Abstract
A theoretical concern exists that atazanavir (ATV) use during pregnancy may exacerbate physiologic neonatal hyperbilirubinemia. The aim of this substudy was to evaluate patterns of neonatal bilirubin following ATV/ritonavir (RTV) treatment of pregnant mothers and clinical and pharmacogenetic factors that may correlate. The design involved a subanalysis of study AI424182, a multicenter, open-label, prospective, single-arm Phase I study. The study had two treatment arms: (1) ATV/RTV 300/100 mg once daily or (2) ATV/RTV 400/100 mg once daily, both in combination with zidovudine/lamivudine 300/150 mg twice daily. Total bilirubin was assessed at baseline, each visit, and delivery day for mothers and on days 1 (delivery day), 3, 5, and 7 and weeks 2 and 6 for neonates. Blood samples were obtained for UGT1A1 genotyping and ATV cord blood concentration. Bilirubin elevation of any grade occurred in 14/40 neonates (35%). All Grade 3 to 4 bilirubin abnormalities (n=7) occurred after day 14. The pattern of neonatal bilirubin levels reported was consistent with neonatal physiologic elevations of bilirubin. Little correlation was observed between either maternal bilirubin levels over the last 4 weeks of pregnancy (including delivery) or ATV cord concentration and neonatal bilirubin. There was a significant association between UGT1A1 genotype and bilirubin grade in the maternal population (p=0.0006) but not neonates (p=0.49). Neither neonatal UGT1A1 genotype nor cord blood ATV concentration is a good predictor of neonatal hyperbilirubinemia. ATV/RTV treatment of mothers does not appear to exacerbate neonatal physiologic hyperbilirubinemia.
Introduction
Atazanavir (ATV) is a potent, well-tolerated, once-daily protease inhibitor with established efficacy and safety in treatment-naive and experienced adult, HIV-1-infected patients.1,2 ATV has a dose recommendation of 300 mg with ritonavir (RTV) 100 mg in pregnant patients with susceptible HIV-1 strains.3 A recent study (AI424182) assessed ATV pharmacokinetics, safety, and efficacy in HIV-1-infected pregnant women who received ATV/RTV (300/100 mg or 400/100 mg once daily), given with zidovudine/lamivudine (AZT/3TC; 300/150 mg twice daily).4,5 ATV/RTV 300/100 appeared to provide adequate ATV systemic exposure throughout pregnancy and was well tolerated.4 HIV RNA levels were suppressed in all mothers who received ATV 300/100, none of the infants had a positive HIV-1 DNA, and ATV exposure in infants at birth was low.4
ATV inhibits the hepatic UGT1A1 enzyme, causing elevated indirect bilirubin, a benign, reversible condition that rarely leads to discontinuation of treatment.4 Newborns produce bilirubin at a rate of approximately 6–8 mg/kg/day, more than twice the production rate in adults, and neonatal hyperbilirubinemia (total serum bilirubin >5 mg/dl) is commonly encountered.6 A theoretical concern has been raised that ATV use during pregnancy may exacerbate physiologic hyperbilirubinemia in neonates,4,7 via three potential mechanisms: (1) passive back-diffusion of infant bilirubin across the placenta caused by saturation of protein binding of bilirubin in mothers with elevated bilirubin, (2) the effect of UGT1A1 inhibition in the fetus caused by ATV crossing the placenta, and (3) high levels of maternal bilirubin may cross the placenta and remain elevated because of immature UGT1A1 in the newborn.4
The aim of this substudy was to evaluate patterns of neonatal bilirubin following ATV/RTV treatment of mothers during pregnancy, including the relationship between maternal and neonate bilirubin, the relationship between neonate ATV plasma concentration and neonate bilirubin levels, and the effect of maternal UGT1A1 genotype on maternal bilirubin levels and neonate UGT1A1 genotype on neonate bilirubin levels.
