Abstract
Mammalian oocyte meiosis encompasses two rounds of asymmetric divisions to generate a totipotent haploid egg and, as by-products, two small polar bodies. Two intracellular events, asymmetric spindle positioning and cortical polarization, are critical to such asymmetric divisions. Actin but not microtubule cytoskeleton has been known to be directly involved in both events. Recent work has revealed a positive feedback loop between chromosome-mediated cortical activation and the Arp2/3-orchestrated cytoplasmic streaming that moves chromosomes. This feedback loop not only maintains meiotic II spindle position during metaphase II arrest, but also brings about symmetry breaking during meiosis I. Prior to an Arp2/3-dependent phase of fast movement, meiotic I spindle experiences a slow and non-directional first phase of migration driven by a pushing force from Fmn2-mediated actin polymerization. In addition to illustrating these molecular mechanisms, mathematical simulations are presented to elucidate mechanical properties of actin-dependent force generation in this system.
Keywords: symmetry breaking, polarity establishment, spindle migration, actin dynamics
1. Introduction
The mammalian oocyte undergoes two consecutive rounds of extremely asymmetric divisions to generate a large haploid egg and two small polar bodies. Such asymmetric divisions are dictated by intracellular asymmetries developed within the oocyte prior to anaphase onset, which includes asymmetric spindle positioning and cortical polarization [1,2]. Owing to its amenability to optical imaging and genetic manipulations, as well as a robust ability to undergo maturation in culture, mouse oocytes have been recognized as one of the best models to understand these events. When maturing in vitro, the meiotic I (MI) spindle of the mouse oocyte is assembled at or near the cell centre after germinal vesicle breakdown (GVBD). Shortly after chromosome alignment, the chromosomes–spindle complex initiates its migration towards one side of the cortex. Accompanying this process is the establishment of cortical polarity characterized by an actin-enriched domain surrounded with a myosin ring [3–6]. At anaphase, coordinated protrusion of the actin-enriched domain and constriction of the myosin ring lead to extrusion of the first polar body containing one set of homologous chromosomes [7–9]. Following the first polar body extrusion, the oocyte proceeds into the metaphase of meiosis II where an MII spindle is assembled near the first division site beneath the cortex. A cortical actomyosin domain similar to that in the MI is again established above the spindle. The oocyte is now fully mature and arrests at this stage awaiting fertilization (figure 1).
Figure 1.
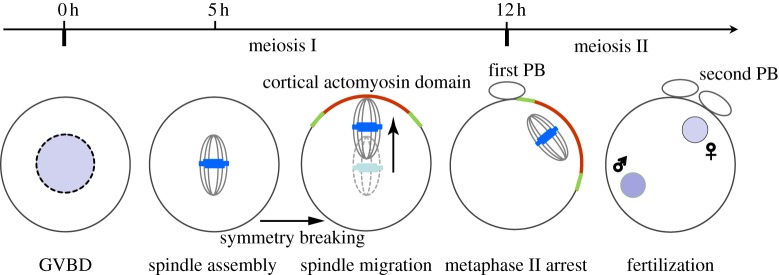
Meiotic maturation of a mouse oocyte. The prophase I-arrested oocyte resumes cell cycle progression and enters its maturation process upon hormone stimulation. The MI spindle is assembled approximately 5 h after germinal vesicle breakdown (GVBD) in cultured oocytes. It migrates towards one side of the cortex along the long axis once the chromosomes are aligned. Immediately after first polar body (PB) extrusion, the MII spindle is formed beneath the cortex, and the subcortical position is maintained through the metaphase II arrest. Later, fertilization triggers MII completion resulting in a second polar body extrusion. In both meiosis I and II, accompanying asymmetric spindle positioning is the formation of a cortical actomyosin cap induced by subcortically located chromosomes. (Online version in colour.)
Both spindle positioning and cortical polarization rely on actin dynamics but not microtubules (see review in Sun & Schatten [10]). Recent works have revealed the underlying mechanisms that drive these events during meiosis I and II [11–15]. In this review, we present a comprehensive working model for spindle positioning and cortical polarization during mouse oocyte maturation, mainly based on our recently published works [5,13,16,17]. We begin by describing a positive feedback loop that connects cortical polarity and asymmetric spindle positioning. We discuss how this feedback loop is critical for maintaining oocyte polarity during prolonged MII arrest, and how it is used, together with Fmn2-mediated actin polymerization, to transport the MI spindle in meiosis I. Finally, we introduce a simple mechanical model that explains how the actin-dependent forces are generated during these processes.
