Abstract
Background
Neural mechanisms of decision-making and reward response in adolescent cannabis use disorder (CUD) are underexplored.
Methods
Three groups of male adolescents were studied: CUD in full remission (n=15); controls with psychopathology without substance use disorder history(n=23); and healthy controls(n=18). We investigated neural processing of decision-making and reward under conditions of varying risk and uncertainty with the Decision-Reward Uncertainty Task while participants were scanned using functional magnetic resonance imaging.
Results
Abstinent adolescents with CUD compared to controls with psychopathology showed hyperactivation in one cluster that spanned left superior parietal lobule/left lateral occipital cortex/precuneus while making risky decisions that involved uncertainty, and hypoactivation in left orbitofrontal cortex to rewarded outcomes compared to no-reward after making risky decisions. Post-hoc region of interest analyses revealed that both control groups significantly differed from the CUD group (but not from each other) during both the decision-making and reward outcome phase of the Decision-Reward Uncertainty Task. In the CUD group, orbitofrontal activations to reward significantly and negatively correlated with total number of individual drug classes the CUD patients experimented with prior to treatment. CUD duration significantly and negatively correlated with orbitofrontal activations to no-reward.
Conclusions
The adolescent CUD group demonstrated distinctly different activation patterns during risky decision-making and reward processing (after risky decision-making) compared to both the controls with psychopathology and healthy control groups. These findings suggest that neural differences in risky decision-making and reward processes are present in adolescent addiction, persist after remission from first CUD treatment, and may contribute to vulnerability for adolescent addiction.
Keywords: cannabis use disorder, decision-making, reward response, orbitofrontal cortex, adolescence, behavioral risk
1. Introduction
Marijuana, whose active component is delta-9 tetrahydrocannabinol (THC)(Ashton, 2001), is the most commonly used illicit drug in the United States (Substance Abuse and Mental Health Services Administration, 2010). Cannabis is an addictive drug (Budney et al., 2001; Gardner, 2005) that leads to cannabis use disorders (CUD; defined as DSM-IV cannabis dependence or abuse). Substance use disorders (SUD; defined as DSM-IV substance dependence or abuse) such as CUD can alter the neurobiology of decision-making and reward evaluation (Bechara, 2005; Ernst and Paulus, 2005; Volkow et al., 2003). THC acts directly as an exogenous agonist for cannabinoid 1 receptors located in the brain’s decision-making and reward circuits by enhancing dopamine tone and causing psychoactive effects (Iversen, 2003). In adults with CUD these processes are further complicated by lower IQ (Fried et al., 2002), poorer executive functions, visual-spatial deficits, and psychomotor slowing (Jacobus et al., 2009; Meier et al., 2012; Schweinsburg et al., 2008).
The cognitive and neural effects of CUD in abstinent adolescents and adults are understudied (Crean et al., 2011; Jacobus, et al., 2009). Further, some cognitive and imaging studies have not controlled for drug abstinence. The few neuroimaging investigations of decision-making in abstinent adults with CUD compared with controls without substance use disorders (SUD) have demonstrated dysregulation of the brain regions involved in decision-making and inhibition (Bolla et al., 2005; Eldreth et al., 2004). Studies of adolescent offspring at familial risk for SUD suggest pre-existing vulnerabilities in decision-making, reward evaluation, and inhibition (Andrews et al., 2011; Dawe et al., 2004; Tarter et al., 2004). However, it is unknown if the existing cognitive and neuro-imaging findings are related to vulnerabilities that predate CUD (Macleod et al., 2004; Pope et al., 2003), active cannabis or other substance use during data collection (Fried et al., 2005; Gonzalez and Swanson, 2012; Pope et al., 2001), or the neurobiological consequences of adolescent onset CUD.
CUD is an extremely difficult to treat, persistent, and long-lasting health problem. Therefore, to develop effective early identification, treatment and prevention strategies for youth with CUD, it is important to better characterize brain responses to specific types of decisions and reward in abstinent adolescents with CUD. Immaturity in decision-making and reward circuits (Bjork et al., 2007; Eshel et al., 2007; Galvan et al., 2006; Geier et al., 2010), along with reorganization of dopamine and endocannabinoid circuits during adolescence (Crews et al., 2007; Realini et al., 2009; Wahlstrom et al., 2010), may be responsible for the increased risk for adolescent-onset CUD (Johnston et al., 2008).
Adolescents begin making life decisions that involve uncertainty and experiencing unpredictable outcomes that may involve loss, such as in dating or career choices. The neural circuits recruited for decision-making and reward processing within the context of uncertainty may be altered in adolescent-onset CUD. Decision-making circuits involve a core set of brain structures: the prefrontal cortex; dorsolateral prefrontal cortex; parietal cortex; insular cortex; and anterior and posterior cingulate (Mohr et al., 2010). Reward-related brain circuits include the nucleus accumbens, caudate, putamen, thalamus, orbitofrontal cortex (OFC), bilateral anterior insula, anterior cingulate cortex, and posterior cingulate cortex (Liu et al., 2011). Reward circuits involve structures that receive dopaminergic input from the midbrain and include the ventral striatum (i.e., the nucleus accumbens), and ventromedial prefrontal cortex (Schott et al., 2008).
To address this issue, the Decision-Reward Uncertainty Task (Huettel, 2006) builds on the fact that abstract rewards, such as winning money, are associated with the same neural substrates that respond to primary reinforcers (e.g., food, love) in animals (Schultz, 2000) and humans (Fisher et al., 2010; Gottfried, O’Doherty, et al., 2003). As such, the Decision-Reward Uncertainty Task is a monetary reward task designed to examine decision-making and reward circuits separately in one task (Huettel, 2006). This methodological feature of the task is important because most previous research has failed to differentiate decisions into risk types and reward evaluation. Thus, in most studies, decision-making was contingent in time upon reward and not separated from reward evaluation (Xiangrui et al., 2010). To address these methodological issues, the Decision-Reward Uncertainty Task examines three types of risk: reward risk; behavioral risk; and no risk. Reward risk is defined as certainty about what decision to make but uncertainty about reward outcomes. In other words, one knows what actions to take for a reward but the reward is probabilistically determined. Reward risk activates decision-making circuits in the parietal cortex, dorsolateral prefrontal cortex, medial frontal lobe, basal ganglia, thalamus, and insula in adults (Huettel, 2006) and adolescents (Yaxley et al., 2011). Behavioral risk is defined as uncertainty about which decisions should be taken to earn a reward or achieve a desired goal. Under these conditions, one is uncertain about what decision to make for a reward. Behavioral risk activates decision-making circuits in additional decision-making circuits in prefrontal, parietal, and insular regions in adults (Huettel, 2006) and adolescents (Yaxley, et al., 2011). In this task, behavioral risk and reward risk conditions are matched on probability and expected value, in that each contain a 50% chance of receiving a constant-size reward. The only difference between these conditions is in whether the participant knows what decision to make (reward risk) or not (behavioral risk). Decision-making under both reward risk and behavioral risk conditions is considered risky because reward is not certain. The Decision-Reward Uncertainty Task includes a no-risk or certainty condition as a control, where the decision required to earn a reward is known and reward is certain.
Since most addiction imaging studies do not control for risk factors such as co-morbid mental illness, co-morbid substance use disorder, or active substance use, we designed this study to control for co-morbid substance use disorder, psychopathology, active substance use, and prenatal factors that may influence adolescent SUD outcomes. Psychopathology is common in adolescent-onset CUD. Co-morbidity may contribute to the neuro-mechanisms leading to addiction and the high relapse rates in adolescents seeking treatment (Kaminer and Bukstein, 2008; Spear et al., 1999). Adolescent CUD is frequently co-morbid with alcoholism (Clark, 2004; Lynskey et al., 2003), conduct disorder (Armstrong and Costello, 2002; Clark et al., 1998; Costello et al., 2003), attention deficit hyperactivity disorder (ADHD)(Armstrong and Costello, 2002), major depression (Degenhardt et al., 2003), trauma history (Dembo et al., 1988), and posttraumatic stress disorder (PTSD)(Clark et al., 1997). Decision-making and reward deficits are seen in conduct disorder (Rubia, 2011; Rubia et al., 2009), ADHD (Rubia, 2011; Volkow et al., 2009), major depression (Rao, 2006) anxiety disorders (Miu et al., 2008), trauma history (Dillon et al., 2009), and PTSD (Admon et al., 2012; Elman et al., 2009; Sailer et al., 2008). Psychopathology may either contribute to or confound the results of previous imaging investigations of decision-making and reward circuits in adolescents with SUD (Clark, 2004; De Bellis, 2002).
