell Sci. 1996;109(Pt 4):787–792. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]
- 48.Commons KG, Beck SG, Bey VW. Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci. 2005;21(6):1577–1586. doi: 10.1111/j.1460-9568.2005.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drew GM, Mitchell VA, Vaughan CW. Glutamate spillover modulates GABAergic synaptic transmission in the rat midbrain periaqueductal grey via metabotropic glutamate receptors and endocannabinoid signaling. J Neurosci. 2008;28(4):808–815. doi: 10.1523/JNEUROSCI.4876-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.