2. A positive feedback loop drives spindle positioning and cortical polarization
(a). Meiotic chromosomes signal the establishment of cortical polarity
The cortical actomyosin cap is a structure essential for polar body extrusion. It is established in meiosis I when the spindle has migrated close to the cortex and during metaphase II arrest where the spindle is asymmetrically positioned beneath the cortex [2,5]. Previous studies suggested that establishment of such a cortical structure depends on signals from chromosomes (figure 2). Each chromosome mass dispersed by nocodazole treatment induced an actin cap at the overlying cortex [1]. Injected sperm heads or DNA-coated beads at the cortex promoted formation of a similar structure [5,18]. It was demonstrated that chromatin induction of the cortical domain is mediated through an ‘at a distance’ effect: chromosomes or DNA stimulate formation of the cortical domain without physically interacting with the cortex, provided that it is within a distance of 20–25 µm. The capacity to induce the cortical domain is inversely related to this distance. The ‘at a distance’ effect from chromosomes on cortical polarity is similar to the one from chromosomes to induce spindle formation, where a Ran guanosine triphosphate (RanGTP) gradient, established by the spatially separated actions of Ran guanine nucleotide exchange factors (GEF) and Ran GTPase activating protein (GAP), is used to transmit the chromosome signal [19–21]. Indeed, the RanGTP gradient is present in both MI and MII oocytes [22]. In oocytes undergoing meiosis II, the RanGTP gradient is involved in mediating the chromosome signal to the cortex, as both dominant negative and constitutively active Ran mutants disrupted DNA-induced cortical polarization. Thus, similar to its role in spindle assembly, the RanGTP gradient also serves as a molecular ruler for cortical polarization in mouse oocytes [5]. It is worth noting that, in both mouse and frog oocytes, mutant Ran perturbed meiotic II spindle assembly but had little effect on meiotic I spindle assembly and first polar body extrusion [22], which raises a question of whether chromosomes in meiosis I signal cortical polarity through molecular mechanisms other than the RanGTP gradient.
Figure 2.
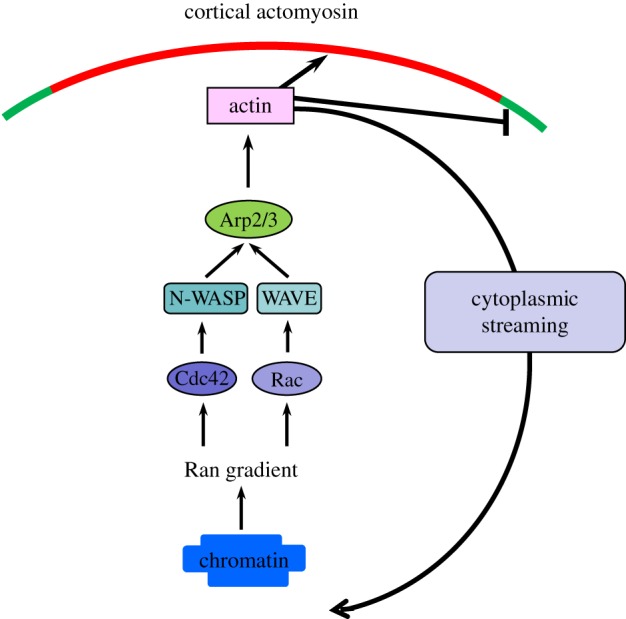
Molecular pathway leading to a positive feedback between chromosomes–spindle positioning and cortical polarization. When the spindle is in the vicinity of the cortex, the chromatin-mediated RanGTP gradient activates N-WASP and the Arp2/3 complex via Cdc42 at the cortex to nucleate actin polymerization, which suppresses premature myosin II ring contraction and promotes cytoplasmic streaming to maintain spindle positioning. Rac1-WAVE2 may serve as a parallel pathway to activate the Arp2/3 complex, and this pathway is also under the control of the Ran gradient. (Online version in colour.)
One of the cytoskeletal targets of the RanGTP gradient is the Arp2/3 complex, as mutant Ran disrupts cortical localization and activation of the Arp2/3 complex in MII mouse oocytes [17] (figure 2). The Arp2/3 complex is a type of actin nucleator that nucleates new F-actin to form a Y-shaped branch off a pre-existing filament. It is well known that Arp2/3-dependent actin dynamics are critical to leading edge protrusion in migrating cells [23]. In MII oocytes, it is localized to the cortical cap and promotes actin polymerization in this region. Inhibition of Arp2/3 or its activator N-WASP diminished actin cap formation [17,24]. Cdc42, a Rho GTPase that regulates Arp2/3 activity through N-WASP in many cell types, was recently found to be accumulated at cortical cap in a Ran-dependent manner [7]. Inhibition of Cdc42 activity led to N-WASP mislocalization, cortical actin cap loss and spindle organization/migration failure [7,25]. These observations indicated that a Cdc42-N-WASP-Arp2/3 pathway operates downstream of Ran signalling to regulate cortical actin cap formation. In addition to Cdc42, another small GTPase, Rac1, is also localized to the cortical cap region under the control of the RanGTP gradient and inhibiting its activity led to similar defects in spindle positioning [26,27]. It is possible that Rac1 regulates cortical actin cap through its specific effector WAVE2, which was indicated to be involved in oocyte polarization in MI, presumably due to failed Arp2/3 localization/activation [28]. These results suggest that multiple signalling pathways are engaged in cortical actin assembly downstream of the master regulator, the RanGTP gradient. As to how RanGTP executes its function to regulate these signalling pathways, in the case of spindle morphogenesis, RanGTP binds to importins competitively, thus triggering release of microtubule polymerization factors to support spindle assembly [19]. A similar mechanism might be at work to enable activation of the Arp2/3 during cortical polarization but the immediate effector of RanGTP remains unknown.
