Abstract
Background
MRI-based selection of patients for acute stroke interventions requires rapid accurate estimation of the infarct core on diffusion-weighted MRI (DWI). Typically used manual methods to delineate DWI lesions are subjective and time-consuming. These limitations would be overcome by a fully automated method that can rapidly and objectively delineate the ischemic core. An automated method would require pre-defined criteria to identify the ischemic core.
Aim
To determine Apparent Diffusion Coefficient (ADC) based criteria that can be implemented in a fully automated software solution for identification of the ischemic core.
Methods
Imaging data from patients enrolled in the DEFUSE study who had early revascularization following tPA treatment, was included. The patients’ baseline DWI and 30-day FLAIR lesions were manually delineated after co-registration. Parts of the DWI lesion that corresponded with 30-day infarct were considered ischemic core, whereas parts that corresponded with normal brain parenchyma at 30 days were considered non-core. The optimal ADC threshold to discriminate core from non-core voxels was determined by voxel-based ROC analysis using the Youden index.
Results
51045 DWI positive voxels from 14 patients who met eligibility criteria were analyzed. The mean DWI lesion volume was 24(±23) mL. Of this, 18(±22) mL was ischemic core and 3(±5) mL was non-core. The remainder corresponded to pre-existing gliosis, CSF, or was lost to post-infarct atrophy. The ADC of core was lower than that of non-core voxels (p<0.0001). The optimal threshold for identification of ischemic core was an ADC ≤620 ×10−6 mm2/s (sensitivity 69% and specificity 78%).
Conclusions
Our data suggests the ischemic core can be identified with an absolute ADC threshold. This threshold can be implemented in image analysis software for fully automated segmentation of the ischemic core.
Keywords: Apparent diffusion coefficient, Ischemic core, Diffusion-perfusion imaging, Diffusion MRI, Ischemic stroke, Neuroimaging of acute stroke
Introduction
There is growing evidence supporting the critical importance of the diffusion lesion volume in selection of ischemic stroke patients for reperfusion therapy. Multiple studies have shown the predictive value of the diffusion lesion volume, either in isolation or in conjunction with angiographic data, perfusion data, or clinical data.(1–8) Both in clinical practice and stroke studies, DWI-based manual delineation has been the method of choice for estimation of the ischemic core. This method is time-consuming and rater-dependent. Automated estimation of the infarct core based on an absolute DWI threshold is inaccurate because the DWI signal intensity varies with magnetic field strength and scanning parameters. Additionally, DWI is susceptible to artifacts such as T2 shine-through and receiver coil-sensitivity-based intensity variation.
The Apparent Diffusion Coefficient (ADC), on the other hand, appears to be a promising candidate for objective and potentially automated delineation.(9, 10) The ADC is a measure of the diffusivity of water molecules in tissue. In ischemic tissue, cytotoxic edema leads to a reduction in the ADC. In an animal stroke model, severe ADC decreases to 75–80% of normal correspond to very low CBF with total breakdown of energy metabolism.(11) The correlation of ADC values with CBF has been observed in human stroke lesions as well(12), suggesting that the ADC may be a good parameter for identification of the ischemic core.
A recent comparison of early DWI-based versus ADC-based prediction of 24-hour infarct volumes in a rat stroke model suggested that ADC-based lesion volume determination is more accurate than DWI-based prediction.(10) This held true for both manual ADC-based delineation and automated thresholding methods. In addition, inter-rater agreement was better with ADC-based methods than with DWI. There is also evidence that ADC values may distinguish between DWI-positive regions that go on to eventual infarction, and those that show sustained recovery, in patients with successful revascularization.(13)
To implement ADC-based delineation of the ischemic core in image processing software, an ADC threshold for the ischemic core needs to be defined.
Aim and hypothesis
We hypothesized that the ADC would provide a good imaging marker for observer-independent identification of the ischemic core in acute stroke patients. Using a unique dataset with sequential MR imaging obtained prospectively as part of the DEFUSE study(1), we sought to determine the optimal ADC threshold for identification of the ischemic core.
