Abstract
Aims: Nitroxyl (HNO) interacts with thiols to act as a redox-sensitive modulator of protein function. It enhances sarcoplasmic reticular Ca2+ uptake and myofilament Ca2+ sensitivity, improving cardiac contractility. This activity has led to clinical testing of HNO donors for heart failure. Here we tested whether HNO alters the inhibitory interaction between phospholamban (PLN) and the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) in a redox-dependent manner, improving Ca2+ handling in isolated myocytes/hearts. Results: Ventriculocytes, sarcoplasmic reticulum (SR) vesicles, and whole hearts were isolated from control (wildtype [WT]) or PLN knockout (pln−/−) mice. Compared to WT, pln−/− myocytes displayed enhanced resting sarcomere shortening, peak Ca2+ transient, and blunted β-adrenergic responsiveness. HNO stimulated shortening, relaxation, and Ca2+ transient in WT cardiomyocytes, and evoked positive inotropy/lusitropy in intact hearts. These changes were markedly blunted in pln−/− cells/hearts. HNO enhanced SR Ca2+ uptake in WT but not pln−/− SR-vesicles. Spectroscopic studies in insect cell microsomes expressing SERCA2a±PLN showed that HNO increased Ca2+-dependent SERCA2a conformational flexibility but only when PLN was present. In cardiomyocytes, HNO achieved this effect by stabilizing PLN in an oligomeric disulfide bond-dependent configuration, decreasing the amount of free inhibitory monomeric PLN available. Innovation: HNO-dependent redox changes in myocyte PLN oligomerization relieve PLN inhibition of SERCA2a. Conclusions: PLN plays a central role in HNO-induced enhancement of SERCA2a activity, leading to increased inotropy/lusitropy in intact myocytes and hearts. PLN remains physically associated with SERCA2a; however, less monomeric PLN is available resulting in decreased inhibition of the enzyme. These findings offer new avenues to improve Ca2+ handling in failing hearts. Antioxid. Redox Signal. 19, 1185–1197.
Innovation.
Our study is the first demonstration that phospholamban can be modified in a redox-dependent manner, leading to improved myocyte and overall cardiac function. HNO donors pose an intriguing alternative to enhance Ca2+ cycling in myocardial cells, in a regulated, reversible and redox-sensitive manner, without compromising normal protein kinase A-regulation.
Introduction
Congestive heart failure frequently involves reduced cardiac contractility and slowed muscle relaxation (20, 21). Abnormalities of the excitation-contraction coupling processes are centrally involved in these adverse outcomes (13, 20, 21). At least in part, these alterations may originate from redox alterations of the myocardium (5). Perturbed Ca2+ cycling into, and out of, the sarcoplasmic reticulum (SR) can result from reduced expression and/or activity of SR Ca2+-ATPase (SERCA2a) or the altered phosphorylation status of phospholamban (PLN). Ongoing efforts to enhance SERCA2a activity include gene transfer (10) or targeted pharmacological therapies (20), with clinical trials currently underway (22).
Nitroxyl (HNO) is the one-electron reduction product of nitric oxide (NO.)(44) that confers in vivo positive inotropy/lusitropy in normal (38) and failing hearts (37) via mechanisms independent of cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) signaling (45). Cardiomyocytes exposed to HNO exhibit enhanced Ca2+ cycling (45) accompanied by augmented SERCA2a activity, which enhances Ca2+ uptake into the SR and increases fractional Ca2+ release. These changes occur without a rise in the trans-sarcolemmal Ca2+ flux via the L-type Ca2+ channels (LTCC) (25) and HNO does not trigger diastolic Ca2+ accumulation or SR Ca2+ overload (45). These properties make HNO an attractive potential therapy for heart failure, particularly when compared to inotropes that stimulate cAMP/PKA signaling, which can induce excess intracellular Ca2+, arrhythmias, and adverse cardiac events (20).
HNO reacts rapidly with protein thiols in vitro (44) and it is thought to induce biochemical effects by changing protein conformation and/or function through thiol modification. The inotropic action of HNO is highly redox-sensitive, being readily inhibited by the addition of a thiol reducing agent, such as dithiothreitol (DTT) (7). HNO, donated by Angeli's salt (AS) modifies cysteine residues in myofilament proteins to enhance force generation (16), and AS/HNO-induced SERCA2a activation appears to involve cysteine modification on either PLN (15) or SERCA2a (28).
This study aimed to elucidate the mechanism(s) underlying PLN/SERCA2a modulation by AS/HNO, and did so by addressing the following three questions: First, is PLN necessary for AS/HNO-induced stimulation of SERCA2a and enhanced contractile function in intact myocytes or hearts? Second, does the impact of the AS/HNO-PLN modification on SERCA2a activity observed in a heterologous expression system (15) apply to intact myocytes or hearts with physiological thiol content, and how does this relate to Ca2+ uptake? Lastly, does AS/HNO impact PLN/SERCA2a protein-protein interactions in a redox-dependent manner and/or alter PLN coupling, such as occurs with PKA phosphorylation?
Results
PLN is central to AS/HNO inotropy and lusitropy in myocytes and for AS/HNO-induced enhancement of left ventricular function in isolated mouse hearts
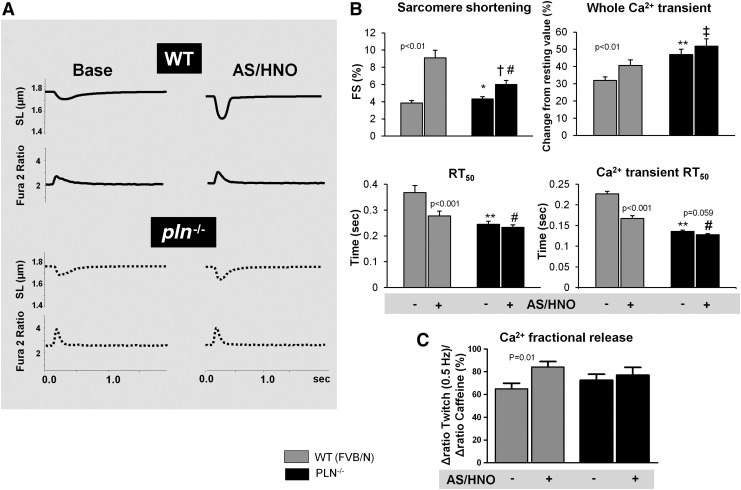
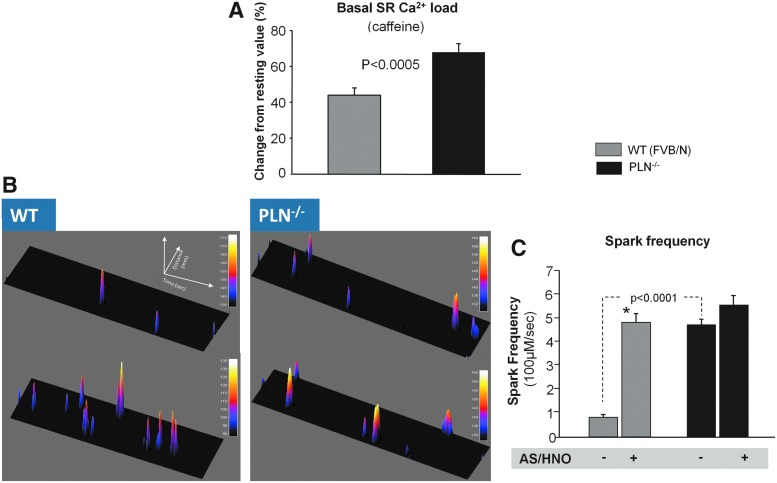
The influence of AS/HNO stimulation was compared between myocytes with or without PLN. In wildtype (WT) cells, 500 μM AS/HNO increased shortening, and whole Ca2+ transient amplitude and shortened the time of sarcomere relengthening and Ca2+ transient decay. AS/HNO also increased Ca2+ fractional release from the SR (Fig. 1A–C). These effects were markedly blunted in myocytes from pln−/− hearts. Analogous disparities were obtained using the β-agonist isoproterenol (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars) that enhances function in part through phosphorylation of PLN. Interestingly, basal SR Ca2+ levels were higher in the pln−/− mice (Fig. 2A). AS/HNO also increased Ca2+ spark frequency in WT mice to a level similar to that observed at baseline in pln−/− mice, while it had no significant impact in pln−/− mice (Fig. 2B, C).
FIG. 1.