(b). Cortical actin assembly promotes chromosome and spindle positioning
It was thought that the sole purpose of the actomyosin cap is to facilitate polar body extrusion. However, inhibition of Arp2/3 or its activator N-WASP induces spindle detachment from the cortex in metaphase-II-arrested oocytes. This suggests that not only are the chromosomes responsible for establishing the cortical actin domain, but also that actin polymerization at the cortex, in turn, impacts chromosomes/spindle positioning [17]. Using a live F-actin probe, GFP-UtrCH, it was observed that actin filaments nucleated by cortical Arp2/3 flow inwardly towards the cell interior [17]. Intriguingly, the actin flow drives cytoplasmic streaming in the mouse oocyte. Cytoplasmic particles flow away from the polar cap along both sides of the cortex towards the opposite side of the oocyte, where the direction is reversed and the particles move towards the spindle from the cell centre. Mathematical simulation and experimental data suggested that cytoplasmic streaming generates a constant pushing force, which is critical to maintain spindle positioning during metaphase II arrest (see next section on force generation). To promote spindle positioning, the cortical Arp2/3-nucleated actin polymerization not only drives cytoplasmic streaming, but just as importantly, it also suppresses premature contraction of the myosin II ring around the actin cap. When the Arp2/3 activity is inhibited, contraction of the myosin II ring results in a reverse streaming that drives the spindle away from the cortex.
As such, asymmetric positioning of the MII spindle is achieved through a dynamic mechanism involving Arp2/3-nucleated actin assembly from the cortical cap. The mechanism is distinct from the one implicated in most other cell types where asymmetric spindle positioning is accomplished by the cortical dynein complex pulling on astral microtubules [29]. This correlates with the fact that meiotic spindle in the mammalian oocyte lacks conventional centrosomes and astral microtubules. One important feature of such a dynamic mechanism is the positive feedback loop between cortical polarization and spindle positioning: the MII chromosomes activate Arp2/3-mediated actin polymerization through Ran signalling to suppress premature myosin II ring contraction and to drive cytoplasmic streaming. The cytoplasmic streaming, in turn, exerts a pushing force on the spindle towards the cortex thus keeping the chromosomes and spindle in place.
(c). Hydrodynamic simulation confirms a pushing force from cytoplasmic streaming
To test whether cytoplasmic streaming can exert a pushing force on the spindle towards the cortex, a hydrodynamic model was proposed [17]. Full-scale simulation of actin flow and cytoplasmic streaming in the oocyte is a demanding computational problem as it requires consideration of two-phase liquid three-dimensional dynamics in a complex geometry. As the observed flow demonstrates rotational symmetry and the cytoplasmic streaming follows the actin flow, one may reduce the simulation to two-dimensional cytosolic flow in a plane produced by the cross section of the oocyte along the animal–vegetal axis. The spindle is represented as an immovable obstacle that can be considered a solid impenetrable body or a partially penetrable object with a specific shape. The flow velocity as well as the fluid pressure distribution is computed as a solution to the Navier–Stokes equation for incompressible fluid
where u denotes the fluid velocity, p is the pressure and ρ and ν are the density and kinematic viscosity of the fluid, respectively. The equation is supplied by the no-slip (zero fluid velocity 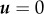 ) condition on the circular boundary of the region representing the oocyte cortex. The flow source mimicking the Arp2/3-complex-dependent actin flow in the oocytes in this geometry is located above the spindle at the symmetry axis between the spindle and the cortical membrane. The simulations show that establishment of a steady-state cytosolic flow accompanied by the pressure difference on the top and bottom surfaces of the spindle obstacle generates a force maintaining the spindle position close to the cortical membrane. In the case of Arp2/3 inhibition, the actomyosin contraction is modelled by a flow sink in the same location between the spindle and the cortical membrane. The cytosolic flow has an opposite direction and the resulting pressure difference drives the spindle away from the cortex.
) condition on the circular boundary of the region representing the oocyte cortex. The flow source mimicking the Arp2/3-complex-dependent actin flow in the oocytes in this geometry is located above the spindle at the symmetry axis between the spindle and the cortical membrane. The simulations show that establishment of a steady-state cytosolic flow accompanied by the pressure difference on the top and bottom surfaces of the spindle obstacle generates a force maintaining the spindle position close to the cortical membrane. In the case of Arp2/3 inhibition, the actomyosin contraction is modelled by a flow sink in the same location between the spindle and the cortical membrane. The cytosolic flow has an opposite direction and the resulting pressure difference drives the spindle away from the cortex.
