Abstract
Background
Bupropion may aid tobacco abstinence by quickly relieving symptoms of nicotine withdrawal, perhaps including impaired cognitive performance. We examined whether bupropion would attenuate abstinence-induced cognitive deficits on the first day of a brief quit attempt, when smokers are most likely to relapse.
Methods
Smokers (N=24) with high quit interest were recruited for a within-subjects cross-over test of bupropion vs placebo on ability to abstain during separate short-term practice quit smoking attempts. After introduction to working memory (N-back) and sustained attention (continuous performance task; CPT) tasks during the pre-quit smoking baseline, performance on these tasks was assessed after abstaining overnight (CO<10 ppm) on the first day of each quit attempt, while on bupropion and on placebo.
Results
Compared to placebo, bupropion after abstinence improved correct response times for working memory (p=.01 for medication by memory load interaction) and for one measure of sustained attention (numbers, but not letters; p<.05).
Discussion
Bupropion may attenuate some features of impaired cognitive performance due to withdrawal on the first day of a quit attempt. Future studies could examine whether this effect of bupropion contributes to its efficacy for longer-term smoking cessation.
Keywords: cognition, working memory, attention, tobacco dependence, smoking cessation, bupropion
1. INTRODUCTION
Smoking abstinence has been shown to impair cognitive performance, including working memory (e.g., Jacobsen et al., 2005; Xu et al., 2005), and this withdrawal-related cognitive impairment can predict smoking relapse (Patterson et al., 2010; Powell et al., 2010). Nicotine reverses abstinence-induced cognitive impairment (Myers et al. 2008), suggesting that smoking lapses after quitting may be aimed partly at moderating this impairment. Moreover, cessation medications that can attenuate such withdrawal-induced impairment during a quit attempt, especially varenicline, may help prevent relapse (Loughead et al., 2010; Patterson et al., 2009).
Bupropion increases ability to quit smoking, likely due to partial relief of withdrawal (Jorenby et al., 1999). This relief clearly includes attenuation of negative affect (Lerman et al., 2002) but also may include reducing impaired cognitive processing, although prior results may be mixed (e.g., Acheson and de Wit, 2008). The current study examined whether bupropion would rapidly attenuate the decline in cognitive performance on the first day of a brief quit attempt, when smokers are at high risk for relapse (e.g., Hughes et al., 2013). We measured working memory with the N-back task and sustained attention using the continuous performance task (CPT) because varenicline (versus placebo) significantly improves performance on both tasks during tobacco abstinence (Patterson et al., 2009). These measures also have been used to assess cognitive performance effects of other medications (e.g., Ashare et al., 2012).
2. METHOD
2.1 Participants
Participants were 25 healthy adult smokers (13 men, 12 women) recruited for a larger, within-subjects test of medication vs placebo on ability to abstain during separate short-term practice quit attempts in a cross-over design, similar to that described in more detail elsewhere (Perkins et al., 2010; see also Loughead et al., 2010). The medication conditions tested included placebo and bupropion, which was selected because it is effective for smoking cessation. A third within-subject condition involved modafinil and was not included in these analyses since modafinil was intended in the larger study as a negative control (i.e., ineffective for cessation) due to its absence of symptom relief (see Schnoll et al., 2008). Participants were required to smoke ≥10 cigarettes per day for ≥1 year and provide a screening expired-air carbon monoxide (CO) of at least 10 ppm. All were planning to quit smoking permanently within three months of entering the study and were offered 12 weeks of free counseling and open-label bupropion to quit smoking after the study, in addition to being paid for participation.
To assess effects of bupropion and placebo on cognitive performance during initial smoking abstinence, only these 25 subjects, able to meet overnight abstinence criteria during the first day of both quit attempts (i.e., CO<10 ppm on bupropion and on placebo), were included in the analyses of medication effects on cognitive tasks of sustained attention and working memory. (In the larger study, 20 others failed to abstain on the first day during one or both conditions and were excluded from these analyses.) One of these 25 participants was excluded from analyses due to missing data while on placebo. Of the 24 included in analyses, 19 were Caucasian, 2 were Asian, 2 were more than one ethnicity and 1 of unknown ethnicity, and mean (SD) characteristics were 36.0±14.0 years old, 16.3±5.9 cigarettes/day, and 4.2±2.0 score on the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991).