Materials and Methods
Study design
This is a subanalysis of study AI424182, a multicenter, open-label, prospective, single-arm Phase I study conducted in South Africa, Puerto Rico, and the United States from June 2006 to September 2008. AI424182 aimed to assess the safety, efficacy, and appropriate dosing regimen for ATV/RTV in HIV-1-infected pregnant women. The study consisted of HIV-1-infected pregnant women (12–32 weeks gestation; CD4+ count ≥200 cells/mm3) and had two treatment arms: (1) ATV/RTV 300/100 mg once daily or (2) ATV/RTV 400/100 mg once daily, both in combination with AZT/3TC 300/150 mg twice daily. Pharmacokinetic parameters (maximum observed plasma concentration, trough observed plasma concentration 24 h postdose, and area under the concentration–time curve in one dosing interval) were determined and compared with historical values (300/100 mg ATV/RTV) for HIV-infected nonpregnant adults. Efficacy analyses were also conducted in treated mothers, and safety was assessed in both treated mothers and neonates. The full study design of AI424182 has been published previously.4
Study assessments
For the purpose of this subanalysis, neonates underwent HIV DNA testing at delivery and at weeks 2, 6, 16, and 24. Total bilirubin was assessed at baseline, each visit, and delivery day for mothers and on days 1, 3, 5, and 7 and weeks 2 and 6 for live-born neonates. For UGT1A1 genotyping, maternal blood samples were obtained at baseline and neonate blood samples were obtained from cord blood at delivery (also used to assess ATV concentrations).
Repeated measures analysis was used to determine the effects of maternal UGT1A1 genotype on maternal bilirubin and neonate UGT1A1 genotype on neonate bilirubin. Fisher's exact test was used to assess the association between the genotype functional groups and bilirubin grades for mothers and neonates. Plasma protein binding was determined by equilibrium dialysis.
Results
Study population
Bilirubin was assessed in 41 HIV-1-infected pregnant women and 40 neonates (40 mother–neonate pairs; one mother withdrew consent after 3 weeks on the study). Baseline demographics were comparable between mothers who received ATV/RTV 300/100 and 400/100 and neonates whose mothers received either regimen.4
Mean baseline HIV RNA levels were 3.52 and 4.02 log10 copies/ml and mean CD4+ counts were 435 and 390 cells/mm3 in the ATV/RTV 300/100 and 400/100 groups, respectively. The proportion of black/African-American women enrolled was 85% (ATV/RTV 300/100) and 90% (ATV/RTV 400/100). At birth, vital signs, physical measurements, and APGAR scores were comparable between neonates whose mothers received ATV/RTV 300/100 and 400/100.
Overall elevations in maternal bilirubin
Maternal bilirubin abnormalities included Grade 3–4 elevation of total bilirubin (see Table 1 for Grade definitions), which occurred in more than twice as many women who received ATV 400/100 (13/20, 62%) than ATV 300/100 (6/19, 30%). The mean change in maternal bilirubin from baseline on the day of delivery was 1.53 mg/dl and 1.83 mg/dl in ATV/RTV 300/100 and 400/100, respectively. During pregnancy, the maximum individual bilirubin levels were 5.8 mg/dl and 6.4 mg/dl, respectively. The maximum individual bilirubin level of 6.4 mg/dl was reported during an episode of preeclampsia.
Table 1.
