Abstract
Objective
To determine the incidence of pathology during routine screening of healthy short children, testing adherence to a consensus statement on the diagnosis and treatment of children with idiopathic short stature, and the cost per identified diagnosis resulting from comprehensive screening.
Study design
Retrospective chart review of 1373 consecutive short stature referrals evaluated at the Cincinnati Children's Hospital Medical Center Pediatric Endocrinology Clinic between 2008 and 2011. We identified 235 patients with a height of <3rd percentile, negative history and review of systems, and normal physical examination. Outcome measures were incidence of pathology detection, diagnostic group characteristics, clinicians' adherence to testing guidelines, and screening costs. ANOVA and χ2 were used to analyze the data.
Results
Nearly 99% of patients were diagnosed as possible variants of normal growth: 23% with familial short stature, 41% with constitutional delay of growth and maturation, and 36% with idiopathic short stature. The incidence of newly diagnosed pathology was 1.3%: 1 patient with biopsy-proved celiac disease, 1 with unconfirmed celiac disease, and 1 with potential insulin-like growth factor I receptor defect. On average, each patient had 64.3% of the recommended tests for age and sex; 2.1% of patients had all of the recommended testing. The total screening tests costs were $315 321, yielding $105 107 per new diagnosis entertained.
Conclusions
Healthy short children do not warrant nondirected, comprehensive screening. Future guidelines for evaluating short stature should include patient-specific testing.
Short stature may be the first presenting sign of an underlying disease and thus warrants careful evaluation by the clinician. Several studies have assessed the diagnostic evaluation for short stature and recommend the use of a standardized screening protocol.1-3 Per the 2008 Consensus Statement on the Diagnosis and Treatment of Children with Idiopathic Short Stature (ISS) guidelines, a diagnosis of ISS requires patients be shorter than 2 SD score below the mean without evidence of systemic, endocrine, nutritional, or chromosomal abnormalities. These guidelines recommends laboratory screening in all patients “for whom the history and physical exam do not suggest a particular diagnosis.”4 However, it is not known how often such routine screening will uncover a pathologic cause for the short stature. A rate up to 5% of detected pathology from short stature referral evaluation has been reported. 1,2,5,6 Some studies included patients with documented growth failure6 or with clinical features clearly indicative of an underlying pathology (eg, skeletal dysplasia or Turner syndrome)1. These studies did not evaluate how often screening tests alone yielded a new diagnosis in patients without any abnormality on history or examination other than short stature. The true incidence of pathology in those patients may, therefore, be lower than previously reported. In addition, no study has presented a cost-analysis associated with such nondiscriminatory screening. In this article, we focus specifically on those patients with short stature without any other signs or symptoms (ie, “asymptomatic short children”). With normal screening test results, these patients would end up being categorized within the heterogenous group of ISS patients, for whom the above noted 2008 consensus guidelines were originally developed.
Methods
A retrospective chart review of 1373 patients newly referred for short stature was performed. The patients were seen in a large academic pediatric endocrinology setting between October 2008 and June 2011. To exclude patients who were not asymptomatic, we excluded patients who had: (1) height >3rd percentile (as measured in the Cincinnati Children's Hospital Endocrinology Clinic [CCHMC]) using a length board if <2 years old or using a wall-mounted stadiometer (Holtain Limited, Crosswell, United Kingdom) with the patient's heels together, buttocks and shoulders touching the wall, and head held in the Frankfurt plane, as described by Tanner et al7; (2) prior height velocity (HV) <5 cm/y; (3) abnormal findings on history, review of systems, or physical examination warranting a specific evaluation; (4) incomplete data in the medical record; or (5) incorrect referral coding. After screening all medical records to exclude patients who fit exclusion criteria 1, 4, or 5 (by M.V.), 2 independent reviewers (M.V. and S.S.) assessed all remaining charts for further exclusion based on criteria 2 and 3. A third reviewer (P.B.) assessed any chart that had led to disagreement about exclusion between the 2 initial reviewers. There were 235 subjects eligible for inclusion in the analysis.
