Abstract
A major cause of morbidity in patients with multiple myeloma is the development and progression of bone disease. Myeloma bone disease is characterized by rampant osteolysis in the presence of absent or diminished bone formation. Heparanase, an enzyme that acts both at the cell-surface and within the extracellular matrix to degrade polymeric heparan sulfate chains, is upregulated in a variety of human cancers including multiple myeloma. We and others have shown that heparanase enhances osteoclastogenesis and bone loss. However, increased osteolysis is only one element of the spectrum of myeloma bone disease. In the present study, we hypothesized that heparanase would also affect mesenchymal cells in the bone microenvironment and investigated the effect of heparanase on the differentiation of osteoblast/stromal lineage cells. Using a combination of molecular, biochemical, cellular and in vivo approaches, we demonstrated that heparanase significantly inhibited osteoblast differentiation and mineralization, and reduced bone formation in vivo. In addition, heparanase also shifts the differentiation potential of osteoblast progenitors from osteoblastogenesis to adipogenesis. Mechanistically, this shift in cell fate is due, at least in part, to heparanase-enhanced production and secretion of the Wnt signaling pathway inhibitor DKK1 by both osteoblast progenitors and myeloma cells. Collectively, these data provide important new insights into the role of heparanase in all aspects of myeloma bone disease and strongly support the use of heparanase inhibitors in the treatment of multiple myeloma.
Keywords: Multiple myeloma, Bone disease, Heparanase, Osteoblastogenesis, Adipogenesis, Bone microenvironment
1. Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by the development of progressive and destructive osteolytic bone disease that is associated with diminished numbers of marrow stromal cells and osteoblasts [17, 27]. Despite recent advances in treatment strategies myeloma remains largely incurable, with renal failure and immunosuppression as well as bone destruction the major causes of morbidity [11, 14, 27]. Numerous studies have shown that the rampant osteolysis in myeloma results from the uncoupling of osteoclastic bone resorption and osteoblastic bone formation [14, 17, 27]. However, the molecular mechanisms regulating these events are not fully understood.
Heparanase is an enzyme that cleaves the heparan sulfate chains of proteoglycans into shorter chain length oligosaccharides [2, 32] and is upregulated in a variety of human tumors, including myeloma [5, 9, 10, 15, 19, 21, 29]. We have demonstrated that increased levels of heparanase dramatically enhance myeloma tumor growth, angiogenesis, and the spontaneous metastasis of tumor cells to bone [18, 26, 33, 35]. Recently, we reported that the expression of heparanase by myeloma cells markedly increased local and systemic osteolysis [36].
However, whether heparanase also contributes to the decreased osteoblast compartment common in myeloma bone disease remains unknown. In the present study, we determined the mechanism(s) by which heparanase modifies the development and/or activity of mesenchymal lineage cells that differentiate into osteoblasts and adipocytes in the bone marrow microenvironment.
2. Methods
2.1. Cells and reagents
The CAG myeloma cell line was established at the University of Arkansas for Medical Sciences (Little Rock, AR) as described previously [3]. CAG cells have a low level of endogenous heparanase expression and were previously transfected with empty vector (HPSE-low) or with vector containing the cDNA for the active form of human heparanase (HPSE-high) [16, 25, 33, 35]. The murine C3H10T1/2 and ST2 pre-osteoblast cell lines were obtained from ATCC (Manassas, VA) and cultured as described below.
Recombinant human heparanase (rHPSE) and heparanase antibodies were kindly provided by Dr. Israel Vlodavsky (Technion, Haifa, Israel). Dickkopf1(DKK1) inhibitor was purchased from Millipore (Billerica, MA); active and total β-catenin antibodies were purchased from Cell signaling (Danvers, MA); human osteocalcin and mouse peroxisome proliferator-activated receptor gamma (PPARγ) antibodies were obtained from ABCAM (Cambridge, MA); and mouse Runt-related transcription factor 2 (Runx2) antibody was purchased from MBL (Woods Hole, MA). Human and mouse DKK1 ELISA kits were obtained from R&D Systems (Minneapolis, MN). ALP and Oil Red O staining kits and β-actin antibody were purchased from Sigma (St. Louis, MO); and the Von Kossa staining kit was from Polysciences (Warrington, PA).
2.2. Animals
All animals were used in this study according to the NIH Guide for the Care and Use of Laboratory Animals and were approved under local institutional guidelines for the humane use of animals in research. SCID (CB.17 scid/scid) and C57BL/6 mice were purchased from Harlan Laboratories, Inc (Indianapolis, IN) and housed in individual cages (5 per cage) in temperature (22°C) and humidity (50%) controlled rooms having a 12 h light/12 h dark cycle with food and water ad libitum. All animal experiments were performed under a UAB IACUC approved protocol.
2.3. SCID-hu model, myeloma bone marrow specimens and Immunohistochemistry
The SCID-hu is a well described animal model in which human fetal long bones (Advanced Bioscience Resources, Inc., Alameda, CA) are implanted subcutaneously on each side of the dorsum of SCID mice [33, 34, 37]. 105 CAG HPSE-low or HPSE-high cells were injected directly into the cut end of one human bone graft (primary bone) in each mouse, whereas the contralaterally implanted human bones were not injected with tumor cells (7 mice in each group). Eight weeks after the injection of tumor cells, mice were euthanized. Tumor-injected human bones and non-injected contralateral human bones were collected and fixed in 10% neutral-buffered formalin and embedded in paraffin as described [36]. The paraffin-embedded bone sections were then stained with human osteocalcin antibody according to the manufacturer’s recommendations and the numbers of osteocalcin positive osteoblasts on the surface of trabecular bones were counted [25, 33].
Twenty eight paraffin-embedded bone marrow core biopsy specimens of myeloma patients, obtained from the Department of Pathology at UAB, were stained for both heparanase and osteocalcin. The experimental procedures and protocols were approved by the UAB Institutional Review Board. Scoring for staining densities was determined in a blinded fashion by two different readers as described previously [18, 36].
2.4. Preparation of conditioned medium (CM) of HPSE-low and HPSE-high cells
HPSE-low and HPSE-high CAG myeloma cells were seeded at a concentration of 5 ×105 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum and incubated for 48 h at 37 °C and 5% CO2 in a humidified chamber. Medium conditioned by the cells was collected at the end of the incubation period and centrifuged at 1000 rpm to remove all the cells. The clarified medium was then aliquoted and stored at 4°C or −20 °C until further use.