Methods
The study design and primary results of the DEFUSE study have been reported previously.(1) The multi-center study was approved by the Institutional Review Boards of the respective institutional sites, and informed consent was obtained from subjects prior to enrollment. Briefly, acute ischemic stroke patients with National Institutes of Health Stroke Scale (NIHSS) scores greater than 5 were treated with IV tPA 0.9 mg/kg between 3 and 6 hours after symptom onset. Patients underwent an MRI of the brain before tPA treatment, as well as 3 to 6 hours afterward. A final follow-up MRI was obtained 30 days after the stroke. MRI scans were obtained on 1.5T scanners. The baseline and early follow-up MRI scans included DWI and 3-D time-of-flight flow-compensated MR angiography of the circle of Willis. MRI scans at day 30 included a T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence. Diffusion-weighted imaging was performed using a spin-echo– echo-planar imaging sequence with a ‘b’ value of 1000 sec/mm2. Axial image slices were 256×256 with voxel dimensions of 0.9–1.1 mm along x and y axes, and 5–7.5 mm along z, in all except 1 subject, who had 128×128 image slices with voxels of 1.9×1.9×7 mm3.
Image analysis
Included subjects had
technically adequate quality scans at each time-point
an initial symptomatic arterial lesion (occlusion or decreased flow), and
demonstrated recanalization (partial or complete) at early follow-up (3–6 hours).
Initial DWI and 30-day FLAIR scans were co-registered using MINC tools (Montreal Neurological Institute, McGill University, Montreal, Canada) and visually checked for accuracy of co-registration. Images that failed to register in the first attempt using a rigid body 3D registration were co-registered using alternate registration procedures, including manual initialization, scaling and shear transforms, to correct for echo-planar imaging artifacts.
The baseline DWI and final FLAIR lesions were delineated according to their maximal visual extent, i.e. the window was carefully adjusted to maximize the extent of the visible lesion before it was manually outlined. This method was used to ensure consistency, and to ensure inclusion of tissue at early stages of injury. Regions of restricted diffusion on the acute scan that were incorporated into the final infarct were termed infarct core. Restricted diffusion regions that did not get incorporated into the final infarct were carefully reviewed. If lack of incorporation was due to infarct atrophy (i.e. ex vacuo dilation of ventricles and sulci) or appeared to be from co-registration inaccuracy (i.e. limited to a thin strip of tissue immediately bordering the perimeter of the core), the regions were discarded. Otherwise, regions with restricted diffusion at baseline that corresponded to normal-appearing brain at 30 days were considered non-core regions. Regions that showed pre-existing gliosis from white matter disease or old infarcts were excluded from the analysis because it was not possible to determine on follow-up imaging whether the tissue should be allocated to core (gliosis plus new infarct) or non-core (T2 hyperintensity from pre-existing gliosis only). Subjects were excluded from analysis if the new DWI lesion completely co-localized with pre-existing gliosis, making any delineation of new from old infarct impossible.
Determination of threshold
A voxel-based analysis scheme was used. Individual subject-wise ADC thresholds were first determined, followed by a pooled analysis across subjects. ADC values outside the 200–1200 ×10−6mm2/s range were considered to be artifactual, and excluded. The baseline ADC of voxels that made up the infarct core was compared to the baseline ADC of non-core voxels using a 2-way ANOVA to account for variability between subjects. The ADC threshold to identify the infarct core was identified using ROC (receiver operating characteristics) analysis in each subject. Finally, data from all subjects was pooled and the Youden index, which weights sensitivity and specificity equally, was maximized to determine the optimal ADC threshold.
Verification
Since the threshold was determined only from regions that exhibited restricted diffusion, the performance of this pooled threshold for core identification within the entire brain was verified within our dataset by applying it to the raw baseline ADC images of each subject. The correlation between automated ADC-based lesion volumes, manual DWI-based lesion volumes and final FLAIR lesion volumes was examined by linear regression.
Results
Of 74 subjects enrolled in the DEFUSE study, 62 had technically adequate MRA scans at both baseline and 3–6 hours after treatment. Of these, 22 had demonstrated recanalization of a major cerebral artery, but five were excluded because they did not undergo a final 30-day scan. Of the remaining, three were excluded due to co-localization of DWI lesion with extensive pre-existing gliosis. Thus, 14 subjects qualified for inclusion in this analysis. Their baseline characteristics are summarized in the Table.
Table.
