Abstract
In the context of HIV, the initiation of effective antiretroviral therapy (ART) has been found to increase the risk of dyslipidemia in HIV-infected individuals, and dyslipidemia has been found to be a risk factor for kidney disease in the general population. Therefore, we examined changes in lipid profiles in HIV-infected men following ART initiation and the association with future kidney dysfunction. HIV-infected men from the Multicenter AIDS Cohort Study initiating ART between December 31, 1995 and September 30, 2011 with measured lipid and serum creatinine values pre-ART and post-ART were selected. The associations between changes in total cholesterol or high-density lipoprotein following ART initiation and the estimated change in glomerular filtration rate (eGFR) over time were assessed using piecewise linear mixed effects models. There were 365 HIV-infected men who contributed to the analysis. In the adjusted models, at 3 years post-ART, those with changes in total cholesterol >50 mg/dl had an average decrease in eGFR of 2.6 ml/min/1.73 m2 per year (p<0.001) and at 5 years post-ART, the average decrease was 2.4 ml/min/1.73 m2 per year (p=0.008). This decline contrasted with the estimates for those with changes in total cholesterol ≤50 mg/dl: 1.4 ml/min/1.73 m2 decrease per year (p<0.001) and 0.1 ml/min/1.73 m2 decrease per year (p=0.594) for the same time periods, respectively. Large decreases in high-density lipoprotein (a decline of greater than 5 mg/dl) were not associated with declines in eGFR. These results indicate that large ART-related increases in total cholesterol may be a risk factor for kidney function decline in HIV-infected men. Should these results be generalizable to the broader HIV population, monitoring cholesterol changes following the initiation of ART may be important in identifying HIV-infected persons at risk for kidney disease.
Introduction
Kidney function generally begins to decline when individuals reach 40–60 years of age. The average decline in glomerular filtration rate (GFR) experienced by individuals after this age is 8 ml/min per decade of life.1 While many factors may contribute to progressive kidney dysfunction, experimental data and the growing knowledge of plausible mechanisms for lipid-induced renal injury support a role for dyslipidemia in the progression of kidney disease.2,3 Dyslipidemia may promote renal injury through damage to glomerular capillary endothelial and mesangial cells as well as podocytes, resulting in glomerulosclerosis and interstitial fibrosis.4
Observational studies implicate dyslipidemia as a risk factor in progressive kidney disease. In the Atherosclerosis Risk in Communities study, which followed participants for approximately 3 years, high triglyceride and low high-density lipoprotein cholesterol (HDLc) levels were associated with observing an increase in serum creatinine of at least 0.4 mg/dl over the study period.5 High non-HDLc levels and the ratio of low-density lipoprotein cholesterol (LDLc) to HDLc as well as low HDLc levels were associated with an increased risk of high serum creatinine (serum creatinine >1.5 mg/dl) after 14 years of follow-up in healthy men participating in the Physicians' Health Study.6 Low HDLc was associated with incident Stage 3 chronic kidney disease (CKD; glomerular filtration <60 ml/min) and faster GFR decline in the Framingham Offspring Study7 and Helsinki Heart Study,8 respectively. Furthermore, data suggest that lipid-lowering strategies ameliorate renal injury9,10 and may possibly benefit patients with CKD by slowing the rate of progression.11–13
In the context of HIV, the initiation of effective antiretroviral therapy (ART) has been found to increase serum lipids,14–16 although these changes may mostly reverse lipid level decline following HIV seroconversion.16 However, postmortem studies indicate that HIV is associated with a high prevalence of renal pathology17 and cross-sectionally, HIV infection has been associated with microalbuminuria with severity dependent upon various cardiovascular risk factors.18 We hypothesized that rapid ART-associated increases in serum lipids could play a role in the kidney damage noted among HIV-infected individuals.
Materials and Methods
Study design and measurements
The Multicenter AIDS Cohort Study (MACS) is an ongoing multicenter prospective observational study of HIV-infected men and HIV-uninfected men who are at risk for infection, which was established by the National Institutes of Health in 1984.19 Four clinical sites contributed data from semiannual visits to the MACS: Baltimore MD, Chicago IL, Los Angeles CA, and Pittsburgh PA. Repository serum samples were collected without regard to fasting status and were tested (Heinz Nutrition Laboratory, University of Pittsburgh, Pittsburgh, PA) for total cholesterol (TC) and HDLc—parameters largely unaffected by recent dietary intake. Total cholesterol level was assessed by an enzymatic method20 while HDLc level was assessed after selective precipitation by heparin/manganese chloride and removal by centrifugation of LDLc.21 CD4+ cell count/μl (CD4) was measured with CD4-specific monoclonal antibodies using two-color flow cytometry.22 Serum creatinine was measured locally at each site primarily using the modified Jaffe method.23 Informed consent has been obtained from each participant and each local institutional review board has approved the study.
