Abstract
Epidemiological studies have shown that a diet rich in fruits and cruciferous vegetables is associated with a lower risk of prostate cancer. Indole-3-carbinol (I3C) and its dimeric product 3,3′-diindolylmethane (DIM) have been shown to exhibit anti-tumor activity both in vitro and in vivo. Recently, we have reported that a formulated DIM (B-DIM) induced apoptosis and inhibited growth, angiogenesis, and invasion of prostate cancer cells by regulating Akt, NF-κB, VEGF and the androgen receptor (AR) signaling pathway. However, the precise molecular mechanism(s) by which B-DIM inhibits prostate cancer cell growth and induces apoptosis have not been fully elucidated. Most importantly, it is not known how B-DIM affects cell cycle regulators and proteasome activity, which are critically involved in cell growth and apoptosis. In this study, we investigated the effects of B-DIM on proteasome activity and AR transactivation with respect to B-DIM-mediated cell cycle regulation and induction of apoptosis in both androgen-sensitive LNCaP and androgen-insensitive C4-2B prostate cancer cells. We believe that our results show for the first time the cell cycle-dependent effects of B-DIM on proliferation and apoptosis of synchronized prostate cancer cells progressing from G1 to S phase. B-DIM inhibited this progression by induction of p27Kip1 and down-regulation of AR. We also show for the first time that B-DIM inhibits proteasome activity in S phase, leading to the inactivation of NF-κB signaling and induction of apoptosis in LNCaP and C4-2B cells. These results suggest that B-DIM could be a potent agent for the prevention and/or treatment of both hormone sensitive as well as hormone-refractory prostate cancer.
Epidemiological studies have shown that a diet rich in fruits and vegetables is associated with a lower risk of cancers, including prostate cancer (Tavani and La, 1995; Cohen et al., 2000). The incidence of prostate cancer is lower in persons who eat large amounts of cruciferous vegetables (Cohen et al., 2000). Indole-3-carbinol (I3C), a naturally occurring component of these plants, arrested the cell cycle in G1, inhibited proliferation of prostate cancer cells and induced apoptosis (Chinni and Sarkar, 2002; Zhang et al., 2003; Brew et al., 2006). More importantly, in a low pH environment like the stomach, I3C is converted to a number of polymers, the most prominent and active of these being 3,3′-diindolylmethane (DIM) which exerts anti-proliferative and pro-apoptotic effects (Ge et al., 1996; Verhoeven et al., 1997; Li et al., 2003, 2005; Nachshon-Kedmi et al., 2003). However, relatively higher concentrations of DIM have been used in most studies, raising questions about their relevance in vivo. Recently, we reported that a lower concentration of a formulated DIM [B-DIM; 50% higher bioavailability in vivo (Anderton et al., 2004)] induced apoptosis and inhibited growth, angiogenesis, and invasion of prostate cancer cells by regulating Akt, NF-κB, VEGF and androgen receptor (AR) signaling pathways (Bhuiyan et al., 2006; Kong et al., 2007; Li et al., 2007), suggesting that B-DIM could act on prostate cancer cells by up-regulating and down-regulating various target molecules.
Ubiquitin/proteasome-mediated protein degradation is a major pathway of intracellular protein catabolism. Proteasome degrades important cellular molecules, including p21Cip1, p27Kip1, IκBα, cyclins (A, B, D, and E), and p53, etc. (Voorhees et al., 2003). Moreover, it is known to be involved in regulation of NF-κB activity through degradation of IκBα (Tanaka et al., 2001). Therefore, an approach that targets proteasome would also be effective for suppressing prostate cancer cell growth and tumor progression. AR, Akt, and NF-κB signaling pathways all play important roles in development of prostate cancer and progression of androgen-sensitive tumors to hormone-refractory state (Chen and Sawyers, 2002; Chen et al., 2004). In hormone-refractory prostate cancer (HRPC), AR gene mutation and amplification have frequently been observed, accompanied by an increased AR mRNA and protein production (Heinlein and Chang, 2004). Even nano-molar concentrations of androgen produced by the tumor itself are sufficient to transactivate AR and promote proliferation of prostate cancer cells (Mohler et al., 2004; Titus et al., 2005). Moreover, AR can also be transactivated by Akt and NF-κB signaling (Wen et al., 2000; Lee et al., 2005). Therefore, targeting AR signaling and its associated molecular events could be a novel means of inhibiting prostate cancer cell growth and tumor progression.
We have previously found that B-DIM significantly inhibited AR and NF-κB activity (Bhuiyan et al., 2006). However, the precise molecular events involved in B-DIM-induced inhibition of NF-κB and AR have not been fully elucidated. Because NF-κB activity is also regulated by proteasome and because AR transactivation participates in the regulation of the cell cycle, we investigated the effects of B-DIM on both proteasome activity and AR transactivation with respect to B-DIM-mediated cell cycle regulation and induction of apoptosis in both androgen-sensitive LNCaP and androgen-insensitive C4-2B prostate cancer cells.
Materials and Methods
Cell culture
LNCaP and C4-2B cells were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS), 100 µg/ml streptomycin, 100 units/ml penicillin, and 2.5 mM glutamine in a humidified incubator with 5% CO2 and 95% air at 37°C. In some experiments the LNCaP medium also contained 10 nM testosterone.