The following information was extracted from the medical record: age, sex, height, weight, parental heights (self-reported), review of systems findings, physical examination findings, laboratory and radiologic test results, and the diagnosis (assigned by the evaluating endocrinologist). Of note, ISS, as a diagnosis of exclusion, was defined individually by the evaluating pediatric endocrinologist. Bone age film interpretation was based on the pediatric endocrinologist's reading. We analyzed data from the ISS statement's recommended tests4: complete blood count, erythrocyte sedimentation rate (ESR), sodium, potassium, chloride, bicarbonate, calcium, phosphate, alkaline phosphatase, blood urea nitrogen, creatinine, insulin-like growth factor I (IGF-1), insulin-like growth factor binding protein-3 (patients <3 years only), immunoglobulin A (IgA), tissue transglutaminase (tTG), thyroid-stimulating hormone, thyroxine (T4), free thyroxine (FT4), and karyotype (female participants only). We included laboratory data from outside institutions if obtained within 6 months of the evaluation. The patient's body mass index (BMI) was calculated as weight in kilograms/height in meters squared.
We used a SAS macro (gc-calculate-BIV.sas, available on the Centers for Disease Control and Prevention website; SAS Institute, Cary, North Carolina) to calculate each patient's height, weight, and BMI SDSs. Target height (TH) was calculated by the method of Tanner et al from the self-reported heights of the biological parents.8 TH SDS was calculated as previously referenced by Grote et al.9 The difference between a patient's height SDS and their TH SDS (height SDS − TH SDS) is referred to as “height deficit.” HV (calculated as change in height in cm/y) was included if ≥ 1 height measurement was available within 6-12 months before the evaluation. Study data were collected and managed using REDCap electronic data capture tools (REDCap; http://project-redcap.org) hosted at CCHMC.10 The protocol was approved by the CCHMC Institutional Review Board, and written consent was waived.
Compendium costs were received from the CCHMC laboratory for all tests. We calculated laboratory charges by multiplying the compendium cost per test by the number of times the test was done. Projected laboratory costs for complete testing on each patient was assessed by calculating the compendium cost per test multiplied by the number of patients, adjusted for age and sex as necessary.
Data were analyzed using SAS version 9.3 (SAS institute). Data were reported as either number and percentage or mean ± SD, as appropriate. ANOVA was used to examine differences between groups for the continuous variables, and χ2 was used for the categorical variables. A post-hoc Tukey-Kramer adjustment was used to compare means between diagnoses. A value of P < .05 was considered statistically significant unless otherwise noted.
Results
We found 235 asymptomatic patients (normal history, review of systems, and examination) who had been referred for short stature and met the ISS consensus statement guidelines criteria for screening evaluation. Patient characteristics are shown in Table I. Patients were, on average, 10 years of age. The majority (73%) were prepubertal boys. Female participants were significantly shorter (−2.6 ± 0.5 SDS) than male participants (−2.5 ± 0.5 SDS) (P = .02). The BMI SDS was on average −0.8 ± 1.3. There were no differences between male and female participants in age, weight SDS, BMI SDS, or TH SDS (n = 227). Only 37% of the patients had longitudinal growth records on referral. For these patients, the mean HV was 6.5 ± 2.8 cm/y over an average interval of 10.2 ± 2.5 months (within 2 SD for age and sex).
Table I. Demographics and auxologic parameters of 235 asymptomatic short children.
| Characteristic | Value |
|---|---|
| Male | 72.8% |
| Age at referral, y | 10.1 ± 4.3 (0.8-17.6) |
| Pubertal status (N = 221) | |
| Tanner I | 60.6% |
| Tanner II | 24.9% |
| Tanner III | 8.6% |
| Tanner IV | 1.8 % |
| Tanner V | 4.1% |
| Height SDS | −2.5 ± 0.5* (−4.8 to −2.0) |
| Weight SDS | −2.2 ± 1.1 (−6.8 to 0.5) |
| BMI SDS | −0.8 ± 1.3 (−5.81 to 2.17) |
| Growth records available | 36.6% |
| Prior HV, cm/y (averaged over 10.6 ± 2.5 mo) | 6.5 ± 2.8 (5 to 14) |
Values are percent or mean ± SD (range).