2.5. Osteoblast and Adipocyte differentiation of primary mouse osteoblastic progenitors
To prepare primary murine osteoblastic progenitors, calvaria were excised from newborn C57BL/6 mice, washed in RPMI 1640 medium, and digested in α-MEM medium containing 0.1% collagenase type A and 0.05% trypsin-EDTA at 37 °C for 20 min, 30 min and 90 min respectively [1]. The supernatant from the first two digestions was discarded, and the cell pellet from the third digestion was resuspended in serum free α-MEM medium, washed and plated onto 100 mm dishes and grown in α-MEM medium supplemented with 10% FCS, 1% glutamine, 1% streptomycin and 1% penicillin until confluent.
Upon reaching confluence, the expanded cells were placed in osteogenic medium (α-MEM medium supplemented with 10% FBS, 1% streptomycin and 1% penicillin, and 10 mM β-glycerophosphate and 50 ug/ml ascorbic acid) in the absence or presence of rHPSE (50 ng/ml) or in a 1:1 mixture of osteogenic medium and conditioned medium (CM) from CAG myeloma HPSE-low or HPSE-high cells. In a separate experiment, the primary murine osteoblastic progenitors were cultured in the above conditions with or without DKK1 inhibitor (3.0 mM). The medium was replaced every 3 days and cell protein was isolated at the times indicated.
The same populations of primary murine osteoblastic progenitors were also cultured in adipocyte differentiation medium (α-MEM medium supplemented with 10% FBS, 1% streptomycin and 1% penicillin, insulin10µg/ml, 0.25 µ M Dexamethasone and 0.5mM 1-methyl-3-isobutylxanthine), in the absence or presence of rHPSE or with the 1:1 addition of the CM of CAG HPSE-low or HPSE-high cells. Culture medium was changed every 3 days and protein and conditioned medium was collected at day 10.
2.6. ALP, Von Kossa and Oil Red O staining
After primary murine calvarial osteoblastic progenitors were cultured in osteogenic medium for 14 days, alkaline phosphatase (ALP) staining for the evaluation of recruitment into the osteoblastic lineage was performed using an ALP kit according to the manufacturer’s instructions (Sigma). Von Kossa staining was performed at day 21 of cell culture for the measurement of matrix mineralization and as a measure of the differentiation of mature osteoblasts. Similarly, Oil Red O staining was performed on the cells cultured toward adipocytes for 10 days. All staining was performed following the manufacturer’s recommendations as we have described [20].
2.7. Western blotting
Equal amounts of protein (80 ug) were subjected to 4–12% gradient SDS-PAGE (BioRad) and transferred to nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) [33]. Transferred proteins were probed with appropriate antibodies and visualized using an enhanced chemiluminescence system (Amersham Biosciences, Buckinghasmshire, UK). Western blots were quantified by NIH ImageJ software version 1.45.
2.8. Enzyme-linked immunosorbent assay (ELISA)
Human DKK1 levels in the conditioned medium from HPSE-low and HPSE-high CAG cells were measured using hDKK1 Quantikine ELISA kit (R&D Systems). Mouse DKK1 levels in conditioned medium from primary murine osteoblastic progenitor cells, C3H10T1/2 pre-osteoblastic cells and ST2 stromal cells cultured in absence or presence of rHPSE were measured by mDKK1 Quantikine ELISA (R&D Systems). All assays were performed according to the protocols provided by the manufacturers.
3. Statistical analysis
Statistical comparisons between two experimental groups were analyzed by Student’s t test. ANOVA was employed for statistical analyses among multiple groups, followed by a post-hoc Bonferroni test. The correlations between heparanase and osteocalcin expression in MM patients’ samples was assessed using Spearman correlation coefficient. P<0.05 was considered statistically significant and is reported as such.
4. Results
4.1. Heparanase suppresses bone formation locally and systemically in the animal model of myeloma
To determine the effects of heparanase expression by myeloma cells on mesenchymal lineage cells, we first examined the effect of heparanase on osteoblastogenesis and parameters of bone formation by evaluating osteoblast parameters in SCID-hu mice [36].
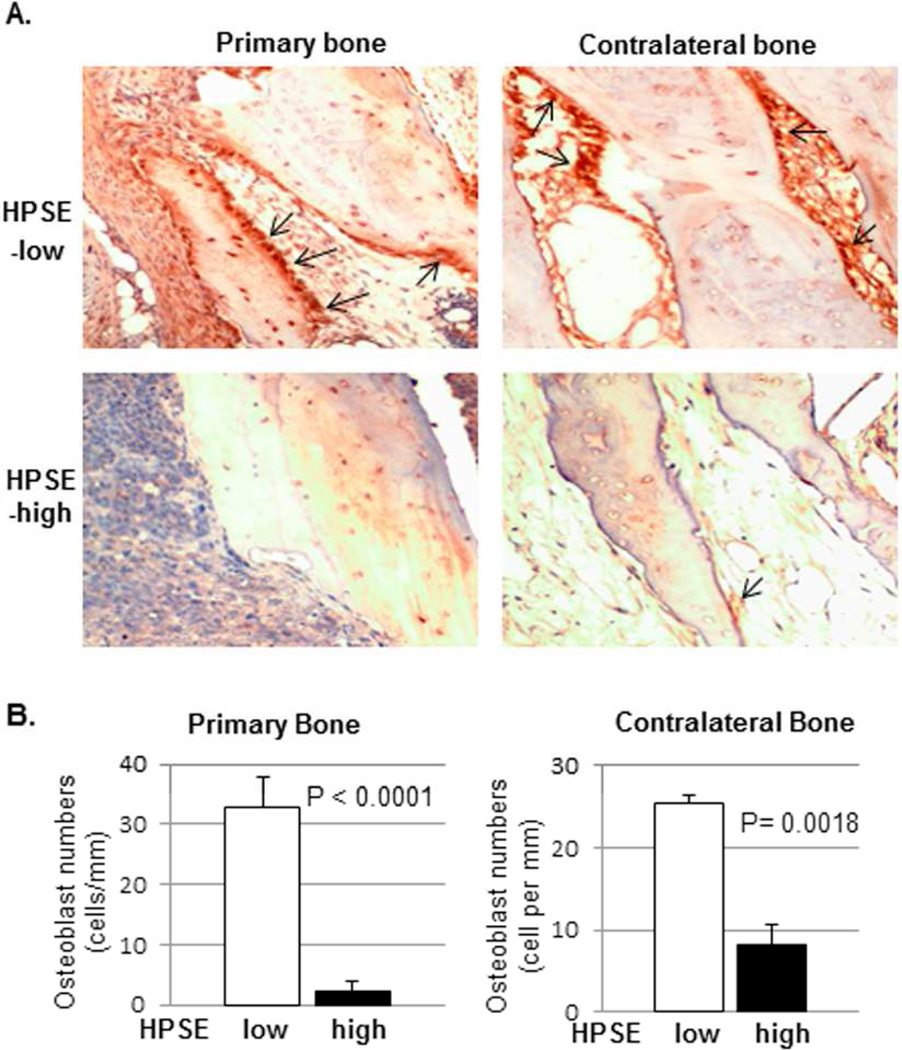
We measured osteocalcin expression, a marker of mature osteoblast differentiation, in histologic sections of engrafted bone [23]. The analysis revealed that the number of osteocalcin-positive osteoblasts was significantly diminished in both primary (injected with tumor cells) and contralateral (not injected with tumor cells) engrafted bone of the mice bearing HPSE-high tumor, compared to animals bearing tumors formed by HPSE-low cells (Figure 1A–B). These data provided the first direct experimental evidence that osteoblastogenesis was inhibited by heparanase in vivo. Interestingly, immunohistochemical staining for human kappa light chain, a specific marker of CAG myeloma cells, revealed no detectable tumor cells in uninjected contralateral grafts (data not shown), suggesting that the inhibition of osteoblastogenesis was humoral and not due to myeloma tumor metastasis to the contralateral graft.