AS/HNO increases contractility but not Ca2+ transient in pln−/− myocytes. (A) Representative traces showing the impact of HNO, generated by AS (500 μM) on sarcomere shortening (sarcomere length [SL]), and whole Ca2+ transients (Fura 2 fluorescence) for WT or pln−/− myocytes; (B) Summary data of the impact of AS on fractional sarcomere shortening (FS) and Ca2+ transient, both expressed as fractional increase from diastolic levels (top), and on time to 50% relaxation (RT50) of SL and of Ca2+ transient (bottom) for WT and PLN−/− myocytes. (C) Ratio of 340:510 emission of Ca2+ fractional release from either WT or PLN−/− mice treated with AS/HNO (n=20 cells for each group, *p<0.05 versus WT;**p<0.001 versus WT *p<0.05 versus WT;**p<0.001 versus Δdrug x genotype interaction; #p<0.01 versus baseline WT; †p<0.05 versus baseline PLN; ‡p<0.05 versus Δdrug×genotype interaction). AS, Angeli's salt; HNO, nitroxyl; PLN, phospholamban; WT, wildtype.
FIG. 2.
AS/HNO has no effect on Ca2+ spark frequency in pln−/− cells. (A) Impact of AS/HNO (500 μM) on SR Ca2+ load and fractional release (via caffeine rapid application of 10 mM caffeine), in isolated ventricular myocytes from WT (FVB/N) and pln−/− hearts (n=20 cells for each group). (B) Example plots of Ca2+ sparks recorded during experiment employing LV myocytes isolated from WT and pln−/− mice. (C) HNO significantly increased Ca2+ sparks frequency in WT heart cells (*p<0.001 versus base, n=4). Conversely, in pln−/− heart cells that already display higher basal frequency as compared to WT cells, HNO did not further increase Ca2+ sparks. LV, left ventricular; SR, sarcoplasmic reticulum.
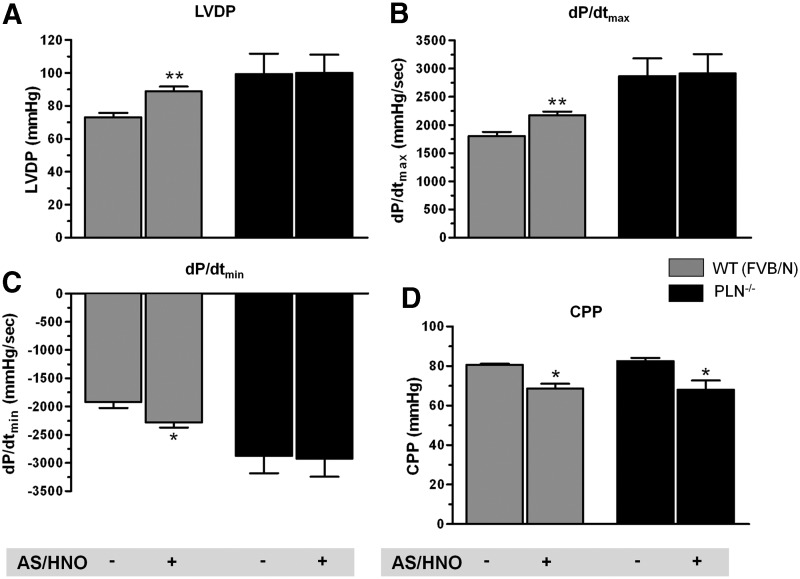
Since the redox state for isolated myocytes can differ from that of an intact heart, and tissue redox balance may impact AS/HNO efficacy, we tested whether PLN is required for AS/HNO inotropy/lusitropy in intact retrogradely perfused mouse hearts. In control hearts, infusion of AS/HNO (500 μM for 10–15 min) enhanced contraction and accelerated relaxation (Fig. 3A–C). These effects were absent in pln−/− hearts. This disparity cannot be attributed to AS/HNO-induced vasodilation because coronary perfusion pressure fell similarly in both groups (Fig. 3D).
FIG. 3.
PLN is essential for AS/HNO to enhance LV function in isolated mouse hearts. Impact of AS/HNO (500 μM) on LV function from WT (FVB/N) and PLN−/− hearts under Langendorff perfusion. Shown are (A) Left ventricular developed pressure (LVDP); Maximal (B) and Minimal (C) rate of pressure changes with time (dP/dt); (D) Coronary perfusion pressure (CPP) (n=3–5 hearts for each group) (*p<0.01; **p<0.001).
AS/HNO enhances Ca2+ reuptake in SR vesicles isolated from WT but not PLN−/− hearts
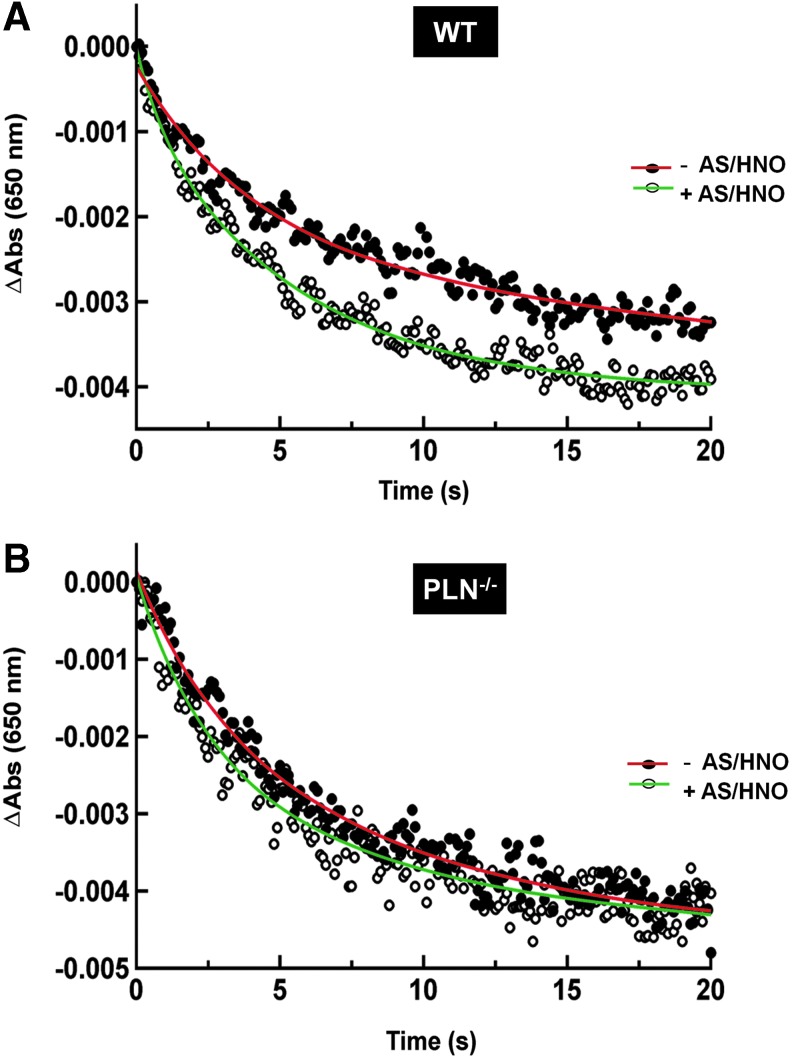
AS/HNO modifies cysteine residues in the PLN transmembrane domain, accelerating SERCA2a enzyme kinetics, and activity in High Five (HF) insect microsomes (15). Here we tested whether AS/HNO increased SERCA2a activity directly in intact WT and pln−/− myocytes. Crude SR vesicles from mouse cardiomyocytes were incubated with AS/HNO (250 μM) before measuring ATP-dependent Ca2+ uptake by stop-flow mixing at 24°C. The time course of Ca2+ uptake monitored at 650 nm was biphasic (Fig. 4A), likely reflecting different populations of vesicles associated with the light and heavy fractions of SR (1). As seen in cardiac SR from C57BL6 mice (45), AS/HNO increased ATP-dependent Ca2+ uptake in SR from WT (FVB/N) hearts, where both the fast and the slow phases of active Ca2+ accumulation into SR vesicles were accelerated more than twofold (Fig. 4A and Supplementary Table S1), without affecting total Ca2+ uptake (Supplementary Table S1). However, AS/HNO did not alter the kinetics of Ca2+ uptake into SR vesicles from pln−/− hearts (Fig. 4B; Supplementary Table S1).
FIG. 4.