P< .05 between boys and girls (−2.5 ± 0.5 SD vs −2.6 ± 0.5 SD, respectively). No other differences existed between sexes.
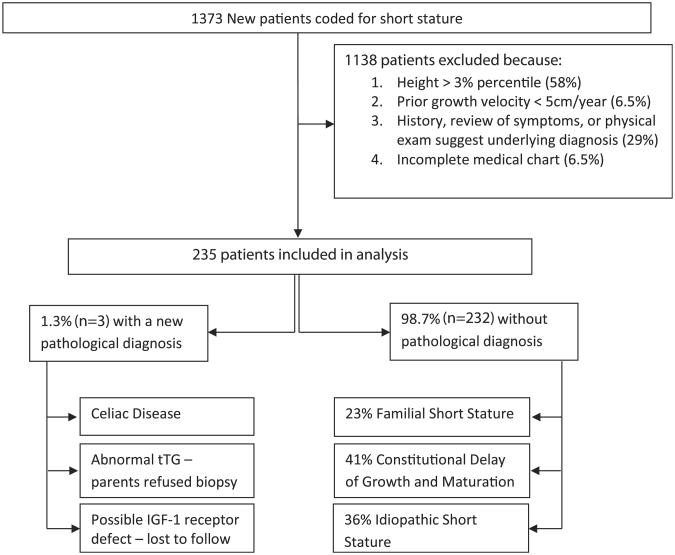
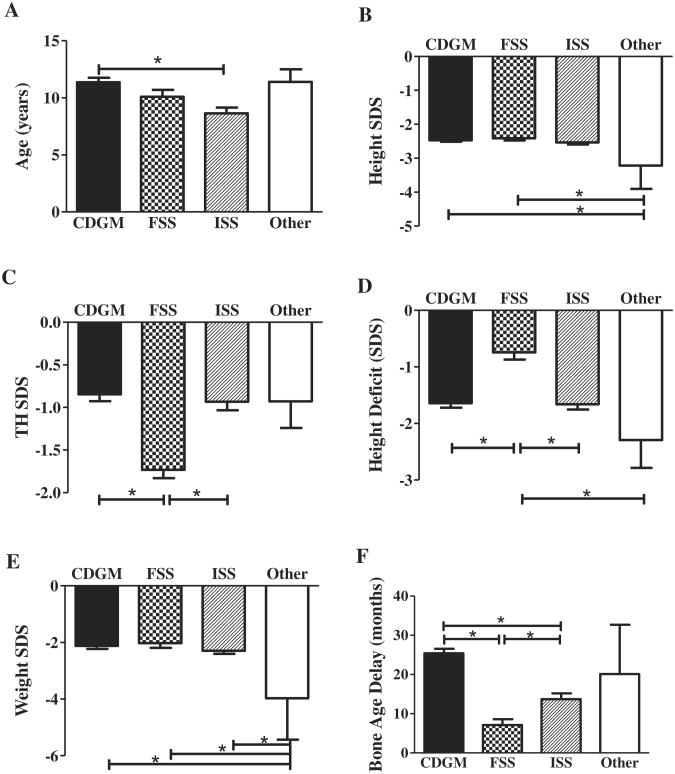
Of the 235 patients included in our analysis, 98.7% (n = 232) did not have any pathology detected on their laboratory and/or radiologic screening. Of these 232 patients, the clinicians diagnosed 23% (n = 53) with familial short stature (FSS), 41% (n = 95) with constitutional delay of growth and maturation (CDGM), and 36% (n = 84) with ISS (Figure 1). Patients with CDGM were significantly older (mean 11.4 years) than patients with ISS (8.6 years) (P < .05) (Figure 2, A). There were no differences in height SDSs between patients with CDGM, FSS, or ISS (Figure 2, B). Patients with FSS had a significantly lower TH SDS (−1.7) than patients with CDGM (−0.9) or ISS (−0.9) (P < .05, Figure 2, C). Additionally, patients with FSS were growing closer to their TH percentile curve, as noted by their smaller height deficit (−0.7 SDS) compared with the CDGM (−1.6) and ISS (−1.6) groups (P < .05) (Figure 2, D). There were no differences in weight SDSs between the CDGM, FSS, or ISS groups (Figure 2, E). Within the ISS group, there was a subset of patients (n = 38) with a significantly lower BMI SDS (−1.4 vs −0.3, P < .05). About one-half of these patients were also receiving medications for attention-deficit/hyperactivity disorder (ADHD). Although they were also considered as variants of normal growth, the low BMI was thought to be a contributing factor to their short stature. Children with CDGM had a greater bone age delay (24.1 months) compared with children with FSS (7.2 months) or ISS (14.2 months) (P < .05, Figure 2, F).
