Abstract
The envelope lipid composition of influenza virus differs from that of the cellular plasma membrane from which it buds. Viruses also appear to fuse preferentially to specific membrane compartments, suggesting that the lipid environment may influence permissiveness for fusion. Here, we investigated the influence of the membrane environment on fusion, focusing on cholesterol composition. Strikingly, manipulating cholesterol levels in the viral membrane had different effects on fusion kinetics compared with analogous changes to the target membrane. Increasing cholesterol content in target vesicles increased lipid- and contents-mixing rates. Moderate cholesterol depletion from the viral membrane sped fusion rates, whereas severe depletion slowed the process. The pleiotropic effects of cholesterol include alterations in both membrane-bending moduli and lateral organization. Because influenza virions have demonstrated cholesterol-dependent lateral organization, to separate these effects, we deliberately selected a target vesicle composition that does not support lateral heterogeneity. We therefore postulate that the monotonic response of fusion kinetics to target membrane cholesterol reflects bending and curvature effects, whereas the multiphasic response to viral cholesterol levels reflects the combined effects of lateral organization and material properties.
Introduction
Membrane fusion is a crucial step in cell entry by enveloped viruses. In influenza virus, endosomal acidification causes rearrangement of the coat protein hemagglutinin, leading to exposure of fusion peptides that insert into the endosomal membranes and drive fusion (1,2). This is a dynamic process, and fusion intermediates are often studied by monitoring lipid and contents exchange between fusion partners (3). Mutagenesis studies have identified changes to the membrane-inserted fusion peptides that block fusion altogether or permit lipid exchange but not contents exchange (4–6). This can be interpreted to indicate at least two free-energy barriers to influenza fusion: one preceding lipid exchange and one after lipid but before contents exchange.
In addition to viral proteins, the lipid environment of both the viral envelope and the host cell can greatly influence the fusion process. Circumstantial evidence for this has long existed, as viral infection alters lipid synthesis pathways (7) and mature virus differs in lipid composition from either the membrane whence it came or uninfected cells (8). Cholesterol in particular is enriched in influenza virus (9). Cholesterol is of special interest because it can stabilize curved negatively membrane structures (10,11), can control lateral membrane organization (12,13), and is readily manipulable (unlike many lipid species, cholesterol can be easily added to or extracted from intact membranes). Cholesterol’s effects on membrane fusion have been extensively studied, and cholesterol has been shown to be necessary for efficient neuronal exocytosis and enveloped viral entry (14–18).
To further probe the effects of cholesterol on influenza viral fusion, we studied how changes to viral or target membrane cholesterol affect fusion kinetics. Here, we report the use of fluorescence dequenching to measure fusion kinetics between live influenza virus and manipulable model membranes as a function of cholesterol content. Viral envelopes contain a complex lipid mixture; therefore, to separate the potential effects of cholesterol on lateral organization and curvature, we either extracted cholesterol from the native viral envelope or constructed simple target liposomes with varying amounts of cholesterol. We chose to use a target liposomal composition that does not support liquid-liquid phase coexistence.
Materials and Methods
We used liposomes composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)/palmitoyloleoylphosphatidylethanolamine (POPE)/cholesterol (Chol)/NBD-dioleyl phosphatidylethanolamine (NBD-DOPE)/Rhodamine-DOPE extruded at 100 nm with aminonaphthalene-trisulfonic acid (ANTS)/p-xylene-bis-pyridinium bromide (DPX) complex encapsulated to monitor lipid and contents mixing simultaneously. Experiments were performed with excess DPX in the fusion buffer to suppress fluorescence signal from leakage and primarily measure contents mixing. We also performed controls without DPX in the buffer to assess leakiness during fusion. The resulting contents-release data (mixing + leakage) produced curve shapes that were indistinguishable from those obtained with mixing alone, indicating an absence of detectable leakage preceding fusion (Fig. 1). Additionally, solution measurements of ANTS fluorescence showed that the ANTS/DPX ratio present after total contents leakage was sufficient to quench >90% of ANTS fluorescence (Fig. S1 in the Supporting Material). Additional controls measuring dequenching of encapsulated ANTS alone with and without DPX in the fusion buffer suggested that minor leakage did occur, but that >90% of the signal in fusion experiments with encapsulated ANTS/DPX was due to mixing (see Supporting Material).
Figure 1.
