Abstract
O6-Benzylguanine (BG) enhances cisplatin [cis-diammine dichloroplatinum (II)]-induced cytotoxicity and apoptosis in head and neck cancer cell lines by an unknown mechanism. We investigated the effect of cisplatin with and without BG on two targets of damage: DNA and the endoplasmic reticulum (ER). We chose three cancer cell lines to ascertain the mechanism of BG-enhanced cytotoxicity: SQ20b head and neck and SKOV-3x ovarian cancer cell lines, where BG enhanced cisplatin cytotoxicity, and A549 nonsmall cell lung cancer line, where BG did not enhance cisplatin cytotoxicity. All three lines had an increase in DNA damage when BG was added to cisplatin treatment, as evidenced by increased platination and phosphorylated histone H2AX formation. The increase in cisplatin-induced DNA damage after treatment with BG plus cisplatin is not sufficient to increase cytotoxicity or apoptosis in A549 cells. We evaluated the effect of cisplatin on the ER and observed increased caspase 12 cleavage in SQ20b and SKOV-3x cells, but not in A549 cells, after treatment with BG plus cisplatin versus cisplatin alone. Growth arrest and DNA damage inducible (GADD) 153, an ER stress-response gene, is up-regulated after treatment with BG plus cisplatin compared with cisplatin alone in SQ20b and SKOV-3x cells, but not in A549 cells. ER stress-induced apoptosis is an integral part of the mechanism by which BG enhances cisplatin. Inhibition of ER stress in the SQ20b cell line by salubrinal, an inhibitor of eIF2α dephosphorylation, or GADD153 small interfering RNA, abrogated BG-enhancement of cisplatin cytotoxicity and apoptosis through caspase 3 and 12 cleavage. These data indicate GADD153 up-regulation plays an important role in BG-enhanced cisplatin cytotoxicity and apoptosis.
Platinating agents have been used extensively over the past 30 years for treatment of carcinomas, including head and neck, lung, testicular, and gynecologic cancers, and relapsed lymphomas (Hartmann and Lipp, 2003). The main cytotoxic effect of cisplatin is attributed to formation of crosslinks on DNA, with the prevalent 1,2,-GpG intrastrand cross-link thought to be the major cytotoxic lesion (Zorbas and Keppler, 2005). Resistance, both acquired and intrinsic, is a major problem of cisplatin treatment. The initial response rate to cisplatin is only 25 to 30% in patients with head and neck cancers (Jacobs et al., 1992), and 48% of responding patients with stage III to IV disease relapse within 5 years (Arnold et al., 2006). Approximately 95% of patients with small cell lung carcinoma relapse after treatment with platinating agents (Siddik, 2003). Even in ovarian carcinomas, in which 70% of patients initially respond to cisplatin, the 5-year survival rate for responding patients is less than 25%. In platinum-resistant recurrent ovarian cancer, the original regimen of paclitaxel plus a platinating agent is ineffective (Moss and Kaye, 2002). These observations underscore the need to develop modulators of platinum agents to effectively overcome resistance.
The guanine analog O6-benzylguanine (BG) enhances cisplatin-induced cytotoxicity in head and neck cancer cell lines (Fishel et al., 2003). BG was originally developed as a potent inactivator of O6-alkylguanine DNA alkyltransferase (Dolan and Pegg, 1997); however, its enhancement of cisplatin cytotoxicity is independent of its ability to inactivate O6-alkylguanine DNA alkyltransferase (Fishel et al., 2003). Structural modifications to BG have resulted in more potent (O6-cyclohexylmethyl guanine) and essentially inactive (9-methyl-O6-benzylguanine) compounds, indicating the importance of various structural features on the ability to enhance cisplatin-induced cytotoxicity (Fishel et al., 2005b). The mechanism by which BG enhances cisplatin-induced cytotoxicity is as yet unknown, but among the mechanisms ruled out are detoxification by GSH in the cytosol and increased DNA repair of platinum adducts through enzymes within nucleotide excision repair (Fishel et al., 2005a).
An additional mechanism by which cisplatin can cause apoptosis is through induction of the endoplasmic reticulum (ER) stress pathway (Mandic et al., 2003; Nawrocki et al., 2005; Fribley et al., 2006). One possible mechanism of inducing the ER stress pathway is through oxidative stress in the ER itself (Liu and Baliga, 2003, 2005). Several laboratories have begun to investigate the effect of cisplatin on the ER stress response and modulation of cisplatin activity via the ER response (Linder and Shoshan, 2005; Nawrocki et al., 2005; Fribley et al., 2006). The proteasome inhibitor bortezomib has been shown to enhance cisplatininduced ER stress in both murine and human cancer cell lines (Nawrocki et al., 2005; Fribley et al., 2006). Bortezomib up-regulates glucose-regulated protein 78 (BiP) and GADD153 (growth arrest and DNA damage 153, also known as CHOP) expression, thereby signaling down-stream in a proapoptotic pathway. Glucose-regulated protein 78 binds to misfolded proteins in the ER during the unfolded protein response, whereas GADD153 acts as a transcription factor that is thought to down-regulate expression of the antiapoptotic protein Bcl-2 (McCullough et al., 2001). GADD34 is a cytoplasmic protein that is involved in potentiation of the ER stress pathway (Kojima et al., 2003). We previously observed that BG plus cisplatin treatment resulted in the up-regulation of GADD34 gene expression in head and neck cancer cell lines (Fishel et al., 2006), further indicating that the mechanism of enhancement of cisplatin-induced cytotoxicity by BG involves potentiation of the ER stress response.
Although DNA lesions have been presumed to be the predominant mechanism for cisplatin-induced cytotoxicity, apoptosis can also be initiated through the ER stress pathway (Mandic et al., 2003). Here, we investigate the differences in damage to nuclear DNA and the ER after treatment with BG plus cisplatin compared with cisplatin alone. We examined the tissue and cell line specificity of these types of damage. Our results provide a better understanding of the mechanism of increased cisplatin-induced cytotoxicity caused by BG and a better understanding of the cellular targets of cisplatin and the importance of the ER in response to cisplatin.
Materials and Methods
Cell Lines
The head and neck cancer cell line, SQ20b, was kindly provided by Dr. Michael Beckett (Department of Radiation and Cellular Oncology, University of Chicago, Chicago, IL). The SKOV-3x ovarian cancer cell line was kindly provided by Dr. Robert Bigsby (Department of Obstetrics and Gynecology, Indiana University School of Medicine, Indianapolis, IN). The following cell lines were purchased from the American Type Culture Collection (Manassas, VA): A549, H460, H520, SKOV-3, C33-A, HEC-1-A, and PaCa-2. Media and serum were purchased from Mediatech (Herndon, VA) and HyClone Laboratories (Logan, UT), respectively. SQ20b cells were maintained in Dulbecco’s modified Eagle’s medium/Ham’s F12 (50:50 mixture), supplemented with 20% fetal bovine serum (FBS) and 0.4 μg/ml hydrocortisone (BD Biosciences, San Jose, CA). The A549 lung carcinoma cell line was maintained in Ham’s F12 medium supplemented with 10% FBS. H460, a large cell lung cancer line, and H520, a squamous cell lung carcinoma cell line, were maintained in RPMI 1640 medium supplemented with 10% FBS, 1.5 g/l sodium bicarbonate (Sigma-Aldrich, St. Louis, MO), 4.5 g/l glucose (Sigma-Aldrich), 10 mM HEPES (Mediatech), and 1.0 mM sodium pyruvate (Mediatech). The C33-A cervical carcinoma line was maintained in Eagle’s minimum essential medium, supplemented with 10% FBS, 1.5 g/l sodium bicarbonate, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate. The HEC-1-A endometrial carcinoma cell line and SKOV-3X and SKOV-3 ovarian carcinoma cell lines were maintained in McCoy’s 5A medium supplemented with 10% FBS, 1.5 g/l sodium bicarbonate, and 1.0 mM sodium pyruvate. The pancreatic cancer cell line PaCa-2 was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. All cell lines were grown as monolayers at 37°C and 5% CO2.