Baseline characteristics
| Mean age (±SD) | 69 (±16) |
| White | 86% |
| Female | 36% |
| Hypertension | 50% |
| Diabetes mellitus | 36% |
| Hyperlipidemia | 36% |
| Median (IQR) baseline NIHSS | 10 (8–16) |
| Median (IQR) time to treatment (min) | 339 (315–349) |
Analyses were performed on 51,045 voxels that were hyperintense on the baseline DWI scans. The mean DWI lesion volume at baseline was 24 (±23) mL. An example of the maximal visual extent method used for demarcation of the baseline DWI lesion is shown in Figs. 1A and B. A mean of 18 (±22) mL of the baseline DWI lesion was incorporated into the final infarct and was designated as the ischemic core. This corresponded to a total of 38,768 ischemic core voxels that were included in the analysis. Of the remainder of the DWI lesion, only 3 (±5) mL corresponded to normal appearing brain tissue at follow-up. This tissue (in total 6,602 voxels) was designated non-core. The remainder of the DWI lesion corresponded to one of the following on follow-up: ventricular CSF, CSF in sulci, leukoariosis, pre-existing infarct, small one-voxel-wide strips that immediately bordered infarct, or was lost to atrophy.
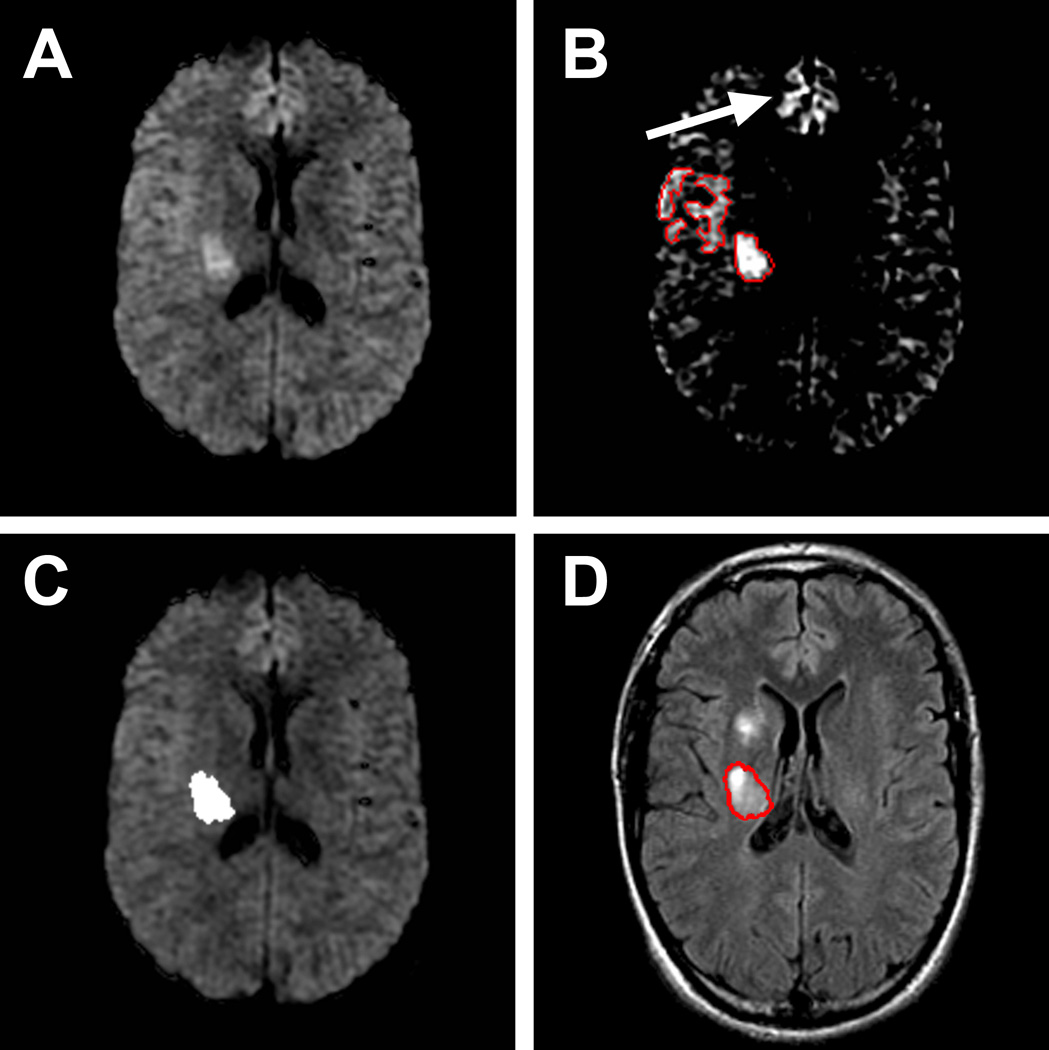
Figure 1.
