Abstract
Background
Nicotine’s acute effects on enhancing reinforcement from sensory rewards, shown in animal models, appear to occur with smoking in humans. These effects may vary due to reinforcer magnitude and amount of acute smoke intake (dose).
Methods
In a fully within-subjects design, dependent smokers (n=23) participated in 3 sessions. Each session followed overnight abstinence and involved 4 trials to assess responding via progressive ratio (PR50%) for sensory reinforcement from high, moderate, or low preference music, or no reward (counter-balanced, 30-sec/reinforcer). Sessions differed in smoking prior to each trial: 8 puffs on arrival and 2 puffs/trial (“8+2”), 2 puffs/trial only (“0+2”), or no smoking. Puffs were consumed via CReSS (Clinical Research Support System) to control topography, and smoking involved own brand to ensure palatability and increase generalizability of results.
Results
Reinforced responding was influenced by main effects of smoking condition (p<.05) and music reward type (p<.001). Compared to no smoking, responding for music was increased after smoking 8+2/trial puffs (p<.005), but not after 0+2/trial puffs. Smoking condition significantly increased reinforced responding only for the high preference music (p=.01), and not for moderate or low preference music, or for no reward. Withdrawal did not differ between the two smoking sessions, ruling out withdrawal relief as an explanation for differential reinforcement enhancement.
Conclusions
Our findings confirm that just one cigarette after abstinence is sufficient for reinforcement enhancing effects and suggest that such enhancement is greater as magnitude of a reward’s reinforcing efficacy increases.
Keywords: nicotine, reinforcement enhancement, smoking, reinforcing efficacy, music, CReSS
1. INTRODUCTION
In addition to nicotine’s primary and secondary reinforcing effects, research with animal models indicates that nicotine has a third reinforcing function, that of enhancing reinforcement from rewards not directly associated with nicotine intake (Caggiula et al., 2001; Chaudhri et al., 2006; Palmatier et al., 2012). Some of this research suggests nicotine’s reinforcement enhancing effects may be specific to certain classes of rewards, such as visual stimuli or others “sensory” in nature (e.g., Weaver et al., 2012). However, nicotine may not enhance reinforcement from primary rewards, perhaps such as food, but this selectivity of nicotine effects may stem from other factors (e.g., Caggiula et al., 2009; Raiff and Dallery, 2008). Even if limited in breadth, nicotine’s enhancement of sensory rewards common to the environment (e.g., Fowler, 1971) could help explain the persistence of nicotine intake in humans via tobacco smoking.
Very few human studies have directly examined nicotine’s reinforcement enhancing effects, particularly for sensory rewards. We recently tested reinforcement enhancing effects of nicotine via smoking, compared with denicotinized smoking, on rewards varying in sensory and other characteristics (e.g., negative vs. positive reinforcer; Perkins and Karelitz, in press). We found that nicotine enhanced responding for a positively reinforcing sensory reward (highly preferred music), but not for a positively reinforcing non-sensory reward (money), a negatively reinforcing sensory reward (termination of aversive noise), or no reward (control for nonspecific behavior). Nicotine’s reinforcement enhancing effect was similar between nondependent and dependent smokers, indicating a response to nicotine that was not due to dependence or withdrawal relief.
Because overall amounts of reinforced responding were similar for the money versus music rewards, our reinforcement enhancing results cannot be explained by differential magnitude of reinforcing efficacy. Yet, nicotine’s reinforcement enhancing effects may differ by the degree of a sensory reward’s reinforcing efficacy (Palmatier et al., 2012). Moreover, we found that modest smoking of roughly half a nicotine cigarette (6 puffs) before each trial produced enhancement of music reinforcement that was equal whether this smoking followed overnight abstinence or no abstinence (i.e., ad lib smoking before the session; Perkins and Karelitz, in press). Thus, such enhancement of reinforcement may occur after very minimal nicotine intake, well below that typically self-administered by smokers from a full cigarette.
The current study used a fully within-subjects design to examine differences in reinforced responding for a sensory reward due to: 1) two different modest amounts of acute nicotine intake via cigarette smoking after overnight abstinence, and 2) three different degrees of preference for a music reward, or no reward. As in our prior research, reinforced responding was assessed on a progressive ratio schedule using a simple operant computer task (e.g., Perkins et al. in press; Perkins and Karelitz, in press). However, here we employed subjects’ preferred brand of cigarettes to increase generalizability and ensure palatability (i.e., avoid or lessen differences in taste or other non-nicotine cigarette characteristics that could cause variable responding between smokers).