Figure 1.
Study population.
Figure 2.
Diagnostic group demographics and auxology. A, Age. B, Height SDSs. C, TH SDSs. D, Height Deficit. E, Weight SDSs. F, Bone age delay. “Other” indicates 3 patients with possible pathology. *Statistical significance; P values denoted in text.
Among the 232 patients, 10 patients (4.3%) had minimally abnormal laboratory results, which returned to normal on repeat testing. Five patients had an abnormal ESR (11-51 mm/h), and 1 patient had elevated bicarbonate (32 mmol/L) and phosphorus (5.1 mg/dL) levels. One patient had an elevated thyroid-stimulating hormone level (5.02 μIU/mL). One patient had a low T4 level (4.8 μg/dL), but free T4 was normal. Two patients had an abnormal IGF-1 for age (36.6 and 106 ng/mL). Both IGF-1 concentrations were normal for bone age and both patients underwent growth hormone (GH) stimulation testing, yielding normal (>10 ng/mL) peak GH values. Finally, one patient had an elevated tTG level (42 units), but endoscopy was subsequently normal and the patient was diagnosed with gastroesophageal reflux disease.
Routine screening after endocrinology referral identified three patients (1.3%) with a possible new diagnosis (Figure 1). There were no differences in age, TH SDS, or bone age delay between these patients and the CDGM, FSS, or ISS groups (Figure 2, A, C, and F, respectively). The patients with potential pathology had a significantly lower height SDS (−3.2) compared with CDGM (−2.5) and FSS (−2.4) groups (P < .05, Figure 2, B). However, the height deficit was only statistically different between the patients with possible pathology (−2.3) and those with FSS (−0.7) (P < .05, Figure 2, D). Weight SDS was different between the 3 patients (−4.0) and all other groups (CDGM −2.2, FSS −2.1, and ISS −2.3) (P < .05, Figure 2, E).
Patient 1 was an 11.6-year-old girl with a normal past medical history. A prior growth record was not available. She had a height SDS of −4.4, BMI SDS of −3.7, Tanner II breasts, and Tanner I pubic hair. Her screening showed an elevated tTG (57 units), normal IgA, and a delayed bone age (7 years 10 months). An endoscopic biopsy confirmed active celiac disease.
Patient 2 was a 9.4-year-old girl with seasonal allergies. She was not receiving any medications. She had a height SDS of −2.0, BMI SDS of −1.5, and a preevaluation growth velocity of 5 cm/y Examination showed an eczematous rash on her posterior neck and Tanner I breasts and pubic hair. Her laboratory testing revealed a minimally elevated ESR (11 mm/h, normal 0-10 mm/h), an elevated tTG (33 units, normal <20 units), normal IgA, and a normal bone age (8 years 10 months). Her parents refused an endoscopy and she did not have further follow-up.
Patient 3 was a 13.2-year-old boy with ADHD receiving treatment with long-acting methylphenidate. He had occasional headaches by history. No prior growth records were available. Physical examination revealed a height SDS of −3.2, BMI SDS of −0.8, extensive acne, Tanner II pubic hair, and 10 cm3 testes bilaterally. No developmental delay or dysmorphic features were present. Laboratory testing showed a minimally elevated IGF-1 (mean ± SD 976.9 + 2.47 ng/mL, normal 71.0-972.0 ng/mL) with a normal bone age (12 years 6 months). Although genetic testing for an IGF-1 receptor defect was considered, he did not follow up for this.