Force generation from cytoplasmic streaming is also supported by the experimental data. For example, the chromosome mass was observed to be pushed towards the cortex once it was released from the spindle by nocodazole treatment [17]. It was also demonstrated that the detached spindle moved back towards the cortex along with the cytoplasmic particles after the streaming resumed in CK-666 wash-out oocytes. Cytoplasmic streaming was first recorded in MII-arrested oocytes, and the hydrodynamic model was originally developed to explain the pushing force accounting for spindle position maintenance in MII-arrested oocytes. However, it was recently revealed that the force generated by Arp2/3-orchestrated cytoplasmic streaming is also involved in moving the spindle towards the cortex during the fast phase of MI spindle migration [13], as discussed in detail below.
3. Biphasic model of symmetry breaking and spindle migration in meiosis I
(a). Fmn2-mediated actin polymerization drives the initial phase of spindle migration
Unlike in meiosis II where the major challenge is to maintain spindle and cortical asymmetry during a prolonged metaphase arrest, in meiosis I, the chromosomes–spindle complex has to migrate to one side of the cortex to prepare for first polar body extrusion. Spindle migration is a critical symmetry breaking step in that it not only establishes its own positional asymmetry, but also in that the chromosomes induce cortical polarization. Previous studies established that actin dynamics and Fmn2, a member of the formin family of actin nucleators, are critical for spindle migration [14,30]. Fmn2 localizes to the oocyte cortex as well as the central region of the oocyte around the spindle [16,31,32]. Correspondingly, intracellular actin filaments were observed to be enriched around the spindle by using multiple probes, including Lifeact (EGFP [33] and FITC labelled [16]), GFP-UtrCH and fluorescently labelled phalloidin [13]. We reported recently that Fmn2 is recruited to the vesicular endoplasmic reticulum (ER) structures around the spindle. This pool of Fmn2 is critical to actin polymerization around the spindle and spindle migration as its disruption causes spindle migration defects [13].
As an actin nucleator, Fmn2 is expected to drive spindle migration through its capability to regulate actin dynamics. Indeed, Fmn2 is responsible for the assembly of actin filaments prior to spindle migration, and lack of a cytoplasmic actin network has been observed in Fmn2 null oocytes [31,32,34]. Importantly, overexpression of Fmn2 evoked bulk accumulation of actin filaments around the spindle [34]. This population of F-actin is absent from Fmn2 null oocytes and is resistant to Arp2/3 inhibitor [13]. These observations suggest that spindle periphery ER-residing Fmn2 nucleates actin polymerization to promote spindle migration. Cortical Fmn2, previously thought to be involved in spindle migration, is associated largely with microvilli. When the spindle approaches the cortex, the cortical Fmn2 is gradually cleared from the area facing the approaching spindle [13]. This is in accordance with exclusion of the microvilli from the cortical cap region, but is in contrast to the view that Fmn2 nucleates cytoplasmic actin filaments from the cortex to pull the incoming spindle.
Although an earlier study proposed that cortical Fmn2-nucleated F-actin provides a track for spindle-pole-associated myosin II to pull the spindle [32], emerging evidence suggests that Fmn2-mediated actin polymerization may instead exert a direct pushing force [13,34]. In GV-arrested oocytes, moderate Fmn2 overexpression induced local accumulation of Fmn2 and actin filaments on the GV membrane, which was observed to push the GV to migrate towards the cortex. In the case of chromosome migration without an intact spindle, Fmn2 remained associated with a spindle remnant structure. It was shown that the chromosomes always migrated towards the cortex with the spindle remnant and Fmn2 lagging behind, further suggesting that Fmn2-mediated actin polymerization pushes the chromosomes forward [13]. It was previously hypothesized that Fmn2-nucleated actin polymerization propels spindle migration by a mechanism similar to the one harnessed by Listeria or verprolin central acidic domain-coated beads [16,35,36]. However, careful examination indicated that, prior to the start of spindle migration, there is no obvious asymmetry of Fmn2 or F-actin distribution when the spindle is intact [13]. Considering this fact, it was proposed that symmetric Fmn2 nucleation generates a random and stochastic pushing force on the spindle, which drives the spindle to migrate in a random manner. Indeed, trajectory tracking and mean square displacement (MSD) analysis of spindle-intact or spindle-less chromosome movement suggested that the first 5–10 µm of migration is slow and non-directional [13].
(b). Arp2/3-orchestrated cytoplasmic streaming drives a phase of fast spindle movement
Following an initial phase of spindle migration characterized as a random walk, the second phase of spindle motion is faster and more directional [13]. Concurrent with the fast-phase spindle movement, cytoplasmic streaming was observed. The streaming lasted after first polar body extrusion and continued to maintain the MII spindle at the cortex during MII arrest. Not surprisingly, the Arp2/3 complex is recruited to the MI cortical cap at the onset of the fast migration phase. Inhibiting Arp2/3 activity abolishes cytoplasmic streaming, thus resulting in abolishment of the second phase of straight and accelerated movement. The facts that streaming speed increases as the chromosome-to-cortex distance shortens and that the spindle migrates at an increasing speed suggest a mutual enhancement between Arp2/3 activation and chromosome migration, similar to the positive feedback loop responsible for spindle position maintenance in MII oocytes. It is of note that the function of Fmn2 may not be to simply generate the first phase of chromosome movement, because in a Fmn2 null oocyte Arp2/3 activation and cytoplasmic streaming were not initiated even when the chromosomes were naturally placed near the cortex.