Synchronization of LNCaP cells by isoleucine deprivation
Cell synchronization was performed essentially as described previously (Cifuentes et al., 2003). Briefly, LNCaP cells (<50% confluence) in culture dishes were washed with isoleucine-free RPMI 1640 (Invitrogen, Carlsbad, CA) and incubated with isoleucine-free RPMI 1640 supplemented with 6% dialyzed FCS (Hyclone, Logan, UT), 100 µg/ml streptomycin, 100 units/ml penicillin, 10 nM testosterone, and 2.5 mM glutamine for 36–40 h. Cells thus arrested in G0/G1 phase were released by adding complete medium containing 10% FCS and 10 nM testosterone. When studying the effect of B-DIM on cell cycle regulatory events, isoleucine-deprived cells were released by adding complete medium containing 10% FCS, 10 nM testosterone, and 50 µM B-DIM (BioResponse, Boulder, CO). Entry of cells into S phase was determined by pulse-labeling the cells at regular intervals after release from isoleucine blockade with 2 µCi/ml 3H-thymidine (ICN Biomedicals, Costa Mesa, CA) and maintaining them at 37°C in a humidified incubator for 30 min. Radioactivity incorporated into acid-precipitable material was then determined as described previously (Cifuentes et al., 2003).
Preparation of cell extracts
LNCaP and C4-2B cells were synchronized at G0/G1 phase, released into the cell cycle, and treated with 50 µM B-DIM as described above. They were then harvested and incubated in ice-cold cell lysis buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 0.5 mg/ml benzamidine) on ice. After 15 min, NP-40 was added to the cell suspension at a final concentration of 0.3% and the samples were vortexed vigorously for 20 sec. After centrifugation, the supernatant was saved as cytoplasmic protein and the nuclear pellet was incubated in ice-cold nuclear extraction buffer (20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 0.5 mg/ml benzamidine) on ice for 30 min. After centrifugation, the supernatant was saved as nuclear protein. Whole cell lysates of LNCaP and C4-2B cells with or without B-DIM were also prepared by sonicating the lysed cells in 62 mM Tris–HCl and 2% SDS. Protein concentration was measured using BCA Protein Assay (Pierce, Rockford, IL) and analyzed by Western blot analysis.
Western blot analysis
An equal amount of protein in each fraction was subjected to denaturing polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes. The membranes were probed with antibodies against AR (Santa Cruz, Santa Cruz, CA), cyclin A (Santa Cruz), p27Kip1 (Santa Cruz), PARP (Biomol, Plymouth Meeting, PA), ubiquitin (Santa Cruz), IκBα (Cell Signaling, Danvers, MA), NF-κB (Upstate, Lake Placid, NY), Bcl-2 (Santa Cruz), or β-actin (Santa Cruz). Immunoreactive bands were developed using horseradish peroxidase conjugated secondary antibodies and SuperSignal WestPico chemiluminescent substrate (Pierce) and visualized using X-ray film.
Assay of proteasomal chymotrypsin-like activity
Chymotrypsin-like activity in nuclear extracts was assayed as described previously (Nam et al., 2001). Briefly, cell lysates (10 µg per reaction) were incubated for 120 min at 37°C in 100 µl assay buffer (50 mM Tris–HCl, pH 7.5) with 20 µM fluorogenic substrate (Suc-LLVY-AMC). After incubation, production of hydrolyzed AMC groups was measured using a Wallac Victor3 multilabel counter with a 365-nm excitation filter and a 460-nm emission filter. In addition, purified rabbit 26S proteasome (17.5 ng) was incubated in 100 µl of assay buffer (50 mM Tris–HCl, pH 7.5) with different concentrations of B-DIM and 10 µM fluorogenic peptide substrate (Suc-LLVY-AMC) for 2 h at 37°C. After incubation, production of hydrolyzed AMC groups was measured.