Figure 1. Heparanase suppresses bone formation in animal model of multiple myeloma.
Human fetal bones were implanted into SCID mice. (A). Primary bone (injected with HPSE-low [top-left] or HPSE-high [bottom-left] myeloma cells) and contralateral bone (uninjected) were harvested and stained for human osteocalcin. The brown-colored cells along the bone surface are osteoblasts (arrows). A-top: Primary and contralateral bones harvested from the mice bearing HPSE-low tumors. Many osteoblasts are visible on the bone surface. A-bottom: Primary and contralateral bones harvested from mice bearing HPSE-high tumors. Original magnification, ×400. (B). The number of osteoblasts on bone surface was measured in both primary and contralateral bones of the mice bearing HPSE-low or HPSE-high tumors (Mean ± SEM per mm of bone surface; n= 7). Significance is indicated for each comparison in each panel.
4.2. The level of osteocalcin expression negatively correlates with the level of heparanase expression in the bone marrow of myeloma patients
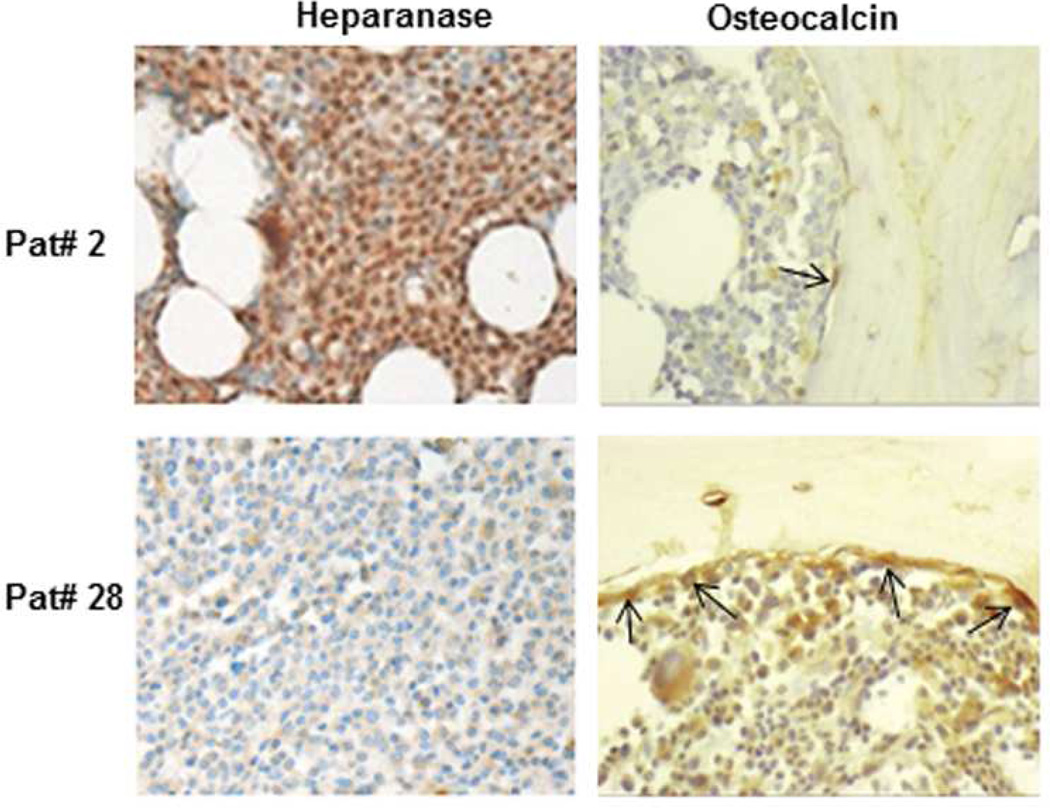
We next investigated the correlation between heparanase expression and osteocalcin expression in myeloma patients, using primary bone marrow core biopsy specimens obtained from myeloma patients. Specific immunohistochemical staining for heparanase and osteocalcin was performed on 28 myeloma patient bone marrow core biopsy specimens. We identified a significant negative correlation (r = −0.62, p< 0.001) between heparanase expression by myeloma cells and osteocalcin expression by bone marrow cells (Figure 2 and Supplementary Table 1). This finding, in conjunction with earlier data that heparanase expression is positively correlated with RANKL expression [36], suggests that heparanase is an important driver of both the proosteoclastic and anti-osteoblastic phenotype characteristic of myeloma bone disease. Interestingly, we also observed a higher population of adipocytes in the bone marrow of myeloma patients with the high levels of heparanase expression (Figure 2).