A and B: DWI-based lesion delineation Example of the maximal visual extent method used for outlining lesions. Only a deep area of restricted diffusion is visible in A, but adequate windowing in B reveals there is some restricted diffusion in the adjoining cortical region that is clearly asymmetric in comparison to the contralateral side. An example of artifactual DWI hyper intensity is seen in the frontal parasagittal region (arrow). C and D: ADC-based lesion delineation and comparison with final FLAIR Applying the ADC threshold of 620 ×10−6 mm2/s to this slice reveals only one critically ischemic area in the posterior corona radiata in C. The 30-day T2-weighted FLAIR image in D reveals the final infarct that corresponds to this area of decreased ADC (red outline). There is a new region of infarction more anteriorly, that we infer to have become critically ischemic at some point in time after the baseline MRI scan.
ADC analysis
The mean ADC values of core and non-core regions were calculated for each subject. The median of these subject-wise means was 605 ×10−6 mm2/s (IQR: 565–648) for the core and 702 ×10−6 mm2/s (IQR: 671–827) for the non-core regions. Compared to non-core regions, core regions had significantly lower ADC values (Fig. 2A) (p < 0.00001; 2-way ANOVA).
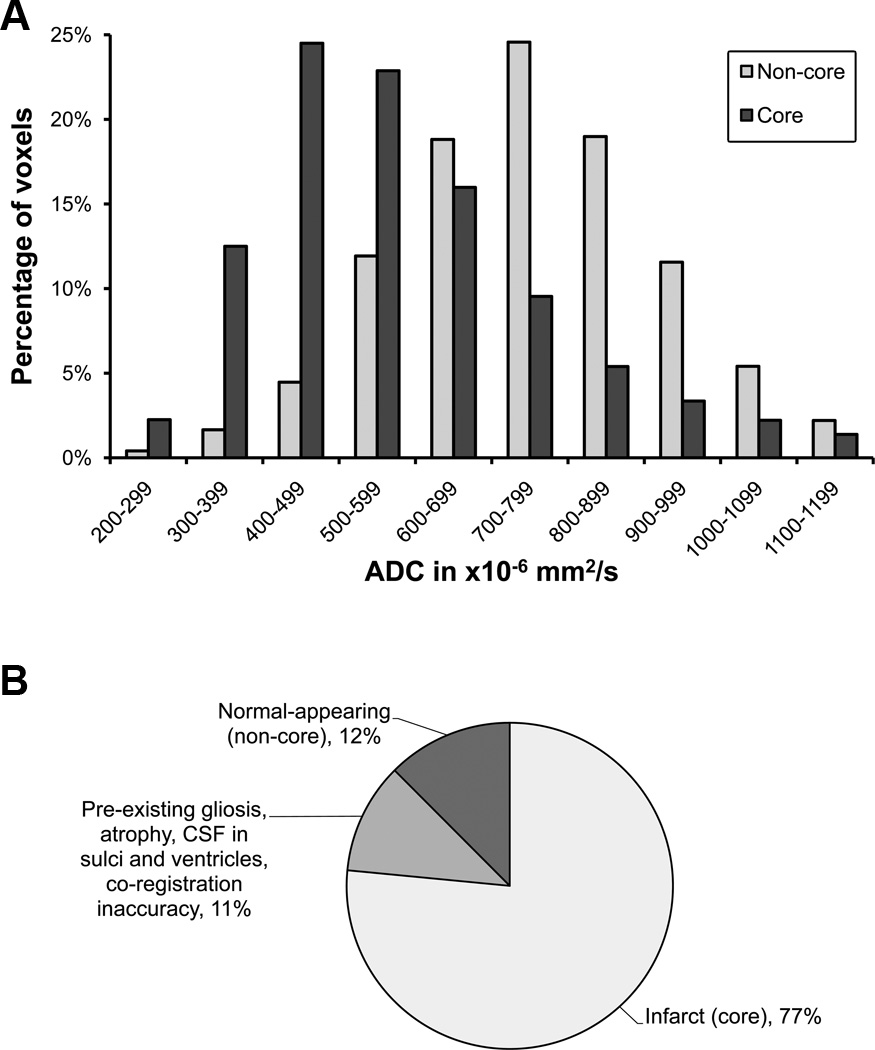
Figure 2.