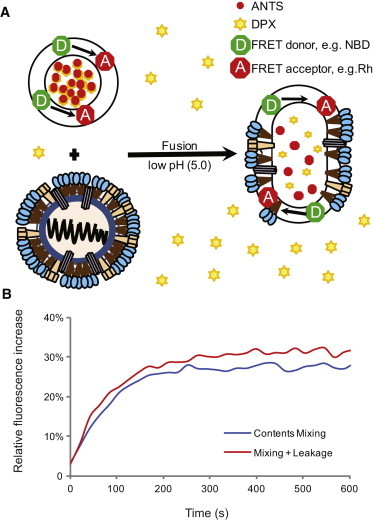
(A) Schema of the fusion assay. Fusion of influenza virus with labeled liposomes is monitored via fluorescence dequenching of lipidic and solution-phase dyes. Lipid mixing causes dilution of the NBD-DOPE and Rh-DOPE FRET pair, and thus an increase in NBD fluorescence. Contents mixing causes dilution of the ANTS/DPX complex and an increase in ANTS fluorescence. Additional DPX is present in the external buffer to quench free ANTS and differentiate mixing from leakage. (B) Representative traces with and without excess DPX in the external buffer.
Influenza virus
Egg-grown influenza virus X:31 (H3N2 A/Aichi/68) was purchased from Charles River Laboratories (Wilmington, MA). Cholesterol was extracted via incubation with 0, 10, 20, or 50 mM methyl-β-cyclodextrin (MβCD) at 37°C for 30 min. Virus was then reisolated from cyclodextrin-cholesterol complexes via centrifugation at 4°C, 14,000 rpm for 40 min, and then resuspended in buffer (10 mM phosphate/90 mM citrate/150 mM NaCl, pH 7.4).
Target liposomes
Large unilamellar vesicles (LUVs) composed of 30 mol % POPE, 1.5 mol % NBD-DOPE, 1.5 mol % Rh-DOPE, 10–40 mol % Chol (as specified), and the remaining 30–67 mol % POPC were extruded at 100 nm with ANTS/DPX contents dye encapsulated (internal concentration: 12.5 mM ANTS/45 mM DPX) and isolated by size-exclusion chromatography. Further details are given in the Supporting Material.
Fusion assay
Virus-liposome fusion was monitored via lipid and contents mixing, and assayed by fluorescence dequenching of the lipidic fluorescence resonance energy transfer (FRET) pair NBD/Rh and dissociation and dequenching of the soluble ANTS/DPX complex. Virus (standardized at 60 μg of viral protein) was mixed with target liposomes (0.2 mM total lipid), adjusted to a final buffer concentration of 30 mM DPX, and incubated for 30 min at 4°C. The reaction mixture was then warmed and equilibrated at 37°C over 15 min. Fusion was triggered by acidification to pH 5.0 using 2 mM citric acid, and fluorescence was monitored in a fluorescence plate reader. Excitation and emission wavelengths were set at 460 nm/538 nm (with a 530 nm cutoff filter) and 360 nm/530 nm (with a 515 nm cutoff filter) for NBD/Rh and ANTS/DPX, respectively. Lipid mixing values were normalized as follows: Inorm = (Iobs – I0) / (Imax – I0), where I0 is the fluorescence reading before acidification and Imax is the fluorescence reading after addition of Triton X-100 to a final concentration of 1%. Contents-mixing values were reported only as the relative increase in fluorescence over baseline: Irel = Iobs / I0 − 1. Fusion rates were determined via fitting to a single-exponential equation with a phase-shift parameter to allow for an experimental time lag between acidification and t = 0 time of fluorescence measurement. This yielded the following fit equation: Inorm(t) = a ∗ (1 − exp(−b ∗ (t + t0))). Lipid dye transfer was observed only under conditions of fusion (Fig. S2), and contents dequenching was almost completely attributable to mixing, as measured by fluorescence of isolated fusion products (Table S1). Further details and dequenching controls are reported in the Supporting Material.
Results and Discussion
To test how fusion kinetics change with target membrane composition, we varied the mole fraction cholesterol in target liposomes from 10 to 40 mol %. Fig. 2 A summarizes the lipid-mixing rates for our virus-liposome fusion experiments; normalized fusion rates are plotted as a function of liposomal cholesterol content in Fig. 3 A. Under all viral conditions tested, increasing liposomal cholesterol increased the fusion rates. We observed a nearly identical effect on contents mixing (Figs. 2 B and 3 B). Lipid-mixing rates of virus that was not treated with methyl-β-cyclodextrin increased 2.4-fold from 10 to 40 mol % liposomal cholesterol (0.014 s−1 to 0.033 s−1). Similarly, the contents-mixing rate increased 2.3-fold (0.013 s−1 to 0.031 s−1). Similar increases were observed at all viral cholesterol levels, indicating that increasing the cholesterol content of target membranes robustly speeds fusion independently of the viral membrane composition. Furthermore, we observed similar trends whether we held the mole fraction POPE in target vesicles constant at 30% or held the POPC/POPE molar ratio constant at 2:1 (Fig. S3).