Figure 2. Myeloma tumor cell expression of heparanase negatively correlates with the level of osteocalcin in the bone marrow of myeloma patients.
Heparanase and osteocalcin staining of representative myeloma patient bone marrow biopsy specimens. Patient 2 (Pat#2): HPSE staining is high (brown stained cells); osteocalcin staining is low (arrows). Patient 28 (Pat#28): HPSE staining is low (brown stained cells); osteocalcin staining is high (arrows). Note: balloon shaped, unstained cells are adipocytes. Images are representative of the 28 biopsy samples examined. Original magnification, ×400.
4.3. Conditioned medium (CM) of HPSE-high cells inhibits osteoblastic differentiation, mineralization and promotes adipocyte differentiation of osteoblast progenitor cells
Primary calvarial osteoblast progenitor cells were cultured 1:1 in the CM from HPSE-low or HPSE-high cells and osteogenic medium as indicated (Figure 3A). Osteoblast differentiation (ALP staining) and mineralization (Von Kossa staining) was significantly suppressed by the CM of HPSE-high cells, compared to CM of HPSE-low cells. Conversely, significantly higher numbers of adipocytes were observed in cells cultured with HPSE-high CM versus HPSE-low CM (Figure 3A), suggesting the CM of HPSE-high cells supports the differentiation of mesenchymal progenitors towards the adipocyte lineage.
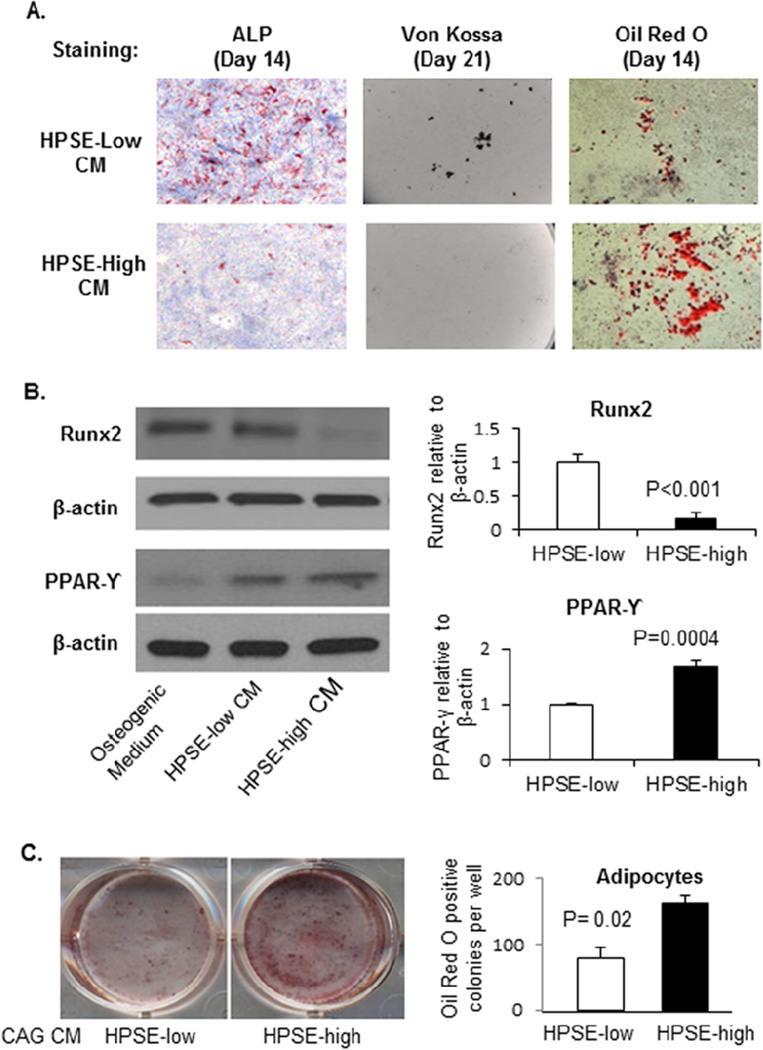
Figure 3. CM from heparanase-high myeloma cells shifts osteoblast progenitor cells from differentiating into osteoblasts to differentiating into adipocytes.
(A). Primary osteoblast progenitor cells harvested from the calvaria of new-born C57BL6 mice were cultured in the CM of HPSE-low or HPSE-high cells with equal volume of osteogenic medium, with medium changed every 3 days. ALP (Day 14), Von Kossa (day 21) and Oil Red O (day 14) staining performed. ALP stained osteoblasts, Von Kossa stained mineralized nodules and Oil Red O stained adipocytes were quantified using ImageJ software. Osteoblast number in HPSE-low CM vs. HPSE-high CM group: 138 ± 44 cells/cm2 vs. 50 ± 17 cells/cm2 (P<0.01); Mineralized nodule number in HPSE-low CM vs. HPSE-high CM group: 847 ± 59 nodules/mm2 vs. 58 ± 26 nodules/mm2 (P<0.0001); and Adipocyte number in HPSE-low CM vs. HPSE-high CM group: 1152 ± 114 cells/mm2 vs. 2869 ± 143 cells/mm2 (P=0.006). (B). Protein was isolated from cultured cells at day 14. Western blots were performed for Runx2 and PPARγ expression in day 14 cells. Left: Western blot; Right: quantification of Runx2 and PPARγ. Each bar represents the average ± SEM from two independent experiments. Significance is indicated for each comparison in each panel. (C). Primary osteoblast progenitor cells were cultured in the CM of HPSE-low or HPSE-high cells with equal volume of adipocyte differentiation medium (12-well plate). Left: Oil Red O staining was performed on day 10 of culture. Right: The number of Oil Red O stained adipocytes in each well was enumerated. Each bar represents the average ± SEM from quadruplicate wells of two independent experiments.