Drugs
Cisplatin was purchased from Sigma-Aldrich and freshly prepared for each experiment by dissolving in 100% dimethyl sulfoxide (DMSO), so that the final DMSO concentration was less than 0.1% for the cell experiments. The structure of cisplatin can be found in Rabik et al. (2007). BG and 9-methyl-O6-benzylguanine (9-methyl-BG) were kindly provided by the late Dr. Robert C. Moschel (National Cancer Institute at Frederick, Frederick, MD). Structures of BG and 9-methyl-BG are found in Fishel et al. (2006). Salubrinal was purchased from Calbiochem (San Diego, CA) and dissolved in 100% DMSO as recommended by the manufacturer, with the stock solution being 10 mM. Salubrinal was used in cells at 25 μM, with the final DMSO concentration being less than 0.1%.
Colony Formation Assay
Cell survival after drug treatment was determined using the colony formation assay as described previously (Fishel et al., 2003). In brief, exponentially growing cells were exposed to BG (2 h, 100 μM) before the addition of increasing concentrations of cisplatin. After a 2-h incubation with BG and cisplatin at 37°C, cells were replated in triplicate at densities varying between 150 and 3000 cells per 100-mm dish. After approximately 10 to 14 days, colonies were stained with methylene blue [0.1% (w/v)] and scored. Percentage survival was calculated based on the plating efficiency of cells exposed to vehicle alone.
DNA Platination Analysis
Atomic absorption spectroscopy was used to quantify total platinum on DNA, as described previously (Fishel et al., 2003). Cells were treated with 9-methyl-BG (50 μM) or BG (100 μM) with or without cisplatin (25 or 50 μM) as described above, and pellets were collected at 0, 24, or 48 h post-treatment. DNA was isolated either using the Invitrogen ChargeSwitch gDNA Mini Tissue Kit (A549) (Invitrogen, Carlsbad, CA) or as previously described using phenol/chloroform/isoamyl alcohol extraction and ethanol precipitation (SQ20b, SKOV-3x) (Fishel et al., 2003). Platinum concentration for all samples was assessed with a PerkinElmer model 1100 flameless atomic absorption spectrometer (PerkinElmer Life and Analytical Sciences, Waltham, MA), monitoring 265.9 nm. Platinum concentrations were determined by comparison with a standard curve of known platinum concentrations performed on the same day as the assay (Erkmen et al., 1995). Because of interexperimental variation, statistical analysis was performed on normalized samples. A two-tailed Student’s t test assuming unequal variance was performed for statistical analysis. All results were obtained from at least three biological replicates.
Western Blots
Cancer cell lines (SQ20b, A549, PaCa-2, SKOV-3x) were treated with vehicle, BG alone, cisplatin alone, and BG plus cisplatin as described above. After drug treatment, exponentially growing cells were harvested and lysed in radioimmunoprecipitation assay buffer containing phosphatase and protease inhibitors. Phosphorylation of histone H2AX at Ser139 (γH2AX) was measured with a phosphorylation-specific H2AX antibody from Cell Signaling Technology Inc. (Danvers, MA) as described previously (Rogakou et al., 1998; Fishel et al., 2007). Mouse monoclonal anti-phosphohistone H2AX (1:1000) or goat anti-actin antibody (1:1000, as a loading control) was used to probe for protein levels. For GADD153 Western blot analysis, rabbit monoclonal anti-GADD153 (1:500) (Abcam Inc., Cambridge, MA) or mouse anti-actin antibody (1:1000, as a loading control) (Abcam Inc.) was used to probe for protein levels. Bands were detected using a chemiluminescence kit from Roche Applied Science (Indianapolis, IN), visualized either from autoradiographic films or directly from the blot using the Bio-Rad ChemiDoc (Bio-Rad, Hercules, CA), and quantified using either QuantityOne (Bio-Rad) or Sigma Scan Pro 5.0 (Aspire Software International, Ashburn, VA) (Vasko et al., 2004).
Caspase 3 and 12 Activity
Apoptosis was determined by measuring cleavage of caspases 3 and 12. Exponentially growing cells were treated with vehicle, cisplatin (25 μM), BG (100 μM), BG plus cisplatin, 9-methyl-BG (50 μM), or 9-methyl-BG plus cisplatin as described above. After treatment, normal growth medium was added back to cells, and cells were incubated for another 24, 48, or 72 h. After the desired incubation, cells were collected and stained by incubating with one of two fluorescein isothiocyanate (FITC)-conjugated small molecules, which bind irreversibly to activated caspases: ZEVD-FITC (caspase 3) or ATAD-FITC (caspase 12) (BioVision, Mountain View, CA). After incubation, cells were washed, and fluorescence was measured using the FL-1 channel of a FACScan (BD Biosciences, Franklin Lakes, NJ).
Quantitative Real-Time PCR
SQ20b, SKOV-3x, and A549 cells were treated for 2 h with BG (100 μM) or vehicle in serum-free media. After 2 h, 25 μM cisplatin was added for 2 h, after which cells were washed in phosphate-buffered saline, trypsinized, pelleted, and flash frozen in liquid nitrogen. Pellets were stored at −80°C until RNA isolation. Total RNA was isolated from cells using a combination of the QIAGEN QiaShredder kit and QIAGEN RNeasy Mini kit (Valencia, CA), following the manufacturer’s protocol. To analyze samples for RNA transcript levels, the LightCycler RNA Amplification SYBR Green I kit for quantitative real-time (qRT)-PCR was purchased from Roche Applied Science, and samples were run on the SmartCycler (Cepheid, Sunnyvale, CA). The protocol used was in accordance with the manufacturer’s indicated specifications. Primers were designed for GADD153 with the forward primer 5′-AACA-GAGTGGTCATTCCC-3′ and the reverse primer 5′-TTCCTGCTT-GAGCCGTTC-3′. β-Actin was used as the endogenous control with forward primer 5′-ATTGCCGACAGGATGCAGA-3′ and reverse primer 5′-GCTCAGGAGGAGCAATGAGCTT-3′. Standard curves for β-actin and GADD153 were prepared from RNA isolated from exponentially growing cells, which ranged in RNA concentration from 0.064 to 1000 ng/μl and had an r2 value of ≥0.985. Thermocycler parameters were as follows: β-actin, 55°C × 1800 s, 95°C × 600 s, cycle of 95°C × 1 s, 58°C × 10 s, 72°C × 6 s (repeated 45 times), followed by a melting curve from 60 to 95°C, moving at 0.1°C/s; and GADD153, 55°C × 1800 s, 95°C × 600 s, cycle of 95°C × 1 s, 58°C × 10 s, 72°C × 6 s, 82°C × 6 s (repeated 45 times), followed by a melting curve from 58 to 95°C moving at 0.1°C/s. Optics were on during the last stage of the cycle and the melting curve. Expression was detected using SYBR Green master mix from the kit. RNA concentration in control and drug-treated samples was calculated using the comparative cycle threshold values. GADD153 expression was normalized using β-actin, and each experiment was conducted in biological triplicate with freshly treated cells. A one-tailed Student’s t test was used to compare the control group with treatment groups, and a two-tailed Student’s t test was used for any comparison between treatment groups. All results were obtained from at least three separate experiments.