Protein Binding Pharmacokinetics of Atazanavir in Maternal Plasma and Cord Blood from the Second Trimester to the Postpartum Period
| |
Maternal plasma |
Cord blood |
||||
|---|---|---|---|---|---|---|
| % Protein-bound, mean (SD) at 3 h postdosea | % Protein-bound, mean (SD) at 24 h postdosea | % Free mean (SD), at 3 h postdosea | % Free, mean (SD) at 24 h postdosea | % Protein-bound, mean (SD) | % Free, mean (SD) | |
| ATV/RTV 300/100 mg second trimester (n=9) | 87.91 (1.74) | 89.06 (1.98) | 12.09 (1.74) | 10.94 (1.98) | ||
| ATV/RTV 300/100 mg third trimester (n=20) | 91.34 (2.32) | 90.37b (2.58) | 8.67 (2.32) | 9.63b (2.58) | ||
| ATV/RTV 300/100 mg peripartum (n=15) | 77.05 (6.88) | 22.95 (6.88) | ||||
| ATV/RTV 300/100 mg postpartum (n=35) | 87.03c (15.81) | 91.02 (3.22) | 10.03c (4.06) | 8.98 (3.22) | ||
| ATV/RTV 400/100 mg third trimester (n=20) | 87.70 (2.69) | 88.89 (2.40) | 12.31 (2.69) | 11.11 (2.40) | ||
| ATV/RTV 400/100 mg peripartum (n=12) | 75.62 (4.34) | 24.38 (4.34) | ||||
Three hours postdose approximates binding at Cmax; 24 h postdose approximates binding at Cmin.
n=19.
n=34.
ATV/RTV, atazanavir/ritonavir.
Overall elevations in neonate bilirubin
Bilirubin elevation of any grade occurred in 14/40 neonates (35%); however, among neonates who had elevated bilirubin in the first 14 days, none was elevated to Grade 1 (see Table 1 for Grade definitions). This is because different grading criteria are employed due to the frequency of bilirubin elevation in normal neonates during this period.4 All Grade 3–4 bilirubin abnormalities (n=7) occurred after day 14. Increased total bilirubin was the only Grade 3–4 liver function abnormality observed (7/40 neonates: ATV/RTV 300/100 group, three neonates; ATV/RTV 400/100 group four neonates); of these, three neonates experienced Grade 4 increased total bilirubin (ATV/RTV 300/100, one neonate; ATV/RTV 400/100, two neonates). American Academy of Pediatrics guidelines recommend phototherapy for neonates with elevated total serum bilirubin based on the age of the neonate (measured in hours or days), the severity of the elevation, and the presence of risk factors.8 Therefore, in this study the decision to treat elevated bilirubin levels with phototherapy was left to the discretion of the study investigator; of the seven neonates with Grade 3–4 bilirubin elevation, six received phototherapy between days 1 and 14. Bilirubin levels associated with these events ranged from 8.1 mg/dl to 13 mg/dl.4
After birth, the mean changes from baseline in total bilirubin on day 3 were 4.32 mg/dl and 5.01 mg/dl, on day 5 were 3.11 mg/dl and 4.28 mg/dl, and on day 7 were 1.98 mg/dl and 2.92 mg/dl (ATV/RTV 300/100 and ATV/RTV 400/100, respectively).
Patterns of bilirubin elevations
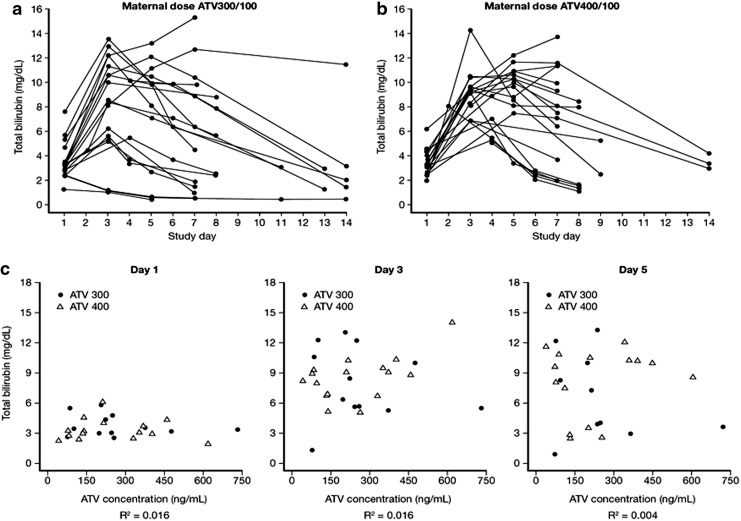
The patterns of changes of bilirubin elevations were comparable over time among neonates whose mothers received ATV/RTV 400/100 compared with ATV/RTV 300/100 (Fig. 1a and b). These patterns are also consistent with neonatal physiologic elevations of bilirubin; bilirubin levels increased from day 1 (date of delivery) to a maximum observed level by day 3, with a subsequent decline to normal levels. Little correlation between maternal and neonatal total bilirubin levels was observed at delivery or over the last 4 weeks of pregnancy (data published in Conradie et al.4).