To begin to understand the mechanism(s) involved in the suppression of osteoblastogenesis, we next performed Western blot analysis. A significant decrease in Runxexpression (a marker of osteoblastogenesis) and increase in PPAR-γ expression (a marker of adipogenesis) was observed in HPSE-high CM treated cells, compared to HPSE-low CM treated cells (Figure 3B). In a separate experiment, primary murine osteoblast progenitor cells were cultured in the CM of HPSE-low or HPSE-high cells with 1:1 adipocyte differentiation medium for 10 days. Oil Red O staining demonstrated a significant increase in adipocytes in the presence of HPSE-high CM, compared with HPSE-low CM (Figure 3C). Taken together, these data suggest that HPSE-high myeloma cells secrete soluble factor(s) to inhibit osteoblastogenesis and promote adipogenesis, even if the cells are in a pro-osteoblastogenic environment in vitro.
4.4. Heparanase enhances DKK1 expression and secretion by myeloma cells, resulting in inhibition of Wnt signaling pathway in osteoblast progenitors
To identify what soluble factor(s) secreted by HPSE-high myeloma cells may be responsible for the decreased osteoblastogenesis and increased adipogenesis, we measured the levels of known regulators transforming growth factor beta (TGF-β), Bone Morphogenetic Protein 2 (BMP-2) and Dickkopf1 (DDK1) in the CM of HPSE-low and HPSE-high cells by ELISA. There was no significant difference in the levels of TGF-β and BMP-2 in the CM of the two types of cell lines (Data not shown). However, a significant increase in DKK1 was found in the CM of three different HPSE-high expressing cell lines, compared to the comparable HPSElow cells (Figure 4A).
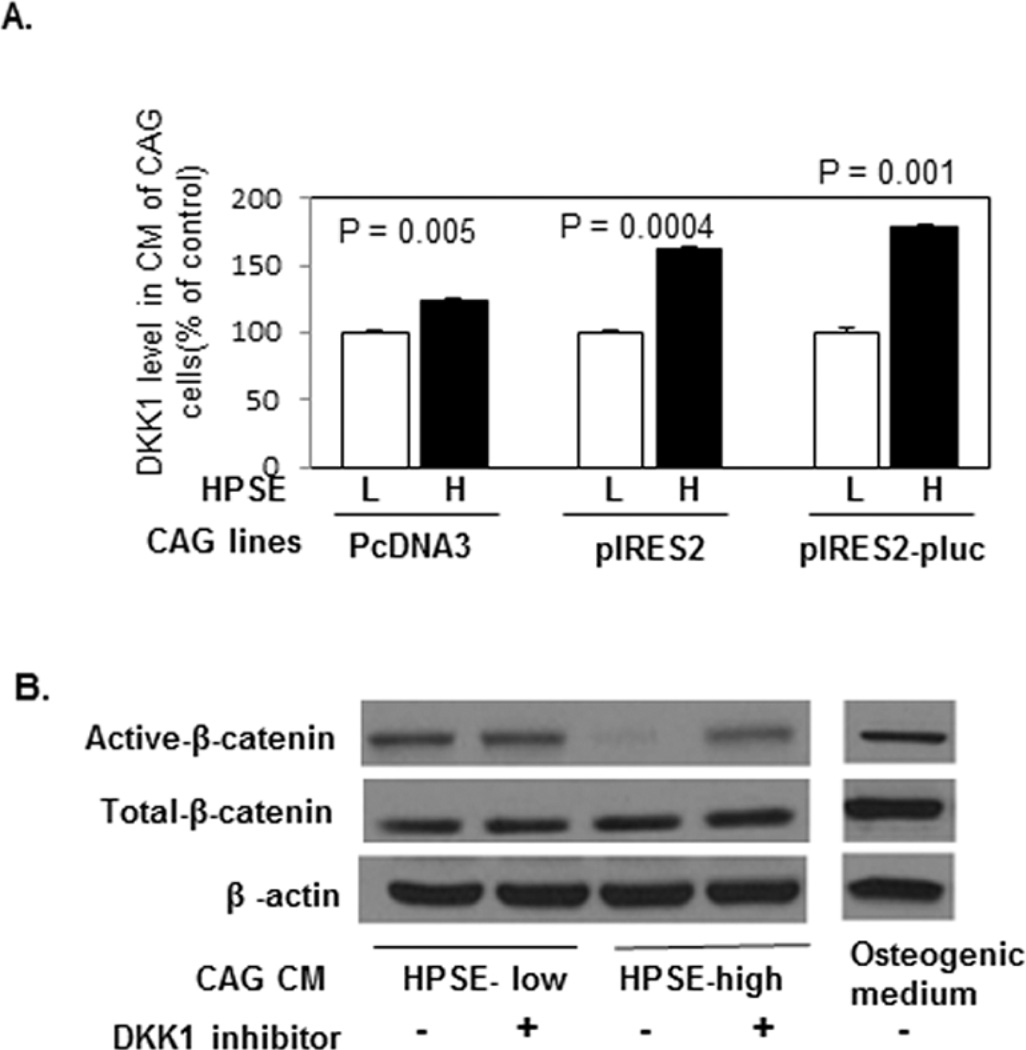
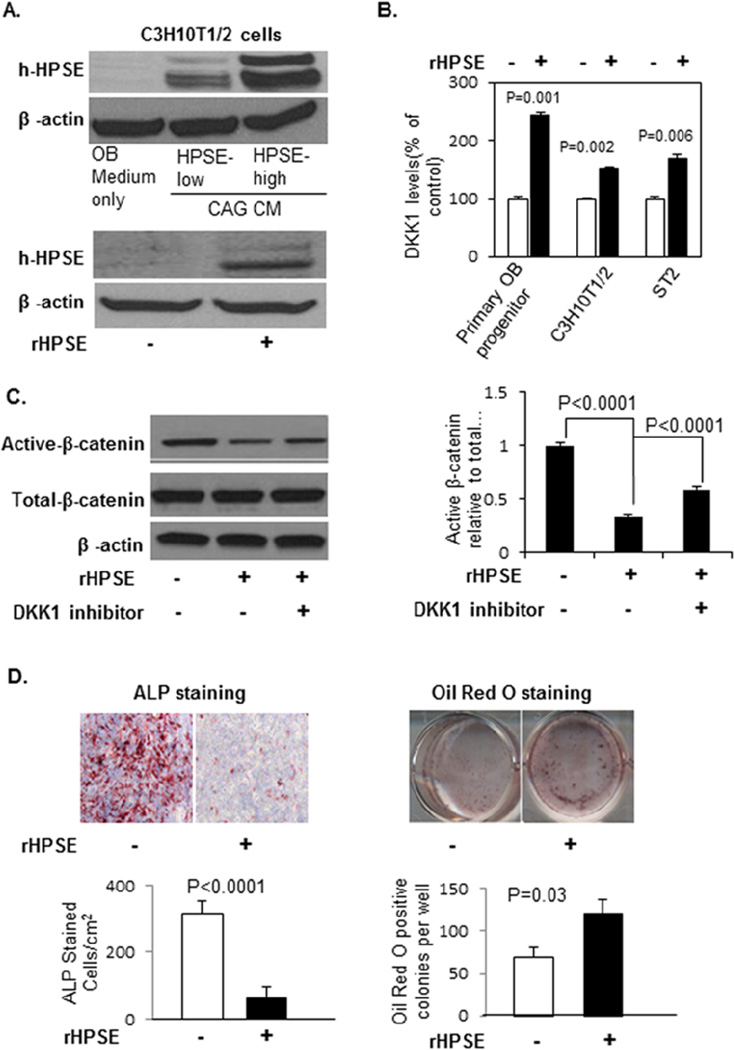
Figure 4. CM from HPSE-high cells inhibits Wnt signaling in osteoblast progenitor cells via DKK1.