(c). A biphasic model for symmetry breaking and spindle migration
Based on the work described above, it was proposed that symmetry breaking and chromosomes/spindle migration are accomplished by a two-phase process (figure 3a). The first phase is driven by a pushing force generated from actin polymerization nucleated by ER-bound Fmn2 at the spindle periphery. Although this movement is non-directional, it can move the chromosomes and spindle to a position closer to the cortex. Once the chromosomes reach a position within a range of 20–25 µm to the cortex, it stimulates an Arp2/3-orchestrated cytoplasmic streaming that further pushes the spindle towards the cortex in a fast and directed manner. In this model, the Fmn2-mediated migration does not result in symmetry breaking, and decisive symmetry breaking occurs when the chromosomes are within a certain distance of the cortex, as a result of a positive feedback between the Arp2/3-dependent cortical actin polymerization and chromosome movement itself. This model of symmetry breaking is different from previous ‘pulling’ and ‘pushing’ models, both of which proposed that symmetry breaking takes place at the onset of spindle migration, with one suggesting it to be a result of one spindle pole winning a tug-of-war by pulling with myosin II and the other emphasizing the importance of Fmn2/F-actin distribution asymmetry [16,32]. By contrast, the biphasic model does not rely on an initial asymmetry in force generation or Fmn2/F-actin distribution. It proposes that establishment of spindle position asymmetry and cortical polarity in MI oocytes is a consequence of Fmn2-mediated perturbation of spindle position and the positive feedback loop described above (figure 3b).
Figure 3.
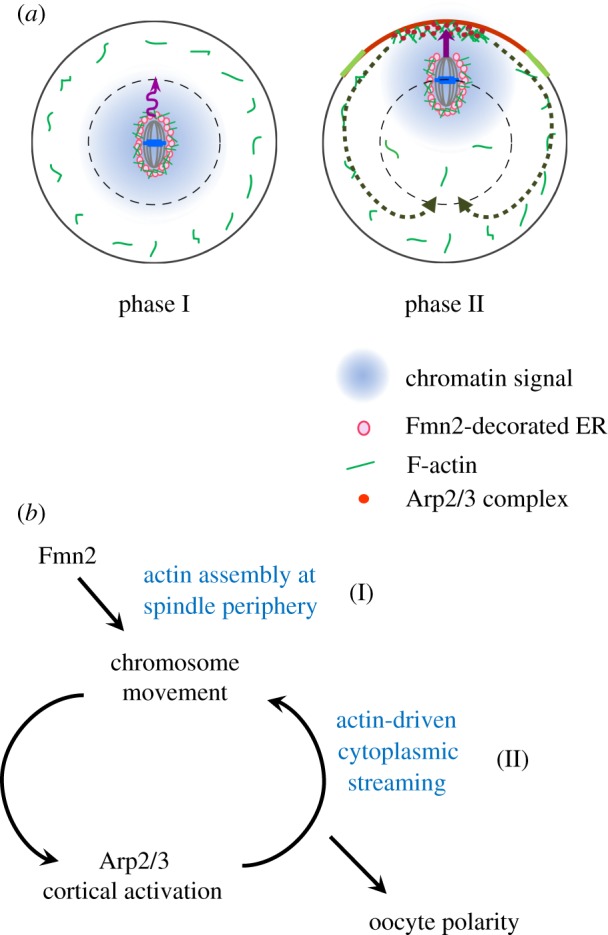
Biphasic chromosome migration and symmetry breaking in mouse oocytes. (a) Consecutive actin-based forces driving chromosome migration. Soon after the formation of the metaphase MI spindle, Fmn2 localized on ER vesicles generates random and slow spindle/chromosome motion via actin polymerization at the spindle periphery (phase I). Once the chromosomes move to a position where a diffusible chromatin-generated signal could reach the cortex, the Arp2/3 complex becomes activated at the cortex and nucleates actin polymerization that drives cytoplasmic streaming, pushing the chromosomes rapidly towards the cortex (phase II). (b) A positive feedback loop drives decisive symmetry breaking in MI oocytes. (Online version in colour.)