Results
B-DIM inhibited the transition of synchronized prostate cancer cells from G1 to S phase
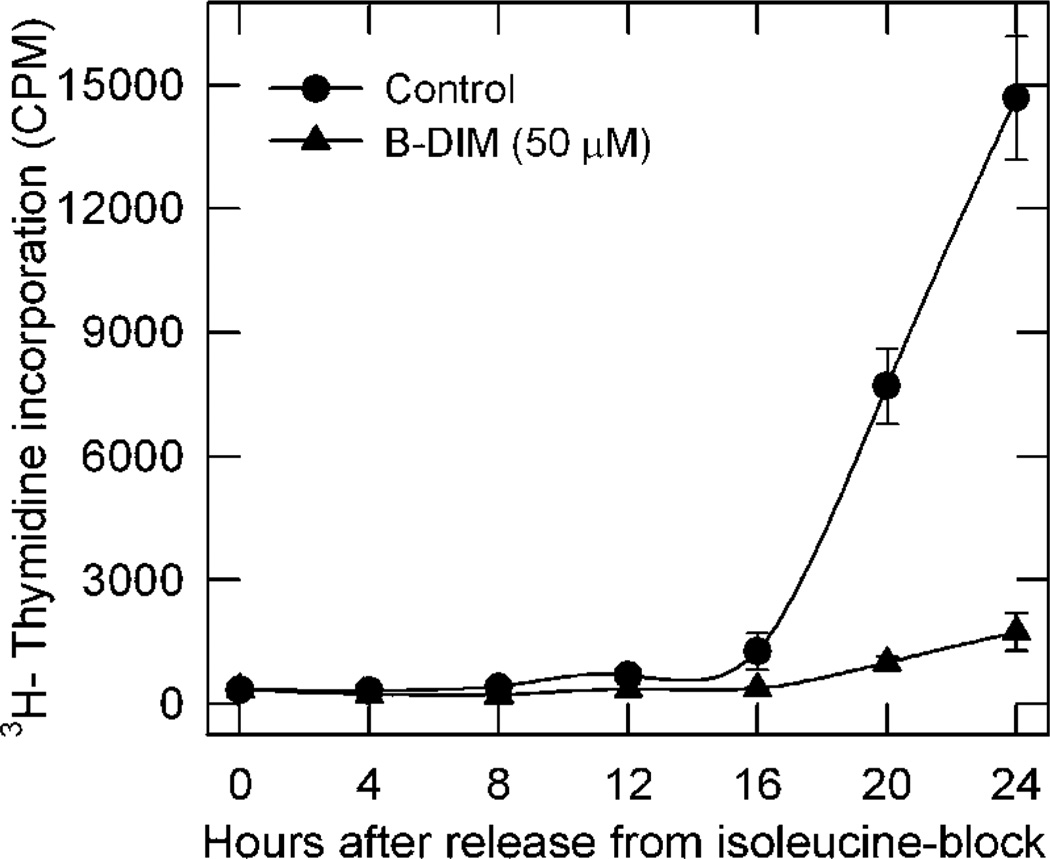
Previous studies (Zhang et al., 2003) reported that DIM, a precursor of I3C, suppressed prostate cancer cell proliferation as demonstrated by inhibition of 3H-thymidine incorporation and accumulation of cells in G1 phase. However, this anti-proliferative effect required prolonged treatment with high doses of I3C that resulted in the over-expression of cyclin-dependent kinase (CDK) inhibitors p21Waf1 and p16INK4a that are often associated with stress-induced checkpoint activation leading to cell cycle arrest and apoptosis. In an attempt to distinguish anti-proliferative from pro-apoptotic actions of this indole compound, we examined the effect of short-term B-DIM treatment on the ability of prostate cancer cells to progress from G1 to S phase. We employed androgen-sensitive LNCaP prostate cancer cells synchronized by isoleucine deprivation. Isoleucine-deprived LNCaP cells are reversibly blocked in G0/G1 phase, and upon release from isoleucine blockade they enter S phase with a lag period (G1 phase) of 15–16 h (Cifuentes et al., 2003). As indicated by the incorporation of 3H-thymidine, solvent-treated (control) LNCaP cells entered S phase starting 16 h after release from isoleucine blockade (Fig. 1). By comparison, LNCaP cells released from isoleucine blockade in the presence of B-DIM failed to enter S phase even after 24 h (Fig. 1). Thus it is evident that the cell cycle regulatory event(s) necessary for progression of cells from G1 to S, fundamental for proliferation, can be inhibited by B-DIM in prostate cancer cells.
Fig. 1.
B-DIM inhibited progression of synchronized LNCaP cells from G1 to S phase after release from isoleucine blockade. LNCaP cells were incubated in isoleucine-free medium for 36 h and then released into complete medium. They were treated with 50 µM B-DIM or left untreated as a control. At given intervals, they were pulse-labeled with 3H-thymidine to measure radioactivity incorporated into DNA. Data represent at least three independent experiments.
B-DIM suppressed the increase in AR protein and induced p27Kip1 expression during G1 phase of synchronized LNCaP cells
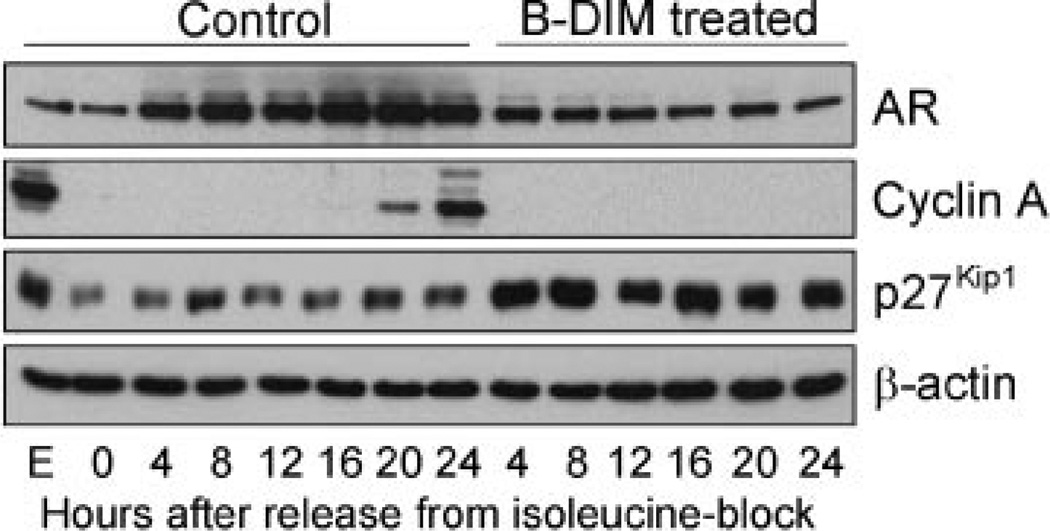
We have previously shown that the AR is required for progression of synchronized LNCaP cells from G1 to S phase (Cifuentes et al., 2003), and that AR increased during G1 phase after they were released from isoleucine blockade (Pelley et al., 2006). Therefore, we examined whether B-DIM-induced blocking entry of LNCaP cells into S phase involves changes in AR expression. We performed Western blot analysis to detect AR levels using equal amounts of proteins from cell extracts prepared at 4-h intervals after release from isoleucine blockade. As shown in Figure 2, we observed that AR in the control cells increased gradually from 4 to 20 h after release, consistent with the time when AR is required for entry of LNCaP cells into S phase (Pelley et al., 2006). Cell entry into S phase was also indicated by the expression of cyclin A (used as a positive control) starting at 16 h after release from isoleucine blockade (Fig. 2). However, in cells treated with B-DIM, the increase of AR between 4 and 20 h after release was eliminated (Fig. 2), consistent with failure to enter S phase as indicated by the absence of detectable cyclin A. Interestingly, the cells treated with B-DIM also showed a noticeable increase in p27Kip1 starting at 4 h after release from isoleucine blockade (Fig. 2). As judged from β-actin levels, equal amounts of proteins were analyzed in samples that showed different AR, cyclin A and p27Kip1 levels between control and B-DIM-treated cells. Thus, B-DIM appears to inhibit progression of LNCaP cells from G1 to S phase by suppressing the increase in AR and inducing expression of the CDK inhibitor p27Kip1 during G1 phase.