Core versus non-core voxel cohorts A. Distribution of ADC values in the core and non-core voxel cohorts. The y-axis represents percentages within each individual cohort. B. Final radiographic fate of the original DWI lesion volume: the absolute number of voxels was much greater in the core than the non-core cohort.
ROC analysis
A pooled ROC analysis was performed by pooling all core and non-core voxels across the subjects (Fig. 3). The optimal ADC threshold for discriminating core from non-core was 620 ×10−6 mm2/s, corresponding to an overall sensitivity of 69% and specificity of 78%.
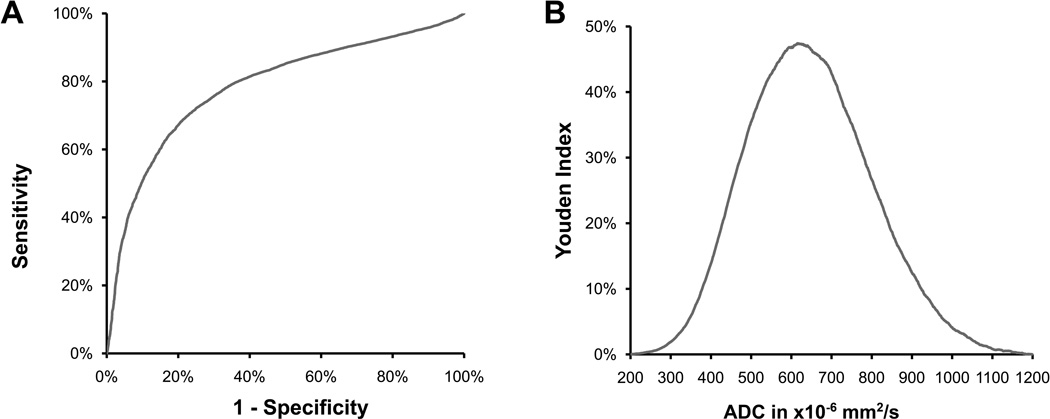
Figure 3.
ROC analysis Left:The ROC curve for pooled data showing the utility of ADC in distinguishing between core and non-core regions. Right: Youden index plotted against ADC to arrive at an optimal threshold for identification of core.
ROC analysis for distinction of core versus non-core regions by ADC values was also performed separately on each of the 11 subjects who had sufficient numbers (>10) of non-core voxels to make such an analysis meaningful. The median area-under-the-curve (AUC) was 0.7 (IQR: 0.65–0.75), while the IQR for the optimal ADC threshold for individual subjects was 582 to 654 ×10−6 mm2/s.
Verification
The optimal ADC threshold (≤620 ×10−6 mm2/s) was applied to the baseline ADC image of each subject in our dataset using an automated algorithm, which included morphological operations (closing, deleting small lesions), and comparison with DWI image to remove false-positives (normal DWI intensity regions). This allowed very good ADC-based delineation of the ischemic core (Fig. 1C).
Comparison of DWI-based manual and ADC-based automated core lesion volumes showed excellent correlation between the two (Fig. 4), with R2 of 0.94 (p<0.0001). There was also good correlation between final FLAIR lesion volumes and ADC-based automated volumes (R2 = 0.78; p<0.0001), barring one outlier subject who had <5 mL core volume by both DWI and ADC methods at baseline, but went on to infarct >100 mL of tissue in spite of demonstrated partial recanalization at early follow-up.