2. METHODS
2.1 Participants
Study participants were 23 dependent smokers (14 M, 9 F) who smoked at least 10 cigarettes per day for at least one year and met DSM-IV criteria for nicotine dependence (APA, 1994), according to a structured interview updated from Breslau et al. (1994). Mean (± SD) smoking characteristics were 14.5±3.6 cigarettes/day, 4.1±1.7 on the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991), 1.0±0.2 mg nicotine yield of preferred brand, and 70% were non-menthol smokers. Participants, recruited from the surrounding community, were 25.8±7.1 years of age and self-identified mostly as Caucasian (82.6%), with 17.4% African-American. Men and women did not differ on any of these smoking characteristics or on age or ethnicity. Excluded were those currently taking medications to treat serious psychological problems (e.g. psychosis, major depression).
2.2 CO, Withdrawal Measures
During the three study sessions, expired-air carbon monoxide (CO) was assessed upon arrival via Breathco CO monitor (Lenexa, KS) to confirm overnight (≥12 hr) smoking abstinence (CO≤ 10 ppm; SRNT Subcommittee, 2002). Nicotine withdrawal was assessed by the Minnesota Nicotine Withdrawal Scale (Hughes and Hatsukami, 2007) using the following 6 items: depressed mood/sad, irritable/angry/frustrated, anxious/nervous, difficulty concentrating, restless/impatient, and drowsiness. Items were each rated on a 0 (“not at all”) to 100 (“extremely”) visual analog scale (VAS) and averaged across symptoms to get a total withdrawal score. CO and withdrawal were obtained at the end of each session to compare effects of the different amounts of intermittent smoking exposure.
2.3 Reinforcement Task
As described in our prior research (Perkins et al., 2009; Perkins et al., in press; Perkins and Karelitz, in press), reinforced responding was assessed with a modified version of a simple operant computer task (“Applepicker”; Norman and Jongerius, 1985). It involved looking for “apples” on a monitor by moving a cursor around a “field” and pressing a button when the cursor landed on one of the “trees.” When an “apple” was found, a red symbol briefly lit up, indicating a unit of the music reward designated for that trial had been earned (i.e., one reinforcer). The number of responses required to find an “apple” constituted the reinforcement schedule, which in our current version of this task (Perkins et al., in press) is a progressive ratio incrementing by 50% after each completed ratio (i.e., PR50%), starting with an FR10.
Each of the four trials per session differed in the preference level of music reward that was available as a reinforcer over the 15-min of task responding: high, moderate, or low preference music, or no reward (control for nonspecific responding), obtained and validated as described below in Procedures. Each earned reinforcer resulted in the immediate playing of 30 sec of the designated music reward, which was not identified before the trial began but only upon earning the first reinforcer and listening to the resulting music via headphones. The experimenter was not in the room during task trials, and playing of music reward was initiated by the computer immediately after a reinforcer was earned. Trials with these different levels of reward were presented within sessions in counter-balanced order between subjects. Upon earning a reinforcer, subjects could continue responding on the task without interruption, to earn additional units of the reinforcer (i.e., extend the continuous time of music, until the PR incremented to higher schedules). They were free to stop responding at any point and read available magazines (intentionally routine in nature) or simply wait for the end of the 15-min task period. However, to eliminate availability of other reinforcers, they were not allowed to bring any of their own reading or other material to sessions.
2.4 Procedure
Participants were first screened by phone on smoking and health history and scheduled for an introductory session in the laboratory to obtain written informed consent, verify eligibility, and obtain their preferred cigarette brand and their different preference levels of music reward. They were instructed to bring with them an unopened pack of their own brand and at least one compact disc (CD) of their favorite music. The pack was used to provide cigarettes for the two experimental sessions involving intake of smoke puffs (see below). Using the CD, they were instructed to listen and select their 5 most highly preferred “tracks”, with 4 tracks each rated at least 75 on a 0–100 VAS of “liking” identified as the high preference music reinforcer. From a library of music compiled by the experimenters, they then listened to additional music tracks (based on their ratings of general preference for different music genres) and rated them on the same 0–100 VAS of “liking”. The moderate preference music comprised 4 tracks rated 40–60, and the low preference music was 4 tracks rated 0–20. For these participants, mean (SD) VAS “liking” ratings were 89.7±5.8, 51.9±7.7, and 7.9±6.2 for their high, moderate, and low preference music, respectively. Each grouping of 4 tracks totaled at least 10 mins of music, the maximum duration of listening possible from responding for music throughout a 15-min trial. They were then briefly introduced to the Applepicker computer task without reinforcement to become familiar with it.