PLN presence is required for AS/HNO to increase ATP-dependent Ca2+ uptake in cardiac SR vesicles. Representative stopped-flow recordings of active Ca2+ accumulation monitored by arsenazo III at 650 nm in the absence (−AS/HNO) and presence of 250 μM AS/HNO (+AS/HNO) in SR vesicles from WT (A) and pln−/− mice (B). The signal decay represents Ca2+ uptake into the SR vesicles from the extra-vesicular medium. The initial absorbance reading on the y-axis was normalized to zero by subtracting the absorbance at t=0 from each of the absorbance readings. The solid curve through the data points represents the best fit of the data to a bi-exponential plus residual equation (n=6; Supplementary Table S1). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
PLN is required for AS/HNO stimulation of SERCA2a activity
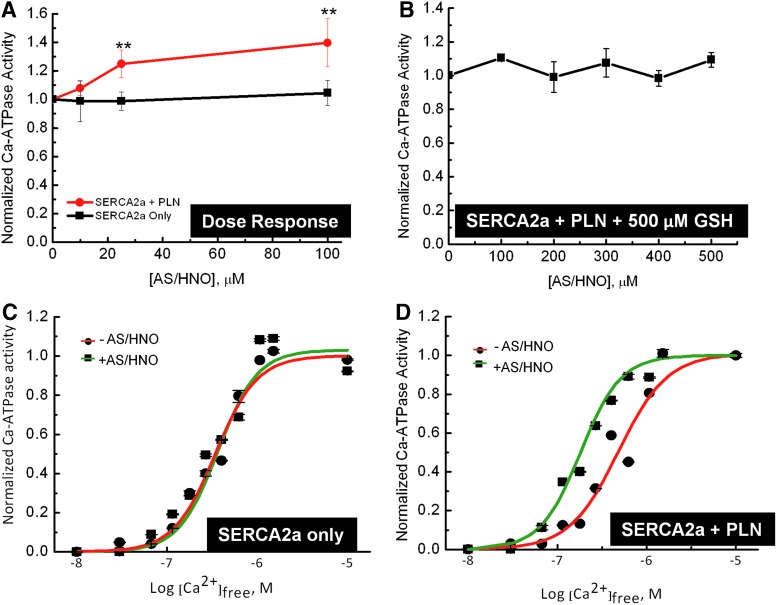
Next, we examined the dose-dependent effects of AS/HNO on SERCA2a Ca2+-ATPase activity in HF insect cell microsomes containing SERCA2a with (SERCA2a+PLN) or without (SERCA2a) PLN (48). In the absence of PLN, AS/HNO was unable to increase SERCA2a activity; but increased when PLN was present in the system (Fig. 5A). The effect of AS/HNO (from 0 to 500 μM) was fully blocked by the addition of 500 μM glutathione (GSH), which we found to be the lowest effective dose for quenching AS/HNO. Interestingly, the addition of GSH itself did not alter SERCA2a activity (Fig. 5B). Additionally, we determined that the effect of AS/HNO on the Ca2+-dependence of SERCA2a activity required coexpression of PLN as the SERCA2a-Ca2+ activity curve was altered after AS/HNO treatment in the presence (ΔK0.5 ∼200 nM) but not in the absence (ΔK0.5 ∼0 nM) of PLN (Fig. 5C, D). The K0.5 for SERCA2a alone was 325±25 nM (Fig. 5C), and was not affected by AS/HNO treatment. In contrast, the K0.5 value for the SERCA2a+PLN sample (Fig. 5D), was 550±50 nM before AS/HNO treatment, and this decreased to 325±25 nM after AS/HNO treatment (a value similar to that of the sample devoid of PLN). Treatment of microsomes containing SERCA2a+PLN with an anti-PLN monoclonal antibody, which functionally uncouples PLN from SERCA2a (3) also shifted the AS/HNO stimulated SERCA2a activity-Ca2+ relationship (Supplementary Fig. S2), similar to that observed for AS/HNO treatment. These data indicate that PLN is centrally involved in HNO-driven enhancement of SERCA2a function.
FIG. 5.
AS/HNO functionally uncouples PLN from SERCA2a in microsome vesicles. (A) Microsomes containing either SERCA2a alone (squares) or SERCA2a+PLN (circles) were suspended (1 mg total protein/ml) in 250 mM sucrose, 10 mM imidazole, pH 7.0, and treated with AS/HNO, at the indicated concentrations. The microsomes were incubated at room temperature for 10 min, after which the microsomes were assayed for ATPase activity in a buffer containing 0.625 μM ionized Ca2+, where PLN has significant effects on SERCA2a activity (n=3–5 repetitions). (B) Experiment repeated using microsomes containing SERCA2a pretreated with 500 μM GSH before AS/HNO addition. Symbols represent the average of two repetitions, and error bars correspond to the standard error of the mean for each point. (C, D) Microsomes containing SERCA2a expressed in the absence (C) or presence of PLN (D). Samples were suspended (0.2 mg total protein/ml) in 250 mM sucrose and 10 mM imidazole, pH 7.0, treated with either vehicle solution (squares, red line), or 100 μM AS/HNO (circles, green line) and incubated at room temperature for 10 min, after which the treated microsomes were assayed for [Ca2+]-dependent ATPase activity at 37°C. The data are shown normalized to their respective maxima to better illustrate the HNO-dependent shift in the [Ca2+]-dependent activity curve and to account for changes in activity due to expression differences in the various samples and thereby better demonstrate the effects of PLN on SERCA2a activity. Symbols are the average of duplicate experiments and the error bars (which are generally smaller than the symbols) represent the high/low values for each point (**p≤0.01). GSH, glutathione. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
AS/HNO increases SERCA2a conformational flexibility in the presence of PLN
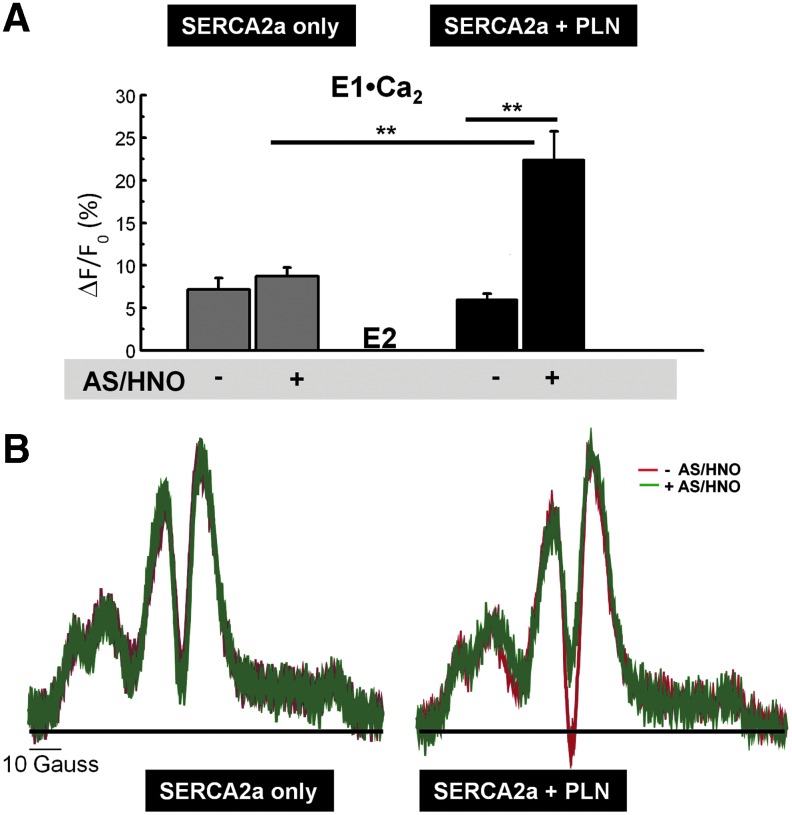
SERCA2a is, in part, inhibited by PLN-stabilization of the Ca2+-free enzyme conformation, which impedes SERCA2a activation by increased Ca2+ (47). Therefore, we tested whether AS/HNO alters the conformational transition of SERCA2a from the Ca2+-free (E2 state) to Ca2+-bound intermediates (E1•Ca2 state), and whether the presence of PLN is required to achieve this effect. Fluorescence spectroscopy was used to monitor changes in fluorescence quenching of fluorescein isothiocyanate (FITC) covalently bound to SERCA2a in microsomes for this purpose. The addition of Ca2+ to microsomes containing SERCA2a without PLN induced the conformational transitions from the E2 to the E1•Ca2 state, as assessed by a 7.8%±1.3% increase in the fluorescence intensity due to altered solvent accessibility to FITC (Fig. 6A) (47). When PLN was present, the amplitude of the E2 to E1•Ca2 conformational change was reduced (6.0%±0.7% increase), consistent with previous findings (47). However, when samples containing no PLN were treated with AS/HNO fluorescence intensity was unaltered (8.7%±1.0% increase); in contrast, when PLN was present, AS/HNO induced a 3.7-fold increase in the intensity (22.4%±3.4%). Since there was no significant effect of AS/HNO on SERCA2a alone, this set of data suggests that AS/HNO alleviates PLN inhibition of SERCA2a [Ca2+]-dependent conformational flexibility, with a concomitant increase in SERCA2a activity.