siRNA Transfection
SQ20b cells were transfected with GADD153 siRNA using the Amaxa 96-well shuttle nucleofection system (Amaxa Biosystems, Gaithersburg, MD) using 250,000 cells/well. The siGENOME ON-Target plus SMARTpool GADD153 siRNA (Dharmacon RNA Technologies, Lafayette, CO) or ON-Target plus Non-Targeting pool was used at a concentration of 1.25 μM, with the total volume of each well being 20 μl. Nucleofection was performed using the Amaxa 96-well Nucleofector Kit SE and nucleofector program DS-113. After nucleofection, cells were allowed to recover for 1 h before addition of BG. Treatment was then performed as described above, with the following exceptions. For the colony-forming assays, higher concentrations of cells were plated to account for the increase in cell death after nucleofection. Cells were plated at concentrations 1.5 times higher than those used for cells that were not transfected. For caspases 3 and 12 assays, apoptosis was evaluated at 48 h post-treatment based on optimization conditions.
Statistical Analysis
All statistical analyses were performed using Student’s t test assuming unequal variance, with the exception of those described below. For colony-forming assays in which the main effect (difference in slopes) was evaluated (salubrinal and siRNA assays), analysis of variance models were used with cisplatin dose (0, 6, 12.5, and 25 μM) and modulator condition as factors. The cisplatin dose by modulator condition interaction was also tested. A significant interaction would indicate that the cell survival rates differ significantly by modulator treatment. Experiment/experiment variability was controlled for by including experiment as a factor in each analysis of variance model. For analytic purposes, the outcome of interest was the proportion of cells surviving (i.e., colony count/number plated). Because of the skewness of the data, the arcsine transformation was employed as appropriate. A p value < 0.05 was considered to be statistically significant. Analyses were performed using Stata, version 10 (Stata Corporation, College Station, TX).
Results
Cell Line Specificity of BG Plus Cisplatin Enhancement
To examine the potential tissue specificity of cisplatin plus BG treatment, we tested a number of cancer cell lines representing tumor types treated with platinating agents in a clinical setting. Using a clonogenic assay, we observed significant enhancement of cisplatin-induced cytotoxicity after BG treatment in the endometrial carcinoma cell line HEC-1-A, the pancreatic tumor cell line PaCa-2, and the ovarian cancer cell line SKOV-3x (p < 0.05; Table 1). In the cervical cancer cell line C33-A and the ovarian cancer cell line SKOV-3, BG enhancement of cisplatin-induced cytotoxicity trended toward significance (Table 1). In two nonsmall cell lung carcinoma cell lines (H460 and H520), there was no enhancement of cisplatin cytotoxicity by BG (Table 1). We previously observed that treatment of five head and neck cancer cell lines with BG before and during cisplatin treatment results in increased cytotoxicity (Fishel et al., 2003), and this enhancement was not observed in the A549 nonsmall cell lung cancer cell line (Fishel et al., 2006).
TABLE 1.
IC50 values for cell lines treated with cisplatin ± BG
| Cell Line | IC50 (Cisplatin) |
Fold Enhancement | p Value | |
|---|---|---|---|---|
| −BG | +BG | |||
| μ M | ||||
| Endometrial | ||||
| HEC-1-A | 20.2 | 10.9 | 1.9 | 0.04 |
| Ovarian | ||||
| SKOV-3x | 11.0 | 6.5 | 1.7 | 0.05 |
| SKOV-3 | 10.0 | 6.2 | 1.6 | 0.07 |
| Cervical | ||||
| C33-A | 5.9 | 3.3 | 1.8 | 0.06 |
| Pancreatic | ||||
| PaCa-2 | 8.2 | 5.4 | 1.5 | 0.01 |
| Lung | ||||
| A549 | 9.7 | 10.8 | 0.9 | 0.76 |
| H460 | 4.7 | 4.5 | 1.0 | 0.28 |
| H520 | 5.2 | 6.2 | 0.9 | 0.29 |
We chose three of these cell lines to act as positive and negative controls for further study to better understand the mechanism of cellular damage caused by cisplatin in the presence of BG. Cell lines chosen as positive for enhancement by BG were the SQ20b head and neck cancer cell line and the SKOV-3x ovarian cancer cell lines, whereas the A549 nonsmall cell lung cancer cell line was chosen as a cell line negative for enhancement by BG.
Effect of BG on Cisplatin-Induced DNA Platination
We previously observed an increase in total DNA platination in the SQ20b head and neck cancer cell line after treatment with BG plus cisplatin compared with cisplatin alone (Fishel et al., 2003). Using atomic absorption spectroscopy, we evaluated DNA platination as a marker for DNA damage in head and neck (SQ20b, positive), ovarian (SKOV-3x, positive), and lung (A549, negative) cancer cell lines (Fig. 1). We observed increased levels of total platination in BG plus cisplatin-treated samples from all three cell lines analyzed, even though cisplatin-induced cytotoxicity is not enhanced in the A549 lung cancer cell line by BG. Directly after 2 h of cisplatin treatment, there were 1.4-, 1.5-, and 1.4-fold increases in total DNA platination in SQ20b, SKOV-3x, and A549 cell lines, respectively, when cells were treated with BG (Fig. 1). This increase in total platination was significant in the SQ20b cell line at all three time points and in the A549 cell line at the latter two time points. We further evaluated platinum levels with a structurally similar agent, 9-methyl-BG, that does not enhance cisplatin cytotoxicity (Fishel et al., 2005b). We observed an enhancement of total DNA platination (1.6-fold at 0 h post-treatment) in the SQ20b head and neck cell line when using 9-methyl-BG, similar to that observed after treatment with BG plus cisplatin (Fig. 1A); however, this enhancement after treatment with 9-methyl-BG plus cisplatin was not significant at the time points evaluated (0, 24, and 48 h post-treatment). We concluded that an increase in total DNA platination in these cell lines did not correlate with an increase in cytotoxicity.