(A). CAG cells from three different heparanase transfections using different vectors (pcDNA3 or pIRES2 or pIRES2-pluc) were cultured in serum free medium for two days. The conditioned medium was collected, and the levels of human DKK1 in the CM were measured by ELISA (HPSE-low [L], HPSE-high [H]). Each bar represents the average ± SD from two independent experiments. Significance is indicated for each comparison. (B). Primary osteoblast progenitor cells were cultured in an equal volume of CM of HPSE-low or HPSE-high cells or osteogenic medium with or without DKK1 inhibitor (3.0 mM) for 10 days (medium changed every 3 days). Cells were harvested for Western blot and stained with antibodies against Active-β-catenin; Total-β-catenin and β-actin.
In addition, decreased β-catenin activation was observed in primary osteoblast progenitor cells cultured in the CM of HPSE-high cells (Figure 4B), which was rescued by the addition of a potent and selective DKK1 inhibitor (Figure 4B). These data demonstrate that the canonical Wnt signaling pathway inhibited by increased DKK1 secretion from HPSE-high myeloma cells.
4.5. Heparanase directly stimulates DKK1 secretion by osteoblast progenitor and shifts these cells from osteoblastogenesis to adipogenesis by inhibiting Wnt signaling
We identified a high level of human heparanase uptake by murine C3H10T1/2 osteoblast precursor cells cultured in CM of human HPSE-high cells or in the presence of exogenous human rHPSE (Figure 5A). Since osteoblast progenitors have been shown to produce DKK[24], we determined whether heparanase uptake by osteoblast progenitors can stimulate DKK1 secretion. Equal numbers of primary mouse osteoblast progenitor cells, C3H10T1/2 and ST2 pre-osteoblast/stromal cells were cultured in osteoblast growth medium with or without rHPSE (100 ng/mL) for 3 days. ELISA analysis revealed a significant increase in the levels of DKK1 in the CM of the cells treated with rHPSE (Figure 5B). Moreover, primary osteoblast progenitor cells cultured in the presence of rHPSE resulted in a dramatic reduction of the levels of the active β-catenin (Figure 5C), and this inhibition was blocked by DKK1 inhibitor (Figure 5C). In addition, ALP and Oil Red O Staining demonstrated a corresponding and significant inhibition of osteoblast differentiation and significant stimulation of adipocyte differentiation (Figure 5D).
Figure 5. rHPSE stimulates DKK1 secretion from osteoblast progenitor and stromal cells, inhibits osteoblastogenesis, and promotes adipogenesis by inhibiting Wnt signaling pathway in these cells.
(A). Human heparanase is detectable in cell lysates of C3H10T1/2 cells after the cells were cultured in CM of HPSE-low and HPSE-high CAG cells (upper panel) or in the presence of human rHPSE (100 ng/ml) (lower panel) for 3 days. (B). Primary murine osteoblast progenitor cells, C3H10T1/2 pre-osteoblastic cells, and ST2 stromal cells were cultured in absence or presence of rHPSE(100 ng/ml) for 3 days, and the level of murine DKK1 in the CM measured by ELISA. Each bar represents the Mean ± SD of 2 measurements. Significance is indicated in the panel. (C). Primary osteoblast progenitor cells cultured in osteogenic medium in absence or presence of rHPSE (100 ng/ml) with or without DKKinhibitor (3.0 mM) for 10 days (medium changed every 3 days). Cells were harvested for Western blot and stained with antibodies against active-β-catenin, total-β-catenin and β-actin (left). The active β-catenin bands from Western blot were quantified by ImageJ from two independent experiments (right). (D). ALP and Oil Red O staining were performed at day 10 for evaluation of osteoblast and adipocyte differentiation. ALP positive osteoblast cells and Oil Red O stained adipocyte colonies were enumerated from two independent experiments (Mean ± SEM). Significance is indicated in each panel.
5. Discussion
Bone is a dynamic tissue that is constantly being remodeled [30]. In normal bone remodeling, osteoclasts resorb old and damaged bone before osteoblasts follow and synthesize and mineralize new bone in an exquisitely balanced or coupled process [31]. The balance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation is the key for maintaining healthy bone metabolism. Myeloma bone disease is the result of an increase in bone resorption and a decrease in bone formation [14, 17, 27], driving a major imbalance in the two processes.
We have shown previously that heparanase enhances the expression and secretion of RANKL by myeloma cells [26, 36], thereby directly stimulating osteoclastogenesis and bone resorption. In the present study, we investigated whether osteoblast differentiation and activity were regulated by myeloma cells expressing heparanase. Strikingly, heparanase expression by myeloma cells that stimulates osteoclastogenesis [26, 36] also decreased osteoblastogenesis (and likely bone formation) by inhibiting osteoblasts and stromal cells in the bone microenvironment.