FIG. 6.
AS/HNO increases SERCA2a flexibility for a more active structure. (A) FITC was used to covalently label SERCA2a in insect cell microsomes containing SERCA2a expressed alone (left) or SERCA2a+PLN (right). The microsomes were pretreated with vehicle (−) or 100 μM AS/HNO (+), after which the Ca2+-dependent conformational transition was measured by monitoring changes in the steady-state fluorescence of SERCA2a upon a [Ca2+] jump from ∼0 to 100 μM Ca2+. Values represent the average of 5–10 independent measurements and error bars represent the standard error of the mean. (B) Maleimide spin label (MSL) was used to covalently label SERCA2a, to measure SERCA2a rotational mobility as affected by PLN and AS/HNO. The ST-EPR spectra of SERCA2a at 4°C in the absence (left) and presence (right) of coexpressed PLN in microsomes pretreated with vehicle (red) or 100 μM AS/HNO (green) were analyzed by the high-field (H″/H) line-height ratio, which was used to determine the rotational correlation time for MSL-SERCA2a in each sample, based on standard curves constructed from a model system. **p≤0.01. FITC, fluorescein isothiocyanate; ST-EPR, saturation transfer electron paramagnetic resonance.
HNO promotes SERCA2a oligomeric rearrangements by reaching the sulfhydryl groups in the transmembrane domains of PLN
PKA-induced phosphorylation of PLN functionally uncouples PLN from SERCA2a, concomitant with decreasing the rotational mobility of SERCA2a (36), consistent with a physical rearrangement of SERCA2a units within the Ca2+ pump oligomeric complex. Therefore, we tested whether AS/HNO has a similar impact on SERCA2a oligomeric interactions, as that affected by PLN. We used conventional and saturation transfer electron paramagnetic resonance (ST-EPR) spectroscopy (33, 36, 42) of a maleimide spin label (MSL) covalently bound to SERCA2a to measure SERCA2a rotational mobility, in the absence and presence of PLN in microsomes (41). Treatment of the microsomes with AS/HNO had no significant effect on the conventional EPR spectrum (data not shown), indicating that AS/HNO had no direct effect on the motion of the spin label in its microenvironment. The corresponding ST-EPR spectrum of SERCA2a+PLN SERCA2a alone is presented in Figure 6B (right and left). Clear PLN-dependent differences in SERCA2a rotational mobility were seen, where the rotational rate (1/τr, the inverse rotational correlation time) (36) for SERCA2a+PLN was approximately (1.66±0.14)×104 s−1 as compared to (1.00±0.06)×104 s−1 for SERCA2a alone. This indicated that the rotational mobility of SERCA2a+PLN was approximately 1.66-fold greater as compared to SERCA2a alone, in keeping with SERCA2a rotational mobility after PLN phosphorylation (32, 36). Treatment of the SERCA2a+PLN sample with AS/HNO (100 μM) produced distinct changes in the ST-EPR spectrum, consistent with a decrease in the SERCA2a rotational rate (1.10±0.10×104 s−1) similar to that observed for SERCA2a alone. In contrast, AS/HNO treatment of SERCA2a alone had essentially no effect on the ST-ER spectrum of SERCA2a, indicating that AS/HNO has little impact on SERCA2a rotational mobility in the absence of PLN. Because integral membrane protein rotational mobility is directly controlled by lipid bilayer fluidity (41), we conducted measurements of lipid hydrocarbon chain mobility, as affected by AS/HNO, using conventional EPR spectroscopy of stearic acid spin-labels incorporated into our microsomes. We found that AS/HNO treatment had no discernible effect on the lipid hydrocarbon chain mobility, and thus, lipid bilayer fluidity (Supplementary Figs. S3 and S4). These findings confirm that the observed changes in SERCA2a rotational mobility are most likely due to changes in the size of the rotating unit and not changes in the bilayer lipid dynamics. Thus, AS/HNO treatment alleviates PLN inhibition from SERCA2a, allowing the pump to undergo rearrangement to form an oligomeric complex, concomitant with AS/HNO-activation of SERCA2a activity. This pattern of change is similar to uncoupling PLN from SERCA2a via PKA-induced PLN phosphorylation (36).
PLN oligomerization is altered by AS/HNO leading to the formation of disulfide bonds among PLN monomers
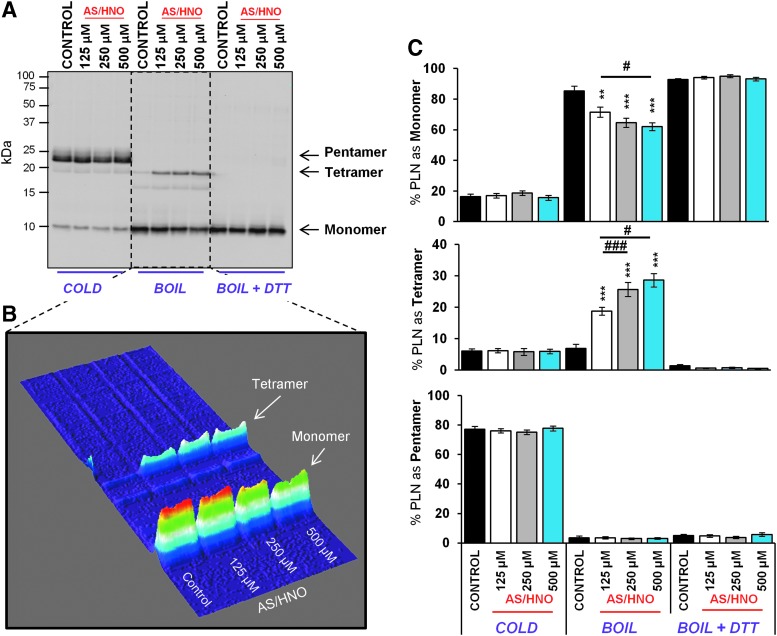
The PLN monomer is regarded as the PLN inhibitory species (31). Therefore, we tested whether AS/HNO alters PLN monomer availability as a potential mechanism for AS/HNO regulation on SERCA2a function. Heart extracts from WT (FVB/N) mice were treated with AS/HNO (125–500 μM) or vehicle (10 mM NaOH) for 1 h at room temperature. Changes in the PLN pentamer/monomer ratio were examined by SDS-PAGE and visualized by Western blot. As expected, in cold samples (4°C) PLN migrated as both pentameric and monomeric species (24). Under these conditions, AS/HNO treatment had no impact on the pentamer/monomer ratio (Fig. 7). However, when samples were boiled to disrupt noncovalent interactions (24), the pentamer dispersed primarily into monomers. A small percentage of PLN (7.0%±1%) migrated at a molecular weight corresponding to a tetrameric form of PLN. When treated with AS/HNO, the amount of the tetrameric form increased to 30%±2.5% of total PLN, with a corresponding decrease in the monomer isoform fraction (86.4%±2.5% to 64.6%±3.3%; Fig. 7). Next, we tested whether AS/HNO-dependent PLN disulfide bond formation was involved in PLN tetramer accumulation. To test this, whole heart extracts pretreated with AS/HNO (as above) were then treated with the reducing agents DTT or tris(2-carboxyethyl)phosphine (TCEP), which are well-known disulfide reducing agents (27). Treatment with DTT or TCEP led to the break-down of the tetrameric form generated upon AS/HNO treatment, with a concomitant rise in PLN monomers (Supplementary Fig. S5). This finding is consistent with previous studies performed in microsomes expressing PLN alone (15).
FIG. 7.
