Abstract
Understanding the variability of the hippocampus in human brain research is essential. The effect of age on the hippocampus has been explored in several studies that have been focused on either normal aging or neural degeneration. Shape analysis of magnetic resonance imaging (MRI) provides morphological measures for brain structures. This study further investigates the age effects on hippocampal morphology in three groups (104 normal controls, 24 Alzheimer’s disease (AD) and 14 vascular dementia (VaD) patients). By utilizing a parametric shape analysis of hippocampal MRI scans, each individual distance map is generated and analyzed statistically. Specifically, after eliminating similarity parameters (rotation, translation, and scaling) effects for each hippocampus, an individual distance map is generated from parametric hippocampal surfaces and medial axes. Then statistical methods, including regression, and permutation tests, are applied to detect the differences in hippocampal distance maps and volumes under the effect of age in each group. Statistical analyses reveal that the loss of hippocampal volume and changes in shape are more significantly related to aging in the control group than in AD/VaD. The results also show that the asymmetry of hippocampus in healthy subjects is greater than that in either of the disease groups. Our study shows that 3D statistical shape analysis could enhance the understanding of age effects on local areas of hippocampi. However, the sample sizes of disease groups are relatively low; further studies with more AD/VaD data are needed.
Keywords: Statistical shape analysis, age, hippocampus, Alzheimer’s disease, vascular dementia
INTRODUCTION
It is well-known that the hippocampus plays an important role in the formation of explicit memory. One of the major factors associated with hippocampal changes is age. The age association has been demonstrated in several studies with cross-sectional (Lim et al., 1990, Coffey et al., 1992, Pfefferbaum et al., 1994, Sullivan et al., 1995, De Leon et al., 1997, Schuff et al., 1999, Jernigan et al., 2001, Scahill et al., 2003, Sullivan et al., 2005) and longitudinal (Jack et al., 1998, Jack et al., 2000, Scahill et al., 2003, Raz et al., 2004b, Du et al., 2006) designs. Recent research based on magnetic resonance imaging (MRI) has also demonstrated that a spatial mapping method could enhance the detection of the subtle changes in volumetric information and the understanding of age effects on specific regions of hippocampi (Janke et al., 2001, Wang et al., 2003, Thompson et al., 2004, Chetelat et al., 2005).
Hippocampal volumes, measured in-vivo, using quantitative 3D MRI have been investigated in many aging and dementia related studies (Seab et al., 1988, Jack et al., 1992, Juottonen et al., 1999, Laakso et al., 2000, Xu et al., 2000, Sencakova et al., 2001, Du et al., 2002). As Alzheimer’s disease (AD) and vascular dementia (VaD) are two common types of dementia, volumetric measures of hippocampus and other medial temporal lobe structures in both disease groups are often compared to non-demented controls. For these comparisons, subject may be matched for age or other factors to determine whether these measures could differentiate between cases with dementia and non-demented controls. These studies found that hippocampal and other temporal structural volumes in the dementia groups were significantly smaller than those in the non-demented group (Kaye et al., 1997, Jack et al., 1999, Fein et al., 2000, Grundman et al., 2002, Jack et al., 2002, Mungas et al., 2002, Jack et al., 2003). Volume reductions of the hippocampus have been observed in cross-sectional studies (Du et al., 2001, Wolf et al., 2001, van de Pol et al., 2006), as well as in longitudinal studies (Kaye et al., 1997, Jack et al., 1998, Jack et al., 2000, Chetelat and Baron, 2003, Jack et al., 2004). These longitudinal studies have also shown that the degree of hippocampal volume atrophy in the subjects with dementia (AD/VaD) was much greater than that in the non-demented controls. Cognitive and functional memory measures were also found to be associated with the changes of hippocampal volumetric measures (Golomb et al., 1993, Golomb et al., 1994, de Leon et al., 1996, Golomb et al., 1996, Petersen et al., 2000, Grundman et al., 2003, Van Petten, 2004, Mungas et al., 2005). In addition, studies of VaD are less frequent compared to AD. Furthermore, most studies of VaD have been compared with other disease groups rather than to non-demented group to find how the different pathologies affect the volumetric differences of brain structures (e.g. hippocampus, entorhinal cortex) (Laakso et al., 1996, Barber et al., 2000, Mungas et al., 2001, Du et al., 2002, Swartz et al., 2002, Wolf et al., 2004).
In addition to treating age as a matching factor, many studies have analyzed the effect of aging on the volume of the hippocampus (Lim et al., 1990, Convit et al., 1995, Sullivan et al., 1995, De Leon et al., 1997, Jernigan et al., 2001, Scahill et al., 2003, Raz et al., 2004a, Raz et al., 2004b, Sullivan et al., 2005, Du et al., 2006). However, these findings in healthy subjects have been inconsistent. Some studies revealed that age was not significantly related to the volume reduction of the hippocampus (Sullivan et al., 1995, Sullivan et al., 2005); others obtained the opposite results (Lim et al., 1990, Convit et al., 1995, De Leon et al., 1997, Jernigan et al., 2001, Scahill et al., 2003, Raz et al., 2004a, Raz et al., 2004b, Du et al., 2006). The discrepancies in the results of these reports might be due to differences in the segmentation of anatomical regions, or in the age range of the subjects studied. Therefore, one of the aims in this study is to evaluate the age effect on the hippocampal volumes. Volumetric measures of the hippocampus alone do not clearly define the specific region of morphological change. Geometric shape analysis methods that capture local changes in specific brain structures can complement volumetric measures (Janke et al., 2001, Wang et al., 2003, Thompson et al., 2004, Chetelat et al., 2005). In previous applications of geometric shape analysis methods in AD studies, the global and morphological changes were demonstrated for a predefined fixed short period (e.g. in Thompson’s paper, hippocampal morphological changes of each subject were evaluated after 2 years). In addition, most previous studies focused on discriminating the diseased groups from the normal clusters and emphasizing the physiological effects. However, normal aging often shows that the hippocampal structural shrinkages increase with the advancement of age (Thompson et al., 2004). Additionally, although age is an important factor in many hippocampal geometry shape studies, it is not clear if the contributions of age on hippocampal shape or volume differ in demented and non-demented groups. A sub-sample of the population-based Honolulu-Asia Aging Study (HAAS) cohort, aged 75 – 95, is chosen for our data pool. The HAAS is a cohort of Japanese-American men, therefore confounding due to sex and ethnicity is minimized. Thus, the further investigation of the effects of age on hippocampal structural changes in a well-chosen sample is another major aim to be pursued in this study.
This study explores the hippocampal changes due to age in non-demented controls, AD and VaD. To obtain the shape change of the hippocampus, parametric-based structure is used to automatically generate the shape map of hippocampus in a large population-based MRI study of the normal and diseased elderly brain. By fitting a statistical model across all of parametric hippocampi, age-related structural changes are investigated in each group. In addition, the association of age to hippocampal volume changes in each group is investigated.
MATERIALS AND METHODS
Subjects and Image Acquisition
Subjects
The details of the HAAS study population have been previously reported, (White et al., 1996). In brief, the cohort includes Japanese-American men born between 1900 and 1919, living on the Oahu Island of Hawaii, who were enrolled in 1965 as part of the Honolulu Heart Program. In 1991, the HAAS was initiated to study brain aging in 3734 men (80% of the surviving cohort). The protocol included measurement of global cognitive function and case finding for prevalent dementia in the 1991 exam and incident dementia in subsequent exams. The Kuakini Medical Center Institutional Review Board approved this study. All subjects gave informed written consent and most of them signed it, except those who showed signs of dementia. In these situations, an informed caretaker signed the consent form.