In this investigation, we compared three groups of adolescent males using the Decision-Reward Uncertainty task: 1) CUD in remission, after successful first-time treatment for CUD; 2) controls with psychopathology similar to the CUD group but without SUD history; and 3) healthy controls. Although there are many youth with psychiatric disorders, most do not suffer from addictions. Thus, we examined decision-making and reward circuits under uncertainty using functional magnetic resonance imaging to examine these neurobiological circuits in healthy adolescents, adolescent with psychiatric disorders, and those with CUD in remission.
We hypothesized that there would be dysregulation in decision-making and reward circuits during risky decision-making in abstinent adolescents with CUD, compared to adolescents with psychopathology and healthy controls. We hypothesized that abstinent adolescents with CUD would show altered brain activations in the key structures described above that are associated with behavioral risk during decision-making and reward processing after making risky decisions compared to both adolescent control groups.
2. Methods
2.1 Participants
Fifteen adolescents with recent outpatient treatment for CUD, in full remission; 23 adolescent control outpatients with psychopathology similar to the CUD group, but without any SUD history; and 18 healthy control adolescent males participated (Table-1). The adolescent controls with psychopathology and CUD group had similar psychopathology and number of biological parents with lifetime SUD, and were recruited through the same outpatient university clinics, where core treatments are cognitive behavioral therapy with family therapy. Healthy adolescents were recruited through local advertisements in the surrounding community. The study was approved by the University Medical Center Institutional Review Board. Adolescents provided written assent and legal guardians provided written informed consent before participation.
Table 1.
Clinical and Demographic Characteristics of 56 Male Adolescents: CUD in full remission (Group 1), Controls with Psychopathology (and without SUD history), (Group 2), and Healthy Controls (Group 3)
| Characteristics | Group, Mean (SD) Score | Test Statistic | p | Pairwise Group Differences | ||
|---|---|---|---|---|---|---|
| CUD (1) | Controls with Psychopathology (2) | Healthy Controls (3) | ||||
| N | (n=15) | (n=23) | (n=18) | F1,53=3.9 | .02 | 1>2* |
| Age, years | ||||||
| Mean (SD) Range | 16.4 ± .73 (14.7–17.2) | 15.4 ± 1.4 (12.9–17.4) | 16.0 ± 1.2 (13.5–17.1) | |||
| IQ | 113.7 ± 11.3 | 106.6 ± 13.5 | 114.4 ± 10.5 | F1,53=2.6 | .08 | |
| Number of Biological Parents with SUD History (0,1,2) | (6/9/0) | (14/7/2) | (13/5/0) | X2=7.1 | .13 | |
| Handedness (right/left) | 13/2 | 19/4 | 15/3 | X2=0.1 | .94 | |
| Ever tried Cannabis? (yes/no) | 15/0 | 4/19 | 5/13 | X2=27.7 | .0001 | 1>2,3** |
| Ever tried Tobacco? (yes/no) | 15/0 | 6/17 | 4/14 | X2=25.5 | .0001 | 1>2,3** |
| Ever tried Alcohol? (yes/no) | 14/1 | 10/13 | 15/3 | X2=13.02 | .002 | 1,3>2** |
| Cannabis/Tobacco/Alcohol/other Substance Use in the 4 weeks prior to MRI scan (yes/no) | 0/15 | 0/23 | 0/18 | __ | ||
| Comparisons between Controls with Psychopathology (2) and CUD group (1) only | ||||||
| Number of Co-morbid Axis I Psychiatric disorders (Range) | 2.13 ± 0.99 (1–3) | 1.8 ± 0.85 (1–3) | 0 - |
t1,36=1.16 | .25 | |
| Any Depressive Disorder (yes/no) | 9/6 | 9/10 | 0/18 | FET | .51 | |
| Any Anxiety Disorder (yes/no) | 11/4 | 11/12 | 0/18 | FET | .18 | |
| Any Disruptive Disorder (yes/no) | 12/3 | 20/3 | 0/18 | FET | .66 | |
| ADHD Combined Type (yes/no) | 3/12 | 3/20 | 0/18 | FET | .66 | |
| ADHD Predominantly Inattentive Type (yes/no) | 3/12 | 8/15 | 0/18 | FET | .47 | |
| ADHD Predominantly Hyperactive/Impulsivity Type (yes/no) | 1/14 | 3/20 | 0/18 | FET | .99 | |
| ADHD NOS | 0/15 | 2/21 | 0/18 | FET | .51 | |
| Oppositional Defiant Disorder (yes/no) | 6/9 | 8/15 | 0/18 | FET | .99 | |
| Conduct Disorder (yes/no) | 8/7 | 2/21 | 0/18 | FET | .006 | 1>2 |
| Major Depression (yes/no) | 7/8 | 8/15 | 0/18 | X2=0.54 | .46 | |
| Dysthymia (yes/no) | 5/10 | 2/21 | 0/18 | FET | .09 | |
| Depressive Disorder, NOS (yes/no) | 1/14 | 1/22 | 0/18 | FET | .99 | |
| Generalized Anxiety Disorder (yes/no) | 5/10 | 5/20 | 0/18 | FET | .47 | |
| Posttraumatic Stress Disorder (yes/no) | 5/10 | 3/20 | 0/18 | FET | .99 | |
| Panic Disorder (yes/no) | 1/14 | 1/22 | 0/18 | FET | .64 | |
| Substance Use Characteristics of the CUD group | ||||||
| Mean age of CUD onset (years) | 14.6 ± 1.32 | |||||
| Mean CUD duration (years) | 1.26 ± 0.83 | |||||
| Mean time between the last cannabis use and MRI scan (days) Range (days) | 133.9 ± 57.9 (60–220) | |||||
| Maximum weekly use (joints) | 19 ± 27.8 | |||||
| Greatest daily use (joints) | 9.14 ± 11.1 | |||||
| History of Nicotine Dependence (yes/no) | 4/11 | 0/23 | 0/0 | __ | ||
| History of Alcohol Abuse (yes/no) | 4/11 | 0/23 | 0/0 | __ | ||
| History of Alcohol Dependence (yes/no) | 0/0 | 0/0 | 0/0 | __ | ||
| History of Other Substance Use Disorder (yes/no) | 0/0 | 0/0 | 0/0 | __ | ||
Abbreviations: CUD, adolescents with cannabis use disorder in full early remission; FET=Fisher’s Exact Test;
Post-hoc LS Means Differences Tukey-Kramer HSD all pairwise comparison, p<.05; **Post-hoc Analysis of Means of Proportion
2.2 Study protocol
Diagnoses were made using the Schedule for Affective Disorders and Schizophrenia for School Aged Children Present and Lifetime Version (KSADS-PL)(Kaufman et al., 1997) administered to all adolescents and their legal guardians as previously described (De Bellis et al., 2009). Disorder onset, defined as the time at which diagnostic criteria were first met, was determined for each disorder. If diagnostic disagreements were not resolved with this method, consensus diagnoses were reached among a child psychiatrist (MDDB) and child psychologist (SRH) using the Best Estimate Method (Clark, 1999; Kosten and Rounsaville, 1992), where a date of onset, defined as the time at which diagnostic criteria were first met, was determined for each disorder(Clark et al., 2001). If disorders occurred within one month of each other, they were considered co-occurring. Subjects underwent a two-subtest (Vocabulary and Matrix Reasoning) IQ test using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). One year after the MRI scan, CUD subjects and their guardians underwent a telephone interview where they were asked about cannabis relapse, other drug initiation, abuse or dependence, and SUD related treatments received since the MRI scan to determine any CUD relapse or new SUD status.