Adherence to Guidelines
On average, each patient had 64.3% of the tests recommended by the ISS consensus statement guidelines. Only 2.1% of the patients had all of testing done as recommended (Table II). Tests for calcium metabolism (calcium, phosphorus, alkaline phosphatase) were done least often (27%-38%). Screening for systemic disease (complete blood count, ESR, or basic metabolic panel) was done in 63%-74% of patients. Celiac disease screening occurred in 59% of patients, although not all patients with a tTG also had an IgA done. Growth factor and thyroid axis testing were done in >80% of patients. Of note, due to an issue with the FT4 laboratory methodology during part of this study interval, some practitioners ordered total T4 instead of free T4 values. Any patient having either a T4 or FT4 was counted as meeting the recommended guideline for thyroid testing. The most common test performed was a bone age (87%).
Table II. Percentage of patients with each test done.
| Test | Obtained, % | Compendium costs,* $ |
|---|---|---|
| Complete blood count | 74 | 56.00 |
| Basic metabolic panel | 71 | 229.32 |
| ESR | 63 | 52.40 |
| Calcium | 38 | 42.29 |
| Phosphorus | 27 | 34.93 |
| Albumin | 38 | 36.57 |
| Alkaline phosphatase | 27 | 40.00 |
| tTG | 59 | 299.83 |
| IgA | 56 | 52.40 |
| IGF-1 | 80 | 295.74 |
| IGFBP-3 | 62 | 298.76 |
| TSH | 86 | 220.96 |
| T4 | 40 | 77.99 |
| FT4 | 57 | 242.91 |
| Karyotype | 41 | 83.30 |
| Bone age | 87 | 267.04† |
BMP, beats per minute; IGFBP-3, insulin-like growth factor binding protein-3; TSH, thyroid-stimulating hormone.
Each test was analyzed for its rate of completion in our patients. IGFBP-3 completion was analyzed only in children <3 y (n = 21), karyotype only in girls (n = 70), and all other tests in all patients (n = 235).
Specific to CCHMC.
Includes reading by radiologist; bone age film alone = $164.14.
Costs
The compendium cost for all of the tests done on our patients was $315 321. Thus, the cost per new diagnosis identified during screening was $105 107. If every patient would have had all testing done as recommended by the ISS consensus statement guidelines, the total cost would have been $560 038 and the cost per new case would have been $186 679.
Discussion
Our findings highlight a need for evidence-based guidelines in the evaluation of children with short stature. Although short stature can occur with or without growth failure, any child who presents with evidence of growth failure clearly warrants a detailed evaluation that will include laboratory and radiology testing. Lindsay et al showed that 14% of elementary school children with a height <3rd percentile and an HV <5 cm/y (identified as “high-risk patients”) had organic pathology.6 However, our study had a different focus in that it specifically analyzed patients without known growth failure. Comprehensive screening for pathology in these otherwise asymptomatic short children yielded only a few patients with a potential pathologic cause for their short stature. In fact, in our study there were more patients with false-positive laboratory results (10 patients) than there were patients in whom a possible new disease was discovered (3 patients). Our findings showed that the 3 patients with possible pathology had a significantly lower height SDS than the other groups. This correlates with the findings by Voss et al, which showed an increase in the prevalence of organic disease as height SDSs decrease.5 To develop evidence-based guidelines for the evaluation of children with short stature, one will likely be required to examine the possibility of underlying pathology in relation to both HV and absolute height SDS criteria.
The number of (potential) new diagnoses resulting from screening of children with short stature in our study is less than that reported in several other studies.1,6,11,12 This is in accord with our expectation that short children, who are asymptomatic, will have a lower incidence of pathology than those children who present with abnormal findings at the time of evaluation. Organic disease was previously found in 7.3% of correctly referred, short-statured patients,1 in 25% of new short stature referrals in a pediatric endocrine clinic by Grimberg et al,11 and in 40% of patients at a specialized growth center.12 All of these prior reports included children with growth failure and/or symptomatic disease and did not assess otherwise asymptomatic patients as a separate group. Many of the included diseases, such as hypothyroidism, GH deficiency, and genetic syndromes, have particular physical signs (eg, delayed deep tendon reflexes, increased central adiposity, disproportionate body segments, delayed puberty) suggestive of an underlying diagnosis. Our study is different because we specifically analyzed a subset of children without abnormality detected by either history or physical examination. Consequently, we observed a much lower incidence of pathology.