The multiple steps have been used to identify sub-type dementia cases, that is described previously (White et al., 1996). In brief, all subjects were first screened with the CASI (Teng et al., 1994), a measure of global cognitive function. Then those who met screen positive criteria were further examined, including a proxy interview, neuropsychological testing based on the CERAD battery (Morris et al., 1989), a neurological exam, and neuroimaging. Finally, diagnosis was made in a consensus conference; DSM-IIIR criteria (American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV., 1994) were applied for dementia, NINDS-ADRDA (McKhann et al., 1984) for AD, and the CADDTC for vascular dementia (Chui et al., 1992). Severity of the cognitive and functional impairment was assessed with the Clinical Dementia Rating scale (Hughes et al., 1982) (see Table 1b).
Table 1.
| a: Description of Study Sample | |||
|---|---|---|---|
| Description | # Subjects | Sex | Age (Mean ± SD) |
| Non-Demented | 104 | Male | 83.2 ± 4.4159 |
| AD | 24 | Male | 82.5 ± 4.5778 |
| VaD | 14 | Male | 80.5 ± 4.4678 |
| b: Neurocognitive status for the AD and VaD cases | |||
|---|---|---|---|
| AD (n=24) | VaD (n=14) | ||
| CASI @ exam 5 (median, [95% CI]) | 67 [58–70] | 67 [58–72] | |
| CDR @ exam 5 (%) | |||
| Stage 1 | 14 (78%) | 11 (85%) | |
| Stage 2 | 3 (17%) | 2 (15%) | |
| Stage 3 | 1 (6%) | 0 (0%) | |
| Missing / UNK | 6 (25%) | 1 (7%) | |
| 18 | (13) 100% | ||
| (100%) | |||
Legend:
AD – Alzheimer’s disease
VaD – Vascular dementia
CASI – Cognitive Abilities Screening Instrument;
CDR – Clinical Dementia Rating Scale
In 1995, a sub-study (n = 575) on MRI was initiated in examination 5. The brain MRI subsample was chosen by use of information from HAAS examinations 4 and 5. In addition to a ≈10% random sample of those participating in examination 5, there was an over-sampling of those with prevalent dementia (excluding the severely demented, who might not be able to undergo the procedure), those who scored poorly on the Cognitive Abilities Screening Instrument (CASI) but did not meet criteria for dementia, those with apoE4 genotype, those with clinical stroke, and those at the oldest ages. As a part of this sub-study, the hippocampus was manually outlined. This analysis is based on 104 non-demented subjects, 24 incident AD cases, and 14 VaD cases whose scans could be successfully processed, as described previously in (Scher et al., 2007). The demographic and group details are summarized in Table 1. The differences in age were not significant between any two groups (Control vs. AD: 0.7675; Control vs. VaD: 0.1430; AD vs. VaD: 0.1984).
Imaging
High-resolution 3D T1-weighted Spoiled Gradient Recalled (SPGR) MRI volumes were acquired on a GE signa 1.5T scanner at Kuakini Medical Center, Honolulu, from all subjects in this study using a protocol described earlier (Scher et al., 2007). Each acquired SPGR sequence was reformatted to oblique coronal sections perpendicular to the long axis of the left hippocampus; every new reformatted T1-weighted MRI image had a 256 × 256 × 124 matrix. In addition, the corresponding left and right hippocampal regions, which included the CA1-4 or CA1-3 regions, the dentate gyrus, the subiculum, fimbria and alveus, were delineated by one reader (intra-rater correlation = 0.97) , who is blinded to dementia diagonosis, using MEDx software (Sensor Systems, Inc., Sterling, Va). The protocol for defining hippocampal borders was described earlier (Scher et al., 2007). The left and right hippocampi were manually determined with Jack’s criteria (Jack et al., 1997). In brief, the most anterior slice of the hippocampal formation was marked as the first slice on which ventral of the amygdale is visible. The ventral border was formed by the white matter of the parahippocampal gyrus. In anterior area, the dorsal border was formed by the amygdala; in more posterior area, cerebrospinal fluid and choroid plexus formed the dorsal border. The most posterior slice of hippocampus was the slice in which the crux of the fornix is visible in its total length.
Image Preprocessing
To improve the accuracy of characterizing the morphological changes of hippocampus, we first applied a skull stripping method (Shattuck et al., 2001) to remove non-brain tissues (skull, skin and fat tissue) and used an inhomogeneity correction method (Sled et al., 1998) in each reformatted T1-weighted MRI image. Then a common space for expressing the coordinates of brain structure from various subjects was created by an automatic registration method (Woods, 1998a, Woods, 1998b). To eliminate the position differences, each hippocampus was then registered to the common space using a linear registration method.
Skull-stripping and Inhomogeneity Correction
Brain Surface Extraction (BSE) (Shattuck et al., 2001) was first used to remove non-brain tissues including the scalp, bone, and meninges, from each reformatted T1-weighted MRI volume. Each individual brain image was further manually edited to eliminate the errors created by the automated segmentation and to remove the spinal cord whose intensity was similar to the intensity of brain tissue. Inhomogeneties in each skull-stripped scan were also eliminated by a radio frequency bias correction method.
3D Image Alignment
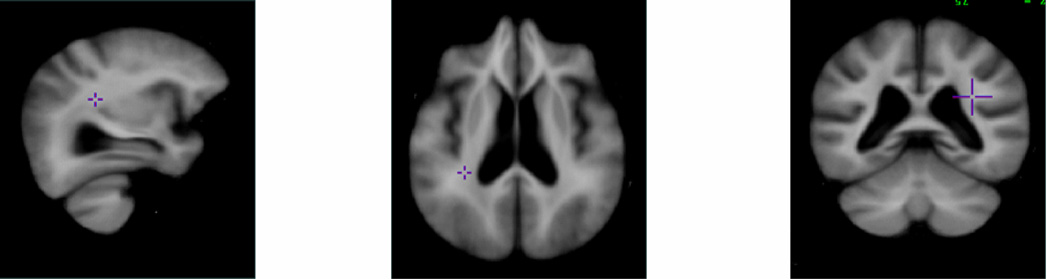
In order to retain quantitative information on inter-subject variations in our population, a study-specific brain atlas was defined using a series of steps. First, to include the population brain information, each skull-stripped and bias corrected brain image was linearly aligned to a randomly selected brain image using the automated image registration (AIR) algorithm (Woods, 1998a, Woods, 1998b) and averaged voxel-by-voxel to create an intermediate average brain image. Then to form an affine average brain, all of the brain scans were again aligned linearly to the intermediate brain image and averaged together. In the last step, in order to register the whole brain including deep structures, a non-linear registration method was then applied to obtain a study-specific atlas (Figure 1) that encompassed the structural information of the study population.
Figure 1.
Study-specific atlases created by 142 brain images manually edited by experts after BSE segmentation
To remove the effect of similarity parameters after the study-specific atlas was obtained, each brain scan was linearly registered to it with the same software used to create the template. As the hippocampus was the major focus of this study, each transformation was saved to align the same subject’s hippocampi to the common space.