Figure 2.
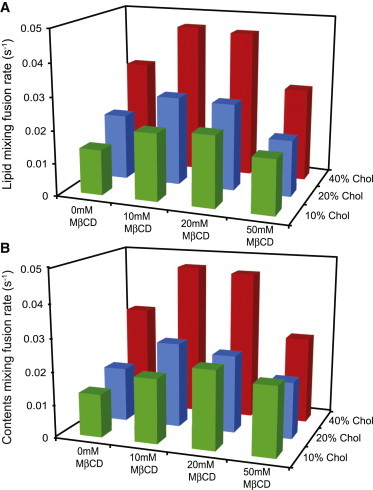
Lipid- and contents-mixing rates at different cholesterol compositions. (A and B) Rates of lipid mixing (A) and contents mixing (B) were determined via single-exponential fits to fluorescence dequenching traces. Mixing rates show a consistent increase with increasing mole fraction cholesterol in target vesicles. A biphasic response is observed with progressive cholesterol extraction from the virus. Each bar represents the median of five to 13 measurements. These trends can be visualized more clearly in Figure 3.
Figure 3.
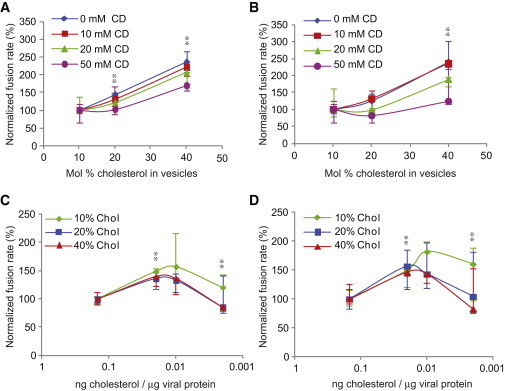
Response of fusion kinetics to altered cholesterol levels in influenza virions or target vesicles. Normalized fusion rates are plotted to visualize trends more clearly; these correspond to the unnormalized rates plotted in Figure 2. (A and B) Rates of lipid (A) and contents (B) mixing are plotted as a function of mol % cholesterol in target vesicles. (C and D) Rates of lipid (C) and contents (D) mixing are plotted as a function of the cholesterol/viral protein ratio after cyclodextrin treatment. Significance was assessed via the Kolmogorov-Smirnov test with a Bonferroni multiple-hypothesis correction; ∗∗ denotes a significant change from both the baseline and the prior condition, p < 0.02. Error bars are plotted as interquartile ranges. Trend lines are placed as visual guides.
According to previous reports (9) and assays we have performed on X:31 virus, influenza virus contains cholesterol/phospholipid in a ∼1:1 ratio. Therefore, we manipulated the viral cholesterol composition via extraction with methyl-β-cyclodextrin (MβCD) rather than by supplementation. We verified the efficiency of extraction using a previously reported enzymatic assay for cholesterol content (18) and found a dose-dependent drop in cholesterol, with 75% depletion for 10 mM MβCD and nearly 90% depletion for 50 mM MβCD (Fig. 4). Normalized fusion rates are plotted as a function of MβCD dose in Fig. 3, C and D. In contrast to the monotonic effect of liposomal cholesterol, cholesterol extraction from the viral envelope yielded a biphasic response: fusion rates increased from 0 to 10 mM MβCD and then slowed down at 50 mM MβCD. For 20 mol % cholesterol vesicles, the lipid-mixing fusion rate increased from 0.02 s−1 without MβCD to 0.027 s−1 when virus was treated with 10 and 20 mM MβCD. Further extraction from the virion reduced the fusion rate to 0.017 s−1 at 50 mM MβCD. Similar trends occurred at 10 and 40 mol % cholesterol in the target vesicles (Figs. 2 and 3) and for lipid mixing as well as contents mixing. Importantly, the extent of both lipid and contents mixing decreased steadily with progressive extraction of cholesterol (Fig. S4). It is the kinetics of the fusion process, rather than the efficiency, that showed a biphasic response.
Figure 4.