FIG. 1.
Patterns of bilirubin elevations in neonates and in mothers. (a) The pattern of elevations in neonate bilirubin levels up to 14 days postpartum at a maternal atazanavir/ritonavir (ATV/RTV) dose of 300/100 mg; (b) The pattern of elevations in neonate bilirubin levels up to 14 days postpartum at a maternal ATV/RTV dose of 400/100 mg. (c) Cord blood ATV concentration vs. neonate bilirubin on days 1, 3, and 5.
Relationship between cord blood ATV concentrations and neonatal bilirubin
No relationship was observed between neonatal total bilirubin on day 1 (delivery day), 3, or 5 and ATV cord blood concentrations for either the ATV/RTV 300/100 or 400/100 groups (Fig. 1c).
The ratio of neonate relative to maternal ATV concentration was approximately 12% and 19% when mothers received ATV/RTV 300/100 and 400/100, respectively. Plasma protein binding in cord blood was lower than in maternal blood at approximately 76% to 77%, compared with 87% to 91% (ATV/RTV 300/100 and 400/100, respectively), with an unbound fraction approximately twice that of maternal. Plasma protein binding in maternal blood was also similar across the second and third trimesters and the postpartum period (Table 1).
Association between UGT1A1 genotypes and bilirubin levels
Subjects were classified into five groups based on the functionality of their UGT1A1 TA-repeat polymorphism type. The effect of maternal UGT1A1 genotype on maternal bilirubin levels and neonate UGT1A1 genotype on neonate bilirubin levels was assessed and the number and frequency of subjects of each genotype and hyperbilirubinemia severity were tabulated for treated mothers and live-born neonates (Table 2).
Table 2.
Summary of Genotype Function and Genotype at Baseline
| |
|
Number of mothers (%)a |
Fisher's exact test for association between genotype and bilirubin gradebAssociation |
|||
|---|---|---|---|---|---|---|
| |
|
|
|
Mothers |
Neonates |
|
| UGT1A1 function and genotypec | Genotype | ATV/RTV 300/100 (n=19) | ATV/RTV 400/100 (n=20) | Number with Grade ≤2 bilirubin | Number with Grade >2 bilirubin | Number with Grade 3–4 bilirubin/number with measurements (%) |
| Increased functionality | 5/6 | 2 (11) | 1 (5) | 3 | 0 | 2/5 (40) |
| Unknown | 5/7 or 5/8 | 3 (16) | 1 (5) | 4 | 0 | 0/1 (0) |
| Normal | 6/6 | 7 (37) | 6 (30) | 9 | 4 | 1/12 (8) |
| Moderate decreased | 6/7 or 6/8 | 4 (21) | 8 (40) | 4 | 8 | 1/7 (14) |
| Major decreased | 7/7 or 7/8 | 3 (16) | 4 (20) | 0 | 7 | 1/3 (33) |
| Fisher's exact test p value | n/a | n/a | n/a | 0.0006 | 0.49 | |
Forty mothers were initially enrolled; however, one mother withdrew from the study after 3 weeks, leaving data from 39 mothers available for analysis.
Grades are based on total bilirubin: normal (Grade 0), <1.1×upper limit of normal (ULN); Grade 1, 1.1 to <1.6×ULN; Grade 2, 1.6 to<2.6×ULN; Grade 3, 2.6 to 5.0×ULN; Grade 4, >5.0×ULN.
UGT1A1 polymorphisms are due to a varying number of TA repeats and functional groups are based on genotype.