Substance use information was gathered by directly interviewing adolescents. For each symptom, ages of onset were estimated to the nearest month. Methods from the Lifetime History of Alcohol Use Interview (Skinner, 1982) were incorporated into the KSADS-PL and used to collect supplemental information on cannabis, alcohol and other abused substances, including nicotine, and seven other drug classes (stimulants, sedatives/anxiolytics, cocaine, opioids, hallucinogens, solvents/inhalants, and other). Additional information included age of onset of regular use (defined as using at least twice a month for 2 months), mean maximum weekly quantity and frequency of use during periods of greatest use, and age of maximum use and quantity of maximum daily use. Cannabis consumption was translated into a standard joint (Chung et al., 2004).
The CUD patients consisted of n=6 with cannabis abuse and n=9 with cannabis dependence (seven had prior cannabis abuse). Six of the CUD subjects met DSM-IV criteria for past history of alcohol abuse without regular drinking or nicotine dependence (n=2 both, n=2 alcohol abuse, n=2 nicotine dependence). CUD patients were in full remission of any SUD prior to study enrollment. No subjects were taking psychotropic medications at the time of the study.
All adolescents received saliva and urine toxicology screens prior to scanning to confirm the absence of THC, alcohol, tobacco or other drug use. Participants with a positive screen were excluded from this investigation (n=4 CUD, n=1 controls with psychopathology). We ran 76 subjects and excluded 20 subjects (n=6 healthy controls, n=10 controls with psychopathology, n=4 CUD) due to excessive head movement (n=10) or scanner related problems (n=10). Data loss did not differ between groups (X2=.56, p=75) and the percent data loss due to motion or scanner problems (26%) was similar to that reported in other pediatric studies that detail this information (Forbes et al., 2011; Forbes et al., 2010). Subjects whose data were not included in the analyses were not reported in Table-1 or in the results section.
2.3 Exclusion Criteria
Exclusion criteria for subjects were: (1) medical, neurological, or pervasive developmental or psychotic disorder; (2) head injury, loss of consciousness; (3) birth weight under 5 lbs., postnatal compromise with neonatal intensive care stay; (4) morbid obesity or growth failure; (5) IQ<80; (6) contraindications to safe MRI research participation; (7) maternal tobacco dependence, alcohol use greater than 4 drinks a month or use of illegal drugs during pregnancy with adolescent participant (these data were collected upon interview with the biological mother and subject birth/prenatal record review); (8) lifetime history of DSM-IV Axis I disorders confirmed by KSADS-PL interview in healthy controls; (9) lifetime SUD in controls with psychopathology; and (10) current SUD in the CUD group.
2.4 Imaging procedures: Experimental task
The Decision-Reward Uncertainty Task was described previously in adolescents (Yaxley, et al., 2011). There are five different cues, with different response-reward contingencies comprising three different task conditions: no risk, reward risk, and behavioral risk. See Figure-1. No-risk cues (i.e., a star or a square) signaled that a correct button press would be rewarded with 100% certainty. Reward risk cues (i.e., a trapezoid or a circle) signaled that a correct button press would be rewarded with 50% probability. In this case, a subject made a decision knowing the “correct” response, but not knowing if they would receive a reward. However, the behavioral risk cue (a triangle) signaled that the response was unknown; only one of the two possible responses (right or left button press) would guarantee a reward, while the other would not. In this case, a subject made a decision without knowing the “correct” response or if they would receive a reward. Decision-making during reward risk and behavioral risk were risky because in both cases, reward was probabilistically determined at 50%, and thus unknown.
Figure 1.
Geometric shape cues, button press response(s), and probabilities of reward for each risk condition for the Decision-Reward Uncertainty Task. No risk cues (left button press on right hand for a star, or right button press on right hand for a square) signaled that the known behavioral response would be rewarded with 100% certainty. Reward risk cues (right button press for a trapezoid, or left button press for a circle) signaled that the known behavioral response would be rewarded with 50% probability. However, the behavioral risk cue (a triangle) signaled that the behavioral response was unknown; either one of the two possible responses (right or left button press) would guarantee a reward, while the other would not. Every trial began with the decision-making phase (in blue), where the cue was presented for 250 milliseconds, followed by a fixed 3 second delay that accompanied a fixation cross. Then participants were prompted with a question mark (“?”) for 1 second in which to make a button press to execute their decision. This was followed by a 1–7 second jittered delay, where the fixation cross was presented. Then the trial outcome phase (in red) was presented for 1 second. The stimuli were either behavioral risk or reward risk, rewarded: “$$”; no risk rewarded: “$”; or no reward for behavioral, reward, or no risk trials, “X” when an incorrect button was pressed. The outcome of each trial was determined by both a correct right or left button response and a probabilistically determined reward. An updated tally of cumulative earnings was displayed in the lower portion of the screen. Finally, the fixation cross was presented again during a 2–8 second jittered inter-trial interval and another decision-making phase began. Participants completed 150 trials on average, split evenly among six 6-minute runs. Optimal performance could yield up to an additional $15 (e.g., $0.15 per correct response for one dollar sign; $0.30 for two dollar signs) in addition to study compensation. Analyses modeled activation during the decision phase and outcome phase separately.
Every trial began with the decision-making phase and ended with the outcome phase. After cue presentation, participants were prompted with a question mark (“?”) for 1 second in which to make a button press to execute their decision. This was followed by a delay. Then the trial outcome phase was presented for 1 second. The outcome stimuli were either behavioral risk or reward risk, rewarded: “$$”; no risk rewarded: “$”; or no-reward for behavioral, reward, or no risk trials, “X” when an incorrect or no button was pressed. Finally, the fixation cross was presented again during the inter-trial interval and another decision-making phase began.
Participants completed 150 trials on average, where the three decision conditions (behavioral risk, reward risk, and no risk) were split evenly among each of six 6-minute runs. The task was presented using Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) for MATLAB (MathWorks, Inc.). In both the decision-making and reward outcome phases, all runs were included that met predetermined standards for quality assurance criteria including signal-to-fluctuation-noise ratio >60 and head motion <2mm. At least two of the six runs had to meet quality assurance criteria for subject inclusion in decision-making and outcome phase analyses. For the outcome phase of the task, all runs were included that contained both behavioral and reward risk rewarded and non-reward outcomes because behavioral risk rewarded outcomes were central to our hypothesis-driven analyses. Since behavioral risk rewarded outcomes were less likely because these were probabilistically determined and would not occur if a subject did not respond “correctly” or failed to press the button, there were fewer outcome trials than decision-making trials included in the analyses.
Participants learned the response-reward contingencies and practiced the task outside of the scanner until they mastered the task rules. Participants were paid for their entire participation in the study but could earn up to an additional $15 if they performed well on the task.
2.5 Image acquisition, processing and data analyses
Images were acquired using a 3.0-T General Electric (Waukesha, WI) MRI scanner. Whole-brain, blood-oxygenated-level-dependent (BOLD) images were collected using a high-throughput T2*-weighted spiral-in pulse sequence with the following acquisition parameters: TR: 2,000 ms; TE: 28 ms, flip angle: 90°; 34 slices; near-isotropic voxel size: 3.75×3.75×3.8 mm comprising 180 volumes. Functional images were normalized and registered to a stereotaxic space with whole-brain T1-weighted high-resolution images acquired with a 3D spoiled gradient-recalled sequence.