Contour Preprocessing
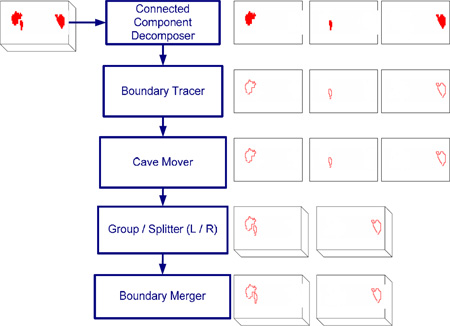
In our study cases, hippocampal volumes from the original delineated images were retained for volumetric statistical analysis. However, not all the manually delineated hippocampal images had a continuous contour on each slice. To make them continuous, each hippocampal region was automatically split into left and right regions corresponding to each hemisphere. Then individual regions were connected to form a continuous 2D contour on each slice with an automated algorithm developed in-house. For a detailed description of this algorithm, please see Appendix D. These automatically split/connected hippocampal contours for each subject were then linearly mapped to the study-specific atlas with the corresponding transformation described above.
Hippocampal Shape Mapping
After each hippocampus was spatially normalized, an anatomical mesh modeling method (Thompson et al., 1996) was then applied to match equivalent hippocampal surface points across subjects in each group.
Detailed morphological parameter mappings were obtained using the following steps. Each individual subject’s spatially digitized and normalized hippocampus was first resampled at the same spatial frequency (that is, the same number of digitized points representing hippocampal contours within and across hippocampal slices) as a 3D parametric surface mesh (Thompson et al., 2004). Each hippocampal surface thus formed a regular parametric grid (100 × 150 surface points) that was used to compare hippocampi across all subjects voxel-by-voxel. Then from that, a “medial curve” was derived by the first moment of each hippocampal surface from each hippocampal slice to obtain the hippocampal structural features. For each hippocampal model, we then measured the distance from each spatially uniform hippocampal surface point to the digitized “medial curve”. Since these distance field maps contained localized structural changes (expansion or constriction), they were used to map the hippocampal shape. In addition, because parametric meshes of all hippocampal surfaces were normalized into a common space, the corresponding distance field maps could be statistically compared across subjects and the relationship between age and hippocampal shrinkage could be evaluated using these distance field maps.
Statistical Analysis
Volumetric Analysis
The absolute volume of each hippocampus in each subject was obtained from the manually segmented hippocampal structure. The means and standard deviations for left and right hippocampus were calculated (in Table 2). Then, to eliminate inter-subject variation of head size, and to consider hippocampus as a part of brain tissues, each normalized hippocampal volume (NH) of each subject was defined as
| (1) |
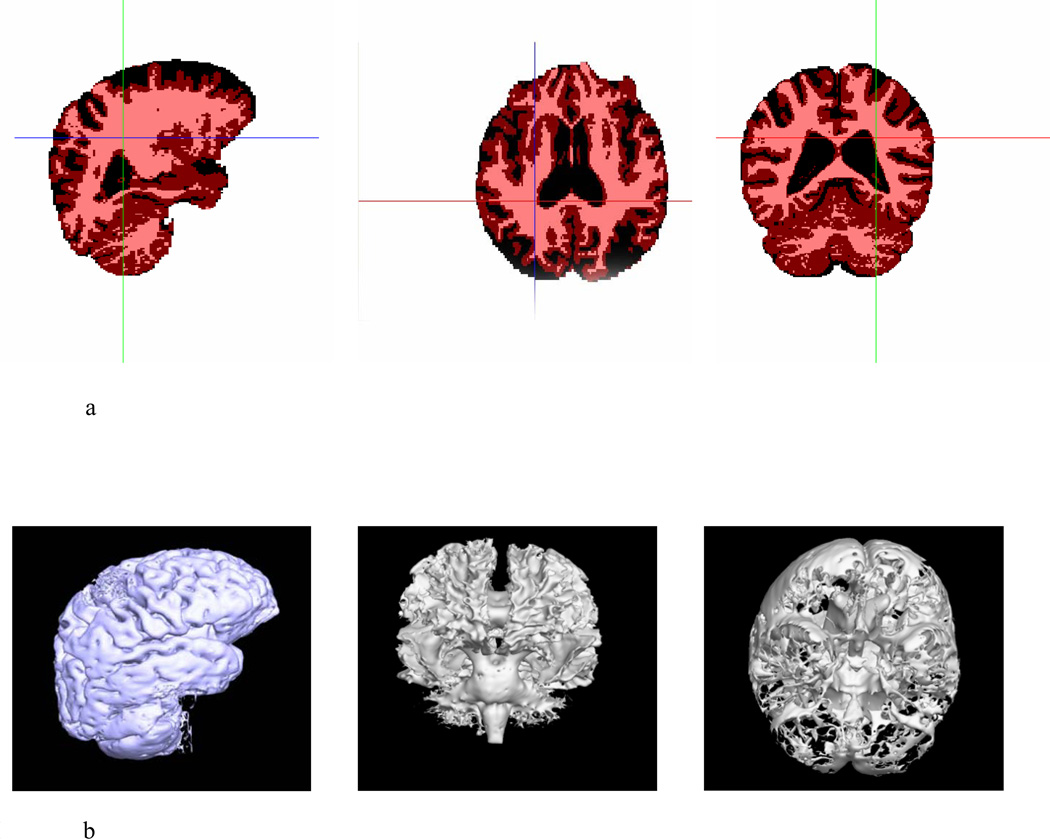
Gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) were classified by partial volume classification method (PVC) (Shattuck et al., 2001) (Figure 2). The means and standard deviations of NH were also calculated (in Table 3). Linear or rank correlation tests and linear regression were used to test the correlations between age and each absolute and normalized volume in each group.
Table 2.
Summary statistics for absolute hippocampal volumes (mm3), normalized hippocampus, and AI information in groups
| Groups |
LHV (Mean ± SD) |
RHV (Mean ± SD) |
NLH (Mean ± SD) |
NRH (Mean ± SD) |
AI (Mean ± SD) |
|---|---|---|---|---|---|
| Non-Demented Controls | 2777.7±474.16 | 2914.2±494.97 | 2.6621±0.4143 | 2.7921±0.4317 | −0.0240±0.0450 |
| AD | 2410.4±429.74 | 2542.5±464.76 | 2.3461±0.3076 | 2.4784±0.3685 | −0.0262±0.0679 |
| VaD | 2692.1±441.80 | 2833.1±548.96 | 2.6056±0.3323 | 2.7438±0.4570 | −0.0255±0.0501 |
Legend:
LHV – Left hippocampus volume
RHV – Right hippocampus volume
AI – Asymmetry index: (LHV − RHV) / (LHV + RHV)
NLH – Normalized left hippocampus: (LHV / * 1000) / (gray matter volume + white matter volume)
NRH – Normalized right hippocampus: (RHV / * 1000) / (gray matter volume + white matter volume)
# Pairwise comparison showed that p values for comparing LHV (p < 0.001), RHV (p < 0.001), NLH (p = 0.001) and NRH (p = 0.001) between control and AD were significant and that p values for comparing LHV (p = 0.040) and RHV (p = 0.005) between control and VaD were significant. Meanwhile, no significant p values were obtained for other comparisons. Since no significant for NLH and NRH between control and VaD were obtained, ANOVA was performed to evaluate VaD-Control differences in total volume of gray and white matters after adjusting for age (GM+WM: F = 10.079, p = 0.008).
Figure 2.
a: Brain tissue classification (BTC) maps for one study subject (gray matter, white matter and CSF). Red was chosen to color gray matter in three cardinal views; areas in pink represent white matter; black areas encode CSF.
b: 3D volume representations of brain tissues generated by PVC from one study subject. Left fig is the right lateral view of gray matter; middle fig is the anterior view of white matter; right fig is inferior view of CSF to illustrate lateral ventricles.
Table 3.
