Abstract
Since its discovery in 1989 as a substrate of the Src oncogene, p120catenin has been revealed as an important player in cancer initiation and tumour dissemination. p120catenin regulates a wide range of cellular processes such as cell–cell adhesion, cell polarity and cell proliferation and plays a pivotal role in morphogenesis, inflammation and innate immunity. The pleiotropic effects of p120catenin rely on its interactions with numerous partners such as classical cadherins at the plasma membrane, Rho-GTPases and microtubules in the cytosol and transcriptional modulators in the nucleus. Alterations of p120catenin in cancer not only concern its expression level but also its intracellular localization and can lead to both pro-invasive and anti-invasive effects. This review focuses on the p120catenin-mediated pathways involved in cell migration and invasion and discusses the potential consequences of major cancer-related p120catenin alterations with respect to tumour spread.
Keywords: cadherin, catenin, adherens junctions, migration, invasion, rho-GTPases
1. Introduction
Discovered in 1989 as a novel substrate for oncogenic membrane-associated Src [1], mouse p120catenin was named after its 120 kDa molecular weight and its further identification as a major cadherin partner in 1994 [2]. Like all catenins (from catena, Latin for chain), it is a core protein of adherens junctions that links cadherins to the underlying actin cytoskeleton. The name ‘catenin’ is perfectly suited as it reflects the dual function of this protein: bridging the transmembrane protein cadherin to the internal cytoskeleton and mediating cadherin intracellular signalling. Over the years, the spectrum of p120catenin functions has extended beyond its role in adherens junction stability to the remodelling of the cytoskeleton and to genetic regulation (see [3–5] for reviews).
In mammals, p120catenin is the prototypic member of a larger protein family which includes the closely related armadillo repeat proteins δ-catenin/neural plakophilin-related armadillo repeat protein (NPRAP), armadillo repeat protein deleted in velo-cardio-facial syndrome (ARVCF) and p0071 [6]. p120catenin is composed of four distinct functional regions, including a coiled-coil domain, a regulatory or phosphorylation domain, an armadillo domain containing nine armadillo repeats and a short C-terminal tail (figure 1a). p120catenin interacts with its partners mainly via its central armadillo domain (e.g. for cadherins, RhoA, Kaiso) and its regulatory domain (e.g. for Fer, kinesin, PLEKHA7, RhoA), even though a catenin-RhoGAP-association-domain (CRAD) has been identified recently in the C-terminus part (figure 2). Although all p120catenin family members share the same mode of interaction with the membrane proximal part of classical cadherins, their functions are not fully redundant. p120catenin deletion in amphibians generates severe embryological alterations and p120catenin knockout (KO) mice die during early embryogenesis despite the presence of ARVCF, δ-catenin and p0071 [7]. By contrast, both ARVCF and δ-catenin KO mice are viable and show no obvious morphological defect, except for the severe cognitive dysfunctions observed in δ-catenin KO mice [8,9]. In vivo ablation of p120catenin in multiple organs has further demonstrated the vital role of p120catenin in morphogenesis, although its precise function appears tissue-dependent (see [10,11] and [9,12] for extensive reviews).
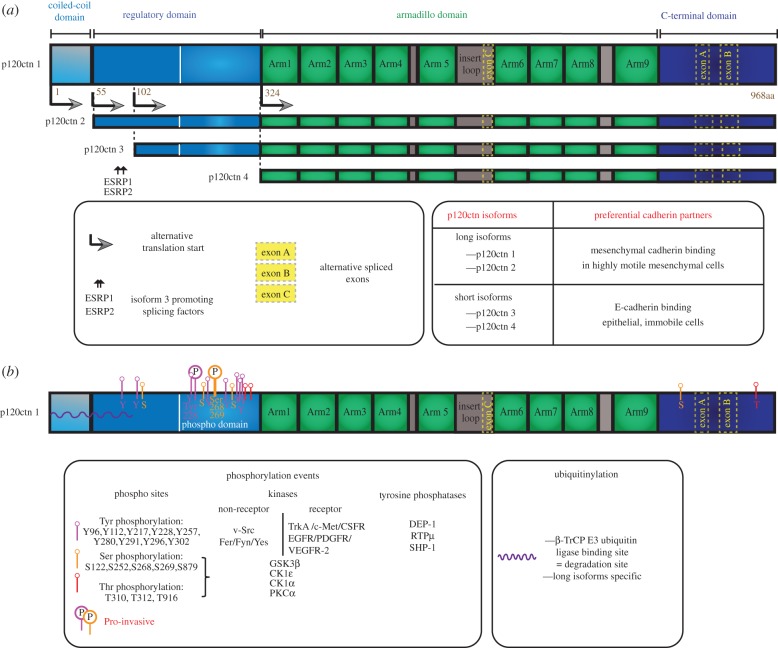
Figure 1.
The molecular diversity of p120catenin: alternative translational isoforms and post-translational modifications. (a) Molecular structure and alternative translational isoforms formation. The four p120catenin isoforms emanate from alternative initiation start codons (the arrows indicate the beginning of the translated region, the number indicates the position on the isoform 1 of the first amino acid of each isoform). The longest isoform (p120catenin1) contains the four domains indicated at the top (coiled-coil domain, regulatory domain, armadillo domain, carboxy-terminal domain). The shortest isoform (p120catenin4) contains only the armadillo and the C-ter domain. (b) Post-translational modifications including phosphorylation events and ubiquitinylation. The position of each modification is shown in the middle panel. Most post-translational modifications take place on the regulatory domain. The phosphorylations that have been shown to promote tumour invasion (pro-invasive) are indicated with a circled ‘P’.
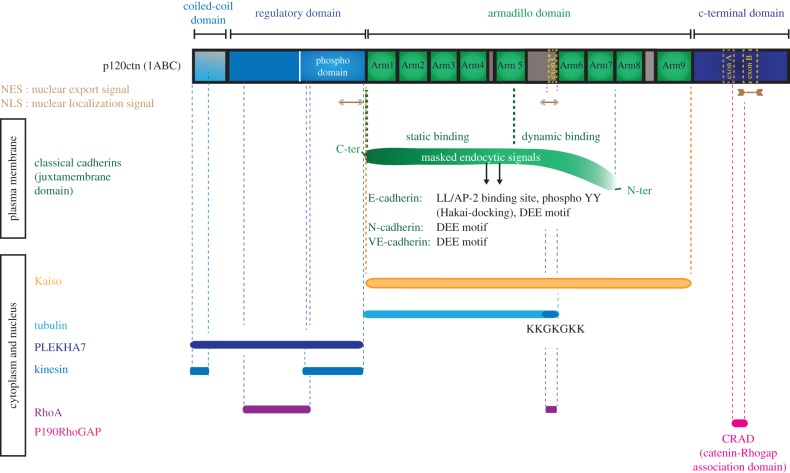
Figure 2.
The main binding partners of p120catenin and their sites of interaction. The main binding partners of p120catenin are listed. A bar of the corresponding colour is positioned above their known binding site on the p120catenin protein. The association of classical cadherins with p120catenin both occurs through a static and a less potent, dynamic binding (indicated by a curve), and is essential to prevent their endocytosis because it masks various endocytic signals (dileucin (LL), di-phosphotyrosines (YpYp) or DEE motif) depending on the classical cadherins. Note the ability of p120catenin to directly bind RhoA and to interact with its GAP, p190RhoGAP. p120catenin could modulate the microtubule cytoskeleton through its direct interaction with tubulin, the heavy chain of the molecular motor kinesin, and PLEKHA7, a protein associated with the microtubule minus-end molecule NEZHA.
In addition to its physiological functions, p120catenin participates in tumour progression. The precise analysis of p120catenin KO mice revealed the presence of a number of cancer hallmarks. Ablation of p120catenin in the salivary gland resulted in morphological defects typically found in high-grade intraepithelial neoplasia, a precancerous stage in humans leading to invasive cancer [13]. Conditional ablation of p120catenin in skin epidermis caused a lethal hyperproliferative disorder and p120catenin-deficient skin grafts revealed early mitotic defects and signs of hyperkeratosis and dysplasia [14,15]. p120catenin deletion alone, with no other genetic alteration, in the oral cavity, oesophagus and squamous forestomach generated alterations in proliferation, differentiation, desmoplasia and neoplastic lesions typically progressing in invasive squamous cell cancer [11]. This study revealed, for the first time, a potent tumour suppressive function for p120catenin in mammals.
The crucial role of p120catenin is also apparent in human tumours where its alterations are associated with poor survival rates [16]. Loss, downregulation or mislocalization of p120catenin is observed in most human cancers [16,17] (figure 3). For example, p120catenin is absent in 48% of skin cancer [18] and 10% of bladder cancer compiled from 228 cases [19–21] and downregulated in 86% of colorectal cancers [16]. Because p120catenin expression is often monoallelic, the mutation of a single allele is sufficient to promote a massive downregulation of the protein [22]. However, to date, CTNND1 (p120catenin gene) mutations have only been reported in breast and colon cancers [23,24], indicating that transcriptional misregulation, epigenetic alterations or miRNA-induced silencing, may also be involved in p120catenin downregulation. In non-small cell lung cancer, p120catenin expression is reduced upon upregulation of the transcription factor FOXC2 [25]. p120catenin also bears an evolutionally conserved seed sequence for miR-197, which is involved in the decrease of p120catenin expression in highly aggressive invasive ductal pancreatic adenocarcinoma [26]. The level of the p120catenin protein may also be controlled by protein degradation. p120catenin degradation by calpain-1 has been observed in response to stress conditions in neuroblastoma, endothelial and epithelial cells [27–29]. Moreover, p120catenin levels may be regulated, such as βcatenin, by Wnt signalling, a known proto-oncogenic pathway. p120catenin interaction with GSK-3β [30] and casein kinase-1α has been shown to promote phosphorylation-dependent ubiquitinylation and degradation of p120catenin [26] (figure 1b). However, in colon cancer cells that bear an APC (adenomatous polyposis coli) mutation, p120catenin level does not always parallel those of βcatenin, suggesting that other pathways may counteract Wnt signalling in these tumours [31].