2.2 Cognitive Tasks
The computerized cognitive testing consisted of the Letter-N-back task (working memory) and the Penn Continuous Performance Task (CPT; sustained attention). Briefly, in the N-back task (Ragland et al., 2002), letters were presented individually on a monitor under three trial conditions varying in memory load. Participants were instructed to respond (button press) when the letter shown was an “X” (0-back; to determine simple response time not involving working memory), or the same as the letter shown immediately prior (1-back condition) or the letter shown two prior (2-back condition). Each letter was shown for 500 msec, followed by a 2500 msec interval until the next letter. The Penn CPT, developed and validated by Gur and colleagues (Gur et al., 2010; Kurtz et al., 2001), used a paradigm involving two separate trials, where subjects viewed a series of 7-segment line displays. They were instructed in two separate runs to respond whenever these lines formed a number (“numbers” trial) or a letter (“letters” trial). Each stimulus was shown for 300 ms, followed by a 700 ms blank screen interval. For both the N-back and CPT, twice as many non-targets as targets were shown in random sequence, and median response time (ms) for correct responses was the primary outcome of interest.
2.3 Procedures
Subjects were first introduced to the tasks while smoking ad libitum during a baseline week prior to receiving any medication. Then, participants were tested in the afternoons on the first day of each of two week-long quit smoking attempts while on bupropion (150 mg b.i.d.) or placebo medication administered double-blind (in counter-balanced order), similar to that described elsewhere (Perkinset al., 2010). Each quit attempt was preceded by a week of medication dose run-up. Overnight abstinence (>12 hr) on day 1 of each quit period was assessed upon arrival by self-report of no smokingsince the previous day and verified by CO<10 ppm. Task sessions were separated by at least ten days, with all participants resuming smoking (if quit) after each medication condition(i.e., washout period). Withdrawal was assessed prior to testing on each session using the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 2007). This research was approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent for participation after the nature and consequences of the study was explained.
2.4 Analyses
Because task orientation performance during the ad lib smoking baseline session always occurred first and could not be counter-balanced with the abstinence periods, those results are presented solely to illustrate responses during non-abstinence, compared with those due to medication effects during abstinence. Repeated-measures multivariate analysis of variance (MANOVA) with follow up ANOVA were used to examine medication (bupropion vs placebo) effects on cognitive task performance. Preliminary comparisons assessed gender, which did not significantly affect responses to both cognitive tasks, either as main effects or in interactions with the medication conditions (all F’s < 1). For all analyses of both tasks, medication condition was a within-subjects factor, and median response time (ms) for correct responses served as the primary dependent variable. Similar analyses were conducted for number of correct responses. For the N-back task, memory load (0-, 1-, 2-back) was included as an additional within-subjects factor, and the follow up analysis focused on medication effects for the largest load difference, between 0- and 2-back (i.e., medication X memory load interaction effects). For the Penn-CPT task, we included type of stimulus (numbers or letters) as a within-subjects factor, with follow-up ANOVA’s exploring medication differences within each type. Medication order (i.e., placebo condition before bupropion, or bupropion condition before placebo) did not interact significantly with these analyses for either task (all p’s >.30). Effect sizes of particular interest are shown by partial eta-squared values (ηp2), indicating the percent of variance explained, with ηp2 values of at least .06 and .14 constituting medium and large effects, respectively (Cohen, 1988).
3. RESULTS
3.1 CO and withdrawal after overnight tobacco abstinence
Mean (SE) COs for those able to abstain overnight were 2.8±0.3 and 3.1±0.4 ppm for placebo and bupropion, respectively, compared to 21.8±1.9 ppm during the smoking baseline session. Similar values for MNWS withdrawal were 26.9±3.9 for placebo and 20.8±3.6 for bupropion, F(1,23)=3.54, p=.07, compared to 14.0±2.8 during smoking baseline.