(d). Mathematical simulation of biphasic spindle migration and symmetry breaking
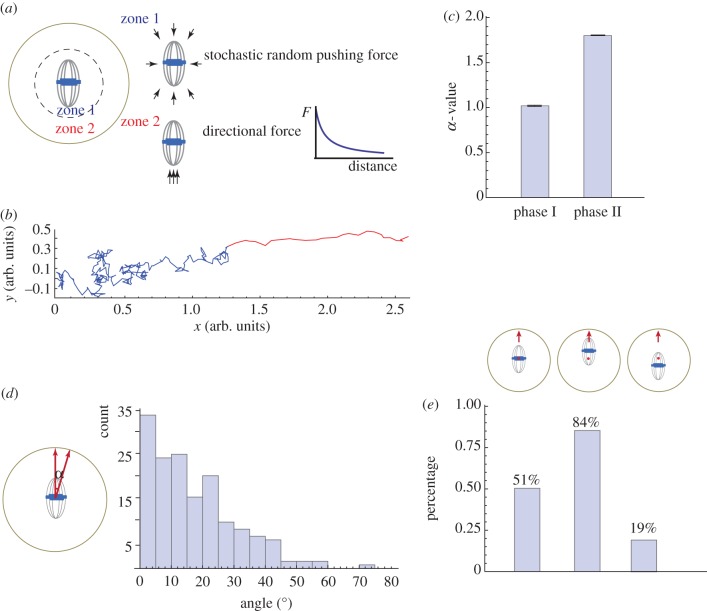
To test the above model, a mathematical model is developed that assumes stochastic forces applied from all directions around the spindle in a zone (zone 1) concentric with the oocyte with a radius at the threshold for cortical Arp2/3 activation by the chromatin signal (figure 4a). Only the net force that goes through the spindle centre is considered because this force does not generate rotation rather displacement. The difference in the drag force when the spindle moves in different directions relative to the spindle axis is estimated by the observed dimension of the spindle. If the chromosomes are beyond zone 1 (in zone 2), the Arp2/3 complex is activated at the proximal cortex to initiate the cytoplasmic streaming, which applies a directional pushing force on the spindle towards the Arp2/3 domain, and the magnitude of this force increases with decreasing distance between the chromosomes and the cortex. Simulation of this mechanical model, indeed, yields biphasic migration trajectories with the first phase close to a random walk and the second phase as straight directional motion (figure 4b,c).
Figure 4.
Modelling biphasic spindle movement in mouse oocytes. (a) Schematic of model assumptions. Stochastic random pushing forces generated by Fmn2-mediated actin polymerization are applied from all directions around the spindle in zone 1. The net force is computed by as a vector sum of individual forces applied to the spindle centroid and split into two components: parallel F1 and normal F2 to the direction of the spindle long axis. The velocity vi in these two directions is computed using the formula: vi = γiFi/(6πμ), where μ is the cytosolic viscosity and γi is the shape factor (γ1 > 1, γ2 < 1) reflecting the motion along and normal to the axis. When the spindle centroid reaches the boundary of Arp2/3 activation by the chromatin (zone 2), cytoplasmic streaming is turned on and produces an additional force parallel to the long spindle axis towards the closest cortex. The dependence of the magnitude F of this force on the distance d between spindle centroid and cortex was modelled using an 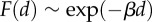 exponential function. All simulations were performed using Mathematica. (b) A representative trajectory generated by the model simulation. (c) α value of the MSD analysis of the trajectories generated from 200 simulations. Histograms show mean and s.e.m. (d) The model predicts that spindle migration is biased along the longer axis. The angle between the long spindle axis and the vector of its migration was calculated from 200 simulated trajectories. The histograms show binned counts of the angles from the 200 simulations. (e) The model predicts that off-centred spindle tends to move in the direction of the proximal cortex. The simulation was run under three situations, where the spindle centroid is at the oocyte centre (red dot), off-centre closer to the upper cortex, or off-centre farther from the upper cortex. The percentage of the cases where the spindle migrates to the upper cortex was calculated from 100 simulations. (Online version in colour.)
exponential function. All simulations were performed using Mathematica. (b) A representative trajectory generated by the model simulation. (c) α value of the MSD analysis of the trajectories generated from 200 simulations. Histograms show mean and s.e.m. (d) The model predicts that spindle migration is biased along the longer axis. The angle between the long spindle axis and the vector of its migration was calculated from 200 simulated trajectories. The histograms show binned counts of the angles from the 200 simulations. (e) The model predicts that off-centred spindle tends to move in the direction of the proximal cortex. The simulation was run under three situations, where the spindle centroid is at the oocyte centre (red dot), off-centre closer to the upper cortex, or off-centre farther from the upper cortex. The percentage of the cases where the spindle migrates to the upper cortex was calculated from 100 simulations. (Online version in colour.)
In addition to the biphasic feature of spindle movement, several other spindle migration characteristics have been described. For example, it has been observed that the direction of the spindle migration is biased along its long axis. This feature can be predicted by the model and is an outcome of the difference in the magnitude of the drag force experienced by the spindle that favours pole-led, as opposed to side-led, movement (figure 4d). Another previously noted trend is that an off-centred spindle tends to move in the direction of the proximal cortex. Again, our mechanical model can recapitulate this trend, which can be rationalized by the increased likelihood for the chromosomes undergoing the first phase of random motion to reach a zone 2 region close to the proximal cortex and therefore initiate the fast migration towards it (figure 4e).