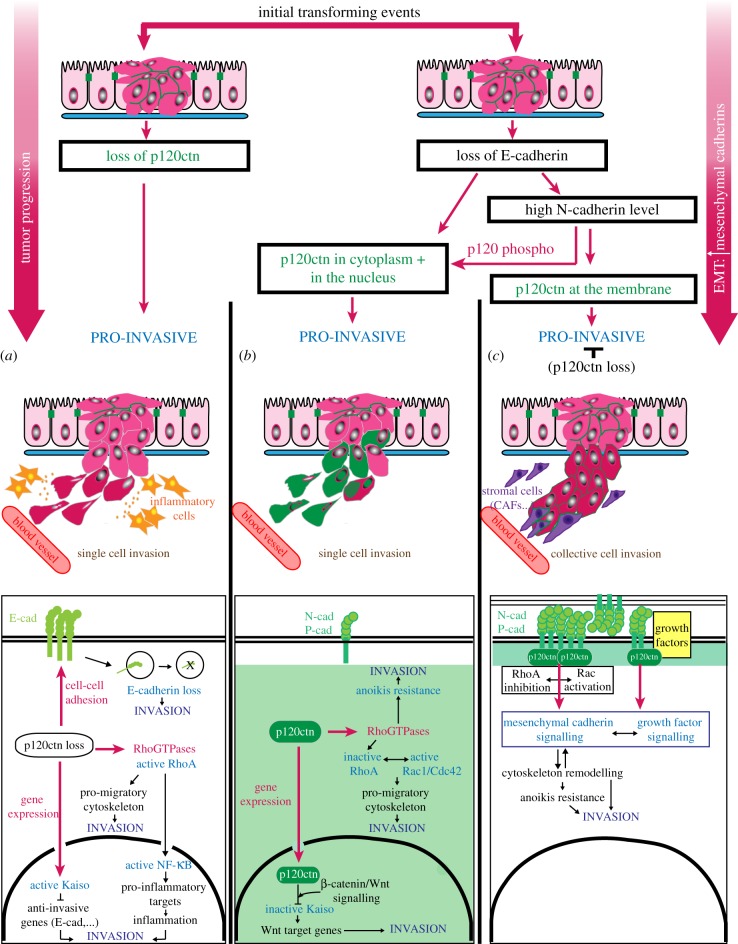
Figure 3.
p120catenin alterations in cancer and their impact on tumour invasion. After the initial transforming events ending up with unpolarized epithelial cells and a relatively organized tumour mass (dark pink cells), the loss or mislocalization of p120catenin promotes tumour invasion (red, green cells) through different pathways depending on the precise timing and the nature of the alteration. (a) If the loss of p120catenin occurs before the loss of E-cadherin, then it promotes invasion by enhancing single cell invasion as a result of (i) E-cadherin internalization and degradation, (ii) misregulation of Rho-GTPases that activates NF-κB to trigger pro-invasive inflammation and remodel the cytoskeleton, and (iii) the interaction of p120catenin with Kaiso and the repression of Kaiso tumour suppressive/anti-invasive target genes. (b) If E-cadherin is altered before p120catenin, two possibilities exist depending first on the advance of the epithelial-to-mesenchymal transition (EMT) and the amount of mesenchymal cadherin produced, and second on the context-dependent phosphorylation of p120catenin. If the level of mesenchymal cadherin is insufficient or if p120catenin is phosphorylated (p120 phospho) on the tyrosine sites highlighted in figure 1, p120catenin relocalizes away from the plasma membrane to the cytoplasm and in rare cases to the nucleus (the green colour indicates the probable localization of p120catenin). As in (a), once in the cytosol, p120catenin can induce migration by regulating the cytoskeleton and participating in anoikis resistance. In the nucleus, and in the presence of active Wnt/beta-catenin signalling, p120catenin interacts with Kaiso and inhibits its repressive role on Wnt target genes expression. As a result it promotes the pro-invasive Wnt signalling. (c) If p120catenin is not phosphorylated and its interaction with the mesenchymal cadherin stabilized, p120catenin can remain at the plasma membrane. Therefore, p120catenin sustains mesenchymal cadherin signalling by regulating Rho-GTPases and mediating growth factor signalling. The functions of p120catenin at the plasma membrane may then favour collective cancer cell invasion by stabilizing mesenchymal cadherin-mediated contacts with stromal cells such as cancer-associated fibroblasts (CAFs, shown in blue). Note that these three different situations (a–c) can happen simultaneously within a tumour with only patches of p120catenin null or cytoplasmic p120catenin cells.
Not only the expression level of p120catenin but also its localization are frequently altered in human tumours (figure 3). Translocation of p120catenin to the cytoplasm and sometimes to the nucleus occurs in a vast proportion of breast cancers, including 90% of invasive lobular carcinomas [32,33]. However, other metastatic breast cancers have been shown to express high levels of E-cadherin and junctional p120catenin [34–37]. These conflicting observations highlight the complexity of p120catenin functions in cancer. In fact, p120catenin appears to have both pro-tumourigenic and anti-tumourigenic effects, depending on the localization and the specific function of p120catenin in each cell compartment. While the anti-invasive function of p120catenin mainly relies on its ability to interact with cadherin at the plasma membrane and to maintain adherens junctions and contact inhibition of locomotion, cytosolic p120catenin influences the cytoskeletal machinery involved in cell migration and nuclear p120catenin indirectly promotes tumour invasion and metastasis by modulating gene expression (figure 3).
2. Membrane-associated p120catenin and the regulation of adherens junctions
In the 1940s, work from Coman [38] showed that tumour cells are less adherent than non-tumour cells. Since then, accumulated evidence demonstrated the essential role of cadherin-mediated cell–cell adhesion in preventing tumour invasion. In the vast majority of epithelial cancers, E-cadherin is altered and associated with pro-invasive behaviour and poor prognosis [39,40]. Early studies revealed that E-cadherin alteration could turn normal epithelial cells into invasive cells and accelerate tumour progression towards a pro-invasive and metastatic behaviour [41,42]. More recently, conditional depletion of E-cadherin in the mammary epithelium of a p53-deleted mouse increased tumour initiation and its invasive and metastatic progression [43]. In non-epithelial cancers such as highly invasive glioma, or glioblastoma, loss of the tissue-of-origin cadherin increases migration speed, alters directionality, and is eventually associated with tumour invasion [44–46]. As a crucial stabilizer of cadherin-mediated cell–cell contacts, the presence of p120catenin at adherens junctions is a key parameter controlling cell dispersion and invasion (figure 3a).
(a). p120catenin controls cadherin endocytosis, recycling and stability
An extensive literature exists on the regulation of cadherin turnover by p120catenin (for a review, see [47]). Early studies by the Reynolds laboratory showed that p120catenin interacts with classical cadherins and this mutual interaction is crucial to maintain the two proteins at the plasma membrane. On the one hand, cadherin binding allows p120catenin recruitment at the membrane as shown by the translocation of p120catenin from the cytoplasm to the plasma membrane upon E-cadherin re-expression in E-cadherin deficient cells [48]. On the other hand, in most tissues, the absence or downregulation of p120catenin leads to a decreased level of classical cadherins and consequently to severe alterations of adherens junctions and loss of compact cell aggregation [49]. p120catenin, but not a p120catenin mutant unable to bind cadherins, was sufficient to restore normal cadherin level by increasing its half-life [23], showing the importance of a direct interaction between p120catenin and cadherins.
The regulation of cadherin stability by p120catenin is due to its precise binding to the juxtamembrane domain (JMD) of the cadherin cytoplasmic tail (figure 2). Mutation of the JMD preventing p120catenin binding considerably increases the rate of cadherin endocytosis [50,51]. These studies led to the hypothesis that some residues lying within the JMD could associate either with p120catenin or with other protein partners known to trigger cadherin internalization, such as the endocytic adaptor AP-2 [50,52]. Recent crystallographic studies of the cadherin-p120catenin complex confirmed this hypothesis by mapping the binding sites between p120catenin and E-cadherin [53] or VE-cadherin [54]. An endocytic dileucine motif, potentially targeted by AP-2 [55], lies within the dynamic binding site of E-cadherin. In addition, two tyrosine phosphorylation sites recognized by the E3 ubiquitin ligase Hakai [56] fall within the core JMD domain of E-cadherin (figure 2). In the absence of p120catenin, this interaction promotes the proteasome-dependent degradation of cadherin [56]. However, in some cell types, such as astrocytes [57] and neurons [58], the expression level of cadherin is not so much impaired by p120catenin downregulation. This may reflect the fact that the two internalization motifs are absent in N-cadherin. Nevertheless, p120catenin depletion has a crucial impact on synaptogenesis [58] suggesting that, in these cells, p120catenin can control cadherin dynamics rather than cadherin expression. In fact, p120catenin may also contribute to cadherin turnover by masking another endocytic DEE signal conserved in the three most studied classical cadherins, E-cadherin, N-cadherin and VE-cadherin [54].
p120catenin also acts indirectly to modulate cadherin endocytosis. By directly binding to and recruiting the plasma membrane p190RhoGAP [59], a RhoA inhibitor, or ROCK1 [60], a Rho effector, p120catenin orchestrates Rho-GTPase signalling at adherens junctions to fine tune cadherin turnover [4] (figures 2 and 4). This indirect effect of p120catenin could be cell-type specific as the expression of a p120catenin mutant unable to regulate RhoA in endothelial cells has no effect on VE-cadherin endocytosis [52]. Finally, cadherin-bound p120catenin associates with FERM-domain containing proteins (EPB4125 and FRMD5) that modulate cadherin stability at the plasma membrane [61,62].
Figure 4.