Pearson correlation between age and hippocampal volumetric information (in each group, the values in the first row are correlation coefficients ρ; the values in the second row are the corresponding p values)
| Variables |
Left Hippocampus |
Right Hippocampus |
NLH | NRH | AI | |
|---|---|---|---|---|---|---|
| Groups | ||||||
| Non-demented Controls | ρ | −0.2643 | −0.3579 | −0.0853 | −0.1881 | 0.1978 |
| p | 0.0067 | 0.00019 | 0.3892 | 0.0559 | 0.0441 | |
| AD | ρ | −0.3252 | −0.2253 | −0.3597 | −0.2074 | −0.1098 |
| p | 0.1210 | 0.2897 | 0.0843 | 0.3309 | 0.6097 | |
| VaD | ρ | 0.1942 | 0.2929 | 0.4360 | 0.4705 | −0.2725 |
| p | 0.5059 | 0.3095 | 0.1191 | 0.0895 | 0.3459 | |
Finally, to check the asymmetry of hippocampus, a linear regression test was used to find the relationship between left and right hippocampus:
| (2) |
Where LHV is the left hippocampal volume; RHV is the right hippocampal volume. The right hippocampal volume was modeled with a constant β (intercept) and a linear coeffienct α (slope). In addition to using the above method, we also calculated the asymmetry index (AI) of left and right hippocampus (Scott et al., 2003) defined as
| (3) |
Nonparametric tests were applied to test the asymmetry in each group (in Table 3).
Statistical Maps and Permutation Tests
We obtained the parametric hippocampal surface model for each subject. These spatially normalized hippocampal surfaces with equivalent points were averaged together across all individuals in a group to generate a mean hippocampal shape map (Thompson et al., 2004). Details are presented in Appendix A.
In addition to mean hippocampal maps, statistical maps were developed to estimate the regional differences in hippocampal shape over age in a group. Briefly, at each hippocampal point, a general linear models (GLM) was performed on hippocampal distance field maps to see if the age effect was significantly linked with the hippocampal shape changes. The P value of the significance of this linkage at each corresponding parametric grid point resulted in a statistical map (Thompson et al., 2004). The details can be found in the Appendix B. To confirm the overall significance of the statistical mapping results and to avoid complex parametric random field correction on surfaces, permutation tests were applied to assess the association of age to hippocampal regions. The details for this permutation tests can be found in Appendix C.
Brain Size Correction
In this study, we focused on age-related differences in hippocampal morphology in normal and demented groups after not only adjusting for rigid movements (rotation and translation) but also removing individual brain size differences. Although the results gave us hippocampal morphological changes, brain size corrections could increase or decrease error variance for regions of interest comparisons (Mathalon et al., 1993). The same anatomical statistical maps were therefore generated in “native scanner space” after correcting with rigid body transformations but retaining the individual brain sizes. This allowed us to see if brain size correction influenced detection of the effects. Similar results were obtained as (Thompson et al., 2004). Therefore, these results are not reported in this study.
RESULTS
Volumetric Analysis
Table 2 shows the average volumes of the hippocampus for the three study groups (non-demented controls, AD cases and VaD cases). The average volumes of the left hippocampus and right hippocampus in each disease group are smaller than those in the control group (among three groups, AD group has the smallest mean volumes of each hippocampus). In addition, normalized hippocampal volumes in AD (Table 2) are the smallest among these three groups. Analysis of variance (ANOVA) adjusted for age was also used to test for pairwise group differences in all absolute and normalized volumes and AIs. As presented in the footnote under Table 2, significant differences for both absolute and normalized volumes comparisons in AD and non-demented control were detected, but only absolute volumes comparisons in VaD and non-demented control showed significant differences. However, no significant differences for AI in any pairwise group comparison were obtained. Since no significant difference for both hippocampi could be detected between controls and VaD after adjusted by age and gray/white matter, ANOVA was also performed to evaluate VaD-Control difference in total volumes of gray and white matters after adjusting for age; significant difference was obtained (GM+WM: F = 10.079, p = 0.008).
Table 3 shows the correlations between hippocampal volumetric information and age in each group. In the non-demented group, all absolute volumes had significant correlations with age (left hippocampus (LH): ρ= −0.2643, p = 0.0067; right hippocampus (RH): ρ= −0.3579, p = 1.911×10−4) by using Pearson correlation tests (see Table 3). There were no significant relationships between normalized hippocampi and age (in Table 3). However, AIs were significantly related to age (ρ= 0.1978, p < 0.05).
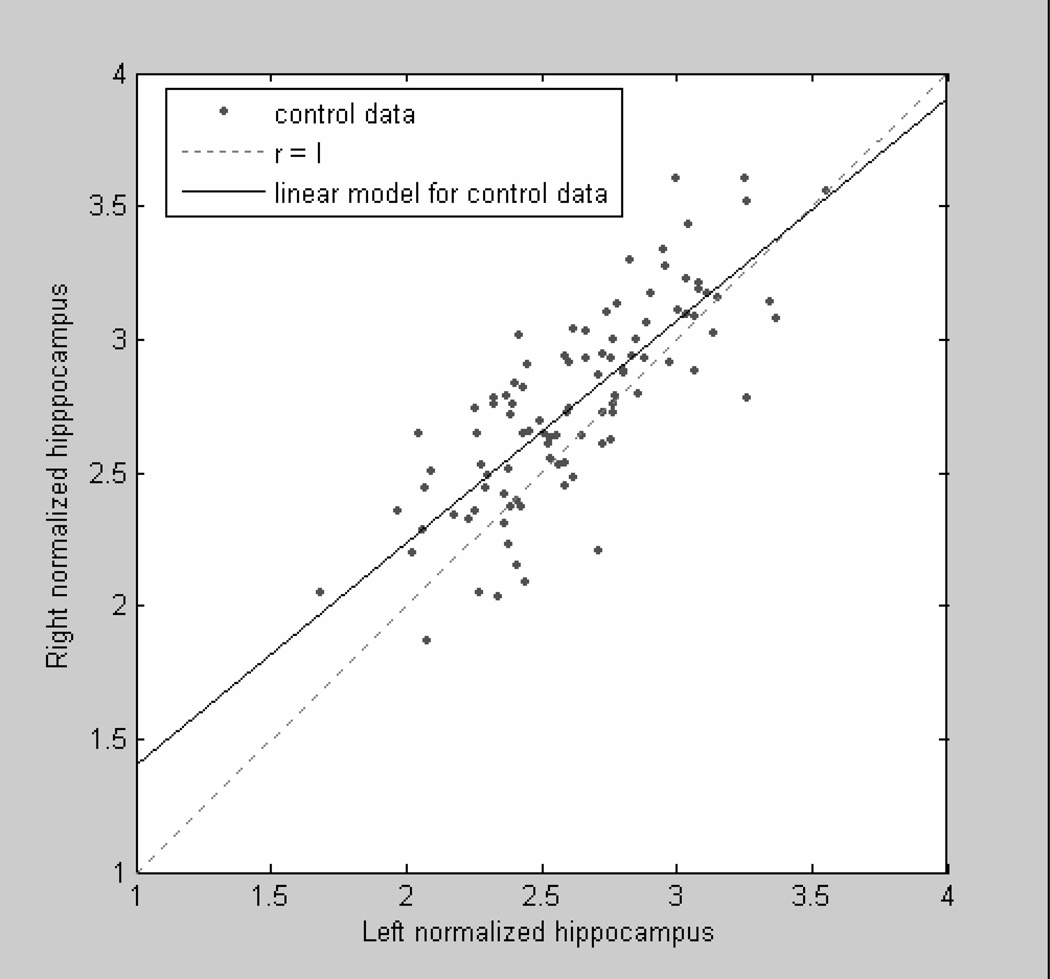
The parameters of the linear regression test describing the hippocampal asymmetry were: slope α = 0.8314 and intercept β = 0.5733 (α: P < 0.0001; β: P = 0.0012 by student’s t-tests). Figure 3 shows that the number of the normal group data above the diagonal line is much greater than below the line and most portions of the estimated linear model are above the line. Therefore, the result from the linear model suggested that there existed an asymmetry between normal right and normal left hippocampal volumes and that the right hippocampi might be larger than left hippocampi in the non-demented control group. Similar results were also obtained with the Wilcoxon sign rank tests to assess normalized volumes and AIs (P < 0.0001).