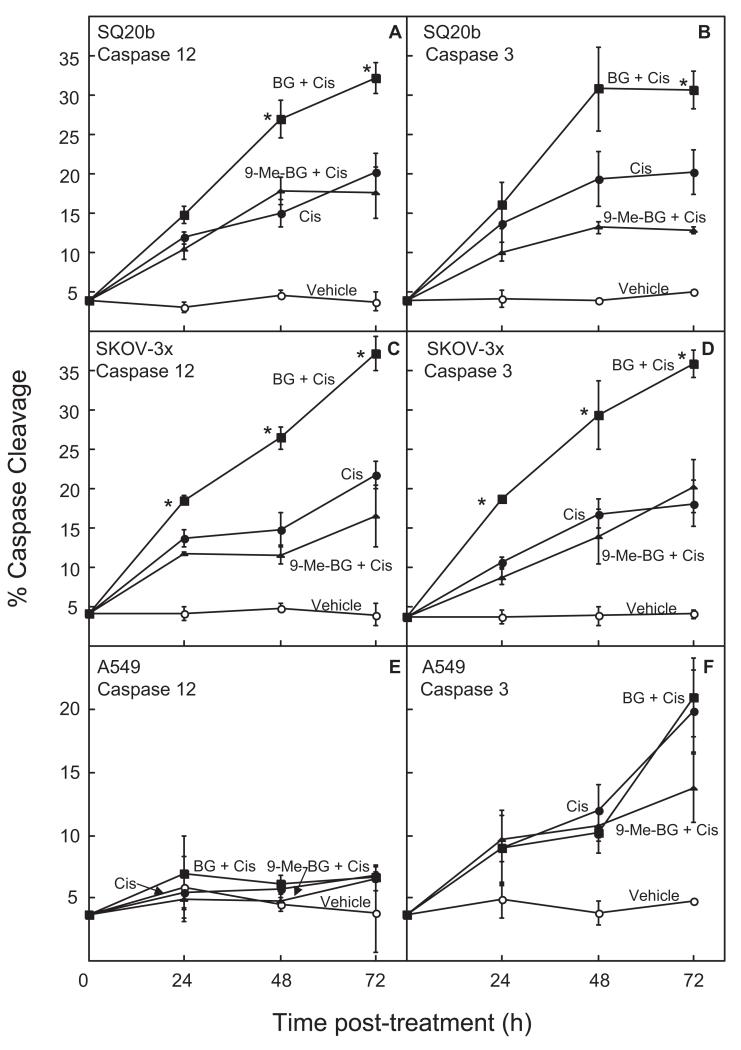
Fig. 1.
Effect of BG on cisplatin-induced DNA platination in SQ20b head and neck, SKOV-3x ovarian, and A549 nonsmall cell lung cancer cell lines. Cells were treated with 100 μM BG or vehicle followed by 50 μM cisplatin. Cells were collected at various time points after treatment. DNA was analyzed for platinum adducts using atomic absorption spectroscopy. Closed circles, cisplatin treatment alone; closed squares, cisplatin plus BG; closed triangles, cisplatin plus 9-Me-BG. A, SQ20b cell line (cisplatin alone, BG plus cisplatin results previously published in Fishel et al., 2003). B, SKOV-3x cell line. C, A549 cell line. Each point represents the mean ± S.E.M. from at least three experiments. Statistical analysis was performed on normalized samples because of interexperiment variation; *, p < 0.05.
Effect of BG on Cisplatin-Induced DSB
To determine whether DSBs were increased upon the addition of BG to cisplatin, we used an antibody specific for γH2AX, which cisplatin alone has been shown to increase (Bosco et al., 2004). Phosphorylation of histone H2AX was used as a marker to quantify formation of DNA double-strand breaks (DSBs). This assay was chosen preferentially over the comet assay (single-cell gel electrophoresis) because of its specificity for DSB. Although the comet assay also measures DSB formation, it also identifies DNA single-strand breaks (Johansson et al., 2008) and apurinic sites (Fatur et al., 2003). In contrast, H2AX phosphorylation is specific for DSB formation and does not measure other types of DNA damage (Johansson et al., 2008). In the three cell lines we tested that were positive for BG enhancement of cisplatin cytotoxicity (SQ20b, SKOV-3x, and PaCa-2), the addition of BG to cisplatin treatment resulted in increased levels of γH2AX formation compared with treatment with cisplatin alone (Fig. 2; data not shown). In SQ20b and SKOV-3x cells, 4- and 3.3-fold more γH2AX formed, respectively, in cells treated with BG plus cisplatin (50 μM) compared with cells treated with cisplatin (50 μM) alone (Fig. 2). To determine whether this was specific to cell lines in which BG enhances cisplatin cytotoxicity, we also examined γH2AX formation in the A549 lung cancer cell line and found 2.5-fold enhanced γH2AX formation with BG plus cisplatin compared with treatment with cisplatin alone (Fig. 2). We also observed that treatment of the SQ20b cell line with cisplatin plus 9-methyl-BG resulted in 2.76-fold enhancement in γH2AX formation compared with cisplatin alone (data not shown).
Fig. 2.
γH2AX Western blot after treatment with BG ± cisplatin. A, cells (SQ20b, SKOV-3x, A549) were treated with 100 μM BG or vehicle followed by cisplatin (25, 50, or 100 μM). Cells were collected 24 h after treatment. Protein was isolated using radioimmunoprecipitation assay extraction, and Western blots were probed using an antibody specific for γH2AX. β-Actin was used as a loading control. B, quantitation of γH2AX formation. Dosimetry was performed using the QuantityOne software. Each bar represents the mean ± S.E.M. for at least three separate experiments. For each cell line, samples were normalized to cisplatin, 50 μM, which was set at 1.0. *, p < 0.05.
Effect of BG ± Cisplatin on Endoplasmic Reticulum Stress
Cisplatin has been shown to result in ER stress leading to apoptosis (Mandic et al., 2003), and we have observed up-regulation of the ER stress gene GADD34 upon treatment with BG plus cisplatin (Fishel et al., 2006). To investigate further the role of ER stress in the response of cancer cell lines to cisplatin in the presence and absence of BG, we used two markers of ER stress: caspase 12 activity and induction of GADD153.
Caspase 3 and 12 Activity Assays
To determine whether the mechanism of enhancement of cisplatin-induced cytotoxicity by BG involved preferentially increasing apoptosis because of ER stress, we investigated cleavage of two different caspases: caspase 12 (ER stress-specific) and caspase 3 (general downstream) in all three cell lines. FITC-labeled small-molecule inhibitors of the active caspases were used to measure caspase cleavage after treatment with BG ± cisplatin. These inhibitors bind irreversibly to activated caspases, providing a measurement of active caspase within the cell. Using flow cytometry, we observed that in the SQ20b and SKOV-3x cell lines, cisplatin alone caused ER stress-induced apoptosis (caspase 12 cleavage) that contributed to cellular apoptosis (caspase 3 cleavage). In contrast, apoptosis in the A549 cell line was independent of ER stress because no caspase 12 cleavage is observed (Fig. 3). Previous reports have shown that A549 cells are capable of activating caspase 12, indicating that this pathway is not defective in these cells (Bitko and Barik, 2001). Cisplatin did induce apoptosis in the A549 cell line, presumably through DNA damage, as indicated by measurable caspase 3 cleavage. However, consistent with cell survival of A549 cells, there was no enhancement in caspase 3 cleavage when comparing samples treated with BG plus cisplatin with those treated with cisplatin alone (Fig. 3F). BG significantly increased the level of cisplatin-induced caspase 12 cleavage only in those cell lines in which BG enhanced cisplatin-induced cytotoxicity (SQ20b and SKOV-3x) (Fig. 3, A and C). Treatment with 9-methyl-BG (negative control) plus cisplatin did not enhance caspase 12 or 3 cleavage over the levels observed with cisplatin alone in any of the three cell lines (Fig. 3). Neither 9-methyl-BG nor BG treatment without cisplatin resulted in increased caspase cleavage over vehicle (data not shown).
Fig. 3.