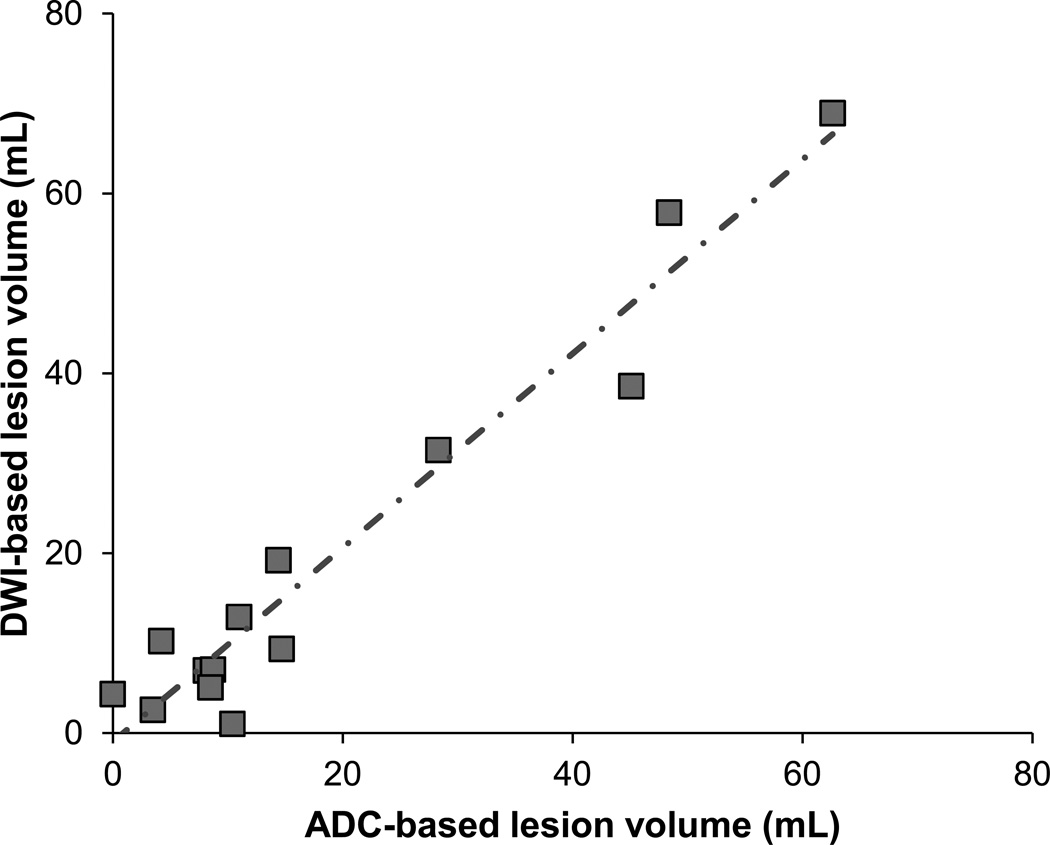
Figure 4.
Automated ADC-based versus manual DWI-based lesion volumes Automated ADC-based and manual DWI-based lesion volumes show excellent correlation. Line of regression (dot-and-dash line) has a slope of 1.08, with R2 = 0.94, and p<0.0001.
Discussion
Manual outlining of the ischemic lesion on DWI is susceptible to artifacts, time-consuming, affected by contrast windowing and operator-dependent. Nevertheless, this has been the method of choice for delineation of infarct core in clinical practice and research. The ADC is objective, less susceptible to artifacts, variations in field strength, and scanning parameters. We found in our dataset that an appropriate ADC threshold can identify the core with fair sensitivity and specificity, lending itself to automated lesion delineation.
Derivation of the ADC threshold was based on the maximum visual extent of the DWI lesion on the baseline scan. Although the majority of the ischemic core would be captured by this method, it is possible that small parts of the core that were not hyper-intense on the baseline DWI were missed. By using this approach, we however avoided erroneously basing the ADC threshold on regions outside of the initial DWI lesion that later infarcted due to recurrent embolization or delay in revascularization. Including such areas that later went on to critical ischemia and infarction, might have resulted in a spuriously high ADC threshold for the core. This is well-illustrated in Fig. 1D by the area that was not part of the initial DWI lesion but went on to infarct later. This area did not show any brightness on DWI, nor a decrease in ADC compared to surrounding tissue. We therefore suspect that this region was not part of the ischemic core initially, but became critically hypo-perfused and went on to infarct at a later time-point.
Given the limitation of our DWI-based approach for derivation of the ADC threshold, a verification step was undertaken. The optimal ADC threshold (620 ×10−6 mm2/s) was applied to the baseline ADC image of each subject that was included in our dataset. The volume of the lesion identified with this ADC threshold correlated well with the final infarct volume assessed on FLAIR images. This internal verification was promising, but further prospective investigation of the performance of this threshold in a larger data set is warranted.