3.2 N-back task
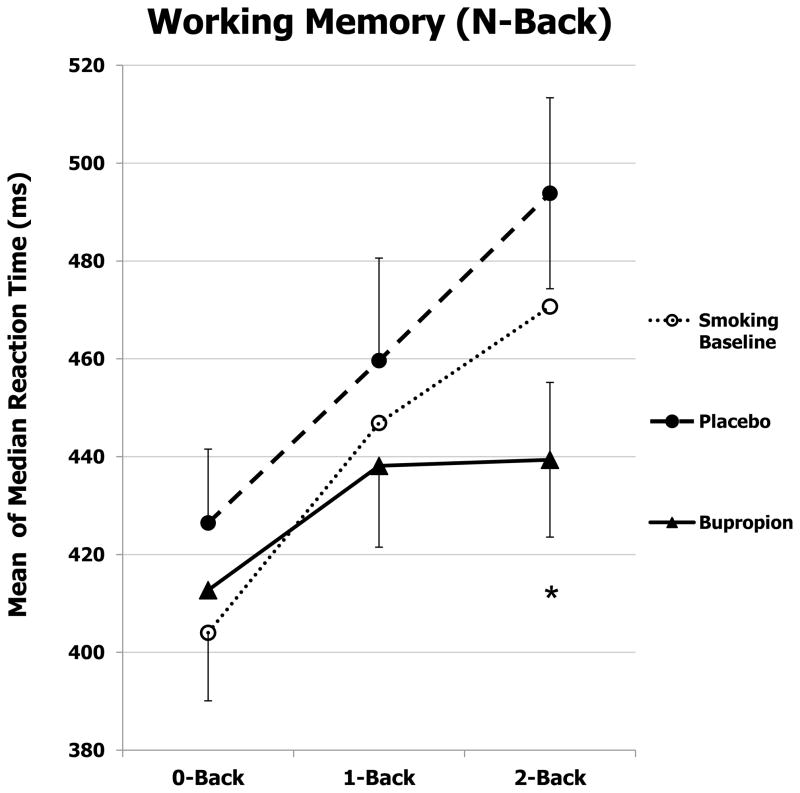
Mean of the median response time (RT) on correct responding for the working memory task during abstinence, by medication (bupropion and placebo) and memory load, is shown in Figure 1, along with RT at initial smoking baseline. For RT, there was a significant main effect of medication, F(1,23)=8.25, p<.01, ηp2 =.26, as overall RT was faster under bupropion vs. placebo. As expected, RT was also influenced by memory load (0-, 1-, 2-back), F(2,46)=11.31, p<.001, ηp2=.33. Notably, there was also a significant interaction between medication X memory load, F(2,46)=5.15, p=.01, ηp2=.18. Follow up ANOVA on the difference between 0- and 2-back showed that bupropion, vs. placebo, improved processing efficiency (reduced median response time) for high-load working memory performance, F(1,23)=7.03, p=.01, ηp2 =.23. Again as expected, number of correct responses was affected by memory load, F(2,46)=6.55, p=.003, as mean (SE) number correct was less for 2-back vs 0-back, 14.25±.26 vs 14.98±.02, respectively, but not different by medication, F(1,23)<1, or medication X memory load, F(2,46)<1.
Fig. 1.
Mean±SE of the median response time for correct responses on N-back task of working memory, by memory load, during initial smoking baseline to learn the task and due to placebo or bupropion after overnight tobacco abstinence (N=24). * p=.01 for the difference between bupropion and placebo in responding to 2-back vs 0-back.
3.3 Penn-continuous performance task
There was no main effect of medication on median RT on the CPT sustained attention task during abstinence, F(1,23)=2.34, ns. As expected, since the number task is easier than the letter task given the vastly higher number of potential foils for letters, there was a significant main effect of stimulus type (numbers/letters), F(1,23)=69.75, p<.001, ηp2 =.75; RT was faster for responses to numbers compared to letters (Table 1). Notably, we found a significant interaction between medication X stimulus type, F(1,23)=4.83, p<.05, ηp2=.17. Bupropion improved median RT for the number stimulus, F(1,23)=4.42, p<.05, ηp2 =.16, but not for the letter stimulus, F(1,23)=.58, ns. Number correct was also greater for the number vs letter stimulus, 59.8±0.1 vs 59.0±0.3, respectively, F(1,23)=7.11, p<.02 for stimulus type, but did not differ by medication or by medication X stimulus type, both F(1,23)<1 (see Table 1).
Table 1.
Number and response time for correct responses on the Continuous Performance Task (Letters, Numbers) during smoking baseline and due to placebo or bupropion after overnight abstinence (N=24).
| Measure | Mean (SE)
|
|||
|---|---|---|---|---|
| Baseline | Placebo | Bupropion | ||
| CPT True Positives (# correct) | ||||
| Letters | 57.7 (0.4) | 59.2 (0.3) | 58.9 (0.4) | |
| Numbers | 58.9 (0.4) | 59.8 (0.1) | 59.8 (0.1) | |
|
| ||||
| CPT Correct Response Time (ms) | ||||
| Letters | 423.1 (10.2) | 438.1 (10.5) | 433.1 (10.8) | |
| Numbers | 394.8 (9.8) | 418.3 (12.5) | 402.4 (11.3)* | |
p<.05 for difference between placebo and bupropion.