AS/HNO stabilizes a homotetramer releasing less inhibitory PLN species. Western blots for PLN in heart extracts from WT (FVB/N) mice were treated with increasing concentrations of AS/HNO and incubated at room temperature. Samples were kept under cold conditions, or broken into its component structures through boiling (with and without the presence of DTT to break up disulfide complexes). In its free form (not bound to SERCA2a), PLN forms pentamers in its storage state as observed under cold conditions. Under boiling conditions, the presence of stabilized tetrameric PLN is observed after increasing treatment of AS/HNO (B) A 3D surface plot of the boiled samples in (A), demonstrates a decrease in monomeric PLN (inhibitory PLN) with increasing AS/HNO. (C) Quantitation of multiple treatments (n=3 mice, two to three replicate treatments per mouse) demonstrate a significant reduction in monomeric and a corresponding increase in the tetrameric form of PLN with AS/HNO treatment. Brief (5 min) incubation of samples with DTT abolished this effect demonstrating that the formation of tetrameric form of PLN is through the formation of disulfide bonds. **p<0.01; ***p<0.001 (all relative to control), #p<0.05; ###p<0.001 (relative to 125 μM AS/HNO). DTT, dithiothreitol.
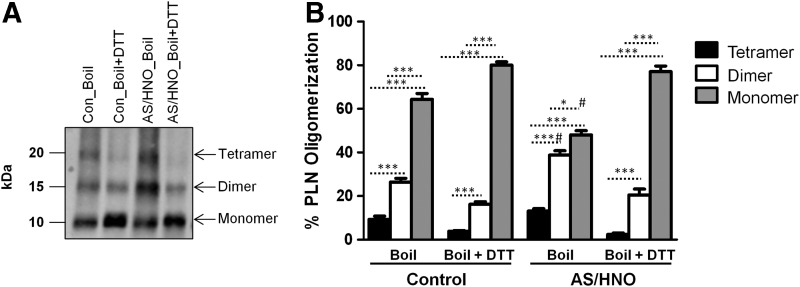
To validate these results in intact cardiomyocytes, freshly isolated adult murine cardiomyocytes were treated with AS/HNO (500 μM) or vehicle (10 mM NaOH), and then SDS-PAGE/Western blot analysis for PLN was performed. Upon boiling, monomeric, dimeric, and tetrameric PLN were detected. Under these conditions, AS/HNO treatment increased PLN tetramers and dimers, while a concomitant decrease in the amount of available monomeric PLN was evident when compared to control conditions (Fig. 8). This effect was reversed by the addition of DTT that led to a substantial decrease in dimer and tetramer levels, with a marked rise in monomeric PLN. The PLN oligomerization profile obtained with AS/HNO+DTT is superimposable to that found under control conditions with DTT alone. Repeating these experiments using TCEP instead of DTT led to a similar breakdown in these oligomeric forms although with less pronounced reduction in monomer levels (Supplementary Fig. S6), likely reflecting the fact that DTT is more effective than TCEP in reducing thiols under the present experimental conditions. Thus, treating left ventricular extracts or isolated intact myocytes with AS/HNO equally result in an increase of the oligomeric PLN forms at the expense of monomeric PLN. The fact that DTT fully reversed AS/HNO-induced changes is highly suggestive of the existence of disulfide bonds between monomers that aggregate either in tetramers or dimers. The possibility that the amount of dimer (or tetramer) increases after treatment with AS/HNO in a redox(thiol)-dependent manner is also supported by prior evidence obtained in microsomes expressing PLN. In those studies, we demonstrated that AS/HNO increased PLN dimerization (15) and that changes in PLN oligomerization are absent when PLN cysteines are mutated to alanines or blocked by pretreatment with N-ethylmaleimide (15).
FIG. 8.
In isolated intact murine cardiomyocytes, AS/HNO decreases the amount of monomeric PLN, favoring dimer and tetramer formation, in a thiol-sensitive manner. Western blots for PLN oligomerization profile in cardiomyocytes from WT (FVB/N) mice treated with 500 μM AS/HNO and incubated at room temperature. Samples were boiled in the absence or the presence of the reducing agent DTT). Data were obtained from n=4 mice (two to three replicate treatments per each mouse). (A) Representative Western. (B) Quantitation of PLN oligomerization. *p<0.05, ***p<0.001; #p<0.001 (relative to control under the same condition).
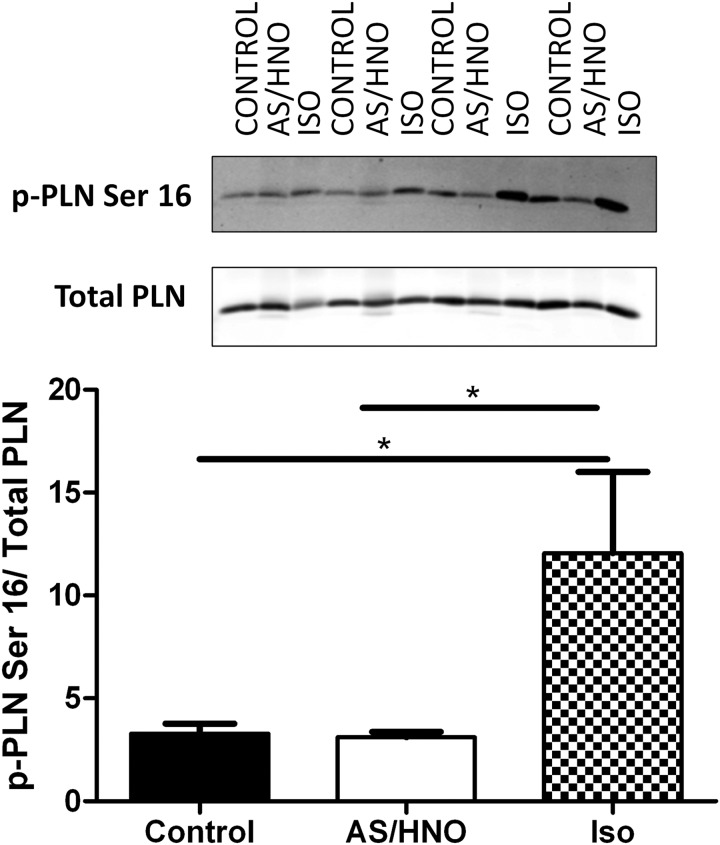
Lastly, since PLN oligomerizes in the presence of AS/HNO, we used coimmunoprecipitation to test whether AS/HNO influences the physical association of PLN to SERCA2a. Our coimmunoprecipitation studies indicate that the relative fraction of PLN associated with SERCA2a is effectively unchanged after exposure to AS/HNO (Supplementary Fig. S7A–C). This outcome is consistent with similar studies on the effects produced by PKA-induced PLN phosphorylation on PLN-SERCA2a association (9, 29). However, AS/HNO action occurred independently from PKA signaling because HNO, unlike the β-agonist ISO, did not alter the phosphorylation status of the PKA-sensitive Ser16 on PLN (Fig. 9).
FIG. 9.
AS/HNO modulation of PLN/SERCA2a interaction occurs independently from PKA phosphorylation. Western blots for PLN in myocytes from WT (FVB/N) mice that were treated either with 500 μM AS/HNO or 10 nM isoproterenol. Samples were boiled in the presence of DTT (50 mM). Phosphorylated PLN at Ser16 increased with the treatment of isoproterenol but not AS/HNO. *p<0.05. PKA, protein kinase A.
Discussion
This study demonstrates that the inotropic action of AS/HNO is markedly blunted in pln−/− myocytes and whole hearts, confirming a central role of PLN to AS/HNO-induced inotropy/lusitropy. To establish how AS/HNO activates SERCA2a, kinetic and spectroscopic techniques were undertaken to assess the impact of AS/HNO on PLN's regulation on SERCA2a (6, 33, 47). We determined that AS/HNO affected the oligomerization of PLN, altering the PLN-SERCA2a inhibitory interaction; thus, enhancing the SERCA2a Ca2+-dependent E2 to E1·Ca2 conformational transition, with a concomitant increase in SERCA2a Ca2+ transport activity. The AS/HNO-induced changes on PLN are thiol-sensitive and involve the formation of cysteine-based disulfide bonds between PLN monomers, favoring PLN oligomer formation.