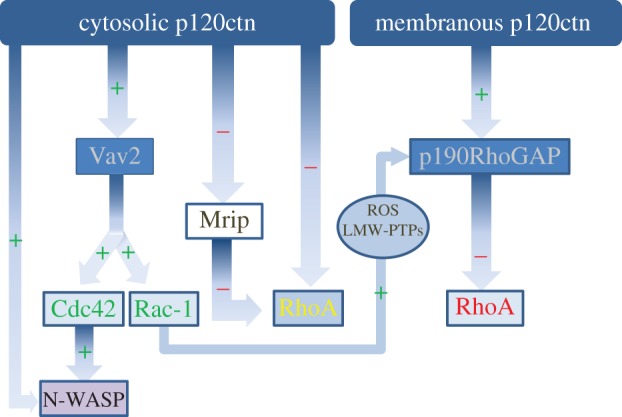
p120catenin regulation of Rho-GTPase signalling. Depending on its localization, p120catenin shows distinct modes of regulation of Rho-GTPases. Cytosolic p120catenin regulates Rho-GTPases by direct association with RhoA. p120catenin functions as a RhoGDI, inhibiting RhoA by sequestering it in its inactive form (RhoA-GDP). Membrane-associated p120catenin inhibits RhoA by interacting with p190RhoGAP, a protein which increases RhoA GTPase activity. In parallel, cytosolic p120catenin activates Cdc42 and Rac1 by associating with the exchange factor Vav2, which promotes the GTP loading on these two GTPases. Moreover, p120catenin can indirectly decrease RhoA activity via Rac1. GTP-bound Rac1 activates p190RhoGAP via a pathway involving reactive oxygen species (ROS) and low molecular weight protein tyrosine phosphatases (LMW-PTPs). Moreover, p120catenin regulates Rho-GTPase signalling by interacting with downstream targets. For instance, cytosolic p120catenin interacts with N-WASP to enhance Cdc42-dependent actin polymerization. It also inhibit myosin phosphatase rho-interacting protein (Mrip), an antagonist of Rho/ROCK signal.
Furthermore, p120catenin influences cadherin delivery to the plasma membrane. First, p120catenin plays a role during cadherin biogenesis of certain types of cadherins by interacting with PTP1B and the cadherin precursor in the ER and favouring its translocation to the Golgi apparatus [63], although this mechanism does not seem to apply to E-cadherin [49]. Second, p120catenin interacts with conventional kinesins (figure 2), which promote p120catenin recruitment to the plasma membrane and prevent its accumulation in the nucleus [64]. Via its interaction with kinesins, p120catenin may support cadherin transport along microtubules to the site of adherens junction assembly [65]. Nevertheless, p120catenin can also reach the plasma membrane, through microtubule transport, independently of cadherin [66]. This suggests that the cadherin–β-catenin complex and p120catenin can be independently recruited to the plasma membrane where they interact to stabilize the cadherin–catenin complex. Once at the plasma membrane, p120catenin mediates the interaction of adherens junctions with microtubule minus-ends by binding PLEKHA7 which itself binds the minus-end protein NEZHA [67]. This connection may facilitate the rapid polarized transport of additional cadherin to strengthen adherens junctions.
(b). The p120catenin-dependent control of adherens junctions can have opposite effects on tumour invasion
In most epithelial cancers (lung, prostate, invasive breast adenocarcinomas, colorectal), p120catenin deletion facilitates tumour dissemination and metastasis formation [68,69] and is correlated with a poor outcome and metastasis in patients [70,71], revealing the anti-invasive function of p120catenin (figure 2a). p120catenin downregulation in cancer cells is often associated with the loss of E-cadherin. The function of p120catenin in the regulation of cadherin levels suggests that loss of p120catenin may be responsible for E-cadherin downregulation during tumour progression, but direct evidence is still lacking [11]. Conversely, membrane-associated p120catenin restrains the invasion of tumour cell lines derived from breast, kidney and vulva cancers [72,73]. Another scenario is at play in highly lethal forms of breast tumours, in which membrane-associated p120catenin appears to be pro-invasive (figure 3c). In this case, the stabilization of E-cadherin mediated by p120catenin and its progressive overexpression are essential to support the progression of a highly lethal form of breast tumour [37]. This may reflect the necessity of intercellular adhesions and cadherin signalling during migration through the surrounding microenvironment [72], supporting the idea that cancer cells migrate collectively [74]. Alternatively, the scaffolding function of p120catenin may be required to control signalling cascades important for cell migration (figure 3c). For instance, in ErbB2 overexpressing breast cancers, p120catenin was shown to act as an obligate intermediate between ErbB2 and Rac1/Cdc42 to enhance the metastatic potential of breast cancer cells [75].
As epithelial cancers progress, the decrease of E-cadherin expression is frequently associated with a cadherin switch, resulting in the concomitant increase in the expression of N-cadherin and other mesenchymal cadherins (P-cadherin, cadherin-11) [76]. N-cadherin expression has been shown to promote the invasive potential of carcinoma cells by modulating growth factor signalling and promoting the pro-migratory interaction of cancer cells with the surrounding stromal cells [77]. The pro-invasive function of p120catenin could then be explained by its ability to sustain the expression of mesenchymal cadherins (figure 3c). Indeed, in some cellular contexts, stabilizing the highly labile P/N-cadherin-p120catenin interaction could favour tumour cell invasion because of the pro-invasive functions of P/N-cadherin, and the regulation of a pro-migratory Rho-GTPase signalling [73,78].
3. Cytosolic p120catenin, Rho-GTPases and cytoskeleton rearrangements
During carcinoma progression, cancer cells of epithelial origin undergo an epithelial-to-mesenchymal transition (EMT) during which they acquire invasive properties. EMT is associated with remarkable changes in cadherin expression and frequently correlates with p120catenin accumulation in the cytosol (figure 3b). Cytosolic p120catenin is found in highly motile mesoderm cells lacking E-cadherin expression during mouse early embryogenesis [33]. Similarly, cytosolic p120catenin is generally considered as an indicator of invasive tumours (figure 3b). Its accumulation has been reported in late-stage tumours and associated with metastatic progression and reduced survival in cohorts of pancreatic, lung and colorectal cancers, suggesting a pro-tumourigenic and pro-invasive role of cytosolic p120catenin [16]. In agreement with this idea, downregulation of p120catenin is both necessary and sufficient to prevent the invasion of E-cadherin-deficient breast cancer cells [73].
(a). The mechanisms of p120catenin relocalization to the cytosol
Cadherins are necessary and sufficient to maintain p120catenin at the plasma membrane [48]. Thus, the pool of cytoplasmic p120catenin has been proposed to serve as a rheostat to measure the ‘density’ of cells in subconfluent cells [79]. In absence of cell–cell contacts, the localization of p120catenin in the cytosol would activate cell migration, increasing the chance of forming new cellular interactions. Upon contact, cells form adherens junctions. Cadherin then recruits p120catenin at the plasma membrane and limits migration. In cancer cells, loss of E-cadherin during EMT leads to the cytoplasmic translocation (without downregulation) of p120catenin [32,33,48,80] (figure 3b). The cadherin switch, together with the expression of mesenchymal cadherins, should, in principle, prevent p120catenin cytosolic accumulation. Nevertheless, p120catenin accumulates in the cytosol of N-cadherin-expressing cancer cells, possibly reflecting the weaker affinity of p120catenin for N-cadherin than for E-cadherin [81].
The N-terminal regulatory domain of p120catenin, which contains most phosphorylation sites, is essential for the control of p120catenin functions [7] (figure 1b). Although the armadillo domain alone is sufficient for cadherin binding and stabilization, changes in the p120catenin N-terminal domain may modulate the affinity of p120catenin for cadherins [49]. Phosphorylation or another post-translational modification of p120catenin may affect cadherin binding and lead to p120catenin accumulation in the cytoplasm. Tyrosine phosphorylated p120catenin was shown to reduce cell–cell adhesion [82,83]. Phosphorylation of p120catenin on tyrosine Y228 was recently shown to be increased in oral cavity neoplastic lesions. The progression of these tumours toward oral squamous carcinoma was associated with the cytoplasmic relocalization of phosphorylated p120catenin, suggesting that Y228-phosphorylated p120catenin could serve as a marker for high risk of aggressive progression in malignant oral lesions [84]. Similarly, Y228 phosphorylation is also associated with the increased invasiveness of glioblastomas [85]. In addition to Src [86], other tyrosine kinases (Yes, Fer and Fyn) [87] and tyrosine phosphatases (RTPµ, DEP-1 and SHP) [88,89] can modulate p120catenin phosphorylation on multiple tyrosine residues. Serine and threonine residues can be phosphorylated by PKC and GSK3 kinases [30,90]. Phosphorylation events influence interactions between p120catenin and its partners, and directly impact on p120catenin functions.
Changes in p120catenin isoforms might also influence p120catenin association with the plasma membrane. Multiple p120catenin isoforms are produced from a single gene (CTNND1) as a result of four different start codons (isoforms 1–4), and three alternatively spliced exons (A, B and C; figure 1a). Even though 32 possible different isoforms have been identified [91], the most commonly found versions of p120catenin are the long isoform 1 and the short isoform 3 which lacks the coiled-coil domain and a portion of the regulatory domain. The presence of distinct isoforms is dependent on the cell type and on the cellular context. Whereas p120catenin short isoforms (3 and 4) are predominant in epithelial and immobile cells, approximately 120 kDa long isoforms (1 and 2) are more abundant in highly motile mesenchymal-like cells [92–94]. EMT is frequently associated with an upregulation of isoform 1 and a downregulation of isoform 3 [95–97]. This isoform switch has been observed in Src-transformed MDCK cells [92] and in advanced prostate [97], thyroid [96], pancreatic [98] and lung cancers [99]. The mechanism allowing the downregulation of the short isoforms involves the downregulation of epithelial splicing regulatory proteins 1 and 2 (ESRP1/2) that favour translation from the third start codon of p120catenin [100]. Known EMT-inducing transcriptional factors such as Snail or Slug can promote these changes [101]. Some reports suggest that cadherins may preferentially interact with a specific p120catenin isoform. In particular, in pancreatic cancer cells, E-cadherin binds the short isoform 3, whereas N-cadherin associates with the long hyperphosphorylated isoform 1 of p120catenin [98]. However, re-expression of E-cadherin after Snail-induced EMT of mesenchymal cells does not rescue the epithelial morphology nor the expression of the epithelial isoform 3 of p120catenin, indicating that the expression of different p120catenin isoforms is mainly due to the expression of specific splice factors rather than to the expression of specific cadherins [101].