Functional images were analyzed using FMRI Expert Analysis Tool (FEAT) (Version 5.98, Analysis Group, FMRIB, Oxford, UK). These images were corrected for slice acquisition time (interleaved ascending), corrected for motion with MCFLIRT, normalized into the standard Montreal Neurological Institute stereotaxic space (MNI, Montreal, QC, Canada), and subjected to a high-pass filter (pass frequency>1/100 Hz). FSL’s Brain Extraction Tool (BET) was used to exclude non-brain voxels from analyses. Images were spatially smoothed using a full-width half-maximum kernel of 5 mm.
As designed, image analyses modeled activation during the decision and outcome phases separately. In the decision-making phase, we contrasted behavioral risk versus no risk and reward risk to measure decision-making under uncertainty. We collapsed no risk and reward risk together because in both these conditions what decision to make was known, compared to behavioral risk, for which the correct action was uncertain. In the outcome phase, we measured brain response to reward in only behavioral risk and reward risk trials because these trials involved probabilistically determined or “risky” rewards.
For the decision phase, first-level (i.e., within-run) regression analyses included three regressors time-locked to 2 seconds of the onset of the decision cue for each trial type (i.e., behavioral risk, reward risk, and no risk), one nuisance regressor for all responses, and one nuisance regressor for missed responses. For the outcome phase, we modeled five regressors of interest time-locked to the first second of outcome presentation (i.e., no-risk rewarded, reward-risk rewarded, behavioral-risk rewarded, behavioral-risk unrewarded, and reward-risk unrewarded). Additionally, a nuisance regressor was included for the presentation of the outcome stimulus (i.e. reward, no-reward). We did not directly contrast the decision and reward phases because the phases necessarily differed in their stimulus and response requirements. Our primary interest was in the effects of experimental condition within the decision phase and outcome phase, separately (Huettel, 2006).
For both the decision and reward phases, second-level analyses collapsed across runs, within each subject, using a fixed-effects model. Third-level analyses collapsed across all subjects in all three subject groups that included an additional regressor for between-group comparisons using a random effects model (FLAME 1). Reported results focus on the whole brain between-group comparisons to examine the differences in the CUD group compared to each of the two comparison groups. All statistical results of whole-brain voxelwise analyses reported in figures and tables were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of p=0.05 (Worsley, 2001).
Results from the whole-brain voxelwise analyses included all 56 individuals in the decision-making phase of the task and 44 individuals (n=13 controls, n=17 controls with psychopathology, n=14 CUD) in the outcome phase of the task because 12 subjects lacked behavioral risk rewarded outcomes in at least two of the six runs that meet predetermined standards for quality assurance criteria, which was our minimal criteria for inclusion in our analyses. Groups did not significantly differ by the number of runs included in the analyses (X2=2.6, p>.26).
To examine the relationship between brain regions of interest (ROI) and clinical variables, we extracted mean BOLD activation in all 56 subjects for the significant clusters derived from third-level whole-brain analyses to illustrate the brain activation patterns during each event using linear regression models. These statistical analyses were carried out controlling for age, using jmp 9.0.2 software (www.jmp.com) (2010 SAS Institute Inc.).
3. Results
3.1 Behavioral analysis
Response times showed no group effects of behavioral risk (F2,53=.1, p=.91), reward risk (F2,53=.32, p=.73), and no risk (F2,53=.02, p=.98) conditions. There were no differences in number of successful runs (that met predetermined standards for quality assurance criteria) between groups during the decision-making phase (F2,53=.21, p=.80). Mean response times analyzed by subsequent reward outcome showed no significant group effects for reward (F2,53 =.02, p=.98) and no-reward outcomes (F2,53=.55, p=.58). At least one reward and no-reward event in each risky condition per run was considered for data analyses. There were no differences in number of successful runs between groups during the reward outcome phase (F2,44=.06, p=.93). These above measures indicated that task performances between groups were similar.
3.2 Regions Activated by Behavioral Risk During Decision-Making
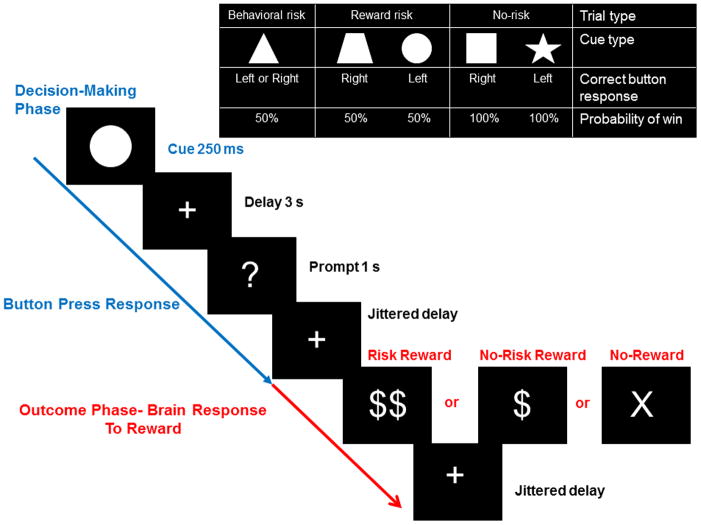
The whole-brain voxelwise analyses of the main effects of behavioral risk versus reward risk and no risk conditions demonstrated activations in parietal cortex, anterior and posterior cingulate gyrus, frontal gyrus, and insula cortex (see Supplementary Figure-1 and Table-1) as predicted from our previous study in healthy adolescents (Yaxley et al., 2011). The whole-brain voxelwise analyses contrasting the activation between risky decisions involving uncertainty (behavioral risk versus reward risk and no risk conditions) revealed greater activations in one cluster that included the left superior parietal lobule, but also left lateral occipital cortex, and left and right precuneus in the CUD group compared with controls with psychopathology (Table-2A and Figure-2A). To understand the relationship of this region during decision-making between the healthy controls and the CUD group, we examined the left superior parietal lobule activations during behavioral risk in all 56 subjects. Post-hoc analyses co-varying for age and age x group interaction revealed that the CUD patients’ superior parietal lobule mean response to behavioral risk significantly differed from both control groups (F5,50=11.36, p<.0001). Post-hoc analyses also showed that CUD behavioral risk activations were elevated compared to controls with psychopathology (LS Means Differences Tukey-Kramer HSD, p<.05) and healthy controls (LS Means Differences Tukey-Kramer HSD, p<.05) (Figure-2B). Age (F5, 50=2.7, p=.1) and age x group (F5, 50=2.5, p=.09) interactions showed a trend for significance in this model. In order to examine if the other decision-making conditions contributed to the results, we also examined the superior parietal lobule ROI for reward risk > baseline (F3,52=.34, p=.79) and no risk > baseline (F3,52=.15, p=.92) in the 3 groups, these results were not significant.
Table 2A.
Whole Brain Analyses: Main Effects of Behavioral Risk (Making a Risky Decision under Uncertainty) > No Risk and Reward Risk Trials
| Regions | Cluster Voxel Size | Peak Z value | XMNI | YMNI | ZMNI | |
|---|---|---|---|---|---|---|
| CUD > Controls with Psychopathology | Left superior parietal lobule/ Left lateral occipital cortex/ Precuneus | 809 | 3.45 | |||
| Left Superior parietal lobule | 3.45 | −26 | −58 | 64 | ||
| Left Superior parietal lobule | 3.33 | −34 | −46 | 52 | ||
| Left Lateral occipital cortex | 3.16 | −18 | −60 | 70 | ||
| Left Precuneus | 3.08 | −12 | −60 | 54 | ||
| Right Precuneus | 2.97 | 6 | −56 | 68 | ||
| Right Precuneus | 2.93 | 8 | −60 | 66 |
Figure 2. The Decision-Making Phase.