Summary in mothers with HIV-1 infection treated with ATV/RTV combination therapy during and after pregnancy and Fisher's exact test for testing the association between genotype and bilirubin grade association for mothers and neonates.
n/a, not applicable.
Mothers with increased and unknown functionality had hyperbilirubinemia ranging from normal to Grade 2, mothers with normal functionality had all grades of hyperbilirubinemia, mothers with moderate decreased functionality had Grade 1–3 hyperbilirubinemia, and mothers with major decreased functionality had Grade 3–4 hyperbilirubinemia (Table 2). Fisher's exact test showed that there was a significant association (p=0.0006) between UGT1A1 genotype and bilirubin grade in the maternal population in both ATV/RTV treatment groups but no significant genotype effects were identified for the neonates (p=0.49; Table 2).
Discussion
In major clinical trials of ATV/RTV in adults with HIV-1, the most common laboratory abnormality was elevated indirect (unconjugated) bilirubin, due to UGT1A1 inhibition.1,7,9,10 A correlation between increased plasma ATV concentrations, UGT1A1 genotype, and hyperbilirubinemia is well established,1,3,11,12 and, as expected, this substudy identified a significant association between UGT1A1 genotypes and bilirubin levels in pregnant women receiving ATV/RTV (300/100 mg and 400/100 mg doses).
In a recent small observational study in 22 HIV-1-infected women treated with ATV/RTV (300/100 mg) during pregnancy and their 23 infants, some association was observed between ATV/RTV and neonatal hyperbilirubinemia, although no causal relationship was confirmed [the median total bilirubin concentration at birth was 43 μM/liter (2.5 mg/dl), range 24–129 (1.4–7.5), four missing data]; 10 infants had normal bilirubin levels at birth, five had mild elevations, and four had high-intermediate to high levels. Four neonates had jaundice and required phototherapy at birth.13 There was no significant correlation between maternal and cord blood concentrations.13 However, in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network Protocol 1026s prospective study in 38 pregnant women with HIV-1 infection, there were no reports of excessive bilirubin levels in neonates (n=38) born to women treated with ATV/RTV (300/100 mg), with or without tenofovir, during pregnancy.14 The results reported in this substudy agree with the findings of IMPAACT and indicate that bilirubin levels are not appreciably more frequently elevated in neonates whose mothers received ATV/RTV during pregnancy.
No apparent relationship was observed between exposure to ATV and neonatal bilirubin level and no significant relationship between neonatal UGT1A1 genotypes and bilirubin levels was demonstrated, based on the Fisher's exact test. Although ATV does not freely cross the placenta, plasma protein binding of ATV in cord blood is less than in maternal blood, which results in the fetus being exposed to approximately 2-fold higher unbound ATV concentrations than those achieved in the mothers at the same total (bound and unbound) plasma ATV concentration.4 However, the greater proportion of unbound ATV does not appear to be associated with higher bilirubin levels in the newborn. Possible explanations for this are that fetal UGT1A1 is not fully functioning and/or neonatal bilirubin is eliminated in some other way, 15–17 A potential benefit of ATV crossing the placenta is that the unbound concentrations achieved in the cord blood may provide some antiviral protection to the fetus.18
Most neonates in this substudy had some elevation of bilirubin. However, the pattern of bilirubin elevation and normalization observed was consistent with physiologic elevations of bilirubin; approximately 60% of otherwise healthy neonates will have jaundice, with about 10% of these requiring phototherapy or exchange transfusions.4,6,10 In this study, 15% of neonates required phototherapy (across both ATV/RTV doses), similar to the standard frequency; no neonates required transfusions. Of the seven neonates with Grade 3–4 bilirubin elevation, six had subsequent phototherapy at the investigators' discretion. According to the American Academy of Pediatrics' (AAP) Guideline for the management of hyperbilirubinemia in the newborn infant, phototherapy is not automatically indicated for all neonates with Grade 3–4 hyperbilirubinemia, but rather, whether or not to initiate phototherapy involves a more complex treatment algorithm based on risk levels, time since birth, and total serum bilirubin levels.8 Therefore, it would be possible for two neonates to have the same bilirubin level, but only one would require phototherapy.