Our study concurs with other published reports regarding sex differences in short stature evaluation.6,11,12 First, our subject population was predominantly male. Second, we found that girls were very slightly but significantly shorter than boys when referred for short stature, as also noted by Grimberg et al.11 Although one could infer from this that girls are less likely to be referred for short stature, boys may actually also be at greater risk for having pathologic short stature than girls. Lindsay et al prospectively measured elementary school children and found a disproportionate number of boys than girls (64% vs 36%, respectively) in their high-risk group (described earlier).6 However, girls seem to be more likely to present with pathology. Grimberg et al showed that girls, on referral, were 2.7 times more likely to have organic disease than boys.11 Given that 70% of our population were boys, it is interesting that 2 of our 3 patients with possible pathology were female. Although this finding is not statistically significant given the small number of patients, it correlates with the previous reports.
This study also underscores the high numberofchildren inappropriately referred for short stature. Twenty-three percent of patients were diagnosed with FSS, which can be easily diagnosed by calculating a TH. However, the authors acknowledge that the mid-parental heights for our patients were based on self-report and not on actual measurements. This may result in inaccuracies, as tall mothers may underestimate and short fathers may overestimate their heights; 60% of our subjects were initially excluded because their heights were not <3rd percentile. The importance of accurate height measurement is paramount to proper growth evaluation. Correct measuring technique can be achieved with basic machinery. Under the age of 2 years, supine length is measured. For older children, a standing height is measured with a stadiometer or another rule. Given that growth failure is a better indicator of abnormal growth than short stature alone, it is important to have consistent height records to reliably calculate a HV. Only 37% of the patients had appropriately maintained growth records forwarded (even after requests from the endocrine division). Unfortunately, the poor quality of electronic medical record–generated growth charts often prevented an accurate assessment of the patients' growth pattern. Although it is possible that some patients had growth records that were not forwarded upon referral, this points towards a lack of appreciation by primary care physicians for documenting longitudinal growth as part of the standard assessment of children with short stature. The pediatrician or family practitioner provides the first contact with the patient and their family. They are vitally important for the initial evaluation of a patient with short stature. Even when a pediatric endocrinologist referral is considered, the patient may not necessarily have follow-up. For example, Wyatt et al reported that 40% of children with short stature do not return to the pediatric endocrinologist for follow-up.13 This underscores the importance of keeping good growth records in the primary care office, to allow proper identification of the specific patients in need further evaluation, and avoid unnecessary referrals of children with normal variants of growth. This is true in particular for children receiving medications for ADHD, who are further at risk of stimulant drug-associated growth deceleration.
Parental anxiety may also be a reason for referring a child with short stature. Contrary to common belief, short stature has not been shown to result in impaired quality of life.14,15 However, patients who are referred for short stature evaluation were reported to have more externalizing behaviors and poorer social skills than nonreferred children.15 Although this is not meant to establish a causal relationship between a referral and impairment in social life, it should cause pediatricians and families to pause before reflexively referring all short children for further evaluation.
Our study also shows that pediatric endocrinologists do not necessarily follow the ISS testing guidelines for evaluation of asymptomatic short children. The number of tests ordered in this study was higher than that reported by Wyatt et al,11 possibly due to awareness of the ISS consensus statement guidelines.4 Not surprisingly, tests more likely obtained by pediatric endocrinologists include those screening for endocrine disease. The relatively low observed testing rates may be indicative of a perceived lack of utility of the tests in this patient population. Additionally, test ordering rates were unlikely to be artificially lower due to the patients' socioeconomic status, because most children in our region have health insurance.