2A) The whole-brain voxelwise analyses contrasting the activation between risky decisions involving uncertainty (the behavioral risk versus the reward risk and no risk condition) revealed greater activations in the cluster involving regions of the left superior parietal lobule, left lateral occipital cortex, and left and right precuneus in the CUD group compared with the controls with psychopathology. Figure-2B. Superior parietal lobule (SPL) mean percent BOLD signal change (and standard error bars) to behavioral risk > baseline (i.e., the jittered fixation between trials) in the three groups. Region of interest analyses demonstrated that the CUD group significantly differed from controls with psychopathology and healthy controls (F1,56=8.70, p=.0005); post-hoc analyses showed CUD behavioral risk activation (purple column) was higher compared to controls with psychopathology (light blue column) and healthy controls (green column). Post-hoc pairwise comparisons were made with LS Means Differences Tukey’s HSD, *p<.05.
3.3 Regions Activated by Rewarded versus Non-rewarded Contrast Outcomes
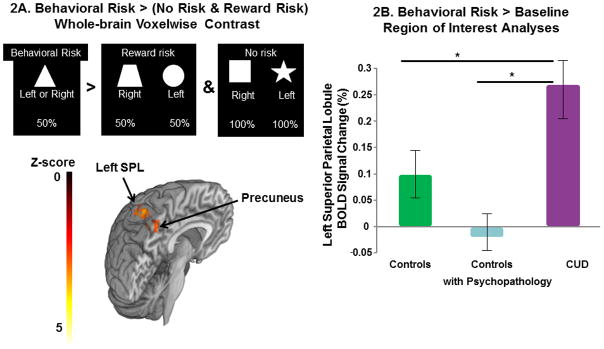
The whole-brain voxelwise analyses of outcomes contrasted the activation between the reward and no-reward outcomes after making risky decisions. This analysis contrasted rewarded outcomes from the behavioral risk and reward risk conditions with the unrewarded outcomes in the same conditions. The whole-brain voxelwise analyses of the main effects of reward versus no-reward outcomes after making risky decisions demonstrated activation in visual cortex, striatum, cingulate gyrus, and frontal cortex (see Supplementary Figure-2 and Table-2) as predicted from our previous study in healthy adolescents (Yaxley et al., 2011). The left OFC revealed significant hypoactivation to reward minus no-reward in the CUD group compared to the controls with psychopathology for rewarded risk trials (Reward Risk + Behavioral Risk)> unrewarded risk trials (Reward Risk + Behavioral Risk) (Table-2B and Figure-3A).
Table 2B.
Whole Brain Analyses: Main Effects of the Outcome Phase: Risky Trials: Reward > No-Reward
| Controls with Psychopathology > CUD | Left Frontal Lobe/Middle Frontal Gyrus/ OFC | 562 | 3.58 | |||
|---|---|---|---|---|---|---|
| Left Frontal Lobe/Middle Frontal Gyrus | 3.58 | −34 | 50 | −10 | ||
| Left Middle Frontal Gyrus | 3.47 | −34 | 46 | −10 | ||
| Left Frontal Pole/OFC | 3.14 | −42 | 42 | −10 | ||
| Left Middle Frontal/ OFC | 2.94 | −28 | 36 | −4 | ||
| Left Frontal Pole/ OFC | 2.9 | −22 | 42 | −12 | ||
| Left Superior Frontal Gyrus | 2.79 | −32 | 58 | −4 | ||
| Left Middle Frontal Gyrus | 3.47 | −34 | 46 | −10 |
Shown for each cluster of significant activation (Z > 2.3) are the coordinates (mm within MNI space) of the peak voxel and sub-voxels within that cluster. OFC=orbital frontal cortex
Figure 3.
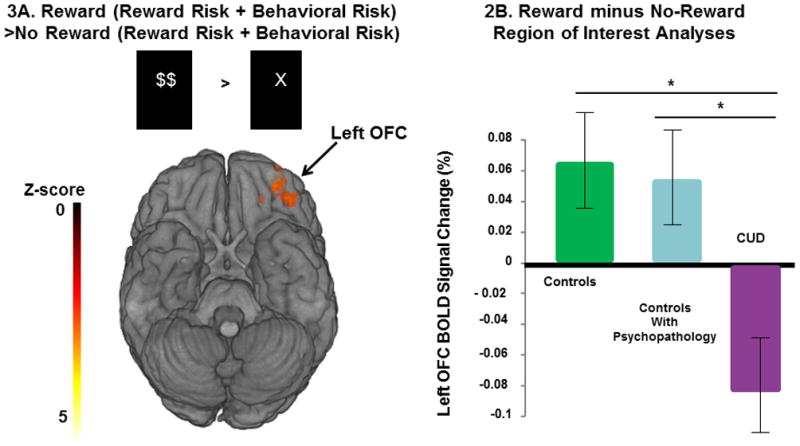
Figure-3A. The Reward Outcome Phase Whole-brain analysis of behavioral and reward risk rewarded > behavioral and reward risk no-reward. Decreased left OFC activations were seen in the CUD group compared to controls with psychopathology. Figure-3B. Region of interest measures showing mean left OFC percent signal change (and standard error bars) to reward minus no-reward after making a risky decision in the three groups. OFC activation was lower in the CUD group (purple column) compared to controls with psychopathology and healthy controls (F2,53=4.8, p<.01); post-hoc analyses showed that CUD activations (purple column) were lower compared to controls with psychopathology (light blue column) and healthy controls (green column). Post-hoc pairwise comparisons were made with LS Means Differences Tukey’s HSD, *p<.05.
To understand the relationship of OFC function in all 3 groups, we examined the left OFC activations during reward versus no-reward in all 56 subjects. Post hoc analyses revealed that the CUD patients’ OFC mean response to reward minus no-reward after making a risky decision was significantly lower compared to both control groups (F2,53=4.8, p<.01). Individual contrasts showed that CUD group activation was lower compared to both controls with psychopathology (LS Means Differences Tukey-Kramer HSD, p<.05) and healthy controls (LS Means Differences Tukey-Kramer HSD, p<.05). Age (p>.2) and age x group interaction (p>.2) were not significant covariates and thus not included in this analyses (Figure-3B).
3.4 Clinical Correlations
No significant relationships were seen between any clinical variables and left superior parietal lobule response to behavioral risk. However, left OFC activations to reward (rs=−.60, p<.02) and reward minus no-reward (rs=−.63, p=.01) significantly and negatively correlated with the total number of individual drug classes the CUD patients experimented with prior to treatment. Lower OFC reward activation correlated with more drug experimentation (Figures-4A & 4B). The left OFC showed a significant interactions between both control groups and condition (reward versus no-reward) (F2,53=4.8, p=0.01; Figure-5A). Follow-up contrasts revealed that both controls groups reward and no-reward activations were significantly different than the CUD group (F1,53=4.8, p=0.003) but similar to each other. CUD duration significantly and negatively correlated with left OFC response to no-reward correcting for the participant’s current age (Partial correlation= −0.7620, p<.009). This suggests that the longer participants had a CUD diagnosis, the more the CUD participant’s response to no-reward appeared similar to both control groups (Figures-5A & 5B).
Figure 4.
Figures-4A & 4B The total number of individual drug classes experimented with prior to CUD treatment was associated with lower left OFC BOLD response to reward (rs=−.60, p<.02) and reward minus no-reward (rs =−.63, p=.01).
Figure 5.