Effect of BG, 9-methyl-BG, and cisplatin alone and in combination on cleavage of caspases 12 and 3. Cells (A and B, SQ20b; C and D, SKOV-3x; E and F, A549) were treated with 100 μM BG, 50 μM 9-methyl-BG, or vehicle, followed by cisplatin (25 μM). Cells were collected 24, 48, or 72 h after treatment and caspase 12 (A, C, and E) and caspase 3 (B, D, and F) cleavage was ana-lyzed by flow cytometry. Open circle, vehicle only; closed circle, cisplatin; closed square, BG plus cisplatin; closed triangle, 9-methyl-BG plus cisplatin. Each point represents the mean ± S.E.M. from at least three experiments. In the SQ20b (caspases 3 and 12) and SKOV-3x (caspases 3 and 12) cell lines, all samples treated with cisplatin, regardless of modulator, were significantly higher than in control cells treated with vehicle only (p < 0.05). Likewise, in the A549 cell line, treatment with cisplatin alone resulted in significantly higher levels of caspase 3 cleavage at 48 and 72 h than observed in control cells treated with vehicle only (p < 0.05).
Induction of GADD153 mRNA
To confirm further the role of ER stress, we evaluated the up-regulation of an important gene in the ER stress pathway, GADD153, immediately after treatment of SQ20b, SKOV-3x, and A549 cell lines with BG plus cisplatin. Upon treatment of SQ20b cells with BG alone, there was an 11-fold increase over vehicle-treated cells in GADD153 expression (p < 0.05). GADD153 RNA levels significantly increased over vehicle control from 2-fold for treatment with cisplatin alone to 23-fold for BG plus cisplatin treatment (p < 0.05) (Fig. 4). Consistent with caspase results, 9-methyl-BG plus cisplatin did not up-regulate GADD153 expression in the SQ20b head and neck cancer cell line compared with vehicle control (data not shown). In the SQ20b cell line, up-regulation of GADD153 was initiated during treatment with BG alone and was further up-regulated after the addition of cisplatin to BG treatment; however, this difference was not significant (Fig. 4).
Fig. 4.
Effect of BG and cisplatin, alone and in combination, on GADD153 mRNA expression in SQ20b (A), SKOV-3x (B), and A549 (C) cancer cell lines. Cells were treated with 100 μM BG or vehicle, followed by cisplatin (25 μM). Cells were collected immediately after treatment and GADD153 mRNA expression was assessed by RT-PCR. Each bar represents the mean ± S.E.M. for at least three replicates. *, treatment is significantly different from vehicle (p < 0.05); †, treatment is significantly different from cisplatin alone (p < 0.05).
Results similar to those in SQ20b cells were observed in the SKOV-3x ovarian cancer cell line. The following increases in GADD153 expression in SKOV-3x cells were observed: 1.4-fold (cisplatin alone), 28-fold BG alone, and 39-fold BG plus cisplatin upon comparison with vehicle control (p < 0.05). Furthermore, there was a significant increase of GADD153 in cells treated with BG plus cisplatin versus cisplatin alone in both SQ20b and SKOV-3x cells. In the A549 lung cancer cell line, BG in the presence or absence of cisplatin did not up-regulate GADD153 expression (Fig. 4).
Induction of GADD153 Protein
Because up-regulation of mRNA transcripts does not always correlate to increases in protein, we confirmed the above results by performing Western blots on SQ20b, SKOV-3x, and A549 cell lines treated with BG plus cisplatin. Although we did not observe the same fold increase observed using qRT-PCR, the overall trends remained the same. Immediately after treatment, SQ20b cells treated with BG plus cisplatin had 2.2-fold more GADD153 protein than did those treated with cisplatin alone (Fig. 5A); in SKOV-3x cells, BG plus cisplatin-treated cells had 1.8 times more GADD153 than did the corresponding cells treated with cisplatin alone (p < 0.05) (Fig. 5B). In SQ20b cells, this increased expression was also observed when comparing BG plus cisplatin-treated cells with vehicle-treated controls 6 h after treatment (data not shown). Notably, we did not observe a continued up-regulation of GADD153 protein in SKOV-3x cells at 6 h (data not shown), potentially indicating a different timeframe of ER stress induction than in the SQ20b cell line. No increase in GADD153 expression was observed in A549 lung cancer cells treated with BG plus cisplatin compared with control at both the 0- and 6-h time points, further indicating that BG plus cisplatin did not cause ER stress in this cell line (Fig. 5C; data not shown).
Fig. 5.
Effect of BG and cisplatin, alone and in combination, on GADD153 protein expression in SQ20b (A), SKOV-3x (B), and A549 (C) cancer cell lines. Cells were treated with 100 μM BG or vehicle, followed by cisplatin (50 μM). Cells were collected immediately after treatment, and GADD153 protein expression was assessed by Western blot. Each bar represents the mean ± S.E.M. for at least three replicates. Cell lines were normalized to β-actin expression and then to vehicle. *, treatment is significantly different from vehicle (p < 0.05); †, treatment is significantly different from cisplatin alone (p < 0.05).
Inhibition of ER Stress by Salubrinal and GADD153 siRNA
To determine whether the increased enhancement of cisplatin-induced cytotoxicity was because of activation of the ER stress pathway, we evaluated how inhibiting the ER stress pathway affected the treatment of SQ20b cells with BG plus cisplatin. We used two different approaches. The first involved pretreatment with salubrinal, a small-molecule inhibitor of eIF2α dephosphorylation, and the second used siRNA targeted against GADD153.
Salubrinal
Salubrinal acts by inhibiting the dephosphorylation of eIF2α (Boyce et al., 2005). Therefore, treatment with salubrinal should provide a translational repression of GADD153 induction. We confirmed significant levels of translational repression of the ER stress-specific protein, GADD153, after treatment with BG, cisplatin, and salubrinal and in samples treated with salubrinal plus cisplatin (Supplemental Fig. 1). Cells treated with salubrinal alone did not experience a significant decrease in GADD153 protein levels, which was expected because salubrinal would prevent induction of GADD153 protein but would not affect the baseline level already present in the cell (Supplemental Fig. 1). Cells treated with either cisplatin alone or BG plus cisplatin exhibited significantly lower levels compared with vehicle control. This is probably because of the complete inhibition of protein translation during eIF2a phosphorylation, which would prevent both baseline and induced expression of GADD153 (Supplemental Fig. 1). Cells were evaluated for the effect of salubrinal on cytotoxicity of BG ± cisplatin (Fig. 6A). Salubrinal treatment did slightly decrease cisplatin cytotoxicity in cells without BG; however, this decrease was not statistically significant. Salubrinal significantly decreased, albeit did not eliminate, the enhancement in cytotoxicity observed after treatment with BG plus cisplatin (Fig. 6A).
Fig. 6.
Effect of salubrinal on BG-enhanced cisplatin cytotoxicity and apoptosis. A, SQ20b cells were treated with cisplatin alone (closed square), BG (100 μM) plus cisplatin (open square), salubrinal (25 μM) plus cisplatin (closed circle), or salubrinal plus BG plus cisplatin (open circle). Total treatment was 5 h, with salubrinal alone for 1 h, salubrinal plus BG for 2 h, and salubrinal, BG, and cisplatin for 2 h. Each point represents the mean ± S.E.M. from at least three experiments, with each experiment representing six dishes per treatment group. p < 0.05 for the following comparisons of cell survival rates: vehicle versus BG, vehicle versus Sal + BG, Sal versus Sal + BG, and BG versus Sal + BG. B and C, SQ20b cells were treated with vehicle (closed squares) or salubrinal (25 μM) (open squares), cisplatin (25 μM) (closed circles), salubrinal plus cisplatin (open circles), BG (100 μM) plus cisplatin (closed triangles), or salubrinal plus BG plus cisplatin (open triangles). Each point represents the mean ± S.E.M. from at least three separate experiments. Cells were analyzed for caspase 12 (B) and 3 (C) cleavage by flow cytometry.*, p < 0.05 BG plus cisplatin versus salubrinal plus BG plus cisplatin samples. †, p < 0.01 BG plus cis versus salubrinal plus BG plus cis samples.