The immunostaining of osteocalcin in engrafted bones harvested from SCID-hu mice and in primary bone marrow core biopsies from myeloma patients demonstrated a significant negative correlation between heparanase expression by myeloma cells and the numbers of osteocalcin-positive osteoblast cells in bone. Importantly, the inhibition of osteocalcin-staining and bone formation observed in the engrafted bones occurs not only in primary tumor-injected bones, but also in contralateral bones where tumor cells were not injected or detected. This strongly suggests that heparanase-expressing myeloma cells decrease the numbers of osteocalcin-positive cells and induce the inhibition of osteoblastogenesis in distal bones prior to the arrival of tumor cells by secreting soluble inhibitor(s) of osteoblastogenesis. This hypothesis was confirmed by culturing primary osteoblast progenitor cells with the conditioned medium of HPSE-high or HPSE-low myeloma cells. The results demonstrated that the conditioned medium of HPSE-high cells was capable of inhibiting both osteoblast progenitor differentiation and mineralization. Perhaps most surprisingly, we found that the conditioned medium of HPSE-high cells also drives these same progenitor cells toward adipocytes. Further studies demonstrated that the shift in cell fate was induced by increased dickkopf1 (DKK1) secretion by HPSE-high cells.
DKK1 is a well-characterized inhibitor of canonical Wnt/β-catenin signaling [24]. Wnt/β-catenin is a critical signaling pathway considered essential for osteoblastogenesis [6, 8]. DKK1 selectively binds to the Wnt receptors Lrp5 or Lrp6, thereby blocking the ability of Wnt ligands to interact with these receptors, specifically blocking the canonical Wnt signaling pathway and thus inhibiting osteoblast differentiation and bone formation [22]. In contrast to the function of Wnt signaling to enhance osteoblast differentiation, Wnt /β-catenin signaling inhibits adipocyte differentiation [7, 12, 13]. In the present study, a significant increase of DKK1 secretion in HPSE-high myeloma cells was observed. Subsequently, a significant inhibition of stable (active) β-catenin expression [8] in osteoblast progenitors treated with conditioned medium from HPSEhigh cells was observed. Importantly, the inhibition of β-catenin expression was completely rescued by the addition of a specific DKK1 inhibitor, confirming that HPSE-high myeloma cells induce the inhibition of osteoblastogenesis and the promotion of adipogenesis via suppression of the canonical Wnt signaling pathway by DKK1.
In addition to myeloma cells, it has been demonstrated that pre-osteoblasts and preadipocytes also secrete DKK1 [24]. Our data demonstrate that the heparanase secreted by HPSE-high myeloma cells can be taken-up by osteoblast progenitors and bone marrow cells, and in turn, stimulate DKK1 secretion by these normal cells. The osteoblast progenitor secreted DKK1 inhibits Wnt signaling in osteoblast progenitors in an autocrine/paracrine fashion, thereby contributing to the inhibition of osteoblastogenesis and the promotion of adipogenesis. Indeed, ALP and Oil Red O staining revealed a remarkable decrease in the number of osteoblasts and an increased number of adipocytes in progenitor cells cultured with either conditioned medium of HPSE-high cells or rHPSE. If recapitulated in vivo, this process, regulated by heparanase, could directly and/or indirectly contribute to the imbalance between bone resorption and bone formation characteristic of myeloma bone disease. In addition, recent evidence suggests that bone marrow adipocytes are an endocrine organ, secreting growth factors and cytokines that regulate many physiological and pathological events [4, 28]. The role of adipocytes in multiple myeloma progression, besides its contribution to bone destruction, is currently the focus of intense scrutiny in our laboratory.
6. Conclusion
The results presented here demonstrate that heparanase expression by myeloma cells can alter the fate of osteoblast progenitor cells, directing cellular differentiation toward adipocytes rather than osteoblasts. The switch in differentiation involved enhanced PPAR-γ expression, decreased Runx2 and high levels of DKK1 secretion from both myeloma cells and bone marrow cells, resulting in the inhibition of Wnt/β-catenin signaling in osteoblast progenitors. Collectively, these data, together with our previous report on the role of heparanase in stimulating bone resorption [36], demonstrate that myeloma bone disease is the result of a combination of enhanced bone resorption and reduced bone formation, both driven by heparanase. Thus, these data also provide the rationale to target heparanase with heparanase inhibitors for the treatment of myeloma bone disease, which may go beyond conventional approaches that target bone resorption. Further studies will determine the extent to which enhanced adipogenesis contributes to myeloma bone disease and tumor progression.
Supplementary Material
Research highlights.
Heparanase inhibits osteoblastogenesis and bone formation in multiple myeloma.
Heparanase shifts the differentiation potential of osteoblast progenitors from osteoblastogenesis to adipogenesis.
Heparanase stimulates DKK1 production and secretion by myeloma cells and bone marrow stromal cells.
Acknowledgements
This work was supported by grants from the National Cancer Institute (CA151538 [YY], CA138340 and CA135075 [RDS], the Multiple Myeloma Research Foundation (Senior Research Award [YY]), the Carl L. Nelson Chair of Orthopaedic Creativity (LJS) and the UAMS Translational Research Institute (TRI) (CTSA grant Award #1 UL1TR000039). The authors also thank Dr. Israel Vlodavsky (Technion, Haifa, Israel) for providing rHPSE and heparanase antibodies, Dr. Shi Wei for help with osteocalcin staining on the bone marrow specimens of myeloma patients, Dr. Majd Zayzafoon and Dr. Yi-ping Li for providing primary murine osteoblastic progenitors, and Dr. Kun Yuan for the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
The authors disclose no potential conflicts of interest.