4. Summary and perspective
As the critical events in asymmetric divisions during mouse oocyte maturation, asymmetric spindle positioning and cortical polarization are interdependent: establishment of the two relies on a feedback loop between chromatin-induced cortical Arp2/3 activation and the Arp2/3-orchestrated cytoplasmic streaming that exerts a force on the chromatin. The feedback loop either maintains asymmetric spindle positioning at metaphase II or pushes the spindle to move towards the cortex during meiosis I. The fact that cytoplasmic streaming continues from MI when the chromosomes are close to the cortex to MII arrest indicates that a singular molecular mechanism underlies cytoplasmic streaming. Indeed, the Arp2/3 complex is activated at the cortex of both MI and MII oocytes and is essential for the streaming. In metaphase II, the Arp2/3 complex is activated through a chromatin-dependent RanGTP gradient; however, it is not known whether a similar pathway activates Arp2/3 in meiosis I. As a profound characteristic in mature oocytes, cytoplasmic streaming was speculated to be an indicator for oocyte quality, which calls for further investigation into whether the extent of streaming correlates with the oocyte's developmental potential. Further, cytoplasmic streaming apparently has an influence on distribution of the proteins and organelles within a mature egg. It is tempting to speculate that it has an impact on pre-cleavage patterning of these intracellular materials.
Spindle migration in MI provides an example of how forces generated by actin dynamics promote symmetry breaking. It is interesting that two types of actin nucleators were sequentially used to nucleate actin filaments in order to propel spindle migration in oocytes, and that symmetry breaking occurs as a result of positive feedback initiated after partial chromosomes–spindle migration. Mathematical simulation of the biphasic model captures the main features of chromosome movement, thus providing a simple platform for further exploration of the mechanical properties of this motility. Future work should also elucidate how Fmn2 is recruited to the vesicular ER and how this process is temporally regulated during meiotic divisions.
Acknowledgements
We thank Brian Slaughter, Jay Unruh, Fengli Guo and colleagues from the R. Li laboratory for discussions.
Funding statement
This work is supported in part by National Institutes of Health grant no. P01 GM 066311.
References
- 1.Longo FJ, Chen DY. 1985. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev. Biol. 107, 382–394 (doi:10.1016/0012-1606(85)90320-3) [DOI] [PubMed] [Google Scholar]
- 2.Maro B, Johnson MH, Webb M, Flach G. 1986. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J. Embryol. Exp. Morphol. 92, 11–32 [PubMed] [Google Scholar]
- 3.Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B. 2000. Asymmetric division in mouse oocytes: with or without Mos. Curr. Biol. 10, 1303–1306 (doi:10.1016/S0960-9822(00)00753-3) [DOI] [PubMed] [Google Scholar]
- 4.Deng M, Williams CJ, Schultz RM. 2005. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev. Biol. 278, 358–366 (doi:10.1016/j.ydbio.2004.11.013) [DOI] [PubMed] [Google Scholar]
- 5.Deng M, Suraneni P, Schultz RM, Li R. 2007. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev. Cell 12, 301–308 (doi:10.1016/j.devcel.2006.11.008) [DOI] [PubMed] [Google Scholar]
- 6.Van Blerkom J, Bell H. 1986. Regulation of development in the fully grown mouse oocyte: chromosome-mediated temporal and spatial differentiation of the cytoplasm and plasma membrane. J. Embryol. Exp. Morphol. 93, 213–238 [PubMed] [Google Scholar]
- 7.Dehapiot B, Carriere V, Carroll J, Halet G. 2013. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev. Biol. 377, 202–212 (doi:10.1016/j.ydbio.2013.01.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu XJ. 2012. Polar body emission. Cytoskeleton 69, 670–685 (doi:10.1002/cm.21041) [DOI] [PubMed] [Google Scholar]
- 9.Deng M, Li R. 2009. Sperm chromatin-induced ectopic polar body extrusion in mouse eggs after ICSI and delayed egg activation. PLoS ONE 4, e7171 (doi:10.1371/journal.pone.0007171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun QY, Schatten H. 2006. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 131, 193–205 (doi:10.1530/rep.1.00847) [DOI] [PubMed] [Google Scholar]
- 11.Yi K, Li R. 2012. Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation. Cytoskeleton 69, 727–737 (doi:10.1002/cm.21048) [DOI] [PubMed] [Google Scholar]
- 12.Azoury J, Verlhac MH, Dumont J. 2009. Actin filaments: key players in the control of asymmetric divisions in mouse oocytes. Biol. Cell 101, 69–76 (doi:10.1042/BC20080003) [DOI] [PubMed] [Google Scholar]