From the MACS cohort, HIV-infected men initiating ART between December 31, 1995 and September 30, 2011 with measured lipid and serum creatinine values pre-ART and post-ART were included in the present study.
Statistical analysis
Comparisons between those included in the analysis and those excluded were made using two-sample median tests and Chi-square tests. Mean comparisons of cholesterol changes were made using t-tests. Estimated GFR (eGFR) was calculated at each 6-month visit using a serum creatinine-based estimating equation, the CKD-EPI equation.24 A linear mixed model was fit to the eGFR data assuming a normal distribution with a random intercept and slope across time to account for the correlation between repeated measures. The changes in lipid levels (TC and HDLc) over approximately a 3-year period following ART initiation were calculated for each participant to capture changes associated with ART initiation. Changes in TC levels were dichotomized using mean ART-associated changes reported by Riddler et al.16 of 50 mg/dl. While Riddler et al. did not report significant ART-associated changes in HDLc, −5 mg/dl was used as the threshold, based on the observed 25th percentile of the distribution of HDLc changes, to represent changes toward a more harmful lipid profile. Changes over time in eGFR (the eGFR slope) were assessed by adding a term for time since the pre-ART visit to the model. In addition, the eGFR slope was allowed to vary at 3 years and 5 years post-ART initiation by adding spline terms to the eGFR–time relationship in order to assess the timing of any potential impact of ART-associated lipid changes on kidney function. Differences in the eGFR slope between those with large and small cholesterol changes were assessed using interaction terms with time. Final models were adjusted for age centered at 45 years, body mass index (BMI) centered at 25 kg/m2, black race (versus white race), smoking history (ever versus never), statin use (ever versus never), tenofovir/truvada use history (ever versus never), diabetes (defined as a fasting glucose >126 mg/dl or diagnoses of diabetes with medication use), nadir CD4 <200 cells/ml, pre-ART creatinine level centered at 1 mg/dl, pre-ART hypertension (defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90 or a diagnosis of hypertension and use of medications), and pre-ART cholesterol level >220 mg/dl. These covariates were only allowed to have an effect on the average level of eGFR across time, as no interaction terms with time were included in the model for these factors. All variables except those related to pre-ART status and black race were time-varying and, thus, were updated at each visit.
Results
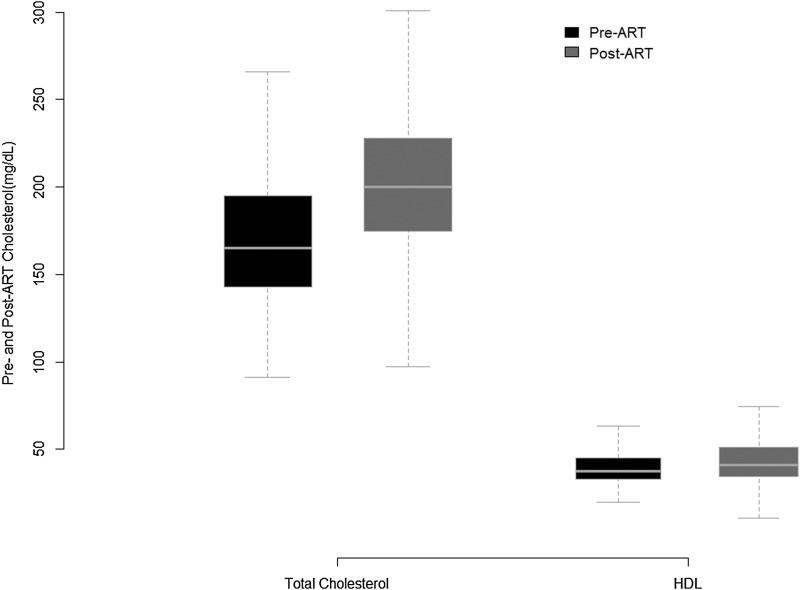
Out of 6,992 HIV-infected men in the MACS, 1,523 initiated ART under observation, of whom 413 (27%) had measured lipid and serum creatinine values pre-ART and post-ART. Of these, 25 were hepatitis C positive and 23 had a diagnosis of diabetes prior to ART initiation and were excluded leaving 365 men (24%) for the analysis. The baseline characteristics of the cohort at the pre-ART visit are shown in Table 1 along with the characteristics of HIV-infected men who were eligible but were not included due to missing lipid measurements at either the pre-ART or post-ART time points. The median age of our study sample was 42 years, the median BMI was 24 kg/m2, and the median nadir CD4 was 249 cells/ml. Among the included men 16% were black, whereas those excluded due to a lack of lipid measurements were 31% black. The majority had hypertension prior to initiating ART and had a history of smoking (∼70% for both), but there was a noticeably lower prevalence of hypertension in the excluded HIV-infected men (56%). We found that 70% of the initial regimens contained a protease inhibitor (PI) in our sample versus 43% among the men excluded. PI-containing initial regimens were associated with greater increases in TC in our sample (a mean change of 37 mg/dl in the PI group versus 24 mg/dl in the no PI group; p=0.03) and smaller gains in HDLc (a mean change of 3 mg/dl in the PI group versus 10 mg/dl in the no-PI group; p<0.001). The pre-ART and post-ART cholesterol distributions for the 365 men are shown in Fig. 1. There was a statistically significant increase in TC (a mean change 34 mg/dl; p<0.001) and HDLc (5 mg/dl; p<0.001) following ART initiation.