We then determined apoptosis by measuring both caspase 3 and 12 cleavage in SQ20b cell lines treated with or without salubrinal, BG, and cisplatin (Fig. 6, B and C). In samples treated with BG, cisplatin, and salubrinal, no enhancement of cisplatin-induced apoptosis was observed in either caspase 12 or 3 cleavage. This indicates that the enhanced apoptosis observed in Fig. 3 was due primarily to ER stress-induced apoptosis because salubrinal is specific for inhibition of ER stress-induced cell death. Corresponding to the results observed in cytotoxicity assays, there was a slight, but not significant, reduction in caspases 3 and 12 cleavage in samples treated with cisplatin plus salubrinal compared with those treated with cisplatin alone at 72 h (Fig. 6, B and C). The combination of cisplatin plus salubrinal did not eliminate the induction of apoptosis via caspase 12 or 3. Therefore, cisplatin-induced apoptosis is more dependent upon DNA damage and is less affected by inhibition of the ER stress pathway. This indicates a potential difference in the mechanism by which cells undergo apoptosis and cytotoxicity between cells treated with cisplatin alone and those treated with BG plus cisplatin.
GADD153 siRNA
Previous research has indicated the importance of GADD153 expression to potentiation of the ER stress pathway (McCullough et al., 2001). To verify the importance of GADD153 in the mechanism of BG-enhanced cisplatin cytotoxicity and because of the potential off-target effects of salubrinal in the cell, we evaluated how down-regulating GADD153 induction via siRNA would affect the ability of BG to enhance cisplatin-induced cytotoxicity and apoptosis. Because we had demonstrated a correlation between increased GADD153 mRNA expression and protein expression (Figs. 4 and 5), we used qRT-PCR to evaluate the effect of siRNA on GADD153. As a control, a scrambled, nontargeting (NT) siRNA was used as a determinant of any off-target effects caused by the siRNA molecule. qRT-PCR indicated that SQ20b cells transfected with NT siRNA before BG with or without cisplatin treatment had up-regulated GADD153, similar to that observed in untransfected cells (Supplemental Fig. 2). As in mock and untransfected cells, treatment with BG in NT-transfected cells resulted in up-regulation of GADD153, but there was not a significant difference between cells treated with BG alone and those treated with BG plus cisplatin (Supplemental Fig. 2). However, cells transfected with siRNA against GADD153 showed significant down-regulation of GADD153 expression, beginning immediately after treatment and continuing through at least 24 h post-treatment (Supplemental Fig. 2).
The ability of GADD153 siRNA to abrogate the enhancement of cisplatin cytotoxicity by BG was evaluated using long-term clonogenic assays. Although NT-transfected cells showed significant enhancement of cisplatin-induced cytotoxicity when treated with BG plus cisplatin versus cisplatin alone (p < 0.05), cells transfected with GADD153 siRNA and treated with BG plus cisplatin did not exhibit any enhanced cytotoxicity compared with treatment with cisplatin alone (Fig. 7, A and B). It is noteworthy that there was no significant difference in cytotoxicity between NT- and GADD153-transfected cells treated with cisplatin alone, nor was there a significant difference between NT-transfected cells treated with cisplatin alone and GADD153 siRNA-transfected cells treated with BG plus cisplatin. There were significant differences between NT- and GADD153-transfected cells treated with BG plus cisplatin at 6, 12.5, and 25 μM cisplatin (p < 0.05).
Fig. 7.
Effect of GADD153 knockdown on enhancement of cisplatin cytotoxicity and apoptosis by BG. A and B, SQ20b cells transfected with either NT (A) or GADD153 (B) siRNA were treated with either vehicle or BG (100 μM) plus cisplatin. Each point represents the mean ± S.E.M. from at least three experiments, with each experiment representing six dishes per treatment group. p < 0.05 for the following comparisons: NT versus NT-BG. C and D, SQ20b cells were transfected with either nontargeting (black bars) or GADD153 (white bars) siRNA. One hour post-transfection, SQ20b cells were treated with vehicle, cisplatin (25 μM), BG (100 μM), or BG plus cisplatin. Forty-eight hours after treatment, cells were analyzed for caspase 12 (C) and 3 (D) cleavage by flow cytometry. Each bar represents the mean ± S.E.M. from at least three separate experiments. *, p < 0.05.
ER stress-induced apoptosis was evaluated after transfection with GADD153 siRNA. Untransfected cells (Fig. 4) and cells transfected with either NT or GADD153 siRNA were analyzed for caspase cleavage after treatment with BG, cisplatin, or the combination (Fig. 7, C and D). Similar to the salubrinal experiments, there was not a significant difference in the percentage of cells undergoing apoptosis between cells transfected with GADD153 siRNA, NT siRNA, or untransfected cells (data not shown) after cisplatin treatment (Figs. 4 and 7, C and D). We observed no difference in caspase 3 or 12 activation between NT-transfected cells and untransfected cells with any of the treatments (data not shown). However, for both caspases 12 and 3 cleavage, cells with reduced levels of GADD153 that were treated with BG plus cisplatin showed significantly lower caspase cleavage than did untransfected or NT-transfected cells, indicating a reduction in the activation of apoptotic pathways (Fig. 7, C and D).
Discussion
Cisplatin remains a vital component of chemotherapy, and overcoming its intrinsic and acquired resistance is important for improving patient outcome. Cisplatin cytotoxicity can be modulated in head and neck, gynecologic, and pancreatic carcinoma cell lines by administration of BG. This effect is not observed in nonsmall cell lung cancer cell lines. Irrespective of BG-enhanced cytotoxicity, we observed an increase in total DNA platination and in formation of DSB as measured by γH2AX after treatment with BG plus cisplatin compared with treatment with cisplatin alone in SQ20b, SKOV-3x, and A549 cell lines. In contrast, we observed a significant increase in ER stress-induced apoptosis as measured by induction of GADD153 and caspase 12-specific cleavage specific in cell lines that demonstrated enhancement of cisplatin-induced cytotoxicity by BG. Inhibition of this pathway by either salubrinal or down-regulation of GADD153 expression significantly diminished this effect, indicating the importance of ER stress-induced damage in the mechanism of enhanced cisplatin cytotoxicity by BG.
The primary mechanism of cisplatin-induced cytotoxicity is presumed to be through the formation of platinum adducts on DNA. Our data indicate that increased platination of DNA and an increase in DSB are effects that may not result in greater cytotoxicity because lung cancer cells exposed to BG plus cisplatin exhibit increases in DNA platination and DSB similar to those observed in SQ20b and SKOV-3x cells, with-out a corresponding increase in cytotoxicity. Therefore, the enhancement of cisplatin-induced cytotoxicity by BG cannot be attributed simply to greater platinum-associated DNA damage and subsequent double-strand break as a result of the damage. In addition, the rates of decrease in DNA platination levels after cisplatin exposure were not significantly different between cells treated with BG plus cisplatin and those treated with cisplatin alone, implying that inhibition of repair is unlikely involved in the mechanism.