AS/HNO and PLN/SERCA2a coupling: a PLN redox-modulation affecting SERCA2a oligomeric interactions
PLN inhibits SERCA2a Ca2+ sensitivity by disrupting SERCA2a oligomeric interactions that are important for enzyme dephosphorylation and subsequent high affinity Ca2+ binding necessary to activate the enzyme for ATP-dependent phosphorylation (6, 33). In support of this finding, Waggoner et al. (47) reported that uncoupling PLN from SERCA2a stimulates the Ca2+-dependent conformational transitions. Mutation of key leucine and isoleucine residues in the PLN transmembrane domain destabilize the PLN pentameric structure, shifting the pentamer/monomer ratio in favor of the monomer. These mutations have been associated with increased inhibition of SERCA2a, confirming the monomer as the active inhibitory species (3, 14, 19). More recently, Robia's group has elegantly shown that a missense mutation (R9C) in PLN's cytoplasmic domain increases the stability of the PLN pentameric assembly via disulfide bridge formation, preventing its binding to SERCA2a and PKA-induced phosphorylation (18). Based on this evidence and on our previous findings showing that AS/HNO potentially promotes the formation of disulfide bonds in the PLN transmembrane domain (15), we hypothesized that AS/HNO favors formation of PLN oligomers at the expense of PLN monomers, resulting in less inhibitory PLN species.
The current data comparing SERCA2a function in the presence and absence of PLN indicate that AS/HNO stimulation of SERCA2a Ca2+ sensitivity and consequent impact on cell shortening and Ca2+ transients depend upon PLN being present. AS/HNO functionally stimulates SERCA2a activity through a PLN-dependent mechanism, allowing SERCA2a to function as if PLN were absent (33, 47). Since Mahaney et al. (33) have shown that PLN inhibits SERCA2a Ca2+ sensitivity, if AS/HNO alters the PLN-SERCA2a interaction; one would predict increases in Ca2+ sensitivity of SERCA2a, which our microsomal sample activity data supports. Likewise, Negash et al. (36), showed that regulatory PLN decreases SERCA2a rotational motion by decreasing the average cross-sectional area (i.e., oligomeric size) of the rotating unit, and Waggoner et al. (47) reported that uncoupling PLN from SERCA2a stimulates the Ca2+-dependent conformational transitions. Therefore, if AS/HNO functionally uncouples PLN from SERCA2a, we would expect to observe AS/HNO-dependent increases in Ca2+ sensitivity for SERCA2a activity, enhanced Ca2+-dependent conformational transitions and changes in SERCA2a oligomeric interactions in the presence of PLN, which our fluorescence and EPR spectroscopy data also supports. Taken together, these data support the conclusion that SERCA2a in the presence of PLN, treated with AS/HNO, has physical and functional properties as the pump in the absence of PLN, or like SERCA2a in the presence of PLN uncoupled from SERCA2a by PLN phosphorylation or treatment with anti-PLN monoclonal antibody (33, 36, 47).
Recently, Lancel and coworkers (28) showed that AS/HNO may directly stimulate SERCA2a activity by promoting reversible S-glutathiolation on SERCA2a at C674. SERCA2a with a C674S mutation did not display either behavior. However, the link between this modification and myocyte or heart function was not explored, nor the potential interaction of this modification and simultaneous changes in PLN. Our results in the pln−/− mouse strongly support a mandatory role of PLN modification, but do not mean that a C674 modification is not involved. Undeniably, the former may be an important direct modification of SERCA2a. In addition to elucidating HNO pharmacology, the current findings are the first to show that PLN function can be modified in a redox-dependent manner that augments rather than detracts from cardiac contraction and relaxation, and is independent from changes in the phosphorylation status of its PKA sites.
Impact of HNO-induced modulation of SERCA/PLN on myocardial contractility
Impaired contractility in heart failure is partially due to depressed SR Ca2+ cycling (20) in which the expression level/activity of the SR Ca2+ pump may be decreased (2, 8, 30, 40). The ratio of PLN/SERCA2a is a critical factor of cardiac Ca2+ homeostasis. Since the protein level of PLN remains effectively unchanged in heart failure, this fact results in an amplified fraction of SERCA2a inhibited by PLN (26), thereby contributing to increased diastolic Ca2+ and depressed cardiac function (46). Both SERCA2a and PLN are targets for gene therapies, and improving Ca2+ transport is central to this strategy (39). Overexpression of SERCA2a in failing human ventricular myocytes can increase SERCA2a activity, enhancing contraction and relaxation velocity (11, 12). As such, SERCA2a gene transfer may benefit patients (22). However, like any other protein, overexpression of SERCA2a could still remain subject to redox-induced post-translational modifications due the oxidative environment present in the failing heart (5, 23); thus, partially jeopardizing the effectiveness of this approach. In contrast to changes in SERCA2a, the ablation of PLN in mice augments SR and myocardial function (14, 19, 49); however, this removes the normal physiologic regulation of SR Ca2+ cycling by PLN, and may not be an optimal approach. Inotropic agents that rely on cAMP/PKA signaling also enhance Ca2+ cycling, but must confront downregulation of β-adrenergic signaling (35) and the toxicity from long-term administration (20). HNO donors pose an intriguing alternative, enhancing this cycling in a regulated and reversible manner, without compromising normal PKA-regulation. Pursuant to this, a Phase IIa clinical study of the hemodynamics, safety, and tolerability of a novel HNO donor (CXL-1020) in patients with acute decompensated heart failure (NCT01096043) was recently completed, with results due soon.
Limitations
It remains uncertain whether deletion of PLN maximally activates SERCA2a or whether post-translational modifications further increase SERCA2a activity. A limitation of the present study is that if PLN deletion does lead to maximally activated SERCA2a, then any HNO-induced post-translational modification of SERCA, as reported for instance by Lancel et al. (28) would not be observed. Furthermore, it is theoretically possible that AS/HNO directly increases SERCA2a expression, or causes other compensatory changes in the excitation-contraction coupling machinery. Additional studies are needed to address these remaining questions. Notwithstanding, the present evidence shows that HNO-induced inotropy is markedly reduced in pln−/− mice; this suggests that PLN, to a large part, explains HNO-induced enhancement of SERCA2a activity. Another limitation is that we do not fully understand how local pools of endogenous reducing agents may affect AS/HNO activity and potency in different cellular compartments. The fact that we used a range of AS/HNO concentrations (from 100 to 500 μM) to elicit physical/functional changes in different preparations (whole myocytes/hearts, cellular homogenates, and isolated microsomal samples) reflects the intrinsic limitation of any agent working in a non receptor-dependent manner and likely a different composition in local pools of thiols (or other antioxidants) in a given cellular compartment.
Conclusions
Our study shows that PLN is required to explain, to a large extent, AS/HNO-induced increases in SERCA2a activity and, in turn, induce positive inotropy/lusitropy in intact murine myocytes/whole hearts. AS/HNO rearranges PLN monomers in dimers and tetramers via a thiol-sensitive process, markedly abating the amount of monomeric (inhibitory) PLN and adjusting the regulation of SERCA2a independent of changes in PLN phosphorylation sites. We show that PLN remains physically associated with SERCA2a; however, less monomeric PLN is available to regulate SERCA2a favoring pump activation. Our study unravels a new, redox-based means by which the PLN/SERCA2a interaction can be modulated by thiol-modifying species to improve contractility and relaxation in intact cardiac preparations.
Materials and Methods
Reagents and animals
We used the HNO donor, AS (Na2N2O3), synthesized by Dr. Jon M. Fukuto (Sonoma State University) or in our laboratory. AS/HNO (10 mM) stock solution was freshly-prepared by dissolving AS in 10 mM NaOH. Fura 2-AM and Fluo-4-AM were purchased from Molecular Probes Inc.-Invitrogen and Life Technologies Corp., respectivel. PLN−/− mice were obtained from Dr. Evangelia Kranias. These mice were back-crossed on to FVB/N for at least 12 generations so its genetic background can be considered identical to that of the WT (FVB/N) mouse strain in our studies.
Isolated myocyte studies
Hearts from euthanized WT (FVB/N) and pln−/− mice (2–6 months) were retrogradely-perfused with a collagenase solution at constant flow (1.5 ml/min) to derive isolated myocytes (Supplementary Data) (45). For changes in whole cell Ca2+ transient, cells were incubated for 10 min with 3 μM of Fura 2-AM in DMSO, rinsed in normal Tyrode's solution, and sarcomere shortening and Ca2+ transients measured (Ionoptix) at room temperature (Supplementary Data).
Measurements of ATP-dependent Ca2+ uptake by murine cardiac SR vesicles
Crude cardiac microsomal vesicles containing fragmented SR were prepared as described (45). Briefly, SR membrane vesicles suspended in medium containing 100 mM KCl, 1 mM MgCl2, 50 μM arsenazo III, 5 mM sodium azide, and 20 mM MOPS, pH 7.4, were mixed with an equal volume of an identical medium containing 1 mM ATP at 24°C in a manually-operated stopped-flow apparatus. The total [Ca2+] in the uptake medium was 0.5 μM, yielding a free [Ca2+] in equilibrium with the Ca2+-arsenazo III complex of 0.2 μM (KA=3.3×104 M−1). The change in [Ca2+] was monitored at 0.1 s intervals using a single-beam UV-VIS spectrophotometer (AVIV, Model 14DS) with a monochromator setting of 650 nm. The addition of AS/HNO (250 μM) to the incubation medium had no effect on the spectral characteristics of arsenazo III or its response to Ca2+. The kinetic and thermodynamic parameters for Ca2+ uptake were evaluated by fitting stopped-flow signals to one- and two-exponential decay functions plus a residual term using nonlinear regression (See Supplementary Data).