One argument for generalized testing is to diagnose ISS, which, by definition, is a diagnosis of exclusion. Because ISS is a Food and Drug Administration–approved indication for GH treatment, physicians may feel compelled to order comprehensive screening to arrive at a diagnosis of ISS. We believe ISS is mostly a clinical diagnosis, and the present study demonstrates a very low incidence of pathology in asymptomatic short children.
Two of our 3 patients with potential pathology had abnormal celiac disease screening, although 1 “normal” patient did have a false-positive celiac screen. Bhadada et al reported that 15% of patients with short stature (defined as height <−2.5 SDS, HV <5th percentile, and height deficit ≥2 SDS) had celiac disease.16 However, the prevalence of celiac disease in children with short stature has been reported to range from 0.63%-23.5%.17 Of note, although the Ontario health technology assessment series reported a rate of 1.1% celiac disease in short patients,17 1 study did not disclose the criteria used to define short stature.18 If this study is excluded, the remaining studies average a rate of 8.2% celiac disease in short stature patients, which would be significantly different than the rate of celiac disease in the general population. One argument for screening short-statured patients for celiac disease regardless of associated symptoms is the associated increase in the prevalence of autoimmune disorders with delayed diagnosis.19 Thus; celiac screening in asymptomatic short individuals may still be of some benefit.
We recognize that, if all testing had been done as recommended by the ISS consensus statement guidelines, additional diagnoses may have been uncovered. However, even if the number of new diagnoses would have doubled, the costs would still be almost $100 000 per new diagnosis. It is unlikely that more complete testing would have resulted in additional diagnoses given that all of the included patients were otherwise healthy and asymptomatic. To put this in perspective: congenital hypothyroidism screening costs between $6000 and $12 000 per new case.20 we did not include in our cost-per-case analysis the charges for phlebotomy and/or anesthetic cream, which would have added $11 000-$23 000 to the overall totals. Additionally, we did not include the other costs incurred from repeating minimally abnormal initial tests, from the GH stimulation testing, or the endoscopy to confirm celiac disease.
Our findings indicate a lack of benefit for comprehensive screening of asymptomatic short children. Given the small number of potential new disorders uncovered during comprehensive routine screening of otherwise healthy children and the high cost associated with such screening, we believe that the ISS consensus statement guidelines should be considered for revision. We believe a clinician should use history, review of systems, and physical examination to guide the evaluation of individuals with short stature. We propose that asymptomatic children should have close monitoring of their HV over a period of at least 6 months first. Further testing should proceed only if the HV is abnormal for age. To reinforce this point, consider again that 2 of the patients from our review who had abnormal laboratory results did not have prior growth records. It is possible that, if an underlying disease was present, either patient may have had growth failure identified by a review of their growth pattern. In such a case, an investigation for growth failure would be fully warranted. For a pediatric endocrinologist, a bone age assessment (radiograph of the left hand and wrist) may be of help to distinguish ISS from CDGM. Future studies should also analyze the usefulness of generalized celiac screening in all short-statured patients given the variable rate of celiac pathology in short stature patients and the negative impact of delayed diagnosis.
In conclusion, children referred for short stature should undergo screening for pathology that is guided by positive findings on history or physical examination. Evidence-based algorithms like the one proposed by Grote et al9 could be constructed for different age ranges. The use of such practical evidence-based guidelines will help pediatricians and family practitioners to assess which specific patients need referral and which patients may be monitored conservatively.
Glossary
- ADHD
Attention-deficit/hyperactivity disorder
- BMI
Body mass index
- CCHMC
Cincinnati Children's Hospital Endocrinology Clinic
- CDGM
Constitutional delay of growth and maturation
- ESR
Erythrocyte sedimentation rate
- FSS
Familial short stature
- FT4
Free thyroxine
- GH
Growth hormone
- HV
Height velocity
- IgA
Immunoglobulin A
- IGF-1
Insulin-like growth factor I
- ISS
Idiopathic short stature
- T4
Thyroxine
- TH
Target height
- tTG
Tissue transglutaminase
Footnotes
The authors declare no conflicts of interest.