4. DISCUSSION
Compared to placebo, bupropion improved working memory (N-back), and one measure of sustained attention (RT for numbers on the CPT), after overnight smoking abstinence. These results suggest that one component of bupropion’s mechanisms for aiding smoking cessation could be attenuation of mild cognitive effects that occur early in nicotine withdrawal. Our findings indicate that this potential beneficial therapeutic effect of bupropion may be apparent early in cessation, after less than a full day of abstinence. Thus, bupropion, varenicline (Loughead et al., 2010, Patterson et al., 2009), and nicotine replacement (e.g., Myers et al., 2008), each FDA-approved for cessation, may be efficacious in part because of their ability to moderate cognitive impairment during abstinence. The duration of these therapeutic effects of bupropion on cognitive performance with prolonged cessation may be uncertain and warrant study (e.g. Cinciripini et al., in press).
Regarding strengths of this study, bupropion’s effects were demonstrated following overnight tobacco abstinence and compared to those of placebo in a powerful within-subjects design. This method minimizes error variance between medication conditions, which were presented in counter-balanced order. We also assessed cognitive performance with two widely used tasks, addressing working memory (N-back) and sustained attention (CPT), to show some evidence of generalizability of bupropion’s cognitive processing effects. In terms of limitations, only subjects able to abstain overnight could be included in analyses. This element introduces a potential self-selection bias, since performance during continued smoking would preclude assessing medication effects on cognitive impairment due to withdrawal. Also, although all participants were selected for high interest in quitting, testing here was done during short-term quit attempts for study purposes, and bupropion effects on cognitive performance early in a permanent quit attempt may differ.
Acknowledgments
Role of Funding Source
This research was supported by NIH Grant P50 CA143187. NIH had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
The authors acknowledge the contributions of Carolyn Fonte, Melissa Mercincavage, Erin Stratton, Jacqueline Sun, and Tiona Jones for their helpful assistance during this study.
Footnotes
Contributors
Authors KAP and CL designed the study, oversaw protocol development, planned statistical analyses, and wrote the first draft of the manuscript. Authors JLK and NCJ managed participant recruitment, data collection, and performed statistical analyses. Author RCG designed the working memory and attention tasks, and helped interpret the results. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Perkins has served as a consultant for Embera Neurotherapeutics on research that is unrelated to the current study. Dr. Lerman has served as a consultant for GlaxoSmithKline, Pfizer, and Astra Zeneca. She has received research funding, unrelated to the current study, from Pfizer and Astra Zeneca. No other authors have any potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, de Wit H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Exp Clin Psychopharmacol. 2008;16:113–123. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Ray R, Lerman C, Strasser AA. Cognitive effects of the acetylcholinesterase inhibitor, donepezil, in healthy, non-treatment seeking smokers: a pilot feasibility study. Drug Alcohol Depend. 2012;126:263–267. doi: 10.1016/j.drugalcdep.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, Brown VL, Engelmann JM, Wetter DW. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70:522–533. doi: 10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavior Sciences. 2. Lawrence Erlbaum Associates, Inc; Hillsdale NJ: 1988. [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Instructions for use of the Minnesota Withdrawal Scale-Revised. 2007 Retrieved from www/uvm.edu/~hbpl.
- Hughes JR, Solomon LJ, Fingar JR, Naud S, Helzer JE, Callas PW. The natural history of efforts to stop smoking: a prospective cohort study. Drug Alcohol Depend. 2013;128:171–174. doi: 10.1016/j.drugalcdep.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the α4β2 partial agonist Varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor R, Moolchan E, Heishman S. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacol. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte C, Mercincavage M, Stitzer ML, Chengappa KRN, Jain A. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2010;88:109–114. doi: 10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacol. 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychol. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Pinto A, Leone F, Gariti P, Siegel S, Perkins KA, Dackis C, Heitjan DF, Berrettini W, Lerman C. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alcohol Depend. 2008;98:86–93. doi: 10.1016/j.drugalcdep.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]