Table 1.
Baseline Characteristics of HIV-Infected Men
| Baseline characteristics | Included N=365 | Excluded N=1158 | pa |
|---|---|---|---|
| Age [years; median (IQR)] | 42 (38–48) | 42 (37–48) | 0.329 |
| BMI [kg/m2; median (IQR)] | 24 (23–27) | 24 (22–27) | 0.709 |
| Nadir CD4 [cells/ml; median (IQR)] | 249 (154–353) | 269 (143–397) | 0.154 |
| Black | 16% | 31% | <0.001 |
| ARV naive | 39% | 47% | 0.012 |
| Pre-ART high blood pressure | 69% | 56% | <0.001 |
| Statin use history | 9% | 9% | 0.839 |
| Smoking history | 71% | 74% | 0.363 |
| PI-based initial ART regimen | 70% | 43% | <0.001 |
| Pre-ART serum creatinine [mg/dl; median (IQR)] | 0.9 (0.8–1.0) | 1.0 (0.9–1.1) | 0.179 |
| Pre ART eGFR [ml/min/1.73 m2; median (IQR)] | 101 (89–110) | 96 (80–109) | 0.455 |
| Pre-ART total cholesterol [mg/dl; median (IQR)] | 165 (143–193) | ||
| Pre-ART HDL cholesterol [mg/dl; median (IQR)] | 38 (33–46) | ||
| Pre-ART non-HDL cholesterol [mg/dl; median (IQR)] | 126 (105–154) | ||
| Pre-ART triglycerides [mg/dl; median (IQR)] | 113 (73–154) |
Comparisons between the included and excluded samples were made using two-sample median tests and Chi-square tests.
Baseline characteristics (visit prior to HAART initiation) of HIV-infected men included in the current study and those who were eligible (initiated HAART from 1995 to 2011) but did not have available lipid level measurements pre- and post-HARRT.
IQR, interquartile range; BMI, body mass index; ARV, antiretroviral; ART, antiretroviral therapy; PI, protease inhibitor; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HAART, highly active antiretroviral therapy.
FIG. 1.
The distribution of total cholesterol and high-density lipids levels in the 365 HIV-infected men from the Multicenter AIDS Cohort Study (MACS) at the visit both before antiretroviral therapy (ART) initiation and approximately 3 years later post-ART initiation.
In the adjusted linear mixed effects model examining the impact of TC changes, many factors had notable effects on the eGFR average level across time (i.e., adjusted for time). A history of tenofovir use was associated with a 4.5 ml/min/1.73 m2 lower average eGFR level across time (p<0.001). Having a nadir CD4 level under 200 cells/ml was associated with a 2.6 ml/min/1.73 m2 lower average eGFR level (p=0.011). Statin use was associated with a significantly higher average eGFR level by approximately 2 ml/min/1.73 m2. Time on statins was also assessed as a continuous factor in the model, replacing the dichotomous variable, but was not significant and did not alter inferences. Smoking, black race, and pre-ART hypertension were also associated with a significantly higher average baseline eGFR level while age and BMI were associated with a significantly lower average baseline eGFR level. Pre-ART lipid levels were not associated with eGFR levels in either the TC model or HDLc model. Pre-ART creatinine was highly predictive of eGFR level as expected given that creatinine is the primary biomarker used to estimate GFR.