When applied to the whole brain, the ADC threshold detected the ischemic core, plus scattered noise voxels, particularly in the cerebellum, brainstem, and high cortical gray matter. These could be largely eliminated in our data set using simple morphological operations and comparison to the DWI (b1000 images), as the b1000 values of these false-positive voxels are not elevated relative to normal brain. This strategy has been previously reported, and allowed fully automated delineation.(9)
Our study is the first to demonstrate the utility of an ADC threshold for ischemic core delineation in human stroke, using a voxel-based analysis. An absolute ADC threshold has been used in a rodent stroke model for prediction of infarct volume; the optimal threshold was 530 ×10−6 mm2/s.(14) Our threshold of 620 ×10−6 mm2/s for separation of core from DWI-positive penumbral tissue agrees well with ADC values observed in a study of diffusion reversal after revascularization in humans.(13) Another study identified an ADC threshold for discrimination of benign oligemia from penumbral tissue, also in humans.(15) As expected, that threshold is higher (650 ×10−6 mm2/s). Another study that enrolled patients without regard to reperfusion status, reported a significant overlap of ADC values between DWI lesions that reverse, and that go on to infarct.(16) While we also find some overlap between core and non-core regions, there still is sufficient ADC separation to make an ADC-based threshold useful for lesion segmentation.
Although uncommon, reversal of restricted diffusion lesions has been reported following early revascularization, both in humans(13, 17–20) and animal stroke models.(21, 22) By including only subjects who showed early recanalization in our analysis, we were able to separate portions of the DWI lesion that were reversible (i.e. non-core), from the ischemic core. This allowed us to determine the ADC threshold that optimally discriminates between these two territories.
The gold standard for identification of the ischemic core is tissue with ischemia at baseline that goes on to infarction despite early restoration of blood supply. Determining characteristics of the ischemic core on baseline MRI, therefore, requires a cohort of patients with documented revascularization on an early follow-up image, and a late follow-up MRI for assessment of the final infarct. A unique strength of this study is the sequential MR imaging obtained at baseline, at 6 hours post-tPA, and at 30 days post-tPA in a prospective cohort of acute stroke patients. A limitation is the relatively small number of subjects who satisfied our stringent inclusion criteria – known arterial occlusion at baseline followed by recanalization at early follow-up imaging, and good final imaging. However, because of the strict criteria, we had a homogeneous population with high quality imaging data. Moreover, the analyses were based on a large number of voxels. The high quality data and large number of voxels allowed us to identify an ADC threshold for identification of the ischemic core.
Conclusion
A simple ADC threshold may be used in acute ischemic stroke to delineate the ischemic core with reasonable sensitivity and specificity. This provides the foundation for the development of software that can rapidly and objectively delineate the ischemic core without manual input.
Acknowledgments
Acknowledgement and Funding
The funding for this study was provided by national institutes of health (NIH) grants R01 NS39325 (GWA), R01 EB002711(RB), K24 NS044848 (GWA), and K23 NS051372 (MGL). tPA was supplied at no charge by Genentech (US and Canada sites) and Boehringer Ingelheim (Belgium site). Neither Genentech, Boehringer Ingelheim nor the NIH played a role in the design or conduct of the study, data collection, management, analysis, or interpretation, or preparation, review or approval of the manuscript.
GW Albers and R Bammer are equity shareholders in iSchemaView.