References
- 1.Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol. 2012;816:19–29. doi: 10.1007/978-1-61779-415-5_2. [DOI] [PubMed] [Google Scholar]
- 2.Barash U, Cohen-Kaplan V, Dowek I, Sanderson RD, Ilan N, Vlodavsky I. Proteoglycans in health and disease: new concepts for heparanase function in tumor progression and metastasis. FEBS J. 2010;277:3890–3903. doi: 10.1111/j.1742-4658.2010.07799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borset M, Hjertner O, Yaccoby S, Epstein J, Sanderson RD. Syndecan-1 is targeted to the uropods of polarized myeloma cells where it promotes adhesion and sequesters heparin-binding proteins. Blood. 2000;96:2528–2536. [PubMed] [Google Scholar]
- 4.Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, Defresne MP, Van Riet I, Van Camp B, Vanderkerken K. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21:1580–1584. doi: 10.1038/sj.leu.2404658. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Dang YW, Luo DZ, Feng ZB, Tang XL. Expression of heparanase in hepatocellular carcinoma has prognostic significance: a tissue microarray study. Oncol Res. 2008;17:183–189. doi: 10.3727/096504008785114138. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Alman BA. Wnt pathway, an essential role in bone regeneration. J Cell Biochem. 2009;106:353–362. doi: 10.1002/jcb.22020. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O'Rahilly S, Sethi JK, Vidal-Puig A. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119:2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Cohen E, Doweck I, Naroditsky I, Ben-Izhak O, Kremer R, Best LA, Vlodavsky I, Ilan N. Heparanase is overexpressed in lung cancer and correlates inversely with patient survival. Cancer. 2008;113:1004–1011. doi: 10.1002/cncr.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen I, Pappo O, Elkin M, San T, Bar-Shavit R, Hazan R, Peretz T, Vlodavsky I, Abramovitch R. Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int J Cancer. 2006;118:1609–1617. doi: 10.1002/ijc.21552. [DOI] [PubMed] [Google Scholar]
- 11.Giuliani N, Colla S, Rizzoli V. New insight in the mechanism of osteoclast activation and formation in multiple myeloma: focus on the receptor activator of NF-kappaB ligand (RANKL) Exp Hematol. 2004;32:685–691. doi: 10.1016/j.exphem.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson B, Eliasson B, Smith U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: a link with osteogenesis. Diabetologia. 2010;53:536–540. doi: 10.1007/s00125-009-1615-1. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson B, Smith U. The WNT inhibitor Dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes. 2012;61:1217–1224. doi: 10.2337/db11-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heider U, Hofbauer LC, Zavrski I, Kaiser M, Jakob C, Sezer O. Novel aspects of osteoclast activation and osteoblast inhibition in myeloma bone disease. Biochem Biophys Res Commun. 2005;338:687–693. doi: 10.1016/j.bbrc.2005.09.146. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann AC, Mori R, Vallbohmer D, Brabender J, Drebber U, Baldus SE, Klein E, Azuma M, Metzger R, Hoffmann C, Hoelscher AH, Danenberg KD, Prenzel KL, Danenberg PV. High expression of heparanase is significantly associated with dedifferentiation and lymph node metastasis in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA and via HIF1a to HB-EGF and bFGF. J Gastrointest Surg. 2008;12:1674–1681. doi: 10.1007/s11605-008-0628-2. discussion 1681-1672. [DOI] [PubMed] [Google Scholar]
- 16.Hulett MD, Hornby JR, Ohms SJ, Zuegg J, Freeman C, Gready JE, Parish CR. Identification of active-site residues of the pro-metastatic endoglycosidase heparanase. Biochemistry. 2000;39:15659–15667. doi: 10.1021/bi002080p. [DOI] [PubMed] [Google Scholar]
- 17.Jagannath S. Pathophysiological underpinnings of multiple myeloma progression. J Manag Care Pharm. 2008;14:7–11. doi: 10.18553/jmcp.2008.14.S7-A.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly T, Miao HQ, Yang Y, Navarro E, Kussie P, Huang Y, MacLeod V, Casciano J, Joseph L, Zhan F, Zangari M, Barlogie B, Shaughnessy J, Sanderson RD. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63:8749–8756. [PubMed] [Google Scholar]
- 19.Lerner I, Baraz L, Pikarsky E, Meirovitz A, Edovitsky E, Peretz T, Vlodavsky I, Elkin M. Function of heparanase in prostate tumorigenesis: potential for therapy. Clin Cancer Res. 2008;14:668–676. doi: 10.1158/1078-0432.CCR-07-1866. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Aronson J, Huang S, Lu Y, Czernik P, Rahman S, Kolli V, Suva LJ, Lecka-Czernik B. Rosiglitazone inhibits bone regeneration and causes significant accumulation of fat at sites of new bone formation. Calcif Tissue Int. 2012;91:139–148. doi: 10.1007/s00223-012-9623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, Pantesco V, Baudard M, De Vos J, Larroque M, Moehler T, Rossi JF, Reme T, Goldschmidt H, Klein B. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109:4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcellini S, Henriquez JP, Bertin A. Control of osteogenesis by the canonical Wnt and BMP pathways in vivo: Cooperation and antagonism between the canonical Wnt and BMP pathways as cells differentiate from osteochondroprogenitors to osteoblasts and osteocytes. Bioessays. 2012 doi: 10.1002/bies.201200061. [DOI] [PubMed] [Google Scholar]
- 23.Mundy GR. Regulation of bone formation by bone morphogenetic proteins and other growth factors. Clin Orthop Relat Res. 1996:24–28. doi: 10.1097/00003086-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD., Jr The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008;283:32628–32636. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. doi: 10.1182/blood-2009-07-234757. Epub ahead of print PMID:20097882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 28.Sadie-Van Gijsen H, Crowther NJ, Hough FS, Ferris WF. The interrelationship between bone and fat: from cellular see-saw to endocrine reciprocity. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafat I, Pode D, Peretz T, Ilan N, Vlodavsky I, Nisman B. Clinical significance of urine heparanase in bladder cancer progression. Neoplasia. 2008;10:125–130. doi: 10.1593/neo.07875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone. 2011;48:6–15. doi: 10.1016/j.bone.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Macleod V, Bendre M, Huang Y, Theus AM, Miao HQ, Kussie P, Yaccoby S, Epstein J, Suva LJ, Kelly T, Sanderson RD. Heparanase promotes the spontaneous metastasis of myeloma cells to bone. Blood. 2005;105:1303–1309. doi: 10.1182/blood-2004-06-2141. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, MacLeod V, Dai Y, Khotskaya-Sample Y, Shriver Z, Venkataraman G, Sasisekharan R, Naggi A, Torri G, Casu B, Vlodavsky I, Suva LJ, Epstein J, Yaccoby S, Shaughnessy JD, Jr, Barlogie B, Sanderson RD. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances syndecan-1 shedding: A novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Ren Y, Ramani VC, Nan L, Suva LJ, Sanderson RD. Heparanase enhances local and systemic osteolysis in multiple myeloma by upregulating the expression and secretion of RANKL. Cancer research. 2010;70:8329–8338. doi: 10.1158/0008-5472.CAN-10-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, Epstein J, Sanderson RD. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100:610–617. doi: 10.1182/blood.v100.2.610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.