Figure 3.
Linear model for asymmetry test of control group data. Each black point is a normalized hipppocampal data. Solid line is the estimated linear model. Dashed line is the diagonal line (slope = 1) to separate space.
Age did not have significant correlations with any absolute or normalized volume in the AD group. Wilcoxon sign rank tests were applied to check if asymmetry between right and left hippocampi in AD group existed. The asymmetry was apparent, however, it was not large enough to reach statistical significance (volume compare: P = 0.0593; AIs: P = 0.0556).
Like the AD group, no significant changes of all variables in the VaD group due to age were observed by using Pearson correlation tests. The asymmetry evaluated using Wilcoxon sign rank tests was also apparent, however, it was not large enough to attain statistical significance (volume compare: P = 0.0574; AIs: P = 0.0942).
Statistical Hippocampal maps
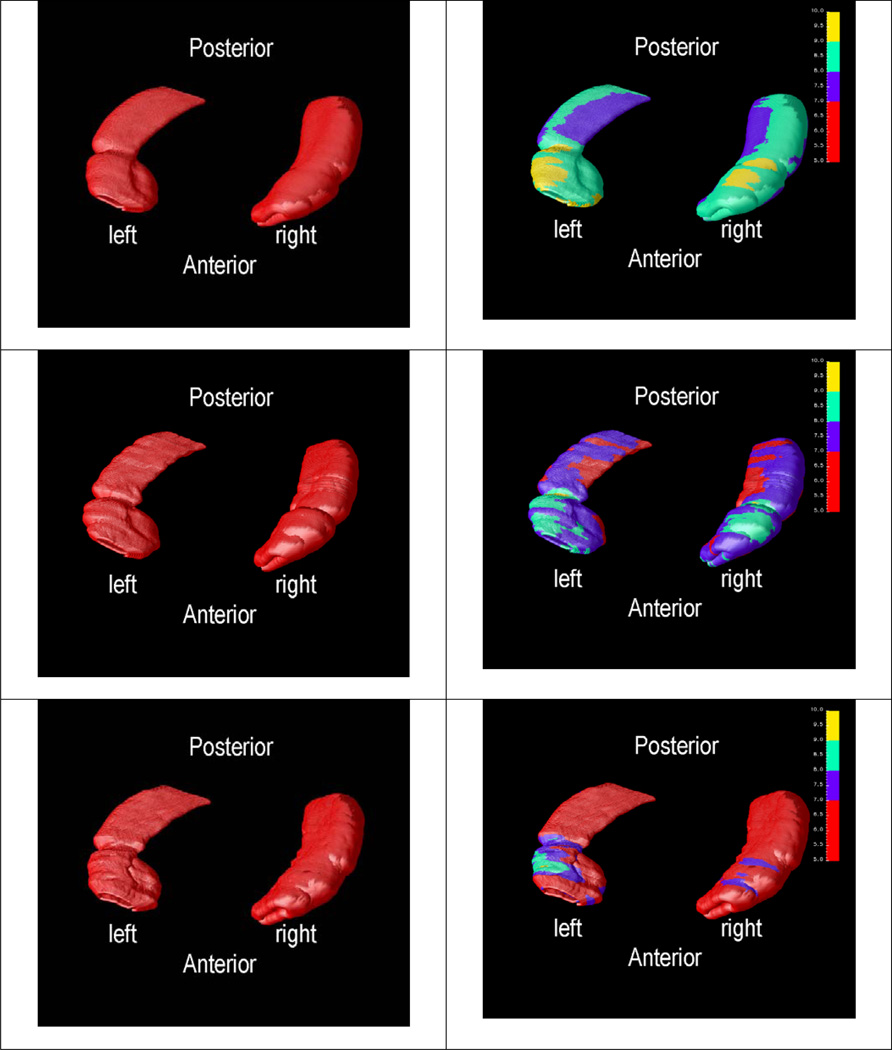
To examine whether regional differences in hippocampus were associated with age, we compared the distance field at each 3D hippocampal surface location in each group. The effects of age were mapped within each group. Probability values, indexed in color, were mapped back onto a group averaged hippocampal surface model at each 3D point.
Figure 4 shows each group’s average and variability mappings for the left and right hippocampus after registration and parametric normalization. The local regional changes of hippocampus in a group could be found in each variability mapping. The figures in the left column show the average mappings in the non-demented group (first row), the AD group (second row), and the VaD group (third row). The figures in the right column are the corresponding variability mappings, representing the variance of each mesh point. The variability mappings show that the variances of the local shape feature in most regions of the non-demented group are greater than those in AD or VaD groups and that the variances in most regions of the VaD group are the smallest.
Figure 4.
Statistical Shape Mapping (inferior view): first row - average (left) and variability (right) mappings of normal aging; second row - average (left) and variability (right) mappings of AD; last row - average (left) and variability (right) mappings of VaD (in all varialbility mappings, the color range is from 5 to 10; red color is marked from 5 to 7; blue is from 7 to 8; green is from 8 to 9; yellow is from 9 to 10.)