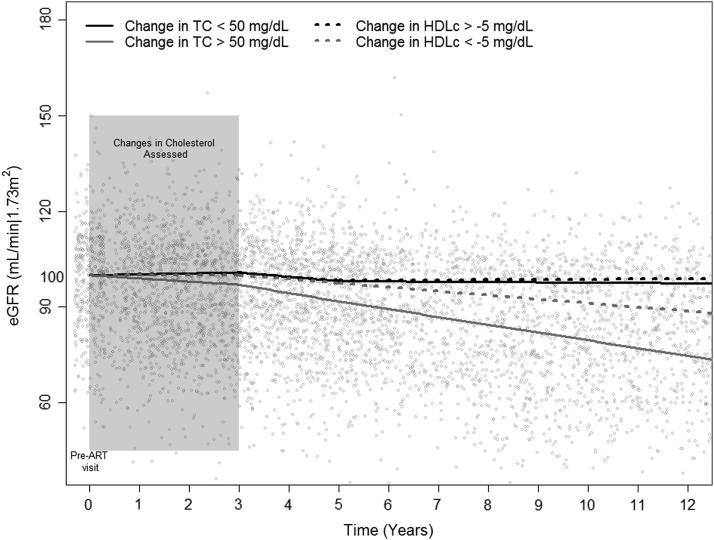
Looking at the impact of changes in TC on the GFR longitudinal trajectory, we found that estimated eGFR slopes over time were more negative across all time points in those with increases in TC >50 mg/dl following ART initiation compared to the group with changes in TC ≤50 mg/dl (Table 2). The former group experiencing a 1.0 ml/min/1.73 m2 decrease in eGFR per year for the first 3 years after the pre-ART visit (coincident with ART-related changes in lipid levels; p=0.007), a 2.6 ml/min/1.73 m2 decrease in eGFR per year from 3 to 5 years (p<0.001), and a 2.4 ml/min/1.73 m2 decrease in eGFR per year after 5 years (p=0.008). In contrast, those with smaller gains of <50 mg/dl experienced an increase in their eGFR of 0.3 ml/min/1.73 m2 per year, a decrease of 1.4 ml/min/1.73 m2 per year, and a decrease of 0.1 ml/min/1.73 m2 per year for the same periods, respectively. The difference in slopes between the groups was significant or marginally significant for all three time periods (p=0.002, p=0,016, and p=0.099, respectively) and resulted in the eGFR trajectories shown in Fig. 2.
Table 2.
Parameter Estimates from the Linear Mixed Effects Model
| Factor | Parameter estimate (95% CI) | p-value |
|---|---|---|
| Intercept | ||
| Pre-ART GFR (ml/min/1.73 m2) | 89 (87, 92) | <0.001 |
| Estimated difference in mean GFR level [ml/min/1.73 m2] | ||
| Age (centered at 45 years; per 10 years) | −6.6 (−7.7, −5.3) | <0.001 |
| BMI (centered at 25 kg/m2) | −0.3 (−05, −0.1) | <0.001 |
| Black race | 16.2 (13.1, 19.2) | <0.001 |
| Smoking history | 2.5 (0.6, 4.4) | 0.009 |
| Statin use history | 1.9 (0.7, 3.1) | 0.002 |
| Tenofovir/truvuda use history | −4.5 (−5.6, −3.4) | <0.001 |
| Incident diabetes | 0.7 (−1.0, 2.3) | 0.438 |
| Nadir CD4 <200 cells/ml | −2.6 (−4.6, −0.6) | 0.011 |
| Pre-ART creatinine (centered at 1 mg/dl; per 0.1 mg/dl) | −5.9 (−6.2, −5.0) | <0.001 |
| Pre-ART hypertension | 2.8 (0.5, 5.1) | 0.018 |
| Pre-ART cholesterol >200 mg/dl | 0.8 (−1.5, 3.1) | 0.502 |
| Estimated mean change in GFR per year [ml/min/1.73 m2/year] | ||
| Among those with an increase in total cholesterol ≤50 mg/dl | ||
| Slope 0<Time<3 years | 0.3 (−0.3, 0.9) | 0.305 |
| Slope 3<Time<5 years | −1.4 (−2.1, −0.6) | >0.001 |
| Slope Time>5 years | −0.1 (−0.4, 0.3) | 0.594 |
| Among those with an increase in total cholesterol >50 mg/dl | ||
| Slope 0<Time<3 years | −1.0 (−1.7, −0.3) | 0.007 |
| Slope 3<Time<5 years | −2.6 (−3.9, −1.4) | <0.001 |
| Slope Time>5 years | −2.4 (−4.2, −0.6) | 0.008 |
Estimates based on N=365 HIV-infected men.