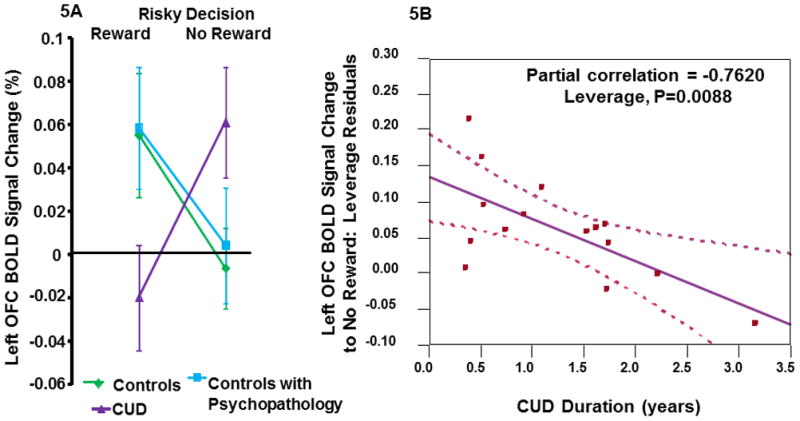
Figure-5A. Mean percent signal change and standard error bars to behavioral and reward risk trials rewarded (reward) and behavioral and reward risk trials not rewarded (no-reward) shown for each group. Percent signal change was extracted from the left OFC cluster found in the whole-brain analysis to illustrate left OFC response of each of the groups to reward and no-reward. The left OFC showed a significant interaction between both control groups and condition (reward versus no-reward) (F2,53 =4.8, p=0.01). Figure-5B. CUD duration significantly and negatively correlated with left OFC response to no-reward covarying for the participant’s current age (Partial correlation= −0.7620, p<.009). This suggests that the longer participants had a CUD diagnosis, the more the CUD participant’s response to no-reward appeared similar to both control groups.
We also investigated the relationship between left OFC reward minus no-reward activity and cannabis relapse. Of the 15 subjects, all but 3 had relapsed by one year follow-up. This rate is similar to high rates of relapse previously described in adolescents treated for SUD (Williams et al., 2000). Mean left OFC reward minus no-reward activation predicted relapse within a year of the MRI scan (X2=10.8, p=.001). Subjects who did not relapse showed higher mean left OFC reward minus no-reward activation (F1,13=11.11, p=.005). Mean left OFC reward minus no-reward activation was higher for those who remained abstinent after additionally controlling for age, SES, handedness, IQ, cannabis consumption variables, and duration of abstinence (t6,13=3.08, p=.02). We emphasize that the sample size is small so results should be considered with caution.
We saw no other significant relationships or group interactions between superior parietal lobule or OFC activations with cannabis consumption variables, age of onset of CUD, age at the time of scan, or between number of days since last cannabis consumption and MRI scan.
CUD patients with histories of alcohol abuse or nicotine dependence and their associated consumption variables did not influence the results.
4. Discussion
We investigated decision-making and reward circuits under uncertainty in abstinent adolescents with CUD compared to adolescent controls with comorbid psychopathology and healthy controls. The whole-brain voxelwise analyses investigating neural responses during risky decisions involving uncertainty revealed hyperactivations in decision circuits that included the left superior parietal lobule, but also the left lateral occipital cortex and bilateral precuneus in the CUD group compared with controls with psychopathology. Post-hoc analyses also revealed significant hyperactivations to decisions involving uncertainty in these brain regions of interest in the CUD patients compared to both control groups. Furthermore, abstinent adolescents with CUD had attenuated left OFC activations to reward but accentuated left OFC activity to no-reward compared to non-SUD adolescents. In the CUD group, left OFC activation was in the opposite direction from both control groups, whose reward activations were similar for both outcomes. Moreover, the nature of our study controlled for non-SUD psychopathology, a common confound in adolescent addiction research, as a possible neural mechanism of CUD vulnerability, and utilized a task that controlled for reward variations. Taken together, these findings suggest that adolescent onset CUD is associated with altered decision and reward circuits that are present early in the life course of addiction and may represent a vulnerability to adolescent addiction.
Meta-analyses show that risky decisions involve superior parietal lobule activity (Krain et al., 2006), particularly in the integration of information (Zysset et al., 2006). As opposed to previous findings in abstinent adults with CUD compared with controls (Bolla, et al., 2005; Eldreth, et al., 2004), we saw no areas of hypoactivations to risky decisions in the CUD group compared to controls at the whole-brain level. Similar to other adolescent SUD researchers, we found hyperactivation in executive regions. Note, the decision-making phase of the task involves decision-making, based on working memory for the association between the different types of cues and correct responses, and response inhibition because subjects had to wait to see a “?” to press the button for their decision to register as a response. The three groups studied here did not differ in their task performance. Although our study involved decision-making under uncertainty, our findings are similar to other investigations of abstinent adolescent marijuana users that demonstrated increased activation in dorsolateral prefrontal, parietal, superior temporal, and posterior cingulate regions during working memory demands and increased parietal and frontal activation during inhibition compared with controls (Gruber and Yurgelun-Todd, 2005; Schweinsburg, et al., 2008; Schweinsburg et al., 2005; Tapert et al., 2007). Hyperactivations in the left superior parietal lobule, left lateral occipital cortex, and bilateral precuneus in the CUD group during behavioral risk suggests that additional neural resources were needed to integrate information to maintain the same level of task performance as both control groups.
Although the CUD group’s activation during risky decisions under uncertainty did not correlate with clinical measures, correlations of left OFC hypoactivation to reward with substance-related clinical variables suggest that adolescent onset CUD may be caused by pre-existing OFC reward dysregulation. In support of this idea, OFC reward and reward minus no-reward response significantly and negatively correlated with the total number of individual drug classes the CUD patients experimented with prior to treatment, indicating that greater OFC hypoactivation to reward under conditions of uncertainty was associated with more drug experimentation. Furthermore, CUD duration correlated with OFC activity to no-reward after risky decisions, such that the longer the CUD patients were using, the lower their OFC responses to no-reward. Additionally, lower left OFC reward minus no-reward activations predicted relapse, suggesting that hypoactivations in reward circuits may contribute to vulnerability for adolescent addiction. This suggests that THC may re-regulate a dysregulated OFC reward system in adolescents with addiction, which is further supported by findings showing that non-abstinent adults with heavy cannabis use show higher OFC activation during wins in the Iowa gambling task (Cousijn et al., 2012).
Several studies suggest that OFC dysfunction creates vulnerability for early substance initiation. A large cross-sectional fMRI study of 1,593 fourteen year old adolescents revealed hypoactivations in lateral OFC during successful inhibition of a stop-signal task in adolescents who showed early substance initiation (alcohol, nicotine or illicit substances)(Whelan et al., 2012). Another longitudinal study reported that smaller OFC volumes at age 12 predicted initiation of cannabis use by age 16 (Cheetham et al., 2012). Healthy adults with a positive parental family history of SUD demonstrated lower OFC activation compared to healthy adults without family history of SUD to anticipation of monetary reward (Andrews, et al., 2011), further suggesting that OFC dysfunction is a vulnerability for SUD. Our three groups did not differ in family history of first degree relatives with a SUD (p>.13). This was particularly true of our controls with psychopathology (40% had at least one parent with SUD history) and the abstinent CUD group (60% had at least one parent with SUD history).
The behaviors characteristic of OFC dysfunction resemble SUD and its treatment-refractory nature. The lateral OFC is involved in drug-seeking behaviors (Roberts, 2006; Rolls, 2000; Schoenbaum and Shaham, 2008). OFC lesions have biased rats toward choices with more immediate access to rewards without regard to effort (Rudebeck et al., 2006), and towards riskier choices(Pais-Vieira et al., 2007). OFC lesions in primates impair their ability to attribute reward delivery or omission to their most recent behavior (Rudebeck et al., 2008). Imaging studies demonstrate that OFC serves to code stimuli into a common currency of relative reward values (Arana et al., 2003; Cox et al., 2005; Elliott et al., 2003; FitzGerald et al., 2009; Gottfried, O’Doherty, et al., 2003; O’Doherty et al., 2001; Plassmann et al., 2007). The region of OFC hypoactivation seen in our CUD subjects is homologous to subregions (frontal polar area 10, area 11 anteriorly, area 13 posteriorly) in primates (Chiavaras and Petrides, 2000). These Areas receive direct connections from the amygdala and integrate data from primary reinforcers (Carmichael and Price, 1995). Specific lesions of areas 11 and 13 in primates cause deficits in the ability to make adaptive choices such as choosing not to eat when satiated (Rudebeck and Murray, 2011).