References
- 1.Grote F, Oostdijk W, De Muinck Keizer-Schrama S, Van Dommelen P, Van Buuren S, Dekker F, et al. The diagnostic work up of growth failure in secondary health care; An evaluation of consensus guidelines. BMC Pediatr. 2008;8:21. doi: 10.1186/1471-2431-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed ML, Allen AD, Sharma A, Macfarlane JA, Dunger DB. Evaluation of a district growth screening programme: the Oxford Growth Study. Arch Dis Child. 1993;69:361–5. doi: 10.1136/adc.69.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu SG, Chen YD, Chi CS. Clinical evaluation of short children referred by school screening: an analysis of 655 children. Acta Paediatr Taiwan. 2002;43:340–4. [PubMed] [Google Scholar]
- 4.Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93:4210–7. doi: 10.1210/jc.2008-0509. [DOI] [PubMed] [Google Scholar]
- 5.Voss LD, Mulligan J, Betts PR, Wilkin TJ. Poor growth in school entrants as an index of organic disease: the Wessex Growth Study. BMJ. 1992;305:1400–2. doi: 10.1136/bmj.305.6866.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125:29–35. doi: 10.1016/s0022-3476(94)70117-2. [DOI] [PubMed] [Google Scholar]
- 7.Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. I. Arch Dis Child. 1966;41:454–71. doi: 10.1136/adc.41.219.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanner JM, Goldstein H, Whitehouse RH. Standards for children's height at age 2 to 9 years allowing for height of parents. Arch Dis Child. 1970;45:819. doi: 10.1136/adc.45.244.819-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grote FK, Van Dommelen P, Oostdijk W, De Muinck Keizer-Schrama SMPF, Verkerk PH, Wit JM, et al. Developing evidence-based guidelines for referral for short stature. Arch Dis Child. 2008;93:212–7. doi: 10.1136/adc.2007.120188. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimberg A, Kutikov JK, Cucchiara AJ. Sex differences in patients referred for evaluation of poor growth. J Pediatr. 2005;146:212–6. doi: 10.1016/j.jpeds.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green AA, MacFarlane JA. Method for the earlier recognition of abnormal stature. Arch Dis Child. 1983;58:535–7. doi: 10.1136/adc.58.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyatt D, Parker KL, Kemp SF, Chiang J, Davis DA. The evaluation and followup of children referred to pediatric endocrinologists for short stature. Int J Pediatr Endocrinol. 2010;2010:652013. doi: 10.1155/2010/652013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coste J, Pouchot J, Carel JC. Height and health-related quality of life: a nationwide population study. J Clin Endocrinol Metab. 2012;97:3231–9. doi: 10.1210/jc.2012-1543. [DOI] [PubMed] [Google Scholar]
- 15.Kranzler JH, Rosenbloom AL, Proctor B, Diamond FB, Watson M. Is short stature a handicap? A comparison of the psychosocial functioning of referred and nonreferred children with normal short stature and children with normal stature. J Pediatr. 2000;136:96–102. doi: 10.1016/s0022-3476(00)90057-x. [DOI] [PubMed] [Google Scholar]
- 16.Bhadada SK, Bhansali A, Kochhar R, Menon AS, Sinha SK, Dutta P, et al. Does every short stature child need screening for celiac disease? J Gastroenterol Hepatol. 2008;23:e353–6. doi: 10.1111/j.1440-1746.2007.05261.x. [DOI] [PubMed] [Google Scholar]
- 17.Clinical utility of serologic testing for celiac disease in asymptomatic patients: an evidence-based analysis. Ontario Health Technol Assess Ser. 2011;11:1–63. [PMC free article] [PubMed] [Google Scholar]
- 18.Giovenale D, Meazza C, Cardinale GM, Sposito M, Mastrangelo C, Messini B, et al. The prevalence of growth hormone deficiency and celiac disease in short children. Clin Med Res. 2006;4:180–3. doi: 10.3121/cmr.4.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 20.Layde PM, Von Allmen SD, Oakley GP. Congenital hypothyroidism control programs A cost-benefit analysis. JAMA. 1979;241:2290–2. [PubMed] [Google Scholar]