(b). Cytosolic p120catenin interacts with and modulates Rho-GTPases
Once in the cytosol, the pro-invasive role of p120catenin is thought to be essentially caused by p120catenin interaction with Rho-GTPase signals (figures 3b and 4), which are essential in cell migration, tumour cell invasion, angiogenesis and anchorage independent growth (for reviews, see [102,103]). This regulation could be an innovation of vertebrate p120catenin as the critical region for Rho-GTPases regulation is missing in Drosophila and the worm Caenorhabditis elegans.
p120catenin inhibits RhoA in vitro by directly interacting and stabilizing an inactive GDP-RhoA (figure 2). p120catenin can also activate Cdc42 and Rac1 through its interaction with their guanine nucleotide exchange factor, Vav2 [79,104,105] (figure 4). Overexpression studies revealed a putative role for p120catenin in orchestrating Rho-GTPases-dependent signalling pathways independently of its function at adherens junctions. More recently, conflicting in vitro and in vivo observations suggested that the role of p120catenin in the regulation of Rho-GTPases may be tissue- and context-dependent [106]. In vivo data in the mouse epidermis [14] and forebrain [58] confirmed the role of p120catenin in RhoA inhibition. This regulatory process can occur in the cytosol through a direct interaction between RhoA and the amino-terminal domain and a central polybasic region of p120catenin [107,108] (figure 2). p120catenin also inhibits RhoA indirectly by interacting with p190RhoGAP (a RhoA-GAP) via its carboxy-terminal-region [109], but this interaction seems to occur only when p120catenin is at the plasma membrane [59]. Alternatively, cytosolic p120catenin may activate Rho by inhibiting the antagonist of Rho/ROCK signalling Myosin phosphatase Rho-interacting protein (Mrip), in an invasive lobular carcinoma mouse model [110] (figure 4). Activation of Rho and ROCK signalling by cytosolic p120catenin can influence cell migration and invasion, and promote anchorage independent survival of E-cadherin-deficient cancer cells [110]. p120catenin can also act downstream of Src and Rac1 to suppress the RhoA-ROCK signalling pathway, highlighting the role of p120catenin in the crucial interplay between Rac and Rho signalling. Thereby, p120catenin mediates Rac-induced anchorage dependent growth/AIG in MDCK cells [111], suggesting that p120catenin may also favour tumour metastasis by promoting anoikis resistance in cells [112].
For Rac and Cdc42 too, the role of p120catenin may vary with the cellular context. p120catenin depletion has no impact on Cdc42 and Rac1 in the mouse epidermis [14], but it is associated with a decrease in Rac1 activity in the mouse forebrain [58], in hormone-dependent ovarian cancer [113] and in cancer cell lines devoid of E-cadherin [114]. Upon GnRH treatment, ovarian cancer cells undergo an E- to P-cadherin switch that promotes p120catenin relocalization to the cytosol and a subsequent increased Rac1 and Cdc42 activity, leading to increased cell migration and invasion in vitro [113]. p120catenin promotes motility of breast cancer cells by inhibiting RhoA and increasing Rac activity [33,73] and p120catenin depletion prevents increased levels of Rac1-GTP in SKBR-3 cells [33]. In the colon cancer cell line SW-40, Wnt3a triggers the dissociation of p120catenin from E-cadherin and its subsequent cytosolic accumulation that mediates Rac1 activation, by a direct interaction and binding to its exchange factor Vav2 [115].
These different and sometimes opposite effects of p120catenin on Rho-GTPases signalling may reflect the specific capacity of the various p120catenin isoforms to regulate Rho-GTPases. p120catenin isoform 1, but not the short p120catenin isoform 4, promotes invasiveness, both in vitro and in vivo, and is associated with renal cancer micrometastasis [108]. This may reflect the inability of p120catenin isoform 4 to bind and to inhibit RhoA (figure 1a). Finally, p120catenin can also interact with downstream effectors of Rho-GTPases, such as N-WASP [116] or cortactin [117], two activators of Arp2/3-dependent actin polymerization. p120catenin interaction with cortactin at the cell leading edge promotes the local accumulation of Rac, cell protrusion formation and migration [117].
(c). p120catenin may regulate the microtubule cytoskeleton
The interaction of p120catenin with the microtubule network may strongly influence Rho-GTPase activity and cell migration. In adherens junctions, p120catenin binds to the heavy chain of microtubule-associated motors kinesins [64,65] to facilitate cadherin trafficking. p120catenin interaction with kinesins suggests that p120catenin may control the distribution of its protein partners (e.g. N-WASP, cortactin…) to specific cell spots to promote localized cell protrusion. Cytosolic p120catenin localizes with microtubules of interphasic cells [118,119]. Direct interaction with tubulin is thought to involve the polybasic microtubule-binding motif (KKGKGKK) contained in the armadillo domain of p120catenin (figure 2) [120]. As this basic motif is also essential for p120catenin-dependent RhoA inhibition [104], the association of p120catenin with microtubules may prevent p120catenin-induced RhoA inhibition, and consequently decrease Rac1 activity. Moreover, the interaction of p120catenin with microtubules is likely to directly contribute to the cell protrusive activity. Cytosolic p120catenin stabilizes the microtubule network [118], as indicated by the increased level of tubulin acetylation [120]. This microtubule-stabilizing activity may involve p120catenin direct interaction with microtubules or its impact on Rho-GTPases activity. Microtubule-associated p120catenin would release and thus activate RhoA, leading to increased microtubule stabilization via RhoA-dependent mDia signalling [121,122]. The localization of p120catenin at the leading ledge of migrating cells (F. Peglion et al. 2013, unpublished data; [117]) may locally promote tubulin acetylation and microtubule stabilization at the protrusions of migrating cells such as fibroblasts, astrocytes or neurons [123]. Changes in cytosolic p120catenin level in cancer may contribute to the alteration of microtubule stability observed in protrusive breast cancer cells [120].
4. Nuclear p120catenin and pro-invasive gene transcription
Although p120catenin is often described in the cytosolic compartment, it is rarely found in the nucleus of tumour cells. However, nuclear p120catenin has been observed in invasive epithelial cancers such as pancreatic tumours [124], skin carcinoma [18] and invasive lobular breast carcinoma [32]. In these cases, p120catenin is likely to profoundly affect gene transcription and tumour spreading (figure 3a,b).
(a). p120catenin translocation to the nucleus
p120catenin bears two putative nuclear localization signals and one nuclear export sequence (figure 2), which allow its transport in and out of the nucleus [125]. However, the nucleoplasmic properties of p120catenin could also be conferred by its Armadillo repeats [119]. The signals that trigger p120catenin import to and export from the nucleus are still largely unknown. The interaction of p120catenin with the microtubule cytoskeleton is likely to impact on p120catenin translocation to the nucleus [119]. Destabilizing the microtubule network enhances nuclear p120catenin, whereas stabilized microtubules, by sequestering more p120catenin in the cytoplasm, prevent nuclear accumulation. As p120catenin promotes microtubule stabilization at adherens junctions via NEZHA and PLEKHA7 [67,126], altered cell–cell adhesion could induce the destabilization of microtubules and an increase in p120catenin translocation to the nucleus. Alternatively, p120catenin could also dock on microtubules either directly or through its interaction with kinesins [64], following a mechanism similar to p53 [127] or smad [128], and could then be transported to the nucleus in response to signalling events [64]. In agreement with this hypothesis, a study using a p120catenin mutant unable to interact with microtubules prevented its localization to the nucleus [120]. These apparently contradictory results reveal that the precise functional link between microtubule binding and nuclear translocation of cytoplasmic p120catenin needs to be further investigated. The transmembrane protein Mucin 1 interacts with p120catenin and β-catenin and has been shown to control both p120catenin and β-catenin nuclear import [129,130]. Mucin-1 overexpression is observed in a number of human carcinomas and correlates with an increase in nuclear catenins. As Mucin-1 is implicated in the alteration of E-cadherin-mediated cell–cell adhesion [131], it is tempting to speculate that increased Mucin-1 expression in carcinomas destabilizes cadherin-mediated junctions by interacting with p120catenin and subsequently promotes the translocation of p120catenin to the nucleus.
(b). p120catenin interacts with Kaiso to control gene transcription
Although β-catenin activates gene expression by interacting with the Lef/TCF family of transcription factors [132], nuclear p120catenin regulates gene transcription by binding to Kaiso, a transcriptional repressor (figure 2). Kaiso is a novel member of the broad complex, Tramtrak, Bric à brac/Pox virus and zinc finger (BTB/POZ)-zinc finger family of transcription factors [133]. Kaiso functions as a transcriptional repressor which recognizes either a sequence-specific DNA consensus site (Kaiso binding site, KBS) or methylated CpG dinucleotide islands [134,135]. By interacting with Kaiso at a specific site encompassing its zinc finger domain, p120catenin inhibits Kaiso functions, thus activating its downstream target genes such as the cancer-related genes Rb, Xist, S100A4 (mts-1) and CDH1 (E-cadherin), metastasis-associated gene 2 (MTA2) and Wnt-11 [135,136].