Footnotes
Conflicts of interest: GW Albers and R Bammer are equity shareholders in iSchemaView
References
- 1.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Annals of Neurology. 2006;60(5):508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 2.Nagakane Y, Christensen S, Brekenfeld C, Ma H, Churilov L, Parsons MW, et al. EPITHET: Positive Result After Reanalysis Using Baseline Diffusion-Weighted Imaging/Perfusion-Weighted Imaging Co-Registration. Stroke. Jan;42(1):59–64. doi: 10.1161/STROKEAHA.110.580464. [DOI] [PubMed] [Google Scholar]
- 3.Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. Journal of cerebral blood flow and metabolism. 2010 Jun;30(6):1214–1225. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lansberg MG, Thijs VN, Bammer R, Kemp S, Wijman CAC, Marks MP, et al. Risk Factors of Symptomatic Intracerebral Hemorrhage After tPA Therapy for Acute Stroke. Stroke. 2007;38(8):2275–2278. doi: 10.1161/STROKEAHA.106.480475. 2007 August 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo AJ, Barak ER, Copen WA, Kamalian S, Gharai LR, Pervez MA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010 Aug;41(8):1728–1735. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]
- 6.Yoo AJ, Gonzalez RG. Clinical applications of diffusion MR imaging for acute ischemic stroke. Neuroimaging clinics of North America. [Review] 2011 Feb;21(1):51–69. doi: 10.1016/j.nic.2011.02.002. vii. [DOI] [PubMed] [Google Scholar]
- 7.Lansberg MG, Thijs VN, Bammer R, Olivot JM, Marks MP, Wechsler LR, et al. The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke. 2008 Sep;39(9):2491–2496. doi: 10.1161/STROKEAHA.107.508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansberg MG, Thijs VN, Hamilton S, Schlaug G, Bammer R, Kemp S, et al. Evaluation of the clinical-diffusion and perfusion-diffusion mismatch models in DEFUSE. Stroke. 2007;38(6):1826–1830. doi: 10.1161/STROKEAHA.106.480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010 Nov;32(5):1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bratane BT, Bastan B, Fisher M, Bouley J, Henninger N. Ischemic lesion volume determination on diffusion weighted images vs. apparent diffusion coefficient maps. Brain Res. 2009 Jul 7;1279:182–188. doi: 10.1016/j.brainres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab. 1995 Nov;15(6):1002–1011. doi: 10.1038/jcbfm.1995.126. [DOI] [PubMed] [Google Scholar]
- 12.Lin W, Lee JM, Lee YZ, Vo KD, Pilgram T, Hsu CY. Temporal relationship between apparent diffusion coefficient and absolute measurements of cerebral blood flow in acute stroke patients. Stroke. 2003 Jan;34(1):64–70. doi: 10.1161/01.str.0000048151.28173.0d. [DOI] [PubMed] [Google Scholar]
- 13.Kidwell CS, Saver JL, Starkman S, Duckwiler G, Jahan R, Vespa P, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002 Dec;52(6):698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]
- 14.Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol. 2004 Feb;55(2):207–212. doi: 10.1002/ana.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Na DG, Thijs VN, Albers GW, Moseley ME, Marks MP. Diffusion-weighted MR imaging in acute ischemia: value of apparent diffusion coefficient and signal intensity thresholds in predicting tissue at risk and final infarct size. AJNR Am J Neuroradiol. 2004 Sep;25(8):1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 16.Loh PS, Butcher KS, Parsons MW, MacGregor L, Desmond PM, Tress BM, et al. Apparent diffusion coefficient thresholds do not predict the response to acute stroke thrombolysis. Stroke. 2005 Dec;36(12):2626–2631. doi: 10.1161/01.STR.0000189688.95557.2b. [DOI] [PubMed] [Google Scholar]
- 17.Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000 Apr;47(4):462–469. [PubMed] [Google Scholar]
- 18.Neumann-Haefelin T, Wittsack HJ, Wenserski F, Li TQ, Moseley ME, Siebler M, et al. Diffusion- and perfusion-weighted MRI in a patient with a prolonged reversible ischaemic neurological deficit. Neuroradiology. 2000 Jun;42(6):444–447. doi: 10.1007/s002340000303. [DOI] [PubMed] [Google Scholar]
- 19.Lutsep HL, Nesbit GM, Berger RM, Coshow WR. Does reversal of ischemia on diffusion-weighted imaging reflect higher apparent diffusion coefficient values? J Neuroimaging. 2001 Jul;11(3):313–316. doi: 10.1111/j.1552-6569.2001.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 20.Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am J Neuroradiol. 2009 Jun;30(6):1206–1212. doi: 10.3174/ajnr.A1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa Y, Fisher M, Latour LL, Dardzinski BJ, Sotak CH. MRI diffusion mapping of reversible and irreversible ischemic injury in focal brain ischemia. Neurology. 1994 Aug;44(8):1484–1490. doi: 10.1212/wnl.44.8.1484. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Liu KF, Silva MD, Omae T, Sotak CH, Fenstermacher JD, et al. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats : correlation with histopathology. Stroke. 2000 Apr;31(4):946–954. doi: 10.1161/01.str.31.4.946. [DOI] [PubMed] [Google Scholar]