SERCA2a/PLN expression, isolation, and characterization
Canine cardiac Ca-ATPase (the SERCA2a isoform) and canine PLN were coexpressed in HF insect cells (48). Microsomes were harvested 48 h after infection with baculovirus and stored in small aliquots at −80°C. Protein concentrations were determined by the Biuret method (17) using bovine serum albumin (Sigma) as a standard. The amount of SERCA2a and PLN in the microsomes was quantified by gel electrophoresis and immuno-blotting, as previously shown (48). Four to five preparations of expressed SERCA2a with or without coexpressed PLN were used in these studies. The Ca-ATPase content of the microsomes was very carefully matched at 16% of the total protein by weight, and the relative proportion of Ca-ATPase to PLN coexpressed in the microsomes was between 1 and 2 mol of PLN/mol of Ca-ATPase (48). For all preparations, the Ca-ATPase was under full regulatory control by PLN when the two proteins were coexpressed, determined by assays of [Ca2+]-dependent ATPase and Ca2+-uptake activity conducted in the presence and absence of anti-PLN antibody, as reported previously for these samples (48) (See Supplementary Data).
SERCA2a ATPase assay studies
[AS/HNO]-dependent activation of SERCA2a ATPase activity was measured colorimetrically using a phosphate liberation assay (malachite green-ammonium molybdate) (48) (See also Supplementary Data). The [Ca2+]-dependent ATPase activity of SERCA2a±PLN in HF insect cell microsomes pretreated with 100 μM HNO was measured colorimetrically at 37°C as described above, but the [Ca2+] of the assay medium was varied from 0–100 μM CaCl2 to give a range of ionized [Ca2+] (3).
Labeling of Ca-ATPase with FITC and fluorescence measurements
See Supplementary Data.
Spin labelling
The overall rotational mobility of the Ca-ATPase was measured by EPR spectroscopy using the short-chain MSL, N-(1-oxyl-2,2,6,6-tetra-methyl-4-piperadinyl)maleimide, covalently bound to the ATPase as previously described (4, 42, 43) (Supplementary Data).
EPR spectroscopy
See Supplementary Data.
EPR spectral analysis
Conventional spectra from SERCA2a and SERCA2a+PLN in HF insect cell microsomes were analyzed by the outer splitting parameter (2TII′). ST-EPR spectra were analyzed by line shape parameter (42), which provided the effective rotational correlation time (τr) for spin-labeled SERCA2a, which was determined from a standard curve constructed from an isotropically tumbling models system (34, 42, 43).
Coimmunoprecipitation studies
See Supplementary Data.
Supplementary Material
Abbreviations Used
- AS
Angeli's salt
- Ca2+
calcium
- cAMP
cyclic adenosine monophosphate
- DTT
dithiothreitol
- E1•Ca2
Ca2+ bound state
- E2
Ca2+ free state
- EPR
electron paramagnetic resonance
- FITC
fluorescein isothiocyanate
- GSH
glutathione
- HF
High Five
- HNO
nitroxyl or nitrosyl hydride
- LTCC
L-type Ca2+ channels
- LV
left ventricular
- MSL
maleimide spin label
- PKA
protein kinase A
- PLN
phospholamban
- SASL
stearic acid spin label
- SERCA2a
sarcoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
- ST-EPR
saturation transfer electron paramagnetic resonance
- WT
wildtype
Acknowledgments
This work is dedicated to the late Dr. Jeffrey P. Froehlich (Johns Hopkins Medical Institutions, Baltimore, MD) who conducted the studies assessing the stimulatory action of AS/HNO on Ca2+ reuptake, and the late Professor Massimo Chiariello (Chief of Cardiology at Federico II University, Naples, Italy) who promoted and coordinated the Naples-Baltimore collaboration.
Author Disclosure Statement
John P. Toscano, David A. Kass, and Nazareno Paolocci are founders and stockholders of Cardioxyl Pharmaceuticals, Inc.
Sources of Funding
This work was supported by the American Heart Association Pre-doctoral Mid-Atlantic Affiliate Fellowship 0815217E and the T32 NIH Training Grant to V.S., the Italian Society of Cardiology and by the ISHR-ES/Servier to C.G.T.; the American Heart Association Post-Doctoral Mid-Atlantic Affiliate Fellowship 10POST4140001 to B.A.S., by the by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to V.C.; by NIH grant R01CA102428 to G.M.W.; by the National Science Foundation grant CHE-1213438 to J.P.T.; by NIH grants K02HL094692, R01HL079283 to M.T.Z.; by the AHA Scientist Development Grant 10SDG2640109 to S.H.; by the NIH grants R01 HL26057 and R01 HL64018 to E.G.K.; by the NIH grant R01 HL101235 to B.O.R.; by the Fondation Leducq, the Peter Belfer Laboratory, and the Abraham and Virginia Weiss Professorship to D.A.K.; by the NIH grant 1 R15 HL091410 to J.E.M.; by the NIH grant R01 HL075265, R01 HL091923 and American Heart Association GIA to N.P.
References
- 1.Antipenko AY. Spielman AI. Sassaroli M. Kirchberger MA. Comparison of the kinetic effects of phospholamban phosphorylation and anti-phospholamban monoclonal antibody on the calcium pump in purified cardiac sarcoplasmic reticulum membranes. Biochemistry. 1997;36:12903–12910. doi: 10.1021/bi971109v. [DOI] [PubMed] [Google Scholar]
- 2.Arai M. Alpert NR. MacLennan DH. Barton P. Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 3.Autry JM. Jones LR. Functional Co-expression of the canine cardiac Ca2+ pump and phospholamban in Spodoptera frugiperda (Sf21) cells reveals new insights on ATPase regulation. J Biol Chem. 1997;272:15872–15880. doi: 10.1074/jbc.272.25.15872. [DOI] [PubMed] [Google Scholar]
- 4.Bigelow DJ. Squier TC. Thomas DD. Temperature dependence of rotational dynamics of protein and lipid in sarcoplasmic reticulum membranes. Biochemistry. 1986;25:194–202. doi: 10.1021/bi00349a028. [DOI] [PubMed] [Google Scholar]
- 5.Burgoyne JR. Mongue-Din H. Eaton P. Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111:1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 6.Chen LTL. Yao Q. Soares TA. Squier TC. Bigelow DJ. Phospholamban modulates the functional coupling between nucleotide domains in Ca-ATPase oligomeric complexes in cardiac sarcoplasmic reticulum. Biochemistry. 2009;48:2411–2421. doi: 10.1021/bi8021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai T. Tian Y. Tocchetti CG. Katori T. Murphy AM. Kass DA. Paolocci N. Gao WD. Nitroxyl increases force development in rat cardiac muscle. J Physiol. 2007;580:951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dash R. Frank KF. Carr AN. Moravec CS. Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001;33:1345–1353. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]
- 9.del Monte F. Hajjar RJ. Harding SE. Inesi G. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure response. Circ Res. 2001;88:e66–e67. doi: 10.1161/hh1101.092004. [DOI] [PubMed] [Google Scholar]
- 10.del Monte F. Harding SE. Dec GW. Gwathmey JK. Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Monte F. Harding SE. Schmidt U. Matsui T. Kang ZB. Dec GW. Gwathmey JK. Rosenzweig A. Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.del Monte F. Williams E. Lebeche D. Schmidt U. Rosenzweig A. Gwathmey JK. Lewandowski ED. Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisner DA. Kashimura T. Venetucci LA. Trafford AW. From the ryanodine receptor to cardiac arrhythmias. Circulation. 2009;73:1561–1567. doi: 10.1253/circj.cj-09-0478. [DOI] [PubMed] [Google Scholar]
- 14.Freeman K. Lerman I. Kranias EG. Bohlmeyer T. Bristow MR. Lefkowitz RJ. Iaccarino G. Koch WJ. Leinwand LA. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest. 2001;107:967–974. doi: 10.1172/JCI12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froehlich JP. Mahaney JE. Keceli G. Pavlos CM. Goldstein R. Redwood AJ. Sumbilla C. Lee DI. Tocchetti CG. Kass DA. Paolocci N. Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 16.Gao WD. Murray CI. Tian Y. Zhong X. DuMond JF. Shen X. Stanley BA. Foster DB. Wink DA. King SB. Van Eyk JE. Paolocci N. Nitroxyl(HNO)-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res. 2012;111:1002–1011. doi: 10.1161/CIRCRESAHA.112.270827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gornall AG. Bardawill CJ. MM D. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–767. [PubMed] [Google Scholar]