The interaction of p120catenin with Kaiso has a strong impact on gene transcription and cell transformation, but its role in cancer invasion remains unclear (figure 3a,b). Kaiso has been shown to promote prostate cancer aggressiveness and cell invasion [137]. By inhibiting Kaiso activity, nuclear p120catenin activates the transcription of a number of potent tumour suppressors, including E-cadherin [42], Xist [138], Rb [139] and mts-1 [140] and could therefore act as a tumour suppressor (figure 3a). On the other hand, the interaction between p120catenin and Kaiso induces the canonical Wnt signalling pathway [141,142], which has been shown to promote tumour cell infiltration and metastasis [143,144], notably through the upregulation of the matrix-degrading enzyme and metastasis-promoting gene MMP-7 (matrysilin; figure 3b) [145]. Interestingly, functional regulator of dishevelled in ontogenesis (Frodo), a downstream target of Wnt/Dsh signalling, associates with p120catenin and reinforces p120catenin-mediated inhibition of Kaiso and thus further increases the expression of Wnt/β-catenin target genes [142]. Another positive feedback loop involves the phosphorylation of p120catenin by CK1ɛ in response to Wnt3a signalling. Phosphorylation on S268 and S269 destabilizes p120catenin–cadherin interactions and leads to p120catenin accumulation in the nucleus. Consequently, it represses Kaiso activity to activate the pro-tumourigenic and pro-invasive Wnt signalling pathway [146,147].
5. p120catenin, inflammation and the pro-invasive tumour microenvironment
Following Virchow's claim more than 150 years ago that chronic inflammation contributes to tumourigenesis, accumulated clinical and experimental data have closely linked inflammation and tumour progression and contributed to describe cancer as a ‘wound that never heals’ [148]. Non-regulated inflammation drives infiltrative tumour progression, through the recruitment and the abnormal activation of neutrophils and phagocytic cells [149]. These immune cells not only contribute to DNA damage and genomic instability through the generation of reactive oxygen and nitrogen species [150], but also promote tumour growth, angiogenesis, EMT and metastasis by secreting cytokines, chemokines, growth factors and matrix metalloproteinases (MMPs) [151–153]. During the past decade, p120catenin has emerged as a suppressor of inflammation, leading to the idea that p120catenin could prevent tumour invasion by inhibiting chronic inflammatory responses at the tumour site.
The loss of p120catenin was shown to activate the key inflammatory transcription factor NF-κB [11,14]. The subsequent secretion of GM-CSF, M-CSF, MCP-1 and TNF-α by p120catenin-negative cancer cells leads to the recruitment of immature myeloid cells to the tumour site [11]. Immature myeloid cells generate a pro-invasive niche by activating cancer-associated fibroblasts (CAFs) which, in turn, in a positive feedback loop, prolong the survival of immature myeloid cells [11]. CAFs are critical actors in collective cancer cell invasion. They invade the surrounding tissue and create tracks in the extracellulair matrix (ECM), physically leading the way for invading chains of carcinoma cells [154]. They also secrete pro-invasive soluble factors, further promoting tumour spread [155,156]. Immature myeloid cells are also known to favour tumour invasion by promoting angiogenesis, secretion of chemokines/cytokines and production of ECM-degrading enzymes [157–160]. The pro-inflammatory microenvironment observed in p120-depleted tissues ultimately evokes mitotic alterations [15], hyperplasia [14] and true invasive malignancy [11], depending on the targeted tissue (figure 2a).
In addition to this cell-autonomous role, p120catenin may indirectly control tissue inflammation by maintaining the barrier function of adherens junctions. Loss of p120catenin in the intestine induces cell–cell adhesion defects, leading to barrier dysfunction, mucosal exposure to internal flora and increased inflammatory response through increased recruitment of neutrophils [161]. One can thus speculate that increased inflammation in the context of tumour development could favour tumour spreading and metastasis.
6. Conclusions
The characterization of p120catenin as a tumour suppressor in mammals is supported by much evidence indicating that alterations of p120catenin expression strongly impact on tumour growth and spread. p120catenin association with cadherins and its ability to stabilize adherens junctions [49] are certainly essential for its tumour suppressor functions. However, p120catenin also plays critical roles in the cytosol where it influences the cytoskeleton, and in the nucleus where it can modulate gene transcription. The pleiotropic effects of p120catenin in different cell compartments add a level of complexity when trying to assess how cancer-related alterations modulate p120catenin-mediated functions (figure 3). It is now clear that the analysis of the global changes in p120catenin expression levels is insufficient to predict how p120catenin alterations affect tumour progression. A careful and systematic analysis of the distribution of p120catenin between the different cell compartments is necessary to gain further insights into how p120catenin contributes to cancer. The high molecular variety of p120catenin owing to alternative splicing and multiple translation initiation codons probably contributes to its wide range of activities and to apparent controversies regarding its precise role. Finally, the variability of p120catenin also involves post-translational modifications. Initially found as an oncogenic tyrosine kinase target, we now may have to assume that p120catenin phosphorylation status is crucial for tumour progression [1]. However, the role of phosphorylated p120catenin in cancer cell invasion still needs to be clarified. The in-depth understanding of the involvement of p120catenin during tumour invasion and cancer progression will require precise analysis of p120catenin isoform expression, p120catenin phosphorylation levels and p120catenin intracellular localization during the course of tumour development. A better characterization of the critical alterations promoting p120catenin pro-invasive or anti-invasive functions will contribute to the identification of new therapeutic strategies targeting specific p120catenin functions.
Acknowledgements
We thank Jean-Baptiste Manneville for his critical reading of the manuscript.
Funding statement
F.P. is funded by the Association pour la Recherche contre le Cancer and the Fondation pour la Recherche Medicale. This work was supported by the Institut National du Cancer, the Fondation ARC, and La Ligue contre le Cancer.
References
- 1.Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. 1989. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol. Cell Biol. 9, 629–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. 1994. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell Biol. 14, 8333–8342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourtidis A, Ngok SP, Anastasiadis PZ. 2013. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog. Mol. Biol. Transl. Sci. 116, 409–432 (doi:10.1016/B978-0-12-394311-8.00018-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menke A, Giehl K. 2012. Regulation of adherens junctions by Rho GTPases and p120-catenin. Arch. Biochem. Biophys. 524, 48–55 (doi:10.1016/j.abb.2012.04.019) [DOI] [PubMed] [Google Scholar]
- 5.Pieters T, van Roy F, van Hengel J. 2012. Functions of p120ctn isoforms in cell–cell adhesion and intracellular signaling. Front. Biosci. 17, 1669–1694 (doi:10.2741/4012) [DOI] [PubMed] [Google Scholar]
- 6.Anastasiadis PZ, Reynolds AB. 2000. The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113, 1319–1334 [DOI] [PubMed] [Google Scholar]
- 7.Reynolds AB, Roczniak-Ferguson A. 2004. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 23, 7947–7956 (doi:10.1038/sj.onc.1208161) [DOI] [PubMed] [Google Scholar]
- 8.Israely I, Costa RM, Xie CW, Silva AJ, Kosik KS, Liu X. 2004. Deletion of the neuron-specific protein delta-catenin leads to severe cognitive and synaptic dysfunction. Curr. Biol. 14, 1657–1663 (doi:10.1016/j.cub.2004.08.065) [DOI] [PubMed] [Google Scholar]
- 9.Pieters T, van Hengel J, van Roy F. 2012. Functions of p120ctn in development and disease. Front. Biosci. 17, 760–783 (doi:10.2741/3956) [DOI] [PubMed] [Google Scholar]
- 10.Marciano DK, et al. 2011. p120 catenin is required for normal renal tubulogenesis and glomerulogenesis. Development 138, 2099–2109 (doi:10.1242/dev.056564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stairs DB, et al. 2011. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell 19, 470–483 (doi:10.1016/j.ccr.2011.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrea PD, Park JI. 2007. Developmental functions of the P120-catenin sub-family. Biochim. Biophys. Acta 1773, 17–33 (doi:10.1016/j.bbamcr.2006.06.009) [DOI] [PubMed] [Google Scholar]
- 13.Davis MA, Reynolds AB. 2006. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev. Cell 10, 21–31 (doi:10.1016/j.devcel.2005.12.004) [DOI] [PubMed] [Google Scholar]
- 14.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. 2006. p120-catenin mediates inflammatory responses in the skin. Cell 124, 631–644 (doi:10.1016/j.cell.2005.11.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. 2008. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc. Natl Acad. Sci. USA 105, 15 399–15 404 (doi:10.1073/pnas.0807301105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hengel J, van Roy F. 2007. Diverse functions of p120ctn in tumors. Biochim. Biophys. Acta 1773, 78–88 (doi:10.1016/j.bbamcr.2006.08.033) [DOI] [PubMed] [Google Scholar]
- 17.Thoreson MA, Reynolds AB. 2002. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation 70, 583–589 (doi:10.1046/j.1432-0436.2002.700911.x) [DOI] [PubMed] [Google Scholar]
- 18.Ishizaki Y, Omori Y, Momiyama M, Nishikawa Y, Tokairin T, Manabe M, Enomoto K. 2004. Reduced expression and aberrant localization of p120catenin in human squamous cell carcinoma of the skin. J. Dermatol. Sci. 34, 99–108 (doi:10.1016/j.jdermsci.2003.12.001) [DOI] [PubMed] [Google Scholar]