- 13.Yi K, Rubinstein B, Unruh JR, Guo F, Slaughter BD, Li R. 2013. Sequential actin-based pushing forces drive meiosis I chromosome migration and symmetry breaking in oocytes. J. Cell Biol. 200, 567–576 (doi:10.1083/jcb.201211068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P. 2002. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat. Cell Biol. 4, 921–928 (doi:10.1038/ncb880) [DOI] [PubMed] [Google Scholar]
- 15.Maro B, Verlhac MH. 2002. Polar body formation: new rules for asymmetric divisions. Nat. Cell Biol. 4, E281–E283 (doi:10.1038/ncb1202-e281) [DOI] [PubMed] [Google Scholar]
- 16.Li H, Guo F, Rubinstein B, Li R. 2008. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat. Cell Biol. 10, 1301–1308 (doi:10.1038/ncb1788) [DOI] [PubMed] [Google Scholar]
- 17.Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R. 2011. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat. Cell Biol. 13, 1252–1258 (doi:10.1038/ncb2320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng M, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, Williams CJ. 2003. Chromatin-mediated cortical granule redistribution is responsible for the formation of the cortical granule-free domain in mouse eggs. Dev. Biol. 257, 166–176 (doi:10.1016/S0012-1606(03)00045-9) [DOI] [PubMed] [Google Scholar]
- 19.Kalab P, Heald R. 2008. The RanGTP gradient: a GPS for the mitotic spindle. J. Cell Sci. 121, 1577–1586 (doi:10.1242/jcs.005959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y. 2004. G protein control of microtubule assembly. Annu. Rev. Cell Dev. Biol. 20, 867–894 (doi:10.1146/annurev.cellbio.20.012103.094648) [DOI] [PubMed] [Google Scholar]
- 21.Caudron M, Bunt G, Bastiaens P, Karsenti E. 2005. Spatial coordination of spindle assembly by chromosome-mediated signaling gradients. Science 309, 1373–1376 (doi:10.1126/science.1115964) [DOI] [PubMed] [Google Scholar]
- 22.Dumont J, et al. 2007. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J. Cell Biol. 176, 295–305 (doi:10.1083/jcb.200605199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard TD, Cooper JA. 2009. Actin, a central player in cell shape and movement. Science 326, 1208–1212 (doi:10.1126/science.1175862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun SC, Wang ZB, Xu YN, Lee SE, Cui XS, Kim NH. 2011. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS ONE 6, e18392 (doi:10.1371/journal.pone.0018392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Na J, Zernicka-Goetz M. 2006. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr. Biol. 16, 1249–1254 (doi:10.1016/j.cub.2006.05.023) [DOI] [PubMed] [Google Scholar]
- 26.Halet G, Carroll J. 2007. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev. Cell 12, 309–317 (doi:10.1016/j.devcel.2006.12.010) [DOI] [PubMed] [Google Scholar]
- 27.Dehapiot B, Halet G. 2013. Ran GTPase promotes oocyte polarization by regulating ERM (Ezrin/Radixin/Moesin) inactivation. Cell Cycle 12, 1672–1678 (doi:10.4161/cc.24901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun SC, Xu YN, Li YH, Lee SE, Jin YX, Cui XS, Kim NH. 2011. WAVE2 regulates meiotic spindle stability, peripheral positioning and polar body emission in mouse oocytes. Cell Cycle 10, 1853–1860 (doi:10.4161/cc.10.11.15796) [DOI] [PubMed] [Google Scholar]
- 29.Siller KH, Doe CQ. 2009. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 11, 365–374 (doi:10.1038/ncb0409-365) [DOI] [PubMed] [Google Scholar]
- 30.Dumont J, Million K, Sunderland K, Rassinier P, Lim H, Leader B, Verihac MH. 2007. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev. Biol. 301, 254–265 (doi:10.1016/j.ydbio.2006.08.044) [DOI] [PubMed] [Google Scholar]
- 31.Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. 2008. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 (doi:10.1016/j.cub.2008.08.044) [DOI] [PubMed] [Google Scholar]
- 32.Schuh M, Ellenberg J. 2008. A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992 (doi:10.1016/j.cub.2008.11.022) [DOI] [PubMed] [Google Scholar]
- 33.Zheng P, Baibakov B, Wang XH, Dean J. 2013. PtdIns(3,4,5)P3 is constitutively synthesized and required for spindle translocation during meiosis in mouse oocytes. J. Cell Sci. 126, 715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azoury J, Lee KW, Georget V, Hikal P, Verlhac MH. 2011. Symmetry breaking in mouse oocytes requires transient F-actin meshwork destabilization. Development 138, 2903–2908 (doi:10.1242/dev.060269) [DOI] [PubMed] [Google Scholar]
- 35.Kuo SC, McGrath JL. 2000. Steps and fluctuations of Listeria monocytogenes during actin-based motility. Nature 407, 1026–1029 (doi:10.1038/35039544) [DOI] [PubMed] [Google Scholar]
- 36.Bernheim-Groswasser A, Wiesner S, Golsteyn RM, Carlier MF, Sykes C. 2002. The dynamics of actin-based motility depend on surface parameters. Nature 417, 308–311 (doi:10.1038/417308a) [DOI] [PubMed] [Google Scholar]