Our data demonstrating that an increased amount of platinum adducts on DNA is not sufficient for the increased cytotoxicity observed with BG treatment led us to investigate the role of ER stress. Several laboratories have demonstrated that the ER plays a role in the cellular response to apoptosis (Mandic et al., 2003; Liu and Baliga, 2005). Cisplatin has been shown to induce apoptosis through ER stress by Mandic et al. (2003), who observed inhibition of cisplatin-induced caspase 12 cleavage and apoptosis in colorectal cancer cell lines after treatment with the small molecule calpeptin. Cisplatin may cause ER stress through oxidative damage (Liu and Baliga, 2003, 2005), and high levels of the cytochrome P450 isoform CYP2E1 correlate with increased levels of cisplatin-induced oxidative stress in the ER because this isoform of cytochrome P450 is present in the ER and generates reactive oxygen species (Liu and Baliga, 2005). Previous data implicated ER stress in the mechanism of BG because GADD34, the regulatory subunit of protein phosphatase 1 (Kojima et al., 2003), was up-regulated in SQ20b cells after treatment with BG plus cisplatin compared with cisplatin (Fishel et al., 2006). This was not observed in SQ20b cells treated with 9-methyl-BG plus cisplatin, nor in A549 cells treated with BG plus cisplatin (Fishel et al., 2006). We now demonstrate that BG plus cisplatin treatment results in ER stress-induced apoptosis and induction of GADD153 expression in head and neck and ovarian cancer cell lines, but not in the A549 nonsmall cell lung cancer cell line. The A549 cell line is capable of undergoing ER stress-induced apoptosis through caspase 12 cleavage when treated with other agents, such as the respiratory syncytial virus (Bitko and Barik, 2001), indicating that the lack of ER stress observed after treatment with cisplatin is not because of an intrinsic defect in this pathway in this cell line.
It is interesting to note that BG alone also increased transcript levels of GADD153 in cell lines where BG is effective in modulating cisplatin activity. BG is not cytotoxic as a single agent (Fishel et al., 2003); therefore, increased GADD153 mRNA levels alone are not sufficient for cytotoxicity. However, increased levels of GADD153 exacerbate the oxidative stress that cisplatin causes in the ER of these cell lines, resulting in cumulative apoptosis through the ER stress, whereas in cells treated with BG alone, the lack of additional stress from cisplatin does not elevate levels of apoptosis, nor does it result in cytotoxicity. One potential mechanism to explain our data is that increased levels of GADD153 exacerbate the oxidative stress that cisplatin causes in the ER, resulting in cumulative apoptosis through the ER stress pathway resulting in enhanced cytotoxicity. In contrast, cells treated with BG alone have increased GADD153 but lack additional stress from cisplatin and therefore do not result in apoptosis or cytotoxicity.
We used two approaches to mechanistically determine the role of ER stress in the response of cells to BG plus cisplatin. We inhibited activation of the ER stress pathway through either pretreatment with salubrinal or down-regulation of GADD153. Salubrinal was discovered as an inhibitor of ER stress in a chemical screen and was observed to specifically inhibit apoptosis caused by known ER stressors, including tunicamycin (Boyce et al., 2005). Salubrinal acts by inducing phosphorylation of eIF2α, then inhibiting its dephosphorylation, most likely through a direct interaction with the GADD34/protein phosphatase 1 complex (Boyce et al., 2005), and is specific for eIF2α (Boyce et al., 2005). In rat hippocampal neurons, salubrinal decreased the level of ER stress-induced apoptosis by reducing caspase 12 cleavage and increasing cell survival (Sokka et al., 2007). In human leukemia cells treated with curcumin, a phytochemical that induces apoptosis through the ER stress pathway in many cancer cell lines, pretreatment with salubrinal resulted in a significant decrease in curcumin-induced apoptosis and down-regulation of GADD153 expression (Pae et al., 2007). In our studies, salubrinal treatment completely ablated enhancement of cisplatin by BG as measured by caspase cleavage, with partial mitigation of enhancement in long-term cytotoxicity experiments.
Salubrinal may also have other effects in the cell because recent work has suggested that salubrinal can also act as a cellular protector of Bcl-2, preventing loss of this antiapoptotic protein (Kessel, 2006). This effect would also lead to a decrease in caspase activity and an increase in cell survival, as we observed after treatment with salubrinal, BG, and cisplatin. Because of this, GADD153 siRNA was also evaluated. GADD153 has been used extensively both as a marker of ER stress-induced apoptosis and as a target for siRNA to inhibit ER stress-induced apoptosis (Pae et al., 2007). In addition to salubrinal, Pae et al. (2007), used siRNA targeted against GADD153 to inhibit curcumin-induced ER stress; GADD153 siRNA was as effective as salubrinal at inhibiting apoptosis after treatment with curcumin (Pae et al., 2007). GADD153 knockdown by siRNA was used in cervical cancer cells to inhibit GADD153-dependent apoptosis after treatment with celecoxib (Kim et al., 2006). Likewise, in a colorectal cancer cell line, GADD153 siRNA attenuated resveratrol-induced apoptosis (Woo et al., 2007).
Inhibition of the ER stress pathway by both approaches at least partially inhibited the enhancement of cisplatin-induced cytotoxicity observed after treatment with BG plus cisplatin compared with treatment with cisplatin alone. Neither down-regulation of GADD153 nor addition of salubrinal significantly affected the percentage of cells undergoing apoptosis in cells treated with cisplatin alone. However, salubrinal resulted in slightly more cellular resistance to cisplatin, as measured by clonogenic assays. This raises the question of the importance of ER stress-induced apoptosis in SQ20b cells treated with cisplatin alone. It may be that the relatively modest amount of caspase 12 cleavage observed after cisplatin treatment is not enough to be affected by salubrinal, which may have other effects in the cell. Therefore, we believe that BG is acting at least partially through augmentation of cisplatin-induced apoptosis via the ER stress pathway.
Although cisplatin remains a vital mainstay of chemotherapy, the incidences of recurrence and platinum resistance lead to poor survival rates for head and neck, ovarian, and nonsmall cell lung cancer patients (Jacobs et al., 1992; Moss and Kaye, 2002; Hartmann and Lipp, 2003; Siddik, 2003; Arnold et al., 2006), underscoring the need to develop effective modulators of platinating agents. We have shown that BG enhances the efficacy of cisplatin by activating the ER stress pathway to increase apoptosis. Further insight into the role of cisplatin in the ER stress response will yield information about the targets of cisplatin and BG in this pathway. The ability to increase apoptosis though the ER stress pathway may circumvent known resistance mechanisms commonly observed with regimens that include platinum-based agents. BG may affect the type and/or quantity of damage that cisplatin causes in the cell; we have shown that BG quantitatively increases the amount of platinum on DNA, and it seems that BG induces greater cisplatin damage in the ER. BG enhances the ability of cisplatin to cause ER stress, leading to increased apoptosis. This should be examined further along with the identification of the cellular target of BG that leads to the increase in ER stress.
Supplementary Material
Acknowledgments
We thank Maria Chidiamara Njoku for excellent technical assistance.
This study was supported in part by the National Institutes of Health Grants CA81485 (to M.E.D.) and CA094025, CA106298, and CA114571 (to M.R.K.), Medical Scientist National Research Service Award Grants 5 T32 GM07281 (to C.A.R.) and CA122298 (to M.L.F.), a Marsha Rivkin Center for Ovarian Cancer Research grant (to M.L.F.), and Riley Children’s Foundation Grant (to M.R.K.).