- 19.Nakopoulou L, Zervas A, Gakiopoulou-Givalou H, Constantinides C, Doumanis G, Davaris P, Dimopoulos C. 2000. Prognostic value of E-cadherin, beta-catenin, p120ctn in patients with transitional cell bladder cancer. Anticancer Res. 20, 4571–4578 [PubMed] [Google Scholar]
- 20.Shimazui T, Schalken JA, Giroldi LA, Jansen CF, Akaza H, Koiso K, Debruyne FM, Bringuier PP. 1996. Prognostic value of cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120cas) in bladder tumors. Cancer Res. 56, 4154–4158 [PubMed] [Google Scholar]
- 21.Syrigos KN, Karayiannakis A, Syrigou EI, Harrington K, Pignatelli M. 1998. Abnormal expression of p120 correlates with poor survival in patients with bladder cancer. Eur. J. Cancer 34, 2037–2040 (doi:10.1016/S0959-8049(98)00279-2) [DOI] [PubMed] [Google Scholar]
- 22.Gimelbrant AA, Ensminger AW, Qi P, Zucker J, Chess A. 2005. Monoallelic expression and asynchronous replication of p120 catenin in mouse and human cells. J. Biol. Chem. 280, 1354–1359 (doi:10.1074/jbc.M411283200) [DOI] [PubMed] [Google Scholar]
- 23.Ireton RC, et al. 2002. A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159, 465–476 (doi:10.1083/jcb.200205115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood LD, et al. 2007. The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113 (doi:10.1126/science.1145720) [DOI] [PubMed] [Google Scholar]
- 25.Mortazavi F, An J, Dubinett S, Rettig M. 2010. p120-catenin is transcriptionally downregulated by FOXC2 in non-small cell lung cancer cells. Mol. Cancer Res. 8, 762–774 (doi:10.1158/1541-7786.MCR-10-0004) [DOI] [PubMed] [Google Scholar]
- 26.Hamada S, et al. 2013. miR-197 induces epithelial-mesenchymal transition in pancreatic cancer cells by targeting p120 catenin. J. Cell Physiol. 228, 1255–1263 (doi:10.1002/jcp.24280) [DOI] [PubMed] [Google Scholar]
- 27.Kusaba T, et al. 2010. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc. Natl Acad. Sci. USA 107, 19 308–19 313 (doi:10.1073/pnas.1008544107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohno H, Uemura K, Shintani-Ishida K, Nakamura M, Inomata M, Yoshida K. 2007. Ischemia promotes calpain-mediated degradation of p120-catenin in SH-SY5Y cells. Biochem. Biophys. Res. Commun. 353, 547–552 (doi:10.1016/j.bbrc.2006.12.061) [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Minshall RD, Schwartz DE, Hu G. 2011. Cyclic stretch induces alveolar epithelial barrier dysfunction via calpain-mediated degradation of p120-catenin. Am. J. Physiol. Lung Cell Mol. Physiol. 301, L197–L206 (doi:10.1152/ajplung.00048.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia X, Mariner DJ, Reynolds AB. 2003. Adhesion-associated and PKC-modulated changes in serine/threonine phosphorylation of p120-catenin. Biochemistry 42, 9195–9204 (doi:10.1021/bi034597h) [DOI] [PubMed] [Google Scholar]
- 31.Smalley-Freed WG, Efimov A, Short SP, Jia P, Zhao Z, Washington MK, Robine S, Coffey RJ, Reynolds AB. 2011. Adenoma formation following limited ablation of p120-catenin in the mouse intestine. PLoS ONE 6, e19880 (doi:10.1371/journal.pone.0019880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarrio D, Perez-Mies B, Hardisson D, Moreno-Bueno G, Suarez A, Cano A, Martin-Perez J, Gamallo C, Palacios J. 2004. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene 23, 3272–3283 (doi:10.1038/sj.onc.1207439) [DOI] [PubMed] [Google Scholar]
- 33.Shibata T, Kokubu A, Sekine S, Kanai Y, Hirohashi S. 2004. Cytoplasmic p120ctn regulates the invasive phenotypes of E-cadherin-deficient breast cancer. Am. J. Pathol. 164, 2269–2278 (doi:10.1016/S0002-9440(10)63783-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debald M, Kaiser C, Abramian A, Schildhaus HU, Locher P, Wolfgarten M, Kuhn W, Braun M. 2013. Evaluation of E-cadherin, Ki-67 and lymphatic vessel invasion in abdominal metastases of human breast cancer. Anticancer Res. 33, 1971–1975 [PubMed] [Google Scholar]
- 35.Ionescu Popescu C, et al. 2013. E-cadherin expression in molecular types of breast carcinoma. Rom. J. Morphol. Embryol. 54, 267–273 [PubMed] [Google Scholar]
- 36.Chu K, Boley KM, Moraes R, Barsky SH, Robertson FM. 2013. The paradox of E-cadherin, role in response to hypoxia in the tumor microenvironment and regulation of energy metabolism. Oncotarget 4, 446–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, Hochman T, Formenti SC, Schneider RJ. 2009. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat. Cell Biol. 11, 903–908 (doi:10.1038/ncb1900) [DOI] [PubMed] [Google Scholar]
- 38.Coman DR. 1944. Decreased mutual adhesiveness, a property of cells from squamous cell carcinomas. Cancer Res. 4, 625–629 [Google Scholar]
- 39.Birchmeier W, Behrens J. 1994. Cadherin expression in carcinomas, role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta 1198, 11–26 [DOI] [PubMed] [Google Scholar]
- 40.Vasioukhin V. 2012. Adherens junctions and cancer. Subcell Biochem. 60, 379–414 (doi:10.1007/978-94-007-4186-7_16) [DOI] [PubMed] [Google Scholar]
- 41.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. 1998. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 (doi:10.1038/32433) [DOI] [PubMed] [Google Scholar]
- 42.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. 1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66, 107–119 (doi:10.1016/0092-8674(91)90143-M) [DOI] [PubMed] [Google Scholar]
- 43.Derksen PW, et al. 2006. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 (doi:10.1016/j.ccr.2006.09.013) [DOI] [PubMed] [Google Scholar]
- 44.Camand E, Peglion F, Osmani N, Sanson M, Etienne-Manneville S. 2012. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J. Cell Sci. 125, 844–857 (doi:10.1242/jcs.087668) [DOI] [PubMed] [Google Scholar]
- 45.Etienne-Manneville S. 2012. Adherens junctions during cell migration. Subcell Biochem. 60, 225–249 (doi:10.1007/978-94-007-4186-7_10) [DOI] [PubMed] [Google Scholar]
- 46.Peglion F, Etienne-Manneville S. 2012. N-cadherin expression level as a critical indicator of invasion in non-epithelial tumors. Cell Adhes. Migr. 6, 327–332 (doi:10.4161/cam.20855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowalczyk AP, Nanes BA. 2012. Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcell Biochem. 60, 197–222 (doi:10.1007/978-94-007-4186-7_9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. 2000. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 148, 189–202 (doi:10.1083/jcb.148.1.189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis MA, Ireton RC, Reynolds AB. 2003. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163, 525–34 (doi:10.1083/jcb.200307111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyashita Y, Ozawa M. 2007. Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J. Biol. Chem. 282, 11 540–11 548 (doi:10.1074/jbc.M608351200) [DOI] [PubMed] [Google Scholar]
- 51.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. 2005. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell 16, 5141–5151 (doi:10.1091/mbc.E05-05-0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. 2009. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol. Biol. Cell 20, 1970–1980 (doi:10.1091/mbc.E08-07-0735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. 2010. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell–cell adhesion. Cell 141, 117–128 (doi:10.1016/j.cell.2010.01.017) [DOI] [PubMed] [Google Scholar]
- 54.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. 2012. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J. Cell Biol. 199, 365–380 (doi:10.1083/jcb.201205029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ. 2008. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456, 976–979 (doi:10.1038/nature07422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. 2002. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4, 222–231 (doi:10.1038/ncb758) [DOI] [PubMed] [Google Scholar]
- 57.Dupin I, Camand E, Etienne-Manneville S. 2009. Classical cadherins control nucleus and centrosome position and cell polarity. J. Cell Biol. 185, 779–786 (doi:10.1083/jcb.200812034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elia LP, Yamamoto M, Zang K, Reichardt LF. 2006. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron 51, 43–56 (doi:10.1016/j.neuron.2006.05.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. 2006. p120-catenin and p190RhoGAP regulate cell–cell adhesion by coordinating antagonism between Rac and Rho. Cell 127, 1027–1039 (doi:10.1016/j.cell.2006.09.046) [DOI] [PubMed] [Google Scholar]
- 60.Smith AL, Dohn MR, Brown MV, Reynolds AB. 2011. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol. Biol. Cell 23, 99–110 (doi:10.1091/mbc.E11-06-0497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirano M, Hashimoto S, Yonemura S, Sabe H, Aizawa S. 2008. EPB41L5 functions to post-transcriptionally regulate cadherin and integrin during epithelial-mesenchymal transition. J. Cell Biol. 182, 1217–1230 (doi:10.1083/jcb.200712086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T, Pei X, Zhan J, Hu J, Yu Y, Zhang H. 2012. FERM-containing protein FRMD5 is a p120-catenin interacting protein that regulates tumor progression. FEBS Lett. 586, 3044–3050 (doi:10.1016/j.febslet.2012.07.019) [DOI] [PubMed] [Google Scholar]
- 63.Hernandez MV, Wehrendt DP, Arregui CO. 2010. The protein tyrosine phosphatase PTP1B is required for efficient delivery of N-cadherin to the cell surface. Mol. Biol. Cell 21, 1387–1397 (doi:10.1091/mbc.E09-10-0880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. 2004. A novel interaction between kinesin and p120 modulates p120 localization and function. J. Biol. Chem. 279, 9512–9521 (doi:10.1074/jbc.M310895200) [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Kojima S, Borisy GG, Green KJ. 2003. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J. Cell Biol. 163, 547–557 (doi:10.1083/jcb.200305137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miranda KC, Joseph SR, Yap AS, Teasdale RD, Stow JL. 2003. Contextual binding of p120ctn to E-cadherin at the basolateral plasma membrane in polarized epithelia. J. Biol. Chem. 278, 43 480–43 488 (doi:10.1074/jbc.M305525200) [DOI] [PubMed] [Google Scholar]