Although the incidence of hyperbilirubinemia in neonates is difficult to estimate due mainly to methodologic differences between studies, it is thought to be high in general.8 A 2003 U.S.-based study found that 4.3% of 47,801 infants had total serum bilirubin levels in a range where phototherapy was recommended by the 1994 AAP guidelines, and 2.9% had values within the range where guidelines suggest considering phototherapy.19 Typically, phototherapy is not considered in neonates until bilirubin levels exceed 12 mg/dl8; however, given the fact that most of the neonates in our study who received phototherapy had lower bilirubin levels (i.e., <12 mg/dl), the investigators may have been more likely to initiate phototherapy at lower bilirubin levels because the mothers were receiving ATV/RTV.
A study limitation that is important to note is that neonates who received phototherapy for elevated bilirubin levels were not excluded from the study analyses. Of the seven neonates who experienced Grade 3–4 bilirubin elevation, three were born to mothers who had received ATV/RTV 300/100 and four were born to mothers who had received ATV/RTV 400/100. Of those who received phototherapy, three were born to mothers who had received ATV/RTV 300/100 and three were born to mothers who had received ATV/RTV 400/100. It is possible that treatment with phototherapy may confound the interpretation of the data presented in this study, although the distribution of these patients was similar across the dosing groups.
In conclusion, neither neonatal UGT1A1 genotype nor cord blood ATV concentration is a good predictor of neonatal hyperbilirubinemia. ATV/RTV treatment of mothers does not exacerbate neonatal physiologic hyperbilirubinemia.
Acknowledgments
The study team would like to thank the mothers and their families for their participation and commitment during the study. This Bristol-Myers Squibb-supported subanalysis is part of Study AI424182, registered with Clinical-Trials.gov (NCT00326716). We thank Bristol-Myers Squibb employee Wenhua Hu for her help with the statistical analysis. Professional medical writing and editorial assistance were provided by Miriam Banner of inScience Communications, Springer Healthcare, funded by Bristol-Myers Squibb.
Data were presented previously at the 12th Adverse Events and Lipodystrophy Workshop (LIPO), London, November 3, 2010 [McGrath D, et al.: Patterns of neonatal bilirubin following atazanavir/ritonavir (ATV/r) treatment of mothers during pregnancy: Clinical and pharmacogenetic factors identified in study AI424182. Abstract 019] and at 1st International Workshop on HIV and Women, Washington DC, January 10–11 2011 [Hardy H, et al.: The safety, efficacy, and steady state pharmacokinetics of atazanavir/ritonavir (ATV/r) once daily given in combination with twice daily AZT/3TC during pregnancy: Results of study AI424182. Abstract 018].
Author Disclosure Statement
F.C. reports receiving research support from Bristol-Myers Squibb, GlaxoSmithKline, Tibotec, Schering Plough, Gilead Sciences, and Abbott Laboratories. C.Z. reports receiving grant support from Tibotec, Pfizer, Bristol-Myers Squibb, Advent, and the NIH institutes: NIAID, NCRR, and NIMH. C.Z. also reports being a member of the Tibotec Presidents Council (advisory group). M.B. reports receiving research support from Pfizer, Boehringer-Ingelheim, and Bristol-Myers Squibb, receiving lecture fees from Bristol-Myers Squibb, Roche, and Aspen, and receiving financial support for conference attendance from Roche. O.O. reports receiving research support from Johnson & Johnson, Tibotec, Bristol-Myers Squibb, Vertex, ViiV, Pfizer, Merck, and Clinlogix, and consulting fees from Gilead Sciences. O.O. also reports being on the speaker bureau for Abbott Laboratories and Jansen. T.E., S.H., H.H., R.B., and D.M. report being employees and shareholders of Bristol-Myers Squibb. D.J. reports no financial disclosure or conflict of interest.