ABBREVIATIONS
- cisplatin
cis-diammine dichloroplatinum (II)
- BG
O6-benzylguanine
- ER
endoplasmic reticulum
- GADD
growth arrest and DNA damage inducible
- FBS
fetal bovine serum
- DMSO
dimethyl sulfoxide
- 9-methyl-BG
9-methyl-O6-benzylguanine
- DSB
double-strand break
- γH2AX
phosphorylated histone H2AX
- FITC
fluorescein isothiocyanate
- PCR
polymerase chain reaction
- qRT
quantitative real-time
- siRNA
small interfering RNA
- NT
nontargeting
Footnotes
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
Contributor Information
Cara A. Rabik, Departments of Molecular Genetics and Cell Biology, University of Chicago, Chicago, Illinois
Melissa L. Fishel, Department of Pediatrics, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, Indiana
Julianne L. Holleran, Departments of Medicine, Pharmacology, and Cancer Institute, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
Kristen Kasza, Department of Health Studies, University of Chicago, Chicago, Illinois.
Mark R. Kelley, Department of Pediatrics, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, Indiana; Departments of Pharmacology and Toxicology and Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, Indiana
Merrill J. Egorin, Departments of Medicine, Pharmacology, and Cancer Institute, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
M. Eileen Dolan, Department of Medicine, University of Chicago, Chicago, Illinois.
References
- Arnold SM, Warren GW, Valentino J, Brill Y, Regine W, Spring P, Given C, Mohiuddin M, Huhn JL, Kudrimoti M. Long term results of regionalcontrol in stage III-IV node positive patients with squamous cell carcinoma of the head and neck using hyperfractionated radiation therapy and intraarterial chemotherapy (HYPERRADPLAT) Int J Radiat Oncol. 2006;66:S446–S447. [Google Scholar]
- Bitko V, Barik S. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J Cell Biochem. 2001;80:441–454. doi: 10.1002/1097-4644(20010301)80:3<441::aid-jcb170>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bosco EE, Mayhew CN, Hennigan RF, Sage J, Jacks T, Knudsen ES. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Dolan ME, Pegg AE. O6-Benzylguanine and its role in chemotherapy. Clin Cancer Res. 1997;3:837–847. [PubMed] [Google Scholar]
- Erkmen K, Egorin MJ, Reyno LM, Morgan R, Jr, Doroshow JH. Effects of storage on the binding of carboplatin to plasma proteins. Cancer Chemother Pharmacol. 1995;35:254–256. doi: 10.1007/BF00686557. [DOI] [PubMed] [Google Scholar]
- Fatur T, Lah TT, Filipic M. Cadmium inhibits repair of UV-, methyl methanesulfonate-, and N-methyl-N-nitrosourea-induced DNA damage in Chinese hamster ovary cells. Mutation Res. 2003;529:109–116. doi: 10.1016/s0027-5107(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Fishel ML, Delaney SM, Friesen LD, Hansen RJ, Zuhowski EG, Moschel RC, Egorin MJ, Dolan ME. Enhancement of platinum-induced cytotoxicity by O6-benzylguanine. Mol Cancer Ther. 2003;2:633–640. [PubMed] [Google Scholar]
- Fishel ML, Gamcsik MP, Delaney SM, Zuhowski EG, Maher VM, Karrison T, Moschel RC, Egorin MJ, Dolan ME. Role of glutathione and nucleotide excision repair in modulation of cisplatin activity with O6-benzylguanine. Cancer Chemother Pharmacol. 2005a;55:333–342. doi: 10.1007/s00280-004-0901-3. [DOI] [PubMed] [Google Scholar]
- Fishel ML, He Y, Smith ML, Kelley MR. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin Cancer Res. 2007;13:260–267. doi: 10.1158/1078-0432.CCR-06-1920. [DOI] [PubMed] [Google Scholar]
- Fishel ML, Newell DR, Griffin RJ, Davison R, Wang LZ, Curtin NJ, Zuhowski EG, Kasza K, Egorin MJ, Moschel RC, et al. Effect of cell cycle inhibition on cisplatin-induced cytotoxicity. J Pharmacol Exp Ther. 2005b;312:206–213. doi: 10.1124/jpet.104.073924. [DOI] [PubMed] [Google Scholar]
- Fishel ML, Rabik CA, Bleibel WK, Li X, Moschel RC, Dolan ME. Role of GADD34 in modulation of cisplatin cytotoxicity. Biochem Pharmacol. 2006;71:239–247. doi: 10.1016/j.bcp.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, Hale TJ, Soengas MS, Kaufman RJ, Wang CY. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–31447. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Lyman G, Velez-García E, Sridhar KS, Knight W, Hochster H, Goodnough LT, Mortimer JE, Einhorn LH, Schacter L. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10:257–263. doi: 10.1200/JCO.1992.10.2.257. [DOI] [PubMed] [Google Scholar]
- Johansson VM, Oredsson SM, Alm K. Polyamine depletion with two different polyamine analogues causes DNA damage in human breast cancer cell lines. DNA Cell Biol. 2008;27:1–6. doi: 10.1089/dna.2008.0750. [DOI] [PubMed] [Google Scholar]
- Kessel D. Protection of Bcl-2 by salubrinal. Biochem Biophys Res Commun. 2006;346:1320–1323. doi: 10.1016/j.bbrc.2006.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hwang CI, Park WY, Lee JH, Song YS. GADD153 mediates celecoxib-induced apoptosis in cervical cancer cells. Carcinogenesis. 2006;27:1961–1969. doi: 10.1093/carcin/bgl027. [DOI] [PubMed] [Google Scholar]
- Kojima E, Takeuchi A, Haneda M, Yagi A, Hasegawa T, Yamaki K, Takeda K, Akira S, Shimokata K, Isobe K. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 2003;17:1573–1575. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- Linder S, Shoshan MC. Lysosomes and endoplasmic reticulum: targets for improved, selective anticancer therapy. Drug Resist Updat. 2005;8:199–204. doi: 10.1016/j.drup.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 2003;63:1687–1696. doi: 10.1046/j.1523-1755.2003.00908.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol. 2005;16:1985–1992. doi: 10.1681/ASN.2004090768. [DOI] [PubMed] [Google Scholar]
- Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C, Kaye SB. Ovarian cancer: progress and continuing controversies in management. Eur J Cancer. 2002;38:1701–1707. doi: 10.1016/s0959-8049(02)00161-2. [DOI] [PubMed] [Google Scholar]
- Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K, Jr, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65:11658–11666. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- Pae HO, Jeong SO, Jeong GS, Kim KM, Kim HS, Kim SA, Kim YC, Kang SD, Kim BN, Chung HT. Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem Biophys Res Commun. 2007;353:1040–1045. doi: 10.1016/j.bbrc.2006.12.133. [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neuroscience. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41:161–170. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo KJ, Lee TJ, Lee SH, Lee JM, Seo JH, Jeong YJ, Park JW, Kwon TK. Elevated gadd153/chop expression during resveratrol-induced apoptosis in human colon cancer cells. Biochem Pharmacol. 2007;73:68–76. doi: 10.1016/j.bcp.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Zorbas H, Keppler BK. Cisplatin damage: are DNA repair proteins saviors or traitors to the cell? Chembiochem. 2005;6:1157–1166. doi: 10.1002/cbic.200400427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.