- 67.Meng W, Mushika Y, Ichii T, Takeichi M. 2008. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell–cell contacts. Cell 135, 948–959 (doi:10.1016/j.cell.2008.09.040) [DOI] [PubMed] [Google Scholar]
- 68.Kumper S, Ridley AJ. 2010. p120ctn and P-cadherin but not E-cadherin regulate cell motility and invasion of DU145 prostate cancer cells. PLoS ONE 5, e11801 (doi:10.1371/journal.pone.0011801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, et al. 2009. Ablation of p120-catenin enhances invasion and metastasis of human lung cancer cells. Cancer Sci. 100, 441–448 (doi:10.1111/j.1349-7006.2008.01067.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gold JS, Reynolds AB, Rimm DL. 1998. Loss of p120ctn in human colorectal cancer predicts metastasis and poor survival. Cancer Lett. 132, 193–201 (doi:10.1016/S0304-3835(98)00190-6) [DOI] [PubMed] [Google Scholar]
- 71.Talvinen K, Tuikkala J, Nykanen M, Nieminen A, Anttinen J, Nevalainen OS, Hurme S, Kuopio T, Krongvist P. 2010. Altered expression of p120catenin predicts poor outcome in invasive breast cancer. J. Cancer Res. Clin. Oncol. 136, 1377–1387 (doi:10.1007/s00432-010-0789-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macpherson IR, Hooper S, Serrels A, McGarry L, Ozanne BW, Harrington K, Frame MC, Sahai E, Brunton VG. 2007. p120-catenin is required for the collective invasion of squamous cell carcinoma cells via a phosphorylation-independent mechanism. Oncogene 26, 5214–5228 (doi:10.1038/sj.onc.1210334) [DOI] [PubMed] [Google Scholar]
- 73.Yanagisawa M, Anastasiadis PZ. 2006. p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness. J. Cell Biol. 174, 1087–1096 (doi:10.1083/jcb.200605022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedl P, Locker J, Sahai E, Segall JE. 2012. Classifying collective cancer cell invasion. Nat. Cell Biol. 14, 777–783 (doi:10.1038/ncb2548) [DOI] [PubMed] [Google Scholar]
- 75.Johnson E, Seachrist DD, DeLeon-Rodriguez CM, Lozada KL, Miedler J, Abdul-Karim FW, Keri RA. 2010. HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. J. Biol. Chem. 285, 29 491–29 501 (doi:10.1074/jbc.M110.136770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. 2004. Cadherin switch in tumor progression. Ann. NY Acad. Sci. 1014, 155–163 (doi:10.1196/annals.1294.016) [DOI] [PubMed] [Google Scholar]
- 77.Christofori G. 2006. New signals from the invasive front. Nature 441, 444–450 (doi:10.1038/nature04872) [DOI] [PubMed] [Google Scholar]
- 78.Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. 2005. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 65, 3092–3099 [DOI] [PubMed] [Google Scholar]
- 79.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. 2001. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell–cell contact formation and regulation of cell locomotion. J. Cell Sci. 114, 695–707 [DOI] [PubMed] [Google Scholar]
- 80.Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. 2005. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 65, 10 938–10945 (doi:10.1158/0008-5472.CAN-05-1947) [DOI] [PubMed] [Google Scholar]
- 81.Anastasiadis PZ, Reynolds AB. 2001. Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13, 604–610 (doi:10.1016/S0955-0674(00)00258-1) [DOI] [PubMed] [Google Scholar]
- 82.Kinch MS, Clark GJ, Der CJ, Burridge K. 1995. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J. Cell Biol. 130, 461–471 (doi:10.1083/jcb.130.2.461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ozawa M, Ohkubo T. 2001. Tyrosine phosphorylation of p120(ctn) in v-Src transfected L cells depends on its association with E-cadherin and reduces adhesion activity. J. Cell Sci. 114, 503–512 [DOI] [PubMed] [Google Scholar]
- 84.Ma LW, Zhou ZT, He QB, Jiang WW. 2012. Phosphorylated p120-catenin expression has predictive value for oral cancer progression. J. Clin. Pathol. 65, 315–319 (doi:10.1136/jclinpath-2011-200516) [DOI] [PubMed] [Google Scholar]
- 85.Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, Giannini C, Galanis E, Sarkaria JN, Anastasiadis PZ. 2013. Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS ONE 8, e56505 (doi:10.1371/journal.pone.0056505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mariner DJ, Anastasiadis P, Keilhack H, Bohmer FD, Wang J, Reynolds AB. 2001. Identification of Src phosphorylation sites in the catenin p120ctn. J. Biol. Chem. 276, 28 006–28 013 (doi:10.1074/jbc.M102443200) [DOI] [PubMed] [Google Scholar]
- 87.Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. 2003. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin interaction. Mol. Cell Biol. 23, 2287–2297 (doi:10.1128/MCB.23.7.2287-2297.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keilhack H, Hellman U, van Hengel J, van Roy F, Godovac-Zimmermann J, Bohmer FD. 2000. The protein-tyrosine phosphatase SHP-1 binds to and dephosphorylates p120 catenin. J. Biol. Chem. 275, 26 376–26 384 (doi:10.1074/jbc.M001315200) [DOI] [PubMed] [Google Scholar]
- 89.Zondag GC, Reynolds AB, Moolenaar WH. 2000. Receptor protein-tyrosine phosphatase RPTPmu binds to and dephosphorylates the catenin p120(ctn). J. Biol. Chem. 275, 11 264–11 269 (doi:10.1074/jbc.275.15.11264) [DOI] [PubMed] [Google Scholar]
- 90.Alema S, Salvatore AM. 2007. p120 catenin and phosphorylation: mechanisms and traits of an unresolved issue. Biochim. Biophys. Acta 1773, 47–58 (doi:10.1016/j.bbamcr.2006.06.001) [DOI] [PubMed] [Google Scholar]
- 91.Keirsebilck A, Bonne S, Staes K, van Hengel J, Nollet F, Reynolds A, van Roy F. 1998. Molecular cloning of the human p120ctn catenin gene (CTNND1): expression of multiple alternatively spliced isoforms. Genomics 50, 129–146 (doi:10.1006/geno.1998.5325) [DOI] [PubMed] [Google Scholar]
- 92.Mo YY, Reynolds AB. 1996. Identification of murine p120 isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res. 56, 2633–2640 [PubMed] [Google Scholar]
- 93.Aho S, Levansuo L, Montonen O, Kari C, Rodeck U, Uitto J. 2002. Specific sequences in p120ctn determine subcellular distribution of its multiple isoforms involved in cellular adhesion of normal and malignant epithelial cells. J. Cell Sci. 115, 1391–1402 [DOI] [PubMed] [Google Scholar]
- 94.Montonen O, Aho M, Uitto J, Aho S. 2001. Tissue distribution and cell type-specific expression of p120ctn isoforms. J. Histochem. Cytochem. 49, 1487–1496 (doi:10.1177/002215540104901202) [DOI] [PubMed] [Google Scholar]
- 95.Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. 2000. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J. Cell Biol. 148, 173–188 (doi:10.1083/jcb.148.1.173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Husmark J, Heldin NE, Nilsson M. 1999. N-cadherin-mediated adhesion and aberrant catenin expression in anaplastic thyroid-carcinoma cell lines. Int. J. Cancer 83, 692–699 (doi:10.1002/(SICI)1097-0215(19991126)83:5<692::AID-IJC21>3.0.CO;2-1) [DOI] [PubMed] [Google Scholar]
- 97.Tran NL, Nagle RB, Cress AE, Heimark RL. 1999. N-cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with stromal cells. Am. J. Pathol. 155, 787–798 (doi:10.1016/S0002-9440(10)65177-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seidel B, Braeg S, Adler G, Wedlich D, Menke A. 2004. E- and N-cadherin differ with respect to their associated p120ctn isoforms and their ability to suppress invasive growth in pancreatic cancer cells. Oncogene 23, 5532–5542 (doi:10.1038/sj.onc.1207718) [DOI] [PubMed] [Google Scholar]
- 99.Miao Y, Liu N, Zhang Y, Liu Y, Yu JH, Dai SD, Xu HT, Wang EH. 2010. p120ctn isoform 1 expression significantly correlates with abnormal expression of E-cadherin and poor survival of lung cancer patients. Med. Oncol. 27, 880–886 (doi:10.1007/s12032-009-9300-2) [DOI] [PubMed] [Google Scholar]
- 100.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. 2009. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol. Cell 33, 591–601 (doi:10.1016/j.molcel.2009.01.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohkubo T, Ozawa M. 2004. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J. Cell Sci. 117, 1675–1685 (doi:10.1242/jcs.01004) [DOI] [PubMed] [Google Scholar]
- 102.Etienne-Manneville S, Hall A. 2002. Rho GTPases in cell biology. Nature 420, 629–635 (doi:10.1038/nature01148) [DOI] [PubMed] [Google Scholar]
- 103.Raftopoulou M, Hall A. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23–32 (doi:10.1016/j.ydbio.2003.06.003) [DOI] [PubMed] [Google Scholar]
- 104.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637–644 (doi:10.1038/35023588) [DOI] [PubMed] [Google Scholar]
- 105.Noren NK, Liu BP, Burridge K, Kreft B. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150, 567–580 (doi:10.1083/jcb.150.3.567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cozzolino M, Stagni V, Spinardi L, Campioni N, Fiorentini C, Salvati E, Alema S, Salvatore AM. 2003. p120 catenin is required for growth factor-dependent cell motility and scattering in epithelial cells. Mol. Biol. Cell 14, 1964–1977 (doi:10.1091/mbc.E02-08-0469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castano J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, Herreros AG, Dunach M. 2007. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol. Cell Biol. 27, 1745–1757 (doi:10.1128/MCB.01974-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yanagisawa M, Huveldt D, Kreinest P, Lohse CM, Cheville JC, Parker AS, Copland JA, Anastasiadis PZ. 2008. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. J. Biol. Chem. 283, 18 344–18 354 (doi:10.1074/jbc.M801192200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zebda N, Tian Y, Tian X, Gawlak G, Higginbotham K, Reynolds AB, Birukova AA, Birukov KG. 2013. Interaction of p190RhoGAP with C-terminal domain of p120-catenin modulates endothelial cytoskeleton and permeability. J. Biol. Chem. 288, 18 290–18 299 (doi:10.1074/jbc.M112.432757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schackmann RC, et al. 2011. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J. Clin. Invest. 121, 3176–3188 (doi:10.1172/JCI41695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dohn MR, Brown MV, Reynolds AB. 2009. An essential role for p120-catenin in Src- and Rac1-mediated anchorage-independent cell growth. J. Cell Biol. 184, 437–450 (doi:10.1083/jcb.200807096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simpson CD, Anyiwe K, Schimmer AD. 2008. Anoikis resistance and tumor metastasis. Cancer Lett. 272, 177–185 (doi:10.1016/j.canlet.2008.05.029) [DOI] [PubMed] [Google Scholar]