Fig. 2.
B-DIM up-regulated p27Kip1 and down-regulated AR and cyclin A in synchronized LNCaP cells progressing from G1 to S phase after release from isoleucine blockade. LNCaPcells were incubated in isoleucine-free medium for 36 h and then released into complete medium. They were treated with 50 µM B-DIM or left untreated as a control. At given intervals, proteins from each sample were extracted. Cell lysates were analyzed by Western blot.
B-DIM suppressed 26S proteasome levels in LNCaP cells at S phase
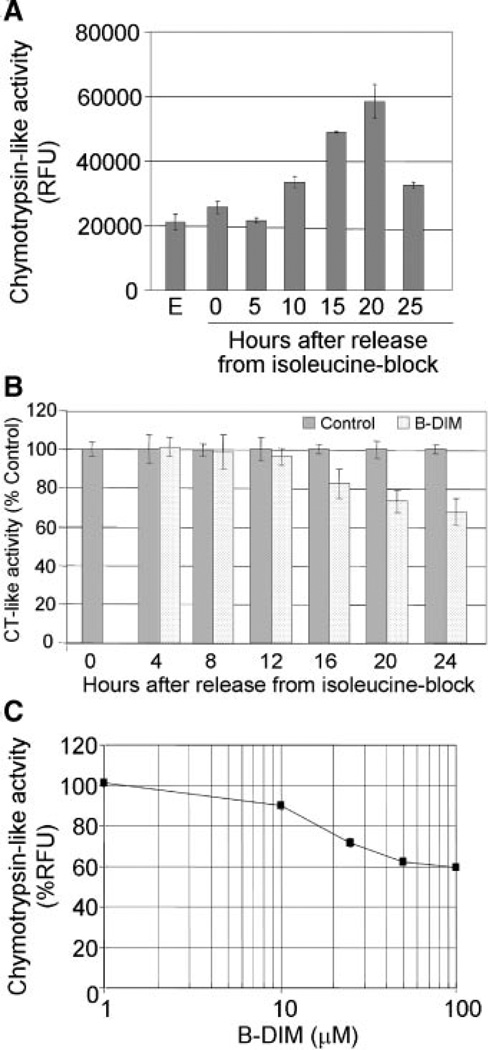
26S proteasome is known to play important roles in cell cycle regulation, and inhibiting it results in the down-regulation of AR and induced p27Kip1 expression in prostate cancer cells (Yang et al., 2008). Therefore, we examined whether B-DIM-induced blockade of cell cycle progression, suppression of AR levels, and induction of p27Kip1 are mediated by inhibition of the proteasome pathway. We observed that 26S proteasome associated chymotrypsin-like activity in synchronized LNCaP cells increased as the cells progressed from G1 to S phase (5–20 h after release from isoleucine blockade), and it decreased once the cells entered S phase (25 h after release) (Fig. 3A). On the other hand, the cells treated with B-DIM exhibited a 40% decrease in the proteasomal chymotrypsin-like activity that was detectable at 16–24 h after release from isoleucine blockade, consistent with the time when the cells would be expected to enter S phase (Fig. 3B). B-DIM also inhibited about 40% chymotrypsin-like activity of a purified 20S proteasome (Fig. 3C). These observations indicate that inhibition of the proteasome activity by B-DIM in B-DIM treated cells is associated with the down-regulation of AR, and induction of p27Kip1 in synchronized LNCaP cells.
Fig. 3.
B-DIM inhibited the chymotrypsin-like activity of proteasome. A: Chymotrypsin-like activity of proteasome in synchronized LNCaP cells progressing from G1 to S phase after release from isoleucine blockade. B: B-DIM (50 µM) inhibited the chymotrypsin-like activity of proteasome in synchronized LNCaP cells progressing from G1 to S phase after release from isoleucine blockade. C: B-DIM inhibited the chymotrypsin-like activity of purified 20S proteasome.