This table shows the difference in the mean level of GFR across time associated with various risk factors for kidney dysfunction as well as the change in the mean eGFR slope comparing those with an increase in total cholesterol of at least 50 mg/dl to those with an increase of less than 50 mg/dl over approximately a 3-year period following ART initiation.
FIG. 2.
The estimated glomerular filtration rate (eGFR) trajectory over time in those with a change in total cholesterol (TC) >50 mg/dl (increase in TC greater than 50 mg/dl) over approximately 3 years following ART initiation compared to those with a ≤50 mg/dl change. The comparison of eGFR trajectories between those with a change in high-density lipoprotein cholesterol (HDLc) <−5 mg/dl (a decrease in HDLc greater than 5 mg/dl) versus ≥−5 mg/dl is also shown. The average eGFR value at the pre-ART visit was approximately 100 ml/min/1.73 m2 and the decrease in eGFR from that value based on the estimated slopes from the piecewise linear models is shown for the exposure groups.
Interestingly, tenofovir use was a confounder in the adjusted models; those using tenofovir-containing regimens compared to those with no tenofovir exposure tended to have smaller mean increases in TC (31 mg/dl versus 43 mg/dl, respectively), though the difference was not significant. Removing person-visits during or after tenofovir use (3,229 person-visits remaining for the analysis out of 5,622 person-visits) did not change the inferences, however. Participants with TC changes >50 mg/dl still had larger significant declines in eGFR for most time periods [1.4 ml/min/1.73 m2 per year (p<0.001), 2.1 ml/min/1.73 m2 per year (p=0.002), and 1.7 ml/min/1.73 m2 per year (p=0.093) from 0 to 3 years, 3 to 5 years, and after 5 years, respectively, from the pre-ART visit]. Similarly, adjusting for PI use in the models did not change the effect estimates of any of the other factors in the model, although PI use was significantly associated with a 3.4 (95% CI: 5.7, 1.6) ml/min/1.73 m2 lower average baseline eGFR level.
We also examined the effect of changes in HDLc cholesterol on the eGFR slope (Table 3). We compared HIV-infected men with decreases in HDLc of greater than 5 mg/dl (i.e., changes of <−5 mg/dl) to men with decreases in HDLc of 5 mg/dl or less (i.e., changes of ≥−5 ml/dl) and we found that those with large decreases in HDLc experienced declines in eGFR of approximately 0 ml/min/1.73 m2 per year for the first 3 years after the pre-ART visit, 1.3 ml/min/1.73 m2 per year from 3 to 5 years, and 1.3 ml/min/1.73 m2 per year after 5 years. However, the estimated eGFR declines were not significantly different between the two groups (Fig. 2).
Table 3.
Parameter Estimates from the Linear Mixed Effects Model
| Factor | Parameter estimate (95% CI) | p-value |
|---|---|---|
| Intercept | ||
| Pre-ART GFR (ml/min/1.73 m2) | 89 (86, 92) | <0.001 |
| Estimated difference in mean GFR level [ml/min/1.73 m2] | ||
| Age (centered at 45 years; per 10 years) | −6.6 (−8.1, −5.1) | <0.001 |
| BMI (centered at 25 kg/m2) | −0.3 (−0.5, −0.1) | 0.002 |
| Black race | 16.0 (12.6, 19.4) | <0.001 |
| Smoking history | 3.3 (1.1, 5.4) | 0.003 |
| Statin use history | 1.6 (0.2, 2.9) | 0.022 |
| Tenofovir/truvuda use history | −4.1 (−5.3, −2.9) | <0.001 |
| Incident diabetes | −0.5 (−2.4, 1.4) | 0.620 |
| Nadir CD4 <200 cells/ml | −2.2 (−4.5, >0.1) | 0.046 |
| Pre-ART creatinine (centered at 1 mg/dl; per 0.1 mg/dl) | −5.5 (−6.1, −4.8) | <0.001 |
| Pre-ART hypertension | 2.6 (>.1, 5.2) | 0.046 |
| Pre-ART cholesterol >200 mg/dl | −0.3 (−2.7, 2.1) | 0.804 |
| Estimated mean change in GFR per year [ml/min/1.73 m2/year] | ||
| Among those with a decrease in HDLc cholesterol ≤5 mg/dl | ||
| Slope 0<Time<3 years | 0.2 (−0.4, 0.8) | 0.557 |
| Slope 3<Time<5 years | −1.1 (−1.8, −0.4) | 0.003 |
| Slope Time>5 years | 0.1 (−0.3, 0.4) | 0.674 |
| Among those with a decrease in HDLc cholesterol >5 mg/dl | ||
| Slope 0<Time<3 years | >0.1 (−1.0, 1.1) | 0.992 |
| Slope 3<Time<5 years | −1.3 (−2.7, 0.1) | 0.079 |
| Slope Time>5 years | −1.3 (−3.7, 1.2) | 0.322 |
Estimates based on N=365 HIV-infected men.