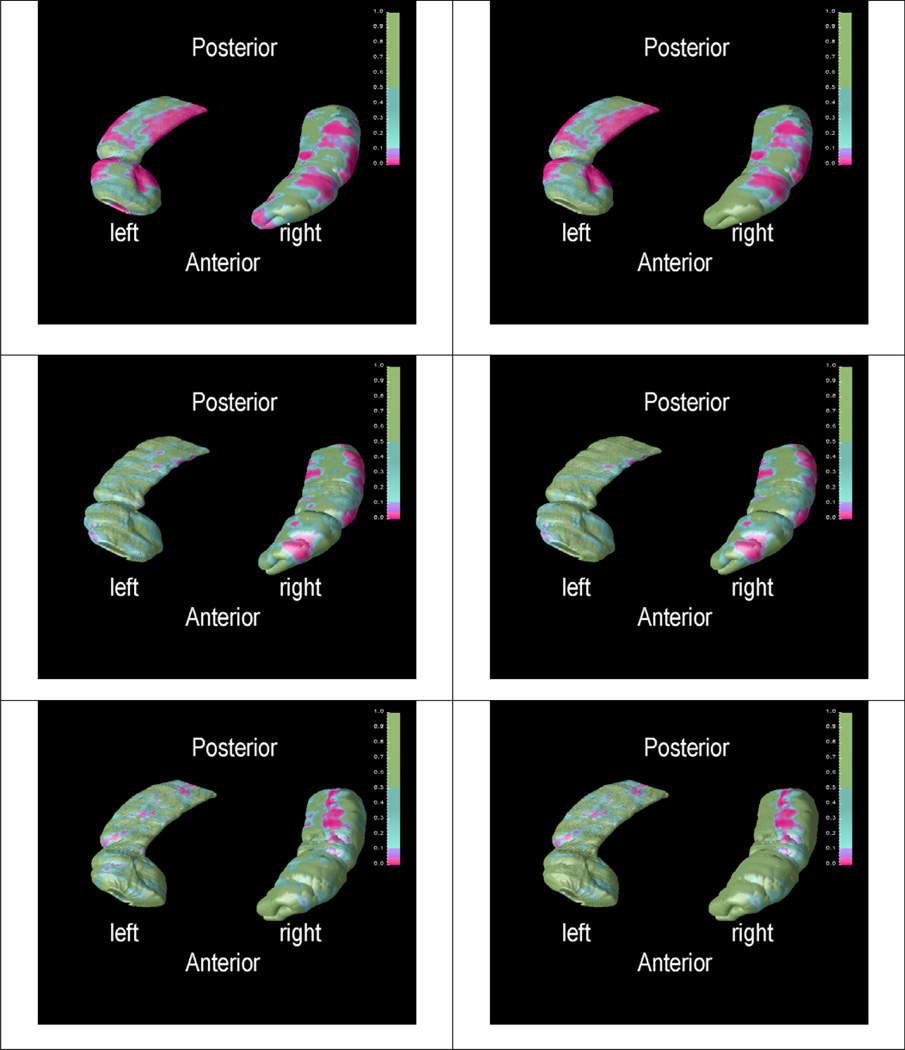
In order to show if age is related with the local shape changes, statistical p maps of the distance field were created by using general linear model. Figure 5 shows the statistical significance structural changes associated with age for the three groups. P-values < 0.05 are colored as pink. Figures in the first row of Figure 5 show that regional hippocampal decreases as age increases in the non-demented group. Furthermore, in the control group, the pink region in the left figure in the first row of Figure 5 suggests that the local changes in those regions either significantly increase or decrease with increasing age; the pink region in the figure on the right in the first row of Figure 5 indicates that the local changes of those regions are inversely and significantly correlated with age. The significant negative linear relationship between hippocampal morphological changes and age were widely distributed in both left and right hippocampi, especially in CA1 and subiculum area. Similarly, the pink region in the left figure in the second row of Figure 5 suggests that the local changes of those regions are significant related to the change of age in AD group; the pink region in the right figure in the second row of Figure 5 indicates that the local changes of those regions are significant decreasing as age is increasing in AD group. Several local areas of significant loss of right hippocampus with increased age in AD are also observed in Figure 5. However, there is no indication of significant age effects to the hippcampi in the VaD (see the third row of Figure 5).
Figure 5.
P maps of age effect (inferior view). First row - absolute P map (left) and negative P map (right) in normal group; second row - absolute P map (left) and negative P map (right) in AD group; third row - absolute P map (left) and positive P map (right) in VaD group (in all images pink part means significant different with P < 0.05)
Permutation Tests
To confirm the overall significance of the statistical mapping results, permutation tests were conducted to correct for possible spatial significance caused by voxel-wise parametric testing. These Permutation tests were applied separately to analyze whether the reduction of the hippocampus were significantly related to age in each group. In the non-demented group, the results indicated that significant relationships between right/left hippocampal reduction and age were widely distributed in the hipppocampus with significant permutation P-values (R: 0.0030; L: 0.00018). This implies that both hippocampi in the non-demented group are deficit with increasing age. In the AD group, since there was no an overall correlation between left hippocampal deficits and age, significant permutation tests’ result could not be obtained. There was an overall correlation between right hippocampi atrophies and age, but it was not strong enough to reach statistical significant (P = 0.0687). In the VaD group, there were no significant relationships between both hippocampi and age.
DISCUSSION
The hippocampus has been analyzed in many studies pertaining to central nervous system diseases and brain development. The MR-based shape analysis method has the ability to extract three-dimensional aspects of hippocampal shape, and therefore may enhance the sensitivity and specificity of structural hippocampal analysis. In the present work, we have demonstrated a complete sequence of conducing shape analysis of MRI scans of human hippocampus with the aim of studying aging effect in a relatively homogenous community-based sample of Japanese American men. Although hippocampal volume reductions with different patterns in normal aging and dementias have already been reported, specific regions of hippocampal volume loss due to age are still not clear. In this study, we employed a study-specific and surface-based anatomical modeling approach to detect the local hippocampal regional changes due to age in non-demented and dementia groups. Significant atrophies in some regions were detected in the non-demented and the AD groups. The main findings are: (1) age was significantly correlated with hippocampal volume only in the non-demented group and not in the two disease groups (AD and VaD); (2) in volume asymmetry tests, we also found that the volume of right hippocampus was significantly larger than that of left hippocampus in the non-demented group; (3) in shape analysis tests, the significant associations between hippocampus and age were in areas consistent with CA1 and subiculum among the non-demented group. Additional follow-up with more detailed anatomical labeling is needed to further explore these findings. However, these current analysis shows that shape analysis can help formulate hypotheses related to different areas of vulnerability in brain structures.
Volumetric Loss
Significant absolute volume reductions related to the age effect were detected in the non-demented group. This is similar to what has been reported in some previous studies (Lim et al., 1990, Convit et al., 1995, De Leon et al., 1997, Jernigan et al., 2001, Scahill et al., 2003, Raz et al., 2004a, Raz et al., 2004b, Du et al., 2006). However, after normalization the relationships were no longer significant. This might be related to the fact that brain tissues (except CSF) decrease with increasing age and the normalization takes out the age-related variability of the measures. In addition, there were no significant relationships between hippocampal volumes and age in either the AD or VaD groups. One explanation might be the relatively small number of subjects in the dementia groups such that there might be the lack of the power to detect the correlations between hippocampal volumes and age in the dementia groups. Furthermore, another factor should be taken into consideration. White matter lesions (WML) are frequently found on MRI images of both demented and non-demented individuals. Some studies found a linear relation between white matter lesions and HA (e.g. (de Leeuw et al., 2004)); some found no relationship between hippocampal volume and WMH volume (e.g. (Du et al., 2005)). However, we do not have volume information of WML for individual subjects in our study. Therefore, unknown WML volume could be a confounding factor that may explain the finding of no significant relationship between hippocampal volumes and age. Yet although the different stages of the disease process could affect the volumes of hippocampi, most of our dementia cases were in CDR stage I (shown in Table 1b). Therefore, the residual confounding by disease stage would not be a big reason in failing to detect significant relationships between hippocampal volumes and age in dementia groups. Hippocampal volume information related to VaD has been reported in a few studies for comparison either with other disease groups (e.g. AD) or with a normal group. In most of those studies, age was treated as a matching factor or a covariate in cognitive function. Bigler et al. (Bigler et al., 2002) found that age alone was associated with hippocampal volume (r = −0.23; p = 0.001). However, this conclusion was obtained from the whole 85 subjects including AD, VaD, cognitive symptoms classified as mild/ambiguous, other neuropsychiatric disorder and normal subjects. Therefore, it is difficult to compare our results in the disease groups with the results from this previous study.
The normalized hippocampal volume of each subject in this study was defined as absolute hippocampal volume divided by the total volume of gray and white matters. Therefore, this normalization method might lead to an over-estimation of hippocampal volumes in these three groups since the volumes of gray and white matters are expected to be lower in older and demented groups. In asymmetry tests, we also found that the volume of right hippocampus was significantly larger than that of left hippocampus in the non-demented group. This is consistent with the findings in (Thompson et al., 2004). However, in AD and VaD groups, the asymmetries of hippocampi were close to significant (AD: P = 0.0593; VaD: P = 0.0574). Laakso et al. (Laakso et al., 2000) found that the change of hippocampal on the right side was significant (P < 0.03) but not on the left (P = 0.16) in 27 AD patients. Our results are compatible with these published studies.