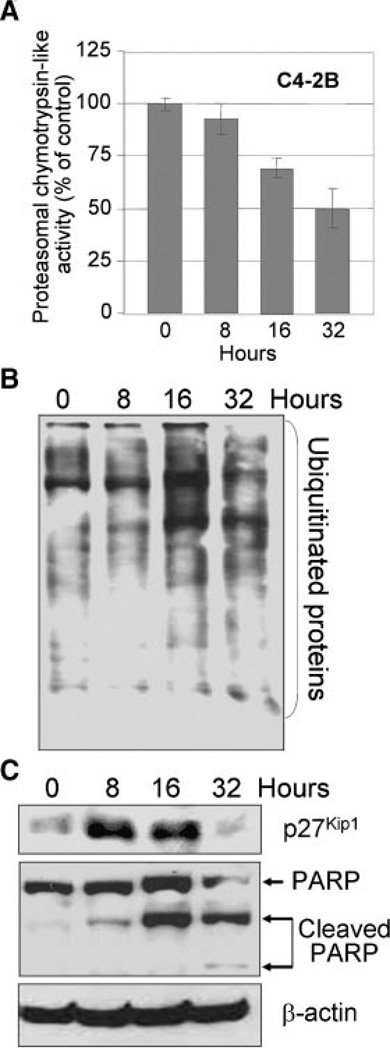
Proteasome inhibition by B-DIM led to apoptosis of prostate cancer cells
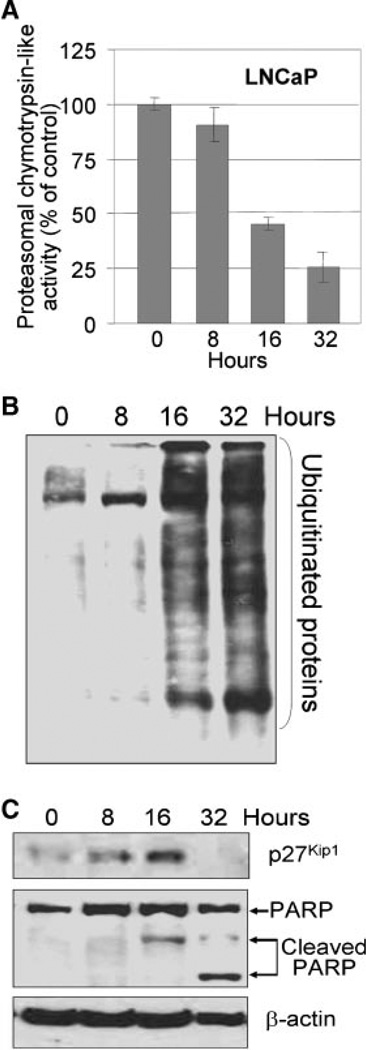
Proteasome also plays important roles in apoptosis, and proteasome inhibitors is known to alter the proteins that regulate apoptosis (Naujokat and Hoffmann, 2002; Yang et al., 2008). Therefore, we examined the effect of B-DIM on proteasome activity and induction of apoptosis in exponentially growing androgen-sensitive LNCaP and androgen-insensitive C4-2B prostate cancer cells. We found that B-DIM treatment for 16 h decreased the cellular proteasomal chymotrypsin-like activity in exponentially growing LNCaP (Fig. 4A) and C4-2B (Fig. 5A) prostate cancer cells. An inhibitory effect of B-DIM on the 26S proteasome activity after 16 and 32 h was also evident since accumulation of ubiquitinated proteins was significantly increased (Figs. 4B and 5B). Also, an increase in p27Kip1 levels was found in both the cell lines, which was more dramatic in C4-2B (Fig. 5C) than in LNCaP cells (Fig. 4C). The levels of p27Kip1 peaked in both the cell lines around 16 h after treatment, and at 32 h p27Kip1 levels dropped below the levels that was observed in untreated control cells, which is perhaps because of the B-DIM mediated induction in cell death. These results are consistent with the previous reports showing that proteasome inhibition results in the accumulation of proteasome target proteins, such as p27Kip1 (Yang et al., 2008). Inhibition of the proteasome activity by B-DIM in both LNCaP and C4-2B cells was associated with apoptotic cell death, as measured by PARP cleavage, a specific marker of apoptosis (Figs. 4C and 5C).
Fig. 4.
B-DIM inhibited proteasome activity and induced p27Kip1 expression and PARP cleavage in LNCaP prostate cancer cells. A: LNCaP cells were treated with 50 µM B-DIM for 8–32 h, and proteasomal chymotrypsin-like activity was measured. B: LNCaP cells were treated with 50 µM B-DIM for 8–32 h, followed by Western blot of ubiquitinated proteins. C: LNCaP cells were treated with 50 µM B-DIM for 8–32 h, followed by Western blot of p27Kip1 and PARP.
Fig. 5.
B-DIM inhibited proteasome activity and induced p27Kip1 expression and PARP cleavage in C4-2B prostate cancer cells. A: C4-2B cells were treated with 50 µM B-DIM for 8–32 h, and proteasomal chymotrypsin-like activity was measured. B: C4-2B cells were treated with 50 µM B-DIM for 8–32 h, followed by Western blot of ubiquitinated proteins. C: C4-2B cells were treated with 50 µM B-DIM for 8–32 h, followed by Western blot of p27Kip1 and PARP.