This table shows the difference in the mean level of GFR across time associated with various risk factors for kidney dysfunction as well as the change in the mean eGFR slope comparing those with a decrease in high-density lipid cholesterol (HDLc) of at least 5 mg/dl to those with a decrease of less than 5 mg/dl over approximately a 3-year period following ART initiation.
To assess the presence of a dose-response, we also estimated the effect of larger changes in TC using a cut point of 90 mg/dl (approximately the 90th percentile of change in the data). The results suggested similar magnitude effects prior to 5 years (from the pre-ART visit) to the 50 mg/dl cut point analysis, but a larger decrease after 5 years. The estimated declines were 1.2 ml/min/1.73 m2 per year (p=0.052), 2.5 ml/min/1.73 m2 per year (p=0.001), and 4.1 ml/min/1.73 m2 per year (p=0.004) from 0 to 3 years, 3 to 5 years, and after 5 years, respectively, from the pre-ART visit.
Discussion
Abnormal lipid levels and other metabolic issues have been associated with ART initiation, particularly regimens containing PIs.16,25–31 The magnitude of the reported changes in lipids following ART is consistent with a return to preseroconversion levels plus normal age-related changes.14,16,26 However, given the relatively short time frame in which these changes occur (less than 3 years), there may be implications for comorbidities such as renal disease that have etiologic links to lipid abnormalities. In this sample of 365 men from the MACS, we examined the association of large changes in lipid levels occurring after ART initiation with changes in eGFR, the primary variable used to assess kidney disease progression. We found that increases in TC of 50 mg/dl (the 65th percentile of change) or more over approximately 3 years from ART initiation were associated with decreases in eGFR that were significantly larger than those seen in men with smaller TC changes. The impact of lipid changes was greatest after 3 years following ART initiation (subsequent to the measured lipid changes) and persisted beyond 5 years following ART initiation with a consistent rate of decline of approximately 2.5 ml/min/1.73 m2 per year. The analysis of HDLc changes yielded more ambiguous results, with a suggestion of similar magnitude eGFR declines in both comparison groups but more persistent declines in those with an HDLc decrease of greater than 5 mg/dl. Tenofovir use, which has been extensively investigated and variably associated with kidney function decline,32–38 was found to have a large effect on the eGFR trajectory. Tenofovir use was also found to weakly correlate with lipid level changes, with smaller TC changes noted in those on tenofovir-containing regimens; however, adjusting for tenofovir use only mildly attenuated effect estimates and restricting to person-visits not on tenofovir did not change inferences, suggesting that lipid changes have an independent association with eGFR trajectory not explained by tenofovir use. A stronger predictor of lipid level changes was PI use, which was associated with larger increases in TC and smaller increases in HDLc cholesterol. PI use had a modest, nonsignificant negative effect on the average eGFR level across time but did not confound the association with lipid changes when added to models; statin use, in contrast, had an independent association, and was associated with a significantly higher average eGFR level across time.
Changes in lipids were assessed at 3 years following ART based on prior studies in the MACS and elsewhere that had evaluated the impact of ART initiation on lipid profiles.14,16,26 However, long-term ART use could result in larger lipid changes, the impact of which on eGFR levels was not assessed in the current study. Data from the Physicians Health Study provide some evidence of longer term elevations in serum creatinine, the primary biomarker used to estimate eGFR, as a result of abnormal baseline lipid profiles.6 Nevertheless, it is likely that the decreases in eGFR noted at 5 years after ART stabilize or moderate at some point in the trajectory. The modeling of the trajectory using only two inflection points at 3 years and 5 years was chosen to examine eGFR changes contemporary with and relatively proximate to the changes in lipid levels. Thus the model was not sensitive to more long-term deviations from linearity, which limits our ability to predict whether these proximate declines represent a future risk of chronic kidney disease.