In the volumetric comparison between VaD and control, we found that there are significant differences for absolute volumes instead of normalized volumes. Since the difference of the total volume of GM and WM between VaD and non-demented control adjusted by age is significant, it might be the reason for non-significant differences in normalized volumes. In addition, since white matter lesion might be related to hippocampal atrophy, it could affect the result of comparison in normalized volumes.
Hippocampal Shape Maps
Several recent studies (Janke et al., 2001, Wang et al., 2003, Thompson et al., 2004, Chetelat et al., 2005) have demonstrated that statistical mapping is a promising approach for analyzing brain structures because it could be used to visualize localized information by color encoding. Similar algorithms were used to evaluate structural changes of hippocampus in different physiological conditions (Thompson et al., 2004, Becker et al., 2006, Frisoni et al., 2006, Scher et al., 2007). All of them found that there was significant local atrophy in the hippocampus in the patients with AD compared with the non-demented controls. The maps for comparing mild cognitive impairment (MCI) with the non-demented controls is also useful to predict future cognitive changes (Apostolova et al., 2006, Becker et al., 2006). Compared with these existing studies, our approach is different in terms of encoding the significance of the correlation between age and the shape feature. This leads to the new discovery that localized significant correlation exists only for the normal aging group. Therefore, statistical mapping is versatile in satisfying the needs for different research hypotheses by encoding different information.
Relation between Volumetric and Shape Analysis
Both volumetric and statistical shape methods were employed to explore the age effects in the different aging groups, each having it own strength. Hippocampal volumes represent the global shape information and hippocampal statistical mappings could visualize the local changes in the hippocampus. Although they characterize different shape information, the more local loss is found, the more global volumetric loss could be detected. Therefore, when permutation tests were applied to check the overall significance of hippocampal decline based shape analysis results, similar conclusions could be reached using hippocampal volume tests. However, the statistical shape analysis of brain structure could help researchers not only measure the global variables, but also visualize, compare and summarize the local variable in brain structures. Although we could not find an overall significant relationship between hippocampal reductions and age, the 3D statistical map showed that the significant changes of the hippocampus due to age could be found in different areas in different aging groups. Our maps also show that the significant hippocampal changes correlated with age is not uniform; this means that statistical shape analysis could be able to give higher signal-to-noise ratio (SNR) than volumes for the effect of age.
Future Studies
Despite the advantage of shape analysis, the current study still has several limitations. First, we are interested in the effects of the age factor on the hippocampus. However there are several other factors that can cause the structural changes of the hippocampus. In the future, a reasonable model incorporating more risk factors (e.g. genetic effect, different genders and etc.) needs to be defined. Second, this study is cross-sectional and not longitudinal, and the age effect may be over-estimated or under-estimated. The longitudinal course of disease and age could improve our understanding of brain structure changes over the clinical stage in order to obtain more sensitive relationship between brain morphological changes and known risk factors. Another limitation of the study is that the available sample size was relatively low for the vascular dementia group, and much greater for the non-demented controls. 4.2% of the HAAS sample are VaD cases (White et al., 1996) and the MRI sub-study is only a sub-sample of the total cohort. As brain mapping studies of VaD are so rare, we chose to report maps based on our limited sample to date.
In this study, several assumptions were made. First, in order to find a common space, we assumed that we had good skull stripping results (to separate brain from non-brain tissue). However, we know that the brain structure is highly variable across a population. When we are interested in a specific brain structure (e.g. hippocampus), we believe that it is better to define a common space for those individual structures instead of the common space defined by the whole brain. Furthermore, the continuous assumption in 2D is not necessarily true in 3D space. Therefore, in order to achieve a more accurate shape analysis, an algorithm to extract 3D surface instead of 2D contour is needed.
Moreover, compared with the over 100 subjects in the healthy control group, the number of each disease group is relatively small especially in the VaD group. The statistical shape analysis is implemented in a relatively low degree of freedom and large variance among individual hippocampi. A larger sample size in each disease group would further improve the strength of statistical mappings. In summary, this quantitative MRI study shows that hippocampal volume, for the normal population between 75 and 95 years old, declines with increasing age and that hippocampal shape shrinks at some local regions. However, there are no significant relationships between normalized hippocampal volumes and age. On the other hand, volume changes associated with age are not significant for the AD and VaD groups in the similar age span. However, age effects on some hippocampal local regions are significant for the AD group.
ACKNOWLEDGEMENT
This work was supported by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB). Additional support was provided by the NIH research grant R01 MH071940 and P01 EB001955, the NIH/NCRR resource grant P41 RR013642. The Honolulu-Asia Aging Study was funded by the National Institute on Aging, Grants U01 AG019349 and R01 AG0-17155S1. This work was also partially supported by the NIA Intramural Research Program.
APPENDIX
A. Hippocampal Surface Averaging
Let Si(u,v) represent the regular parametric meshes on the ith hippocampus (i = 1, …, n). n is the total number of hippocampus. The average hippocampal surface is then given by the following form:
Where each hippocampus is represented by a regular rectangular grid which size is I × J.
B. Statistical Mappings
Statistical maps are used to estimate the significant effect of a variable (e.g age, disease) on the measured change in hippocampal morphology. To obtain it, a series of steps will be preformed:
-
Fit a general linear model (GLM):
Example: x – age; y – shape feature (distance between medial axis and surface)
Where a is the intercept, b is the slope and ε is the error. The method of least squares is used to estimate a and b:Sxy = ∑ (xi − x̄)(yi − ȳ) and Sxx = ∑ (xi − x̄)2
- Statistically significant correlation
- Null hypothesis: there is no linear relationship between the two variables in the population (b = 0)
- Alternative hypothesis: there is a statistical linear relationship between the two variables in the population
-
Calculate F has approximately a F1,n−2 distributionWhere MSR (the mean squares for regression)MSE (the mean squares for error):
- Calculate the p value of the above test
- The statistical map includes the p value of each mesh
C. Permutation Test Algorithm
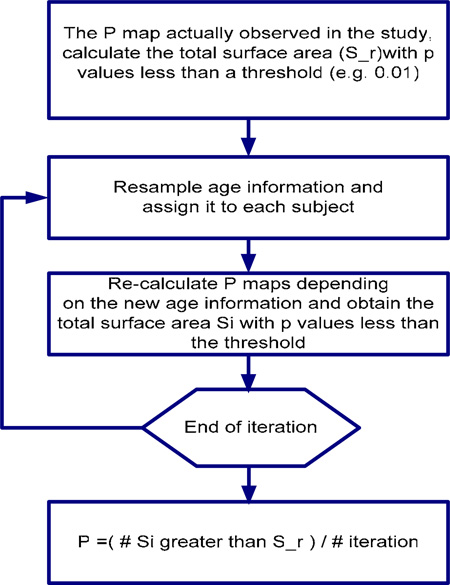
Permutation test is applied to investigate if the correlation between hippocampal structural changes and age occurs randomly or not. The null hypothesis is that there is no relationship between the above two variables. The results of permutation test indicate if the overall structural changes are significantly related with age. Small p value (e.g < 0.05) means that the shapes changes of hippocampi linked with age do not randomly occur. Otherwise, the large p value could not provide the evidence against the hypotheses that the structure changes occur randomly. The detail procedure of our permutation test is listed as following:
Figure 6: Permutation tests flow chart for investigating the relationship between age and overall structural changes.