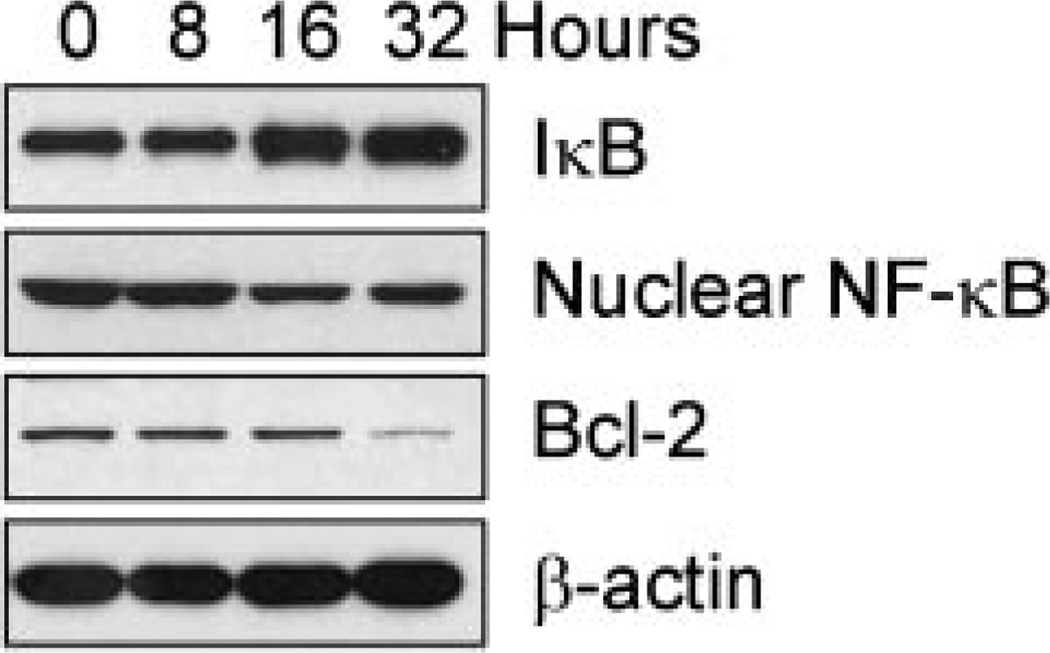
Consistent with the inhibition of proteasome activity by B-DIM, we also found increased IκBα, and decreased nuclear NF-κB, and Bcl-2 (an NF-κB downstream target gene well known as an inhibitor of apoptosis) in B-DIM treated LNCaP cells (Fig. 6). These results are also consistent with our previously published results showing that B-DIM could induce apoptosis in C4-2B and LNCaP prostate cancer cells (Bhuiyan et al., 2006). Taken together, these observations clearly provide data for the first time showing that B-DIM could inhibit the proteasomal activity which, in turn, induces p27Kip1 expression leading to cell cycle arrest, and induction of apoptotic events in androgen-sensitive as well as androgen-insensitive prostate cancer cells.
Fig. 6.
B-DIM inhibited NF-κB signaling and down-regulated Bcl-2 expression in LNCaP cells. LNCaP cells were treated with 50 µM B-DIM for 8–32 h, followed by Western blot of IκB, NF-κB, and Bcl-2.
Discussion
Using isoleucine deprivation to synchronize androgen-sensitive and androgen-insensitive prostate cancer cells is considered the best way to examine the changes in cell cycle regulators during progression from G1 to S phase because AR status will not influence the synchronization caused by isoleucine-deprivation (Cifuentes et al., 2003). The cell cycle is tightly controlled by many regulatory proteins, including cyclins, cyclin-dependent kinases (CDKs), CDK inhibitors (CDKI), and tumor suppressor gene products such as p53 and pRb (Bloom and Cross, 2007). Together, they permit or stop cell cycle transition, checkpoint progression, and cell proliferation. During progression from G1 to S phase, the CDK inhibitors p27Kip1 and p21WAF1 bind to the cyclin/CDK complexes and control the G1/S transition. In addition, cyclin A starts to accumulate during S phase and activates CDK2, suggesting that it too is important for entry into S phase. We tested the effect of B-DIM on several target molecules that are critical for control of G1/S transition in synchronized cells and found that it significantly inhibited the transition of synchronized prostate cancer cells from G1 to S phase. We also observed an increase in p27Kip1 starting at 4 h after release from isoleucine blockade in cells treated with B-DIM, suggesting that B-DIM could have anti-proliferative effect by inducing p27Kip1 and inhibiting CDK4 and CDK6, which are necessary for progression of prostate cancer cells from G1 to S phase. Cyclin A increased 16 h after release from isoleucine blockade in control cells, suggesting that this is when cells normally enter S phase; however, in B-DIM treated cells, cyclin A was undetectable, suggesting failure to enter S phase.
AR is a critical regulator of G1/S phase progression, inducing signals that promote CDK activity and inactivate RB (Balk and Knudsen, 2008). We observed that AR level increased gradually from 4 to 20 h after release from isoleucine blockade, suggesting the importance of AR in G1/S transition. Importantly, in B-DIM treated cells this increase in AR was attenuated. Collectively, these results demonstrate that B-DIM arrests prostate cancer cells at G1 phase of the cell cycle through regulation of p27Kip1, cyclin A and AR, thus blocking prostate cancer cell proliferation and cell growth.