- 18.Ha KN. Masterson LR. Hou Z. Verardi R. Walsh N. Veglia G. Robia SL. Lethal Arg9Cys phospholamban mutation hinders Ca2+-ATPase regulation and phosphorylation by protein kinase A. Proc Natl Acad Sci U S A. 2011;108:2735–2740. doi: 10.1073/pnas.1013987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haghighi K. Schmidt AG. Hoit BD. Brittsan AG. Yatani A. Lester JW. Zhai J. Kimura Y. Dorn GW. MacLennan DH. Kranias EG. Superinhibition of sarcoplasmic reticulum function by phospholamban induces cardiac contractile failure. J Biol Chem. 2001;276:24145–24152. doi: 10.1074/jbc.M102403200. [DOI] [PubMed] [Google Scholar]
- 20.Hasenfuss G. Teerlink JR. Cardiac inotropes: current agents and future directions. Eur Heart J. 2011;32:1838–1845. doi: 10.1093/eurheartj/ehr026. [DOI] [PubMed] [Google Scholar]
- 21.Houser SR. Margulies KB. Is Depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 22.Jaski BE. Jessup ML. Mancini DM. Cappola TP. Pauly DF. Greenberg B. Borow K. Dittrich H. Zsebo KM. Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawase Y. Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–565. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y. Kurzydlowski K. Tada M. MacLennan DH. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem. 1997;272:15061–15064. doi: 10.1074/jbc.272.24.15061. [DOI] [PubMed] [Google Scholar]
- 25.Kohr MJ. Kaluderic N. Tocchetti CG. Dong GW. Kass DA. Janssen PM. Paolocci N. Ziolo MT. Nitroxyl enhances myocyte Ca2+ transients by exclusively targeting SR Ca2+ cycling. Front Biosci (Elite Ed) 2010;2:614–626. doi: 10.2741/e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kranias EG. Hajjar RJ. Modulation of cardiac contractility by the phopholamban/SERCA2a Regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumsta C. Thamsen M. Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antioxid Redox Signal. 2011;14:1023–1037. doi: 10.1089/ars.2010.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancel S. Zhang J. Evangelista A. Trucilla MP. Tong XY. Siwik DA. Cohen RA. Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via S-glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J. Bigelow DJ. Squier TC. Phosphorylation by cAMP-Dependent protein kinase modulates the structural coupling between the transmembrane and cytosolic domains of phospholamban. Biochemistry. 2003;42:10674–10682. doi: 10.1021/bi034708c. [DOI] [PubMed] [Google Scholar]
- 30.Limas CJ. Olivari MT. Goldenberg IF. Levine TB. Benditt DG. Simon A. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy. Cardiovasc Res. 1987;21:601–605. doi: 10.1093/cvr/21.8.601. [DOI] [PubMed] [Google Scholar]
- 31.MacLennan DH. Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 32.Mahaney JE. Albers RW. Kutchai H. Froehlich JP. Phospholamban controls Ca2+ pump oligomerization and intersubunit free energy exchange leading to activation of cardiac muscle SERCA2a. N Y Acad Sci. 2003;986:1–3. doi: 10.1111/j.1749-6632.2003.tb07208.x. [DOI] [PubMed] [Google Scholar]
- 33.Mahaney JE. Albers RW. Waggoner JR. Kutchai HC. Froehlich JP. intermolecular conformational coupling and free energy exchange enhance the catalytic efficiency of cardiac muscle SERCA2a following the relief of phospholamban inhibition. Biochemistry. 2005;44:7713–7724. doi: 10.1021/bi048011i. [DOI] [PubMed] [Google Scholar]
- 34.Mahaney JE. Thomas DD. Effects of Melittin on Molecular Dynamics and Ca-ATPase activity in sarcoplasmic reticulum membranes: electron paramagnetic resonance. Biochemistry. 1991;30:7171–7180. doi: 10.1021/bi00243a019. [DOI] [PubMed] [Google Scholar]
- 35.Mann DL. Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 36.Negash S. Chen LT. Bigelow DJ. Squier TC. Phosphorylation of phospholamban by cAMP-dependent protein kinase enhances interactions between Ca-ATPase polypeptide chains in cardiac sarcoplasmic reticulum membranes. Biochemistry. 1996;35:11247–11259. doi: 10.1021/bi960864q. [DOI] [PubMed] [Google Scholar]
- 37.Paolocci N. Katori T. Champion HC. St. John ME. Miranda KM. Fukuto JM. Wink DA. Kass DA. Positive inotropic and lusitropic effects of HNO/NO− in failing hearts: Independence from β-adrenergic signaling. Proc Natl Acad Sci U S A. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paolocci N. Saavedra WF. Miranda KM. Martignani C. Isoda T. Hare JM. Espey MG. Fukuto JM. Feelisch M. Wink DA. Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Periasamy M. Kalyanasundaram A. SERCA2a Gene therapy for heart failure: ready for primetime. Mol Ther. 2008;16:1002–1004. doi: 10.1038/mt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pieske B. Maier LS. Bers DM. Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 41.Squier TC. Bigelow DJ. Thomas DD. Lipid fluidity directly modulates the overall protein rotational mobilityof the Ca-ATPase in sarcoplasmic reticulum. J Biol Chem. 1986;263:9178–9186. [PubMed] [Google Scholar]
- 42.Squier TC. Thomas DD. Applications of new saturation transfer electron paramagnetic resonance methodology to the rotational dynamics of the Ca-ATPase in sarcoplasmic reticulum membranes. Biophys J. 1986;49:937–942. doi: 10.1016/S0006-3495(86)83721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Squier TC. Thomas DD. Methodology for increased precision in saturation transfer electron paramagnetic resonance studies of rotational dynamics. Biophys J. 1986;49:921–935. doi: 10.1016/S0006-3495(86)83720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tocchetti CG. Stanley BA. Murray CI. Sivakumaran V. Donzelli S. Mancardi D. Pagliaro P. Gao WD. van Eyk J. Kass DA. Wink DA. Paolocci N. Playing with cardiac redox switches: the HNO way to modulate cardiac function. Antioxid Redox Signal. 2011;14:1687–1698. doi: 10.1089/ars.2010.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tocchetti CG. Wang W. Froehlich JP. Huke S. Aon MA. Wilson GM. Di Benedetto G. O'Rourke B. Gao WD. Wink DA. Toscano JP. Zaccolo M. Bers DM. Valdivia HH. Cheng H. Kass DA. Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vafiadaki E. Papalouka V. Arvanitis DA. Kranias EG. Sanoudou D. The role of SERCA2a/PLN complex, Ca2+ homeostasis, and anti-apoptotic proteins in determining cell fate. Pflugers Arch. 2009;457:687–700. doi: 10.1007/s00424-008-0506-5. [DOI] [PubMed] [Google Scholar]
- 47.Waggoner JR. Huffman J. Froelich JP. Mahaney JE. Phospholamban inhibits Ca-ATPase conformational changes involving the E2 intermediate. Biochemistry. 2007;46:1999–2009. doi: 10.1021/bi061365k. [DOI] [PubMed] [Google Scholar]
- 48.Waggoner JR. Huffman J. Griffith BN. Jones LR. Mahaney JE. Improved expression and characterization of Ca-ATPase and phospholamban in high-five cells. Protein Expr Purif. 2004;34:56–67. doi: 10.1016/j.pep.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W. Yuan Q. Qian J. Waggoner JR. Pathak A. Chu G. Mitton B. Sun X. Jin J. Braz JC. Hahn HS. Marreez Y. Syed F. Pollesello P. Annila A. Wang H-S. Schultz JEJ. Molkentin JD. Liggett SB. Dorn GW., II Kranias EG. The presence of Lys27 instead of Asn27 in human phospholamban promotes sarcoplasmic reticulum Ca-ATPase superinhibition and cardiac remodeling. Circulation. 2006;113:995–1004. doi: 10.1161/CIRCULATIONAHA.105.583351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.