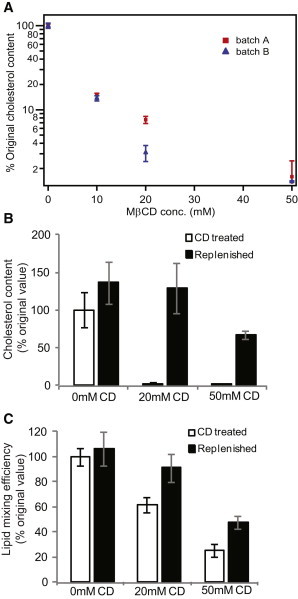
Cholesterol depletion from and replenishment of influenza virions. (A) Relative cholesterol depletion from X:31 influenza virions as a function of MβCD treatment. Error bars are plotted as the SD over four independent measurements per condition. Initial cholesterol levels were measured at 0.15 ± 0.0043 and 0.22 ± 0.0153 ng cholesterol per microgram of viral protein for batches A and B, respectively. (B) Results of cholesterol replenishment experiments after extraction with 0, 20, or 50 mM MβCD. (C) Results of lipid-mixing efficiency experiments on cholesterol-extracted and replenished virus. Extraction with 20 mM MβCD is fully reversible, whereas extraction with 50 mM MβCD is partially reversible. Values and error bars represent the mean and SD calculated from four to six independent experiments per condition in panel B and seven to 11 independent experiments per condition in panel C.
Cyclodextrin can be used as a bidirectional cholesterol carrier, so to test the reversibility of cholesterol removal from influenza virions, we performed replenishment experiments by adding cholesterol back using a cholesterol-MβCD carrier complex as previously described (19). We observed full reversibility of cholesterol extraction, assayed via both cholesterol content and fusion efficiency, at MβCD concentrations as high as 20 mM. At 50 mM MβCD, cholesterol content and fusion efficiency were both partially restored (Fig. 4), indicating a loss of some viable virus. Hemagglutination titers performed on virus treated with 0 mM and 50 mM MβCD yielded measurements of 1:2560 for each sample in two independent experiments, indicating that hemagglutination activity was intact to within the 2-fold sensitivity of the assay (see Supporting Material). Finally, methyl-β-cyclodextrin primarily extracts sterols from lipid membranes, but related cyclodextrins can bind other lipid components (20). We performed assays of phosphate content on the cyclodextrin after extraction. At 10 and 20 mM MβCD, the phosphate content was at the limit of detection, but at 50 mM MβCD, we cannot exclude the possibility that some phospholipid was extracted from the viral membrane. It is therefore possible that the slowing of fusion at 50 mM MβCD treatment may be due to a combination of cholesterol extraction and other, minor changes to membrane composition.
The curvature effects of cholesterol are especially important for fusion because membrane fusion is believed to proceed via highly curved intermediates (21). Low levels of cholesterol impair influenza membrane fusion, as demonstrated by prior work on influenza virus or hemagglutinin-transfected cells (18,22). Similar effects have been seen in model membranes (23). The theoretical explanation for this is that cholesterol has a negative spontaneous curvature (11) and reduces the energy of highly curved fusion intermediates (24). Cholesterol can also be enriched in highly curved regions of model membranes (10). In our experiments, increasing the mole fraction of cholesterol in target liposomes increased the viral fusion rates, which agrees with hypothesis that cholesterol stabilizes the negative curvature of high-energy fusion intermediates.
Cholesterol also controls the spatial localization of viral fusion proteins, providing yet another mechanism to modulate fusion behavior. Influenza hemagglutinin localizes into cholesterol-rich regions of cellular membranes (25), and this localization is believed to be important for efficient fusion (26). Cholesterol content can affect the patterning and dynamics of viral envelopes, particularly because it alters temperature-sensitive lipid-phase behavior (27). Because multiple hemagglutinin trimers are believed to be necessary for efficient fusion (28), changes to hemagglutinin spatial patterning may alter the viral fusion kinetics. In this study, cholesterol depletion from the viral envelope produced a complex effect: moderate depletion increased the rate of fusion and severe depletion slowed it significantly. This biphasic behavior may reflect the different physical roles of cholesterol in the viral membrane. Extraction may alter hemagglutinin lateral distribution, mobility, lipid-phase behavior, and the free energies of fusion intermediates. Recent NMR spectroscopic studies on influenza virus showed the coexistence of ordered and disordered lipid domains, with a gel phase triggered by cholesterol depletion (27). Such phase changes would also alter hemagglutinin mobility.
Conclusions
Our results suggest that sterol-dependent changes to the viral envelope are multifunctional. This complexity itself is not a surprise, but it is striking that the multiple mechanistic roles of cholesterol can be seen in multiphasic fusion kinetics. Consistent with prior observations, we observed that both viral and target membrane cholesterol act to promote fusion efficiency, and depletion of either one reduces efficiency. The complex effects of cholesterol are apparent only in measurements of fusion kinetics. These effects can be deconvolved by further experiments to probe the spatial organization and mobility of hemagglutinin in cholesterol-depleted virus. Functional assays on live virus, as presented here, are necessary to link these physical changes to viral entry kinetics.