Regulated proteolysis plays important roles in both cell physiological and pathological conditions. Ubiquitin- and proteasome-dependent proteolytic system largely contributes to regulated proteolysis. Proteasome-mediated protein degradation is deregulated in a number of human cancers, including prostate cancer, leading to imbalance of proliferation and apoptosis (Nencioni et al., 2007). Accumulated evidence demonstrates that the ubiquitin-proteasome proteolytic system plays important roles in apoptosis through the cellular pathways acted on by the proteasome; therefore, proteasome could be a target for cancer therapy (Nencioni et al., 2007; Orlowski and Kuhn, 2008). Several proteasome inhibitors have been found to strongly induce apoptosis (Daniel et al., 2007; Nencioni et al., 2007; Orlowski and Kuhn, 2008). Proteasome inhibitors block activation of NF-κB, a critical inhibitor of apoptosis, and influence both the proteins that regulate apoptosis and intracellular signals in a number of cancer cell lines, resulting in induction of apoptosis (Tanaka et al., 2001).
In quiescent cells, NF-κB remains in the cytoplasm in an inactive state by binding to its inhibitor, IκBα. Cell growth signaling induces phosphorylation of IκBα, leading to targeted degradation of IκBα by proteasome. This frees NF-κB to translocate into the nucleus where it induces expression of its target genes which inhibit apoptosis. We found that B-DIM inhibited proteasome activity and induced apoptosis. This could involve the NF-κB signaling pathway triggered by the inhibition of 26S proteasome in S phase cells, because we observed increased IκB and decreased nuclear NF-κB after B-DIM treatment. We also observed that down-regulation of NF-κB decreased expression of Bcl-2, which is an NF-κB downstream target gene and is a well known anti-apoptotic molecule. These results demonstrate that B-DIM induces apoptosis through sequential inhibition of proteasome activity, IκB degradation, NF-κB activation, and Bcl-2 expression. A recent study (Domingo-Domenech et al., 2008) reported that inactivation of NF-κB by proteasome inhibition contributed to the increased apoptosis induced by histone deacetylase inhibitors in human breast cancer cells. Proteasome inhibition could also enhance TRAIL-induced apoptosis through regulation of NF-κB (Chen et al., 2008), suggesting that B-DIM mediated proteasome inhibition could be an effective strategy for the treatment of prostate cancer.
The CDK inhibitor p27Kip1, a proteasome target protein, is a critical regulator of cell cycle; however, it also regulates apoptosis (Chen and Lin, 2004; Chu et al., 2008). A recent study by Nickeleit et al. (2008) showed that p27Kip1 is a critical downstream target of proteasome. They identified a new proteasome inhibitor, argyrin A, and showed that it could prevent p27Kip1 degradation by proteasome, induce apoptosis, and inhibit angiogenesis. They also found that argyrin A-induced apoptosis of tumor cells requires stabilization of p27Kip1, confirming the role of p27Kip1 in the induction of apoptosis. Therefore, the induction of apoptosis by B-DIM in our study could be partly due to the induction of p27Kip1 beginning at 4 h after release from isoleucine blockade, because the increase in p27Kip1 was also associated with PARP cleavage. However, it might not be entirely due to the inhibition of proteasome activity, because we observed some increase in p27Kip1 before proteasome inhibition. DIM reportedly induced apoptosis in AR-positive LNCaP and AR-negative DU145 and PC3 prostate cancer cells (Nachshon-Kedmi et al., 2003, 2004), suggesting that it can induce apoptosis independently of its effect on AR in prostate cancer cells. Our studies showed that although B-DIM suppressed AR expression in G1 phase, this might contribute solely to the blockade of cell cycle progression from G1 to S phase, not apoptosis. Therefore, down-regulation of AR could contribute primarily to the cell cycle arrest and the inhibition of prostate cancer cell proliferation.
In summary, we believe that our study is the first to show cell cycle-dependent effects of B-DIM on cell proliferation and apoptosis in synchronized prostate cancer cells. B-DIM mediated inhibition of cell cycle progression from G1 to S phase was due in part by the induction of p27Kip1 and down-regulation of AR. Moreover, here we show for the first time that B-DIM could inhibit proteasome activity in S phase, which in turn leads to the inactivation of NF-κB signaling, and consequently down-regulation of Bcl-2, resulting in the induction of apoptotic cell death. Collectively, these findings suggest that B-DIM could be a potent agent for the prevention and/or treatment of prostate cancer, regardless of whether cells are sensitive to androgen or not.
Acknowledgments
This work was supported by the Department of Defense grant W81XWH-05-1-0071 to GPVR and the National Cancer Institute, NIH, grants 5R01CA108535 to FHS, and 5R01CA120009 to QPD.