D. Contour Preprocessing Algorithm
Figure 7: The procedure of contour preprocessing algorithm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Barber R, Ballard C, McKeith IG, Gholkar A, O'Brien JT. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology. 2000;54:1304–1309. doi: 10.1212/wnl.54.6.1304. [DOI] [PubMed] [Google Scholar]
- Becker JT, Davis SW, Hayashi KM, Meltzer CC, Toga AW, Lopez OL, Thompson PM. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Kerr B, Victoroff J, Tate DF, Breitner JC. White matter lesions, quantitative magnetic resonance imaging, and dementia. Alzheimer Dis Assoc Disord. 2002;16:161–170. doi: 10.1097/00002093-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Baron JC. Early diagnosis of Alzheimer's disease: contribution of structural neuroimaging. Neuroimage. 2003;18:525–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Parashos IA, Soady SA, Sullivan RJ, Patterson LJ, Figiel GS, Webb MC, Spritzer CE, Djang WT. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- Convit A, de Leon MJ, Hoptman MJ, Tarshish C, De Santi S, Rusinek H. Agerelated changes in brain: I. Magnetic resonance imaging measures of temporal lobe volumes in normal subjects. Psychiatr Q. 1995;66:343–355. doi: 10.1007/BF02238754. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, Barkhof F, Scheltens P. White matter lesions and hippocampal atrophy in Alzheimer's disease. Neurology. 2004;62:310–312. doi: 10.1212/01.wnl.0000103289.03648.ad. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, George AE, Golomb J, de Santi S, Tarshish C, Rusinek H, Bobinski M, Ince C, Miller D, Wisniewski H. In vivo structural studies of the hippocampus in normal aging and in incipient Alzheimer's disease. Ann N Y Acad Sci. 1996;777:1–13. doi: 10.1111/j.1749-6632.1996.tb34395.x. [DOI] [PubMed] [Google Scholar]
- De Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Ezekiel F, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiology of Aging. 2005;26:553–559. doi: 10.1016/j.neurobiolaging.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Laakso MP, Zhu XP, Jagust WJ, Yaffe K, Kramer JH, Miller BL, Reed BR, Norman D, Chui HC, Weiner MW. Effects of subcortical ischemic vascular dementia and AD on entorhinal cortex and hippocampus. Neurology. 2002;58:1635–1641. doi: 10.1212/wnl.58.11.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM. In vivo neuropathology of the hippocampal formation in AD: a radial mapping MR-based study. Neuroimage. 2006;32:104–110. doi: 10.1016/j.neuroimage.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging. An association with recent memory impairment. Arch Neurol. 1993;50:967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Convit A, Mittelman MS, Cohen J, Rusinek H, De Santi S, George AE. Hippocampal formation size in normal human aging: a correlate of delayed secondary memory performance. Learn Mem. 1994;1:45–54. [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Mittelman M, Cohen J, George AE. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- Grundman M, Jack CR, Jr, Petersen RC, Kim HT, Taylor C, Datvian M, Weiner MF, DeCarli C, DeKosky ST, van Dyck C, Darvesh S, Yaffe K, Kaye J, Ferris SH, Thomas RG, Thal LJ. Hippocampal volume is associated with memory but not monmemory cognitive performance in patients with mild cognitive impairment. J Mol Neurosci. 2003;20:241–248. doi: 10.1385/jmn:20:3:241. [DOI] [PubMed] [Google Scholar]
- Grundman M, Sencakova D, Jack CR, Jr, Petersen RC, Kim HT, Schultz A, Weiner MF, DeCarli C, DeKosky ST, van Dyck C, Thomas RG, Thal LJ. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci. 2002;19:23–27. doi: 10.1007/s12031-002-0006-6. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, Kokmen E, Petersen RC. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, Xu Y, Shiung M, O'Brien PC, Cha R, Knopman D, Petersen RC. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60:253–260. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke AL, de Zubicaray G, Rose SE, Griffin M, Chalk JB, Galloway GJ. 4D deformation modeling of cortical disease progression in Alzheimer's dementia. Magn Reson Med. 2001;46:661–666. doi: 10.1002/mrm.1243. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Juottonen K, Laakso MP, Partanen K, Soininen H. Comparative MR analysis of the entorhinal cortex and hippocampus in diagnosing Alzheimer disease. AJNR Am J Neuroradiol. 1999;20:139–144. [PubMed] [Google Scholar]
- Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B, Sexton G. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Lehtovirta M, Partanen K, Riekkinen PJ, Soininen H. Hippocampus in Alzheimer's disease: a 3-year follow-up MRI study. Biol Psychiatry. 2000;47:557–561. doi: 10.1016/s0006-3223(99)00167-5. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer's disease, Parkinson's disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- Lim KO, Zipursky RB, Murphy GM, Jr, Pfefferbaum A. In vivo quantification of the limbic system using MRI: effects of normal aging. Psychiatry Res. 1990;35:15–26. doi: 10.1016/0925-4927(90)90005-q. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50:121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, Norman D, Mack WJ, Willis L, Chui HC. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Jagust WJ, DeCarli C, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004a;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004b;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Scher AI, Xu Y, Korf ES, White LR, Scheltens P, Toga AW, Thompson PM, Hartley SW, Witter MP, Valentino DJ, Launer LJ. Hippocampal shape analysis in Alzheimer's disease: A population-based study. Neuroimage. 2007;36:8–18. doi: 10.1016/j.neuroimage.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126:2551–2557. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- Seab JP, Jagust WJ, Wong ST, Roos MS, Reed BR, Budinger TF. Quantitative NMR measurements of hippocampal atrophy in Alzheimer's disease. Magn Reson Med. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- Sencakova D, Graff-Radford NR, Willis FB, Lucas JA, Parfitt F, Cha RH, O'Brien PC, Petersen RC, Jack CR., Jr Hippocampal atrophy correlates with clinical features of Alzheimer disease in African Americans. Arch Neurol. 2001;58:1593–1597. doi: 10.1001/archneur.58.10.1593. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. Ieee Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Swartz RH, Black SE, Sela G, Bronskill MJ. Cognitive impairment in dementia: correlations with atrophy and cerebrovascular disease quantified by magnetic resonance imaging. Brain and Cognition. 2002;49:228–232. [PubMed] [Google Scholar]
- Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- van de Pol LA, Hensel A, Barkhof F, Gertz HJ, Scheltens P, van der Flier WM. Hippocampal atrophy in Alzheimer disease: age matters. Neurology. 2006;66:236–238. doi: 10.1212/01.wnl.0000194240.47892.4d. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage. 2003;20:667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, Rodriguez BL, Blanchette PL, Havlik RJ, Wergowske G, Chiu D, Foley DJ, Murdaugh C, Curb JD. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. Jama. 1996;276:955–960. [PubMed] [Google Scholar]
- Wolf H, Grunwald M, Kruggel F, Riedel-Heller SG, Angerhofer S, Hojjatoleslami A, Hensel A, Arendt T, Gertz H. Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly. Neurobiol Aging. 2001;22:177–186. doi: 10.1016/s0197-4580(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Wolf H, Julin P, Gertz HJ, Winblad B, Wahlund LO. Intracranial volume in mild cognitive impairment, Alzheimer's disease and vascular dementia: evidence for brain reserve? Int J Geriatr Psychiatry. 2004;19:995–1007. doi: 10.1002/gps.1205. [DOI] [PubMed] [Google Scholar]
- Woods R, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated Image Registration: I. General Methods and Intrasubject, Intramodality Validation. Journal of Computer Assisted Tomography. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods R, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated Image Registration: II. Intersubject Validation of Linear and Nonlinear Models. Journal of Computer Assisted Tomography. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jack CR, Jr, O'Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1760–1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]