Acknowledgments
We thank J. White, L. Tamm, C. Stroupe, and D. Steinhauer for many helpful discussions.
This work was supported by NIH R01 GM098304.
Supporting Material
References
- 1.Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez L.D., Hoffman L.R., White J.M. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal R., Gallo S.A., Puri A. Fluorescent lipid probes in the study of viral membrane fusion. Chem. Phys. Lipids. 2002;116:39–55. doi: 10.1016/s0009-3084(02)00019-1. [DOI] [PubMed] [Google Scholar]
- 4.Kemble G.W., Danieli T., White J.M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 5.Melikyan G.B., White J.M., Cohen F.S. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J. Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao H., Armstrong R.T., White J.M. A specific point mutant at position 1 of the influenza hemagglutinin fusion peptide displays a hemifusion phenotype. Mol. Biol. Cell. 1999;10:2759–2769. doi: 10.1091/mbc.10.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munger J., Bennett B.D., Rabinowitz J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aloia R.C., Tian H.R., Jensen F.C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerl M.J., Sampaio J.L., Simons K. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J. Cell Biol. 2012;196:213–221. doi: 10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Yang L., Huang H.W. Evidence of cholesterol accumulated in high curvature regions: implication to the curvature elastic energy for lipid mixtures. Biophys. J. 2007;92:2819–2830. doi: 10.1529/biophysj.106.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Rand R.P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 1997;73:267–276. doi: 10.1016/S0006-3495(97)78067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simons K., Gerl M.J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 13.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 14.Hambleton S., Steinberg S.P., Gershon A.A. Cholesterol dependence of varicella-zoster virion entry into target cells. J. Virol. 2007;81:7548–7558. doi: 10.1128/JVI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linetti A., Fratangeli A., Rosa P. Cholesterol reduction impairs exocytosis of synaptic vesicles. J. Cell Sci. 2010;123:595–605. doi: 10.1242/jcs.060681. [DOI] [PubMed] [Google Scholar]
- 16.Rosa P., Fratangeli A. Cholesterol and synaptic vesicle exocytosis. Commun. Integr. Biol. 2010;3:352–353. doi: 10.4161/cib.3.4.11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H., Li Y., Mori Y. Human herpesvirus 6 envelope cholesterol is required for virus entry. J. Gen. Virol. 2006;87:277–285. doi: 10.1099/vir.0.81551-0. [DOI] [PubMed] [Google Scholar]
- 18.Sun X., Whittaker G.R. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 2003;77:12543–12551. doi: 10.1128/JVI.77.23.12543-12551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pucadyil T.J., Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim. Biophys. Acta. 2004;1663:188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Zidovetzki R., Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernomordik L.V., Kozlov M.M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas S., Yin S.R., Zimmerberg J. Cholesterol promotes hemifusion and pore widening in membrane fusion induced by influenza hemagglutinin. J. Gen. Physiol. 2008;131:503–513. doi: 10.1085/jgp.200709932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque M.E., McIntosh T.J., Lentz B.R. Influence of lipid composition on physical properties and peg-mediated fusion of curved and uncurved model membrane vesicles: “nature’s own” fusogenic lipid bilayer. Biochemistry. 2001;40:4340–4348. doi: 10.1021/bi002030k. [DOI] [PubMed] [Google Scholar]
- 24.Kozlovsky Y., Kozlov M.M. Stalk model of membrane fusion: solution of energy crisis. Biophys. J. 2002;82:882–895. doi: 10.1016/S0006-3495(02)75450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess S.T., Gould T.J., Zimmerberg J. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc. Natl. Acad. Sci. USA. 2007;104:17370–17375. doi: 10.1073/pnas.0708066104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda M., Leser G.P., Lamb R.A. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. USA. 2003;100:14610–14617. doi: 10.1073/pnas.2235620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polozov I.V., Bezrukov L., Zimmerberg J. Progressive ordering with decreasing temperature of the phospholipids of influenza virus. Nat. Chem. Biol. 2008;4:248–255. doi: 10.1038/nchembio.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danieli T., Pelletier S.L., White J.M. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J. Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirst G.K. The quantitative determination of influenza virus and antibodies by means of red cell agglutination. J. Exp. Med. 1942;75:49–64. doi: 10.1084/jem.75.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