- 113.Cheung LW, Leung PC, Wong AS. 2010. Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene 29, 2427–2440 (doi:10.1038/onc.2009.523) [DOI] [PubMed] [Google Scholar]
- 114.Soto E, Yanagisawa M, Marlow LA, Copland JA, Perez EA, Anastasiadis PZ. 2008. p120 catenin induces opposing effects on tumor cell growth depending on E-cadherin expression. J. Cell Biol. 183, 737–749 (doi:10.1083/jcb.200805113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Valls G, et al. 2012. Upon Wnt stimulation, Rac1 activation requires Rac1 and Vav2 binding to p120-catenin. J. Cell Sci. 125, 5288–5301 (doi:10.1242/jcs.101030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajput C, et al. 2013. Neural Wiskott–Aldrich syndrome protein (N-WASP)-mediated p120-catenin interaction with Arp2-Actin complex stabilizes endothelial adherens junctions. J. Biol. Chem. 288, 4241–4250 (doi:10.1074/jbc.M112.440396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, Feinstein E, Geiger B, Bershadsky A. 2007. p120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc. Natl Acad. Sci. USA 104, 10 882–10 887 (doi:10.1073/pnas.0702731104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ichii T, Takeichi M. 2007. p120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells 12, 827–839 (doi:10.1111/j.1365-2443.2007.01095.x) [DOI] [PubMed] [Google Scholar]
- 119.Roczniak-Ferguson A, Reynolds AB. 2003. Regulation of p120-catenin nucleocytoplasmic shuttling activity. J. Cell Sci. 116, 4201–4212 (doi:10.1242/jcs.00724) [DOI] [PubMed] [Google Scholar]
- 120.Franz CM, Ridley AJ. 2004. p120 catenin associates with microtubules, inverse relationship between microtubule binding and Rho GTPase regulation. J. Biol. Chem. 279, 6588–6594 (doi:10.1074/jbc.M312812200) [DOI] [PubMed] [Google Scholar]
- 121.Cook TA, Nagasaki T, Gundersen GG. 1998. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J. Cell Biol. 141, 175–185 (doi:10.1083/jcb.141.1.175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palazzo AF, Cook TA, Alberts AS, Gundersen GG. 2001. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3, 723–729 (doi:10.1038/35087035) [DOI] [PubMed] [Google Scholar]
- 123.Falconer MM, Vielkind U, Brown DL. 1989. Establishment of a stable, acetylated microtubule bundle during neuronal commitment. Cell Motil. Cytoskeleton 12, 169–180 (doi:10.1002/cm.970120306) [DOI] [PubMed] [Google Scholar]
- 124.Mayerle J, Friess H, Buchler MW, Schnekenburger J, Weiss FU, Zimmer KP, Domschke W, Lerch MM. 2003. Up-regulation, nuclear import, and tumor growth stimulation of the adhesion protein p120 in pancreatic cancer. Gastroenterology 124, 949–960 (doi:10.1053/gast.2003.50142) [DOI] [PubMed] [Google Scholar]
- 125.Kelly KF, Spring CM, Otchere AA, Daniel JM. 2004. NLS-dependent nuclear localization of p120ctn is necessary to relieve Kaiso-mediated transcriptional repression. J. Cell Sci. 117, 2675–2686 (doi:10.1242/jcs.01101) [DOI] [PubMed] [Google Scholar]
- 126.Chausovsky A, Bershadsky AD, Borisy GG. 2000. Cadherin-mediated regulation of microtubule dynamics. Nat. Cell Biol. 2, 797–804 (doi:10.1038/35041037) [DOI] [PubMed] [Google Scholar]
- 127.Giannakakou P, Nakano M, Nicolaou KC, O'Brate A, Yu J, Blagosklonny MV, Greber UF, Fojo T. 2000. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc. Natl Acad. Sci. USA 99, 10 855–10 860 (doi:10.1073/pnas.132275599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. 2002. Microtubule binding to Smads may regulate TGF beta activity. Mol. Cell 5, 27–34 (doi:10.1016/S1097-2765(00)80400-1) [DOI] [PubMed] [Google Scholar]
- 129.Li Y, Kufe D. 2001. The human DF3/MUC1 carcinoma-associated antigen signals nuclear localization of the catenin p120(ctn). Biochem. Biophys. Res. Commun. 281, 440–443 (doi:10.1006/bbrc.2001.4383) [DOI] [PubMed] [Google Scholar]
- 130.Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA. 2003. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J. Biol. Chem. 278, 38 029–38 039 (doi:10.1074/jbc.M304333200) [DOI] [PubMed] [Google Scholar]
- 131.Wesseling J, van der Valk SW, Hilkens J. 1996. A mechanism for inhibition of E-cadherin-mediated cell–cell adhesion by the membrane-associated mucin episialin/MUC1. Mol. Biol. Cell 7, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Willert K, Jones KA. 2006. Wnt signaling: is the party in the nucleus? Genes Dev. 20, 1394–1404 (doi:10.1101/gad.1424006) [DOI] [PubMed] [Google Scholar]
- 133.Daniel JM, Reynolds AB. 1999. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol. Cell Biol. 19, 3614–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prokhortchouk A, Hendrich B, Jorgensen H, Ruzov A, Wilm M, Georgiev G, Bird A, Prokhortchouk E. 2001. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 15, 1613–1618 (doi:10.1101/gad.198501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daniel JM, Spring CM, Crawford HC, Reynolds AB, Baig A. 2002. The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 30, 2911–2919 (doi:10.1093/nar/gkf398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J. 2003. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol. Cell 12, 723–734 (doi:10.1016/j.molcel.2003.08.008) [DOI] [PubMed] [Google Scholar]
- 137.Jones J, et al. 2012. Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am. J. Pathol. 181, 1836–1846 (doi:10.1016/j.ajpath.2012.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yildirim E, Kirby JE, Brown DE, Mercier FE, Sadreyev RI, Scadden DT, Lee JT. 2013. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 152, 727–742 (doi:10.1016/j.cell.2013.01.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murphree AL, Benedict WF. 1984. Retinoblastoma: clues to human oncogenesis. Science 223, 1028–1033 (doi:10.1126/science.6320372) [DOI] [PubMed] [Google Scholar]
- 140.Caldas C, et al. 1994. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat. Genet. 8, 27–32 (doi:10.1038/ng0994-27) [DOI] [PubMed] [Google Scholar]
- 141.Kim SW, Park JI, Spring CM, Sater AK, Ji H, Otchere AA, Daniel JM, McCrea PD. 2004. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat. Cell Biol. 6, 1212–1220 (doi:10.1038/ncb1191) [DOI] [PubMed] [Google Scholar]
- 142.Park JI, Ji H, Jun S, Gu D, Hikasa H, Li L, Sokol SY, McCrea PD. 2006. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: distinct catenin subfamilies promote Wnt signals. Dev. Cell 11, 683–695 (doi:10.1016/j.devcel.2006.09.022) [DOI] [PubMed] [Google Scholar]
- 143.Anastas JN, Moon RT. 2013. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (doi:10.1038/nrc3419) [DOI] [PubMed] [Google Scholar]
- 144.DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. 2009. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 69, 5364–5373 (doi:10.1158/0008-5472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spring CM, Kelly KF, O'Kelly I, Graham M, Crawford HC, Daniel JM. 2005. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the beta-catenin/TCF target gene matrilysin. Exp. Cell Res. 305, 253–265 (doi:10.1016/j.yexcr.2005.01.007) [DOI] [PubMed] [Google Scholar]
- 146.Casagolda D, et al. 2010. A p120-catenin-CK1epsilon complex regulates Wnt signaling. J. Cell Sci. 123, 2621–2631 (doi:10.1242/jcs.067512) [DOI] [PubMed] [Google Scholar]
- 147.Del Valle-Perez B, Casagolda D, Lugilde E, Valls G, Codina M, Dave N, Herreros AG, Dunach M. 2011. Wnt controls the transcriptional activity of Kaiso through CK1epsilon-dependent phosphorylation of p120-catenin. J. Cell Sci. 124, 2298–2309 (doi:10.1242/jcs.082693) [DOI] [PubMed] [Google Scholar]
- 148.Dvorak HF. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (doi:10.1056/NEJM198612253152606) [DOI] [PubMed] [Google Scholar]
- 149.Coussens LM, Werb Z. 2002. Inflammation and cancer. Nature 420, 860–867 (doi:10.1038/nature01322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maeda H, Akaike T. 1998. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (Mosc.) 63, 854–865 [PubMed] [Google Scholar]
- 151.Meira LB, et al. 2008. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 118, 2516–2525 (doi:10.1172/JCI35073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Radisky DC, et al. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 (doi:10.1038/nature03688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Williams IR, Parkos CA. 2007. Colonic neutrophils in inflammatory bowel disease: double-edged swords of the innate immune system with protective and destructive capacity. Gastroenterology 133, 2049–2052 (doi:10.1053/j.gastro.2007.10.031) [DOI] [PubMed] [Google Scholar]
- 154.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. 2007. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400 (doi:10.1038/ncb1658) [DOI] [PubMed] [Google Scholar]
- 155.Bhowmick NA, Neilson EG, Moses HL. 2004. Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337 (doi:10.1038/nature03096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. 2009. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 18, 1016–1018 [DOI] [PubMed] [Google Scholar]
- 157.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. 2004. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102 (doi:10.1016/j.ccr.2009.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ferrara N. 2010. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr. Opin. Hematol. 17, 219–224 [DOI] [PubMed] [Google Scholar]
- 159.Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (doi:10.1038/nri2506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ostrand-Rosenberg S, Sinha P. 2009. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182, 4499–4506 (doi:10.4049/jimmunol.0802740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Smalley-Freed WG, et al. 2010. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J. Clin. Invest. 120, 1824–1835 (doi:10.1172/JCI41414) [DOI] [PMC free article] [PubMed] [Google Scholar]