All subsequently completed three 2-hr experimental sessions, each following overnight smoking abstinence (confirmed upon arrival by CO ≤ 10 ppm). Sessions differed only in the acute smoking condition in effect: 1) 8 puffs on arrival (after CO check) and 2 puffs prior to each trial (8+2/trial puffs), 2) no smoking on arrival but 2 puffs prior to each trial (0+2/trial puffs), or 3) no smoking at all throughout the session (0 puffs). Thus, on one session (“8+2”), they took 8 puffs over 4 mins (one every 30 sec) on one cigarette after providing initial CO. Then, on both of the smoking sessions (8+2, 0+2), participants self-administered two puffs over 60 sec prior to each of the 4 task trials. These two smoke amounts provided just over half a full cigarette (10 puffs) versus a small fraction of a cigarette (2 puffs) prior to the first task trial of responding for music reward, and they totaled a full cigarette (16 puffs) versus a half cigarette (8 puffs) by the end of the respective session (e.g. Hatsukami et al., 1990). The order of these smoking conditions across sessions was counter-balanced between subjects, and sessions were separated by at least one day. All smoking during sessions involved their preferred cigarettes, unblinded and provided by the experimenter. This smoking was done via the portable Clinical Research Support System (“CReSS Pocket”; Borgwaldt KC, Inc., Richmond VA; www.plowshare.com), which assesses puff volume (in ml; Lee et al., 2003; Perkins et al., 2012). Exact puff timing and duration were guided by computer-presented puffing instructions to standardize intake at about 60 ml per puff (comparable to ad lib puffing; e.g., Perkins et al., 2012), as in prior studies of controlled smoke exposure (Zacny and Stitzer, 1986), including with the CReSS (Perkins et al., 2010).
During each trial, participants were instructed they were free to work as much or as little as they wanted on the task for the designated music reward (high, moderate, low preference, or no reward) until the end of the 15-min task period. Consistent with the subjective “liking” ratings used to identify each music reward preference type during the introductory session (see above), the mean (SD) duration of task responding per 15-min trial was 6.3±2.8, 4.3±3.7, 1.8±2.1, and 2.0±2.4 mins for high, moderate, and low preference music, and no music reward, respectively. Thus, duration of participant responding was a function of the preference level of music reward, as expected, and they discontinued responding prior to the end of trials. The different orders of music reward across trials were counter-balanced between subjects but remained constant across the three sessions within subjects. Trials lasted 25 mins each, to provide time for controlled intake of 2 puffs, completion of the task period, and brief rest until the next trial. Withdrawal and CO were obtained after the last trial of each session to gauge effects and exposure from these intermittent smoking conditions compared to no smoking. This study protocol was approved by the University of Pittsburgh Institutional Review Board.
2.5 Data Analyses
All analyses were conducted using IBM SPSS 20.0. The primary dependent measure was number of responses on the Applepicker task (i.e. reinforcement), and the independent measures were the smoking condition per session following overnight abstinence and the music reward preference level per trial (high, moderate, and low, as well as no reward). Preliminary analyses showed no effects of smoking condition order across sessions, and so data for the three sessions were collapsed across orders in subsequent analyses. In these completely within-subjects analyses of variance (ANOVAs), which greatly increased statistical power (e.g. Cohen, 1988), the factors were smoking condition (no smoking, 0 puffs+2 puffs/trial, 8 puffs+2 puffs/trial) and type of music reward. Significant effects were followed up with paired comparisons of responses using Fisher’s LSD t-test (Huitema, 1980). Because we previously found enhancement of high preference music reinforcement due to nicotine via smoking (Perkins and Karelitz, in press), we specifically examined responding for each type of music reward preference due to smoking in this study. Effect sizes for reinforced responding of particular interest are given by partial eta-squared values (ηp2), the percent of variance explained.
3. RESULTS
3.1 Control over smoke intake
CO due to overnight abstinence did not vary at the start of each session but did increase differentially due to the manipulation of smoke intake between sessions, as expected. Mean (SD) CO values at baseline and end of session, respectively, were 5.2±2.2 and 5.0±2.1 ppm for no smoking, 5.7±1.9 and 11.6±3.1 ppm for 0 puffs on arrival and 2 puffs/trial (0+2), and 6.8±3.2 and 14.6±4.0 ppm for 8 puffs on arrival and 2 puffs/trial (8+2), F(2,44) = 61.49, p<.001, for the interaction of smoking condition × time.
On the two sessions in which participants took 2 measured puffs from their preferred cigarette prior to each task trial, the total mean (SE) puff volume per session was greater, as expected, following 8+2 puffs vs 0+2 puffs, 878.5±53.1 vs. 518.4±34.2 ml, respectively, F(1,22)=108.9, p<.001. However, the mean volume for the two puffs per task trial was greater during the session following 0 puffs vs. 8 puffs on arrival, 132.7±9.0 vs 116.5±7.7 ml, respectively, F(1,20) = 10.05, p=.005. Yet, also as expected (because smoking preceded music), puff volume per trial did not differ by the type of music reward (range of 124.0 ml for moderately preferred to 125.2 ml for low preferred music), F(3,60)<1, ns, or by the interaction of smoking condition × music reward, F(3,60)=1.37, p=.26.