References
- 1.Croom K. Dhillon S. Keam S. Atazanavir A review of its use in the management of HIV-1 infection. Drugs. 2009;69(8):1107–1140. doi: 10.2165/00003495-200969080-00009. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults, adolescents. Department of Health and Human Services. 2011 Oct 14;:1–167. [Google Scholar]
- 3.Reyataz® (atazanavir sulfate) Prescribing Information. Mar, 2012. http://packageinserts.bms.com/pi/pi_reyataz.pdf. [Jul;2013 ]. http://packageinserts.bms.com/pi/pi_reyataz.pdf
- 4.Conradie F, et al. Safety and exposure of once-daily ritonavir-boosted atazanavir in HIV-infected pregnant women. HIV Med. 2011;12(9):570–579. doi: 10.1111/j.1468-1293.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- 5.Hardy H, et al. The safety, efficacy, steady state pharmacokinetics of atazanavir/ritonavir (ATV/r) once daily given in combination with twice daily AZT/3TC during pregnancy: Results of study AI424182 [poster 018] In 1st International Workshop on HIV and Women. 2011 Jan;:10–11. [Google Scholar]
- 6.Porter ML. Dennis BL. Hyperbilirubinemia in the term newborn. Am Fam Physician. 2002;65(4):599–606. [PubMed] [Google Scholar]
- 7.Panel on Treatment of HIV-Infected Pregnant Women Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Sep 14, 2011. http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf. [Jul;2013 ]. pp. 1–207.http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf 28 February 2012.
- 8.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 9.Molina JM, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53(3):323–332. doi: 10.1097/QAI.0b013e3181c990bf. [DOI] [PubMed] [Google Scholar]
- 10.Watson RL. Hyperbilirubinemia. Crit Care Nursing Clin N Am. 2009;21(1):97–120. doi: 10.1016/j.ccell.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez No'voa S, et al. Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C-T polymorphism at the multidrug resistance gene 1. Clin Infect Dis. 2006;42(2):291–295. doi: 10.1086/499056. [DOI] [PubMed] [Google Scholar]
- 12.Turatti L, et al. UGT1A1*28 variant allele is a predictor of severe hyperbilirubinemia in HIV-infected patients on HAART in Southern Brazil. AIDS Res Hum Retroviruses. 2012;28(9):815–818. doi: 10.1089/AID.2011.0261. [DOI] [PubMed] [Google Scholar]
- 13.Mandelbrot L, et al. Atazanavir in pregnancy: Impact on neonatal hyperbilirubinemia. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):18–21. doi: 10.1016/j.ejogrb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Mirochnick M, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56(5):412–419. doi: 10.1097/QAI.0b013e31820fd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Q. BCRP/ABCG2 in the placenta: Expression, function and regulation. Pharm Res. 2008;25(6):1484. doi: 10.1007/s11095-008-9537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blazquez AG, et al. Characterization of the role of ABCG2 as a bile acid transporter in liver and placenta. Mol Pharmacol. 2012;81(2):273–283. doi: 10.1124/mol.111.075143. [DOI] [PubMed] [Google Scholar]
- 17.Marin JJ, et al. Molecular bases of the fetal liver-placenta-maternal liver excretory pathway for cholephilic compounds. Liver Int. 2008;28(4):435–454. doi: 10.1111/j.1478-3231.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 18.Ripamonti D, et al. Atazanavir plus low-dose ritonavir in pregnancy: Pharmacokinetics and placental transfer. AIDS. 2007;21(18):2409–2415. doi: 10.1097/QAD.0b013e32825a69d1. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson LR, et al. Phototherapy use in jaundiced newborns in a large managed care organization: Do clinicians adhere to the guideline? Pediatrics. 2003;111(5 Pt 1):e555–561. doi: 10.1542/peds.111.5.e555. [DOI] [PubMed] [Google Scholar]