It should also be noted that equations used to estimate GFR in populations with relatively normal kidney function tend to underestimate the true GFR and have not been validated in HIV-infected populations. However, bias associated with estimation should not impact inferences regarding within-person GFR changes, as the bias should be approximately equivalent across all time points. Furthermore, the CKD-EPI was not intended for use with the older creatinine assays such as the Jaffe that were commonly used in MACS. Assuming the assay used for repeated measures of creatinine over time remained consistent for an individual, this should not significantly influence the findings.
Additional limitations of the current study include the inability to assess the association between lipid changes and kidney function changes in HIV-infected women given the all-male composition of the MACS study. Studies in HIV-uninfected populations have included women and found associations between lipid levels and kidney function,5,7 suggesting that this relationship is generalizable across sexes, but studies in HIV-infected women are needed to confirm the relationship noted in the present analysis. Furthermore, very few fasting lipid measurements were collected (N=59) and therefore we could not perform robust analyses on the association of triglycerides or low-density lipids with kidney function decline. Finally, we did not explore additional time-by-covariate interactions that might have improved the model specification and better controlled for confounding of the association between cholesterol changes and the eGFR slope over time due to the added complexity these terms would introduce.
Given suggestions in the literature that lipid-lowering medications may minimize renal injury and slow CKD progression,9–13 HIV-infected individuals initiating ART may be an appropriate high-risk group for such therapies if they develop dyslipidemia. The benefit of statins for kidney disease prevention in this context is not established, however. Those on statins did have significantly higher average eGFRs. An evaluation of proteinuria levels in those individuals with increasing serum creatinine trajectories would be beneficial in identifying accompanying glomerular damage. With support for earlier initiation of ART, continued study of the long-term implications of therapy for kidney dysfunction is warranted.
Acknowledgments
Data in this article were collected by the Multicenter AIDS Cohort Study (MACS) with centers located at the Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD (principal investigator, Joseph Margolick, MD, PhD); Howard Brown Health Center and Northwestern University Medical School, Chicago, IL (principal investigator, John Phair, MD); University of California–Los Angeles (principal investigator, Roger Detels, MD); and University of Pittsburgh, Pittsburg, PA (principal investigator, Charles Rinaldo, PhD); and the Data Coordinating Center, the Johns Hopkins University Bloomberg School of Public Health (principal investigator, Lisa Jacobson, ScD). This work is supported by grants UO1-AI-35042, 5-M01-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI- 37613, UO1-AI-35041, M01 RR00425, and 1K23DK081317 and by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Author Disclosure Statement
No competing financial interests exist. The disclosure statement is accurate.
References
- 1.Lindeman RD. Tobin J. Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 2.Joles JA. Kunter U. Janssen U, et al. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J Am Soc Nephrol. 2000;11(4):669–683. doi: 10.1681/ASN.V114669. [DOI] [PubMed] [Google Scholar]
- 3.Keane WF. Kasiske BL. O'Donnell MP. Lipids and progressive glomerulosclerosis. A model analogous to atherosclerosis. Am J Nephrol. 1988;8(4):261–271. doi: 10.1159/000167599. [DOI] [PubMed] [Google Scholar]
- 4.Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol. 2004;24(1):46–53. doi: 10.1159/000075925. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P. Coresh J. Smith JC. Eckfeldt J. Klag MJ. Plasma lipids and risk of developing renal dysfunction: The atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffner ES. Kurth T. Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14(8):2084–2091. doi: 10.1681/ASN.V1482084. [DOI] [PubMed] [Google Scholar]
- 7.Fox CS. Larson MG. Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 8.Manttari M. Tiula E. Alikoski T. Manninen V. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 1995;26(4):670–675. doi: 10.1161/01.hyp.26.4.670. [DOI] [PubMed] [Google Scholar]
- 9.Bussolati B. Deregibus MC. Fonsato V. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16(7):1936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL. O'Donnell MP. Cleary MP. Keane WF. Treatment of hyperlipidemia reduces glomerular injury in obese Zucker rats. Kidney Int. 1988;33(3):667–672. doi: 10.1038/ki.1988.51. [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M. Moye L. Sacks FM. Cole T. Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14(6):1605–1613. doi: 10.1097/01.asn.0000068461.45784.2f. [DOI] [PubMed] [Google Scholar]