Literature Cited
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE. Physiological modeling of formulated and crystalline 3,3’-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004;32:632–638. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3’-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- Brew CT, Aronchik I, Hsu JC, Sheen JH, Dickson RB, Bjeldanes LF, Firestone GL. Indole-3-carbinol activates the ATM signaling pathway independent of DNA damage to stabilize p53 and induce G1 arrest of human mammary epithelial cells. Int J Cancer. 2006;118:857–868. doi: 10.1002/ijc.21445. [DOI] [PubMed] [Google Scholar]
- Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol. 2002;22:2862–2870. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Chou CW, Chang YF, Chen CC. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: Involvement of NF-kappaB and reactive oxygen species-mediated p53 activation. J Immunol. 2008;180:8030–8039. doi: 10.4049/jimmunol.180.12.8030. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Lin JK. Induction of G1 arrest and apoptosis in human jurkat T cells by pentagalloylglucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/WAF1, and Bax proteins. J Biol Chem. 2004;279:13496–13505. doi: 10.1074/jbc.M212390200. [DOI] [PubMed] [Google Scholar]
- Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin Cancer Res. 2002;8:1228–1236. [PubMed] [Google Scholar]
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: Prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Cifuentes E, Croxen R, Menon M, Barrack ER, Reddy GP. Synchronized prostate cancer cells for studying androgen regulated events in cell cycle progression fromG1 into S phase. J Cell Physiol. 2003;195:337–345. doi: 10.1002/jcp.10317. [DOI] [PubMed] [Google Scholar]
- Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- Daniel KG, Chen D, Yan B, Dou QP. Copper-binding compounds as proteasome inhibitors and apoptosis inducers in human cancer. Front Biosci. 2007;12:135–144. doi: 10.2741/2054. [DOI] [PubMed] [Google Scholar]
- Domingo-Domenech J, Pippa R, Tapia M, Gascon P, Bachs O, Bosch M. Inactivation of NF-kappaB by proteasome inhibition contributes to increased apoptosis induced by histone deacetylase inhibitors in human breast cancer cells. Breast Cancer Res Treat. 2008;112:53–62. doi: 10.1007/s10549-007-9837-8. [DOI] [PubMed] [Google Scholar]
- Ge X, Yannai S, Rennert G, Gruener N, Fares FA. 3, 3’-Diindolylmethane induces apoptosis in human cancer cells. Biochem Biophys Res Commun. 1996;228:153–158. doi: 10.1006/bbrc.1996.1631. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3, 3’-diindolylmethane is mediated by NF-kappaB downstream target genes MMP-9 and uPA regulated bioavailability of VEGF in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- Lee SO, Lou W, Nadiminty N, Lin X, Gao AC. Requirement for NF-(kappa)B in interleukin-4-induced androgen receptor activation in prostate cancer cells. Prostate. 2005;64:160–167. doi: 10.1002/pros.20218. [DOI] [PubMed] [Google Scholar]
- Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci. 2005;10:236–243. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Sarkar FH. Gene expression profiles of I3C- and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J Nutr. 2003;133:1011–1019. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3’-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- Mohler JL, Gregory CW, FordOH III, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3’-diindolylmethane through the mitochondrial pathway. Br J Cancer. 2004;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshon-Kedmi M, Yannai S, Haj A, Fares FA. Indole-3-carbinol and 3,3’-diindolylmethane induce apoptosis in human prostate cancer cells. Food Chem Toxicol. 2003;41:745–752. doi: 10.1016/s0278-6915(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Naujokat C, Hoffmann S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest. 2002;82:965–980. doi: 10.1097/01.lab.0000022226.23741.37. [DOI] [PubMed] [Google Scholar]
- Nencioni A, Grunebach F, Patrone F, Ballestrero A, Brossart P. Proteasome inhibitors: Antitumor effects and beyond. Leukemia. 2007;21:30–36. doi: 10.1038/sj.leu.2404444. [DOI] [PubMed] [Google Scholar]
- Nickeleit I, Zender S, Sasse F, Geffers R, Brandes G, Sorensen I, Steinmetz H, Kubicka S, Carlomagno T, Menche D, Gutgemann I, Buer J, Gossler A, Manns MP, Kalesse M, Frank R, Malek NP. Argyrin a reveals a critical role for the tumor suppressor protein p27(kip1) in mediating antitumor activities in response to proteasome inhibition. Cancer Cell. 2008;14:23–35. doi: 10.1016/j.ccr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: Lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- Pelley RP, Chinnakannu K, Murthy S, Strickland FM, Menon M, Dou QP, Barrack ER, Reddy GP. Calmodulin-androgen receptor (AR) interaction: Calcium-dependent, calpain-mediated breakdown of AR in LNCaP prostate cancer cells. Cancer Res. 2006;66:11754–11762. doi: 10.1158/0008-5472.CAN-06-2918. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kawakami T, Tateishi K, Yashiroda H, Chiba T. Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway. Biochimie. 2001;83:351–356. doi: 10.1016/s0300-9084(01)01237-8. [DOI] [PubMed] [Google Scholar]
- Tavani A, La VC. Fruit and vegetable consumption and cancer risk in a Mediterranean population. Am J Clin Nutr. 1995;61:1374S–1377S. doi: 10.1093/ajcn/61.6.1374S. [DOI] [PubMed] [Google Scholar]
- Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van PG. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact. 1997;103:79–129. doi: 10.1016/s0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- Voorhees PM, Dees EC, O’Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316–6325. [PubMed] [Google Scholar]
- Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- Yang H, Landis-Piwowar KR, Lu D, Yuan P, Li L, Reddy GP, Yuan X, Dou QP. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J Cell Biochem. 2008;103:234–244. doi: 10.1002/jcb.21399. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hsu BAJ, Kinseth BAM, Bjeldanes LF, Firestone GL. Indole-3-carbinol induces a G1 cell cycle arrest and inhibits prostate-specific antigen production in human LNCaP prostate carcinoma cells. Cancer. 2003;98:2511–2520. doi: 10.1002/cncr.11844. [DOI] [PubMed] [Google Scholar]