Although several studies show that ventral striatum activity is increased in adolescents compared to adults, suggesting a developmental immaturity of subcortical areas in adolescents(Chambers et al., 2003; Ernst et al., 2006; Geier, et al., 2010; Leijenhorst et al., 2010), we found main effects of reward in ventral striatum in all 3 groups and no group differences. Our data are inconsistent with the idea that adolescents are vulnerable to SUD because they are seeking more stimulating experiences to compensate for ventral striatum hypoactivity (Bjork et al., 2004; L. P. Spear, 2000). Blunted ventral striatum activation was seen in healthy young adults with positive SUD family history (Andrews, et al., 2011). It is possible that ventral striatum dysfunction may not become evident in participants at risk for SUD until young adulthood due to maturation changes that occur during adolescence. We did not find attenuated reward activity in the nucleus accumbens and caudate nucleus as seen in a study of young adults with CUD who were only one week abstinent from cannabis and had positive urine toxicology (van Hell et al., 2010). The latter study may represent findings of early cannabis withdrawal as our CUD patients were abstinent for at least one month before MRI scan.
Our study had several strengths. Our design insured that our results were not due to greater prenatal exposure of substances, prenatal factors, outpatient clinic status, comorbid psychopathology, child maltreatment and trauma histories, or greater familial risk in the CUD group.
Our data also have several limitations. First, the whole brain BOLD differences were between the CUD group and controls with psychopathology in both the decision-making and reward outcome phases of the task and not the CUD and healthy control groups. Given our region of interest analyses, we believe this was due to lack of power for a 3-cell fMRI study given our group sample sizes. The controls with psychopathology and healthy control groups region of interest data for each phase were similar for the decision-making and reward versus no-reward outcomes and opposite the CUD group data. Our fMRI between-group data is cross-sectional so we cannot prove that the different activation patterns to risky decision-making and reward in the CUD group represent pre-existing vulnerabilities for adolescent onset CUD. However, our clinical correlation and relapse data would support this interpretation. Secondly, because our treatment center had a majority of male adolescents with CUD, it was not feasible to examine females with CUD. Sex differences in the OFC were seen in adults recently abstinent from cocaine where males show lateral and females medial OFC activations (Adinoff et al., 2006). Gender differences may exist that have yet to be explored. Lastly, findings of our cross-sectional study have several untested explanations: 1) An inherent vulnerability for adolescent-onset CUD; 2) An inherent vulnerability for life-long treatment-refractory SUD; or 3) An unmeasured and initial THC-induced neurodevelopmental change in decision-making and reward circuits caused by heavy adolescent THC consumption that lead to adolescent-onset CUD. Since a human lesion fMRI study found that the left OFC was responsible for motivational modulation (Szatkowska et al., 2011) and our findings indicate that adolescents with CUD in full remission showed dysregulation of this motivation region (which can manifest itself as motivational deficits during uncertainty), it is possible that dysfunctional left OFC response to reward may be an initial THC-induced neurodevelopmental change that lead to prolonged cannabis use. The fact that known consequences of cannabis misuse are deficits in new learning and anhedonia (Janiri et al., 2005; Pope and Yurgelun-Todd, 2001) may support a picture of very early THC-induced changes to the OFC (Ameri, 1999) that can contribute to the “gateway drug” effect of cannabis (US Department of Health and Human Services, 1999).
In conclusion, abstinent adolescents with CUD demonstrated hyperactivations in decision-making brain regions to risky decisions and OFC hypoactivations to reward that significantly differed from controls with psychopathology and healthy control adolescents. These findings suggest that dysfunction in decision-making and reward brain regions may be vulnerabilities for adolescent CUD and merit further investigation. Given that the frontal cortex undergoes substantial maturation during adolescence (Paus, 2005), cognitive interventions developed for at-risk adolescents may be implemented to optimize decision-making during uncertain situations by improving neuro-regulation in decision-making and reward brain circuits.
Supplementary Material
The whole-brain voxelwise analyses of the main effects of behavioral risk versus reward risk and no risk conditions demonstrated activations in parietal cortex, anterior and posterior cingulate gyrus, frontal gyrus, and insula cortex (see Supplementary Table-1) in all three groups as predicted from our previous study in healthy adolescents (Yaxley et al., 2011).
Supplementary Figure 2. The whole-brain voxelwise analyses of the main effects of reward versus no-reward outcomes after making risky decisions demonstrated activation in visual cortex, striatum, cingulate gyrus, and frontal cortex (see Supplementary Table-2) n all three groups as predicted from our previous study in healthy adolescents (Yaxley et al., 2011).
References
- Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD. Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gender Medicine. 2006;3(3) doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, et al. Imbalanced Neural Responsivity to Risk and Reward Indicates Stress Vulnerability in Humans. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- Ameri A. The effects of cannabinoids on the brain. Progress in Neurobiology. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, et al. Individuals Family History Positive for Alcoholism Show Functional Magnetic Resonance Imaging Differences in Reward Sensitivity That Are Related to Impulsivity Factors. Biological Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. Journal of Consulting and Clinical Psychology. 2002;70:1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- Ashton CH. Pharmacology and effects of cannabis: a brief review. British Journal of Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of will power to resist drugs: a neurocognitive perspective. Nature neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.200424/8/1793. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. The Journal of Neuroscience. 2007;27(18):4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480– 492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Archives of General Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal Volumes in Early Adolescence Predict Initiation of Cannabis Use: A 4-Year Longitudinal and Prospective Study. Biological Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Chiavaras MM, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. The Journal of Comparative Neurology. 2000;422:35–54. [PubMed] [Google Scholar]
- Chung T, Martin CS, Winters KC, Cornelius JR, Langenbucher JW. Limitations in the Assessment of DSM–IV Cannabis Tolerance as an Indicator of Dependence in Adolescents. Experimental and Clinical Psychopharmacology. 2004;12:136–146. doi: 10.1037/1064-1297.12.2.136. [DOI] [PubMed] [Google Scholar]
- Clark DB. The natural history of adolescent alcohol use disorders. Addiction. 2004;99(Suppl 2):5–22. doi: 10.1111/j.1360-0443.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- Clark DB. Psychiatric Assessment. In: Ott PJ, Tarter RE, Ammerman RT, editors. Sourcebook on Substance Abuse: Etiology, Epidemiology, Assessment, and Treatment. Needham Heights MA: Allyn & Bacon; 1999. pp. 197–211. [Google Scholar]
- Clark DB, Kirisci L, Moss HB. Early adolescent gateway drug use in sons of fathers with substance use disorders. Addictive Behaviors. 1998;23:561–566. doi: 10.1016/s0306-4603(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Clark DB, Lesnick L, Hegedus A. Trauma and other stressors in adolescent alcohol dependence and abuse. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1744–1751. doi: 10.1097/00004583-199712000-00023. [DOI] [PubMed] [Google Scholar]
- Clark DB, Pollock NK, Mezzich A, Cornelius J, Martin C. Diachronic assessment and the emergence of substance use disorders. Journal of Child and Adolescent Substance Abuse. 2001;10:13–22. [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Porrino LJ, et al. Individual differences in decision making and reward processing predict changes in cannabis use: a prospective functional magnetic resonance imaging study. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- Cox SM, Andrade A, Johnsrude IS. Learning to like: a role for human orbitofrontal cortex in conditioned reward. Journal of Neuroscience. 2005;25:2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An Evidence-Based Review of Acute and Long-Term Effects of Cannabis Use on Executive Cognitive Functions. Journal of Addiction Medicine. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsivenss as dimensions of impulsivity: implications for substance misuse. Addictive behaviors. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental Traumatology: A contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper S, DPW, Shenk CE. Demographic, Maltreatment, and Neurobiological Correlates of PTSD Symptoms in Children and Adolescents. Journal of Pediatric Psychological. 2009;35(5):570–577. doi: 10.1093/jpepsy/jsp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Dembo R, Dertke M, Borders S, Washburn M, Schmeidler J. The relationship between physical and sexual abuse and tobacco, alcohol, and illicit drug use among youths in a juvenile detention center. International Journal of the Addictions. 1988;23:351–378. doi: 10.3109/10826088809039203. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood Adversity Is Associated with Left Basal Ganglia Dysfunction During Reward Anticipation in Adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914– 920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JFW. Differential Response Patterns in the Striatum and Orbitofrontal Cortex to Financial Reward in Humans: A Parametric Functional Magnetic Resonance Imaging Study. The Journal of Neuroscience. 2003;23(1):303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional Neuroimaging of Reward Circuitry Responsivity to Monetary Gains and Losses in Posttraumatic Stress Disorder. Biological Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. S0006-3223(05)00710-9 [pii] [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. S0033291705005891 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D. Reward, Addiction, and Emotion Regulation Systems Associated With Rejection in Love. J Neurophysiol. 2010;104:51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- FitzGerald TH, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. Journal of Neuroscience. 2009;29:8388–8395. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural Systems of Threat Processing in Adolescents: Role of Pubertal Maturation and Relation to Measures of Negative Affect. Developmental Neuropsychology. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, et al. Healthy Adolescents’ Neural Response to Reward: Associations With Puberty, Positive Affect, and Depressive Symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marijuana—a comparison with predrug performance. Neurotoxicol Teratol. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, James D, Gray R. Current and former marijuana use: preliminary findings of a longitudinal study of effects on IQ in young adults. Canadian Medical Association Journal of Medicine. 2002;166:887–891. [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: Emphasis on dopamine. Pharmacology Biochemistry and Behavior. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in Reward Processing and Its Influence on Inhibitory Control in Adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Swanson JM. Long-term effects of adolescent-onset and persistent use of cannabis. PNAS. 2012;109:15970–15971. doi: 10.1073/pnas.1214124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding Predictive Reward Value in Human Amygdala and Orbitofrontal Cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Huettel SA. Behavioral, but not reward, risk modulates activation of prefrontal, parietal, and insular cortices. Cognitive Affective Behavioral Neuroscience. 2006;6(2):141–151. doi: 10.3758/cabn.6.2.141. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacology Biochemistry and Behavior. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, et al. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropschobiology. 2005;52:37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings 2007. Bethesda MD: National Institute on Drug Abuse; 2008. [Google Scholar]
- Kaminer Y, Bukstein OG, editors. Adolescent Substance Abuse: Dual Diagnosis and High Risk Behaviors. NY NY: Routledge/Taylor & Francis; 2008. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. American Journal of Psychiatry. 1992;149:1225–1227. doi: 10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: A meta-analysis of decision-making. NeuroImage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Leijenhorst LV, Zanolie K, Van Mee CS, Westenberg PM, Rombouts SARB, Crone EA. What Motivates the Adolescent? Brain Regions Mediating Reward Sensitivity across Adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. Journal of the Amercian Medical Association. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Macleod J, Oakes R, Copello A, Crome I, Egger M, Hickman M, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal general population studies. Lancet. 2004;363:1579–1588. doi: 10.1016/S0140-6736(04)16200-4. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Pro Nat Acad Sc. 2012;109:2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Houser D. Anxiety impairs decision-making: Psychophysiological evidence from an Iowa Gambling Task. Biological Psychology. 2008;77:353–358. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Mohr PN, Biele G, Heekeren HR. Neural processing of risk. Journal of Neuroscience. 2010;30:6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Lima D, Galhardo V. Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience. 2007;145:225–231. doi: 10.1016/j.neuroscience.2006.11.058. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. TRENDS in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The Video Toolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. Journal of Neuroscience. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug & Alcohol Dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Yurgelun-Todd D. The Residual Cognitive Effects of Heavy Marijuana Use in College Students. Journal of the Amercian Medical Association. 2001;275:521–527. [PubMed] [Google Scholar]
- Rao U. Links Between Depression and Substance Abuse in Adolescents: Neurobiological Mechanisms. American Journal of Preventive Medicine. 2006;31(6S1):S161–S174. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacological Research. 2009;60:132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. TRENDS in Cognitive Sciences. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rubia K. “Cool” Inferior Frontostriatal Dysfunction in Attention-Deficit/Hyperactivity Disorder Versus “Hot” Ventromedial Orbitofrontal-Limbic Dysfunction in Conduct Disorder: A Review. Biological Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-Specific Dissociation of Orbitofrontal Dysfunction in Boys With Pure Conduct Disorder During Reward and Ventrolateral Prefrontal Dysfunction in Boys With Pure ADHD During Sustained Attention. American Journal of Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, et al. Frontal cortex subregions play distinct roles in choices between actions and stimuli. The Journal of Neuroscience. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Rudebeck PHS, Murray EA. Dissociable Effects of Subtotal Lesions within the Macaque Orbital Prefrontal Cortex on Reward-Guided Behavior. The Journal of Neuroscience. 2011;31(29):10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer U, Robinson S, Fischmeister FFS, Konig D, Oppenauer C, Lueger-Schuster B, et al. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The Role of Orbitofrontal Cortex in Drug Addiction: A Review of Preclinical Studies. Biological Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elemenhorst D, Lang M, Winz OH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. Journal of Neuroscience. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward systems in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Toronto: 1982. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. S0149-7634(00)00014-2 [pii] [DOI] [PubMed] [Google Scholar]
- Spear SF, Ciesla JR, Skala SY. Relapse patterns among adolescents treated for chemical dependency. Substance Use & Misuse. 1999;34(13):1795–1815. doi: 10.3109/10826089909039427. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings: Office of Applied Studies, NSDUH Series H-38A, HHS Publication No SMA 10–4586 Findings. Rockville MD: 2010. [Google Scholar]
- Szatkowska I, Szymanska O, Marchewka A, Soluch P, Rymarczyk K. Dissociable contributions of the left and right posterior medial orbitofrontal cortex in motivational control of goal-directed behavior. Neurobiology of Learning and Memory. 2011;96:385–391. doi: 10.1016/j.nlm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehaviour disinhibition in childhood predisposes boys to substance use disorder by young adulthood: Direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: An fMRI study. European Neuropsychopharmacology. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. The Journal of Clinical Investigation. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating Dopamine Reward Pathway in ADHD: Clinical Implications. Journal of the Amercian Medical Association. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Whelan R, Conrod PJ, Poline J-B, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience. 2012 doi: 10.1038/nn.3092.. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Chang SY Addition Centre Adolescent Research Group. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7:138–166. [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI. An Introduction to Methods. Oxford: Oxford University Press; 2001. pp. 251–270. [Google Scholar]
- Xiangrui L, Zhing-Lin L, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI Images. Human Brain Mapping. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaxley RH, VanVoorhees EE, Bergman S, Hooper SR, Huettel SA, De Bellis MD. Behavioral risk elicits selective activation of the executive system in adolescents: clinical implications. Frontiers in Psychiatry. 2011;2:Article 68, 1–11. doi: 10.3389/fpsyt.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Wendt CS, Volz KG, Neumann J, Huber O, von Cramon DY. The neural implementation of multi-attribute decision making: A parametric fMRI study with human subjects. NeuroImage. 2006;31:1380–1388. doi: 10.1016/j.neuroimage.2006.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The whole-brain voxelwise analyses of the main effects of behavioral risk versus reward risk and no risk conditions demonstrated activations in parietal cortex, anterior and posterior cingulate gyrus, frontal gyrus, and insula cortex (see Supplementary Table-1) in all three groups as predicted from our previous study in healthy adolescents (Yaxley et al., 2011).
Supplementary Figure 2. The whole-brain voxelwise analyses of the main effects of reward versus no-reward outcomes after making risky decisions demonstrated activation in visual cortex, striatum, cingulate gyrus, and frontal cortex (see Supplementary Table-2) n all three groups as predicted from our previous study in healthy adolescents (Yaxley et al., 2011).