- 12.Collins R. Armitage J. Parish S. Sleigh P. Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 13.Fried LF. Orchard TJ. Kasiske BL. Effect of lipid reduction on the progression of renal disease: A meta-analysis. Kidney Int. 2001;59(1):260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 14.Mulligan K. Grunfeld C. Tai VW, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23(1):35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Carr A. Samaras K. Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: A cohort study. Lancet. 1999;353(9170):2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 16.Riddler SA. Smit E. Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289(22):2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 17.Wyatt CM. Morgello S. Katz-Malamed R, et al. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2009;75(4):428–434. doi: 10.1038/ki.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szczech LA. Grunfeld C. Scherzer R, et al. Microalbuminuria in HIV infection. AIDS. 2007;21(8):1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaslow RA. Ostrow DG. Detels R, et al. The Multicenter AIDS Cohort Study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 20.Allain CC. Poon LS. Chan CS. Richmond W. Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 21.Warnick GR. Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19(1):65–76. [PubMed] [Google Scholar]
- 22.Giorgi JV. Cheng HL. Margolick JB, et al. Quality control in the flow cytometric measurement of T-lymphocyte subsets: The multicenter AIDS cohort study experience. The Multicenter AIDS Cohort Study Group. Clin Immunol Immunopathol. 1990;55(2):173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 23.Osberg IM. Hammond KB. A solution to the problem of bilirubin interference with the kinetic Jaffe method for serum creatinine. Clin Chem. 1978;24(7):1196–1197. [PubMed] [Google Scholar]
- 24.Levey AS. Stevens LA. Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behrens G. Dejam A. Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13(10):F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 26.Berthold HK. Parhofer KG. Ritter MM, et al. Influence of protease inhibitor therapy on lipoprotein metabolism. J Intern Med. 1999;246(6):567–575. doi: 10.1046/j.1365-2796.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 27.Carr A. Samaras K. Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Dong KL. Bausserman LL. Flynn MM, et al. Changes in body habitus and serum lipid abnormalities in HIV-positive women on highly active antiretroviral therapy (HAART) J Acquir Immune Defic Syndr. 1999;21(2):107–113. [PubMed] [Google Scholar]
- 29.Levy AR. McCandless L. Harrigan PR, et al. Changes in lipids over twelve months after initiating protease inhibitor therapy among persons treated for HIV/AIDS. Lipids Health Dis. 2005;4:4. doi: 10.1186/1476-511X-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahajan AP. Tashima KT. Bausserman LL. Flynn MM. Carpenter CC. Plateau in body habitus changes and serum lipid abnormalities in HIV-positive women on highly active antiretroviral therapy: A 3.5-year study. J Acquir Immune Defic Syndr. 2001;28(4):332–335. doi: 10.1097/00126334-200112010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Stein JH. Komarow L. Cotter BR, et al. Lipoprotein changes in HIV-infected antiretroviral-naive individuals after starting antiretroviral therapy: ACTG Study A5152s Stein: Lipoprotein Changes on Antiretroviral Therapy. J Clin Lipidol. 2008;2(6):464–471. doi: 10.1016/j.jacl.2008.08.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mocroft A. Kirk O. Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24(11):1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 33.Gallant JE. Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23(15):1971–1975. doi: 10.1097/QAD.0b013e32832c96e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izzedine H. Hulot JS. Vittecoq D, et al. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20(4):743–746. doi: 10.1093/ndt/gfh658. [DOI] [PubMed] [Google Scholar]
- 35.Horberg M. Tang B. Towner W, et al. Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2010;53(1):62–69. doi: 10.1097/QAI.0b013e3181be6be2. [DOI] [PubMed] [Google Scholar]
- 36.Calza L. Trapani F. Tedeschi S, et al. Tenofovir-induced renal toxicity in 324 HIV-infected, antiretroviral-naive patients. Scand J Infect Dis. 2011;43(8):656–660. doi: 10.3109/00365548.2011.572906. [DOI] [PubMed] [Google Scholar]
- 37.Longenecker CT. Scherzer R. Bacchetti P, et al. HIV viremia and changes in kidney function. AIDS. 2009;23(9):1089–1096. doi: 10.1097/QAD.0b013e32832a3f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ando M. Yanagisawa N. Ajisawa A. Tsuchiya K. Nitta K. Kidney tubular damage in the absence of glomerular defects in HIV-infected patients on highly active antiretroviral therapy. Nephrol Dial Transplant. 2011;26(10):3224–3229. doi: 10.1093/ndt/gfr020. [DOI] [PubMed] [Google Scholar]