3.2 Reinforcing value of rewards
The amount of responding for music reinforcement was significantly influenced by the main effects of smoking condition, F(2,44)=3.27, p= .048, ηp2 = .129, in addition to music reward type, F(3,66)=32.03, p<.001, ηp2 = .593. The interaction of smoking condition × music reward type was not significant, F(6,132)=1.21, p=.31, ηp2 = .052. In paired comparisons, mean (SE) task responding per trial after smoking 8+2/trial puffs was greater than responding after no smoking during the session, 331±50 vs. 244±31, respectively, t(22)=3.25, p=.004, but responding after 0+2/trial puffs, 259±32, did not differ from 8+2/trial puffs, t(22)=1.55, p=.14, or after no smoking, t(22)=0.47, p=.65. Similar paired comparisons showed significantly different task responding between all 4 music reward types (513±46, 334±55, 119±30, and 146±36 for high, moderate, low, and no reward; t(22)’s > 3.25 for all comparisons, all p<.005), except between low preference music and no reward, t(22)=0.78, p=.45.
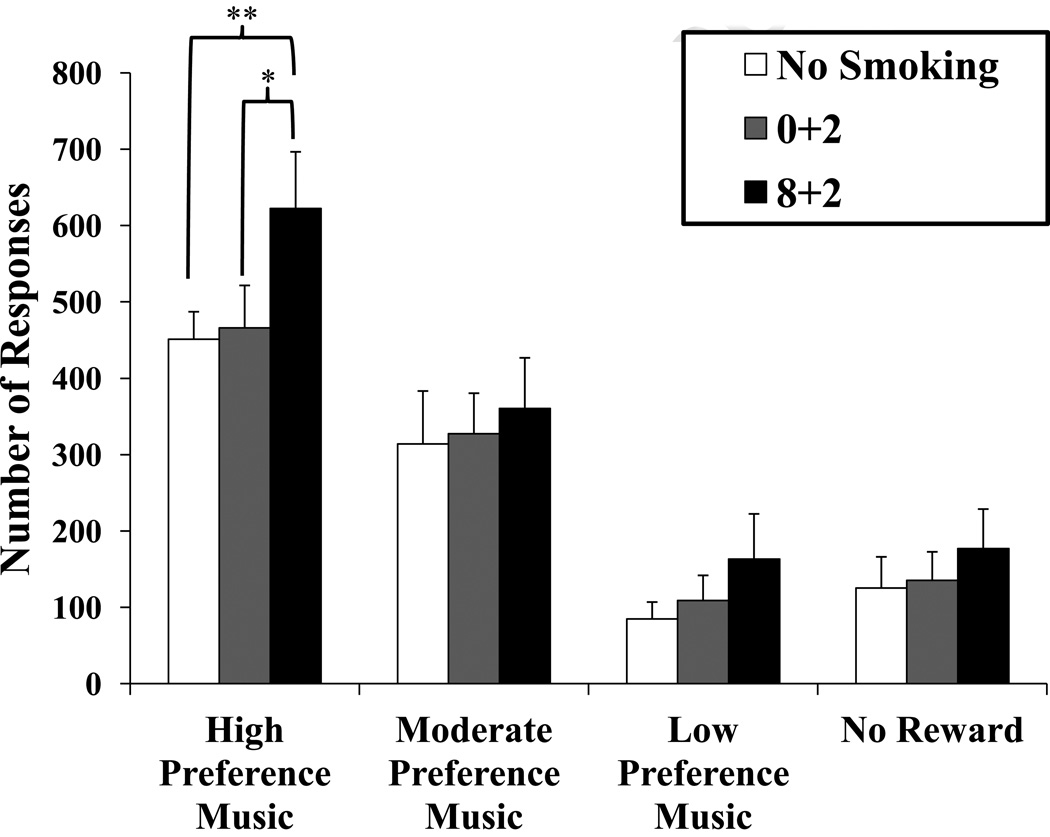
Because our prior research showed significant enhancement of reinforcement from high preference music due to nicotine via smoking (Perkins and Karelitz, in press), we examined smoking effects on responding separately for each type of music reward in this study. As shown in Figure 1, the effect of smoking condition significantly increased reinforced responding for the high preference music, F(2,44)=5.00, p=.01, ηp2 = .185. Session smoking condition had no influence on responding for moderate preference music, F(2,44)=.37, p=.69, ηp2 = .017, low preference music, F(2,44)=1.37, p=.27, ηp2 = .058, and no music reward (control), F(2,44)=.84, p=.44, ηp2 = .037. Responding for high preference music increased as smoking intake increased, 8+2 vs. 0+2 puffs, t(22)=2.30, p=0.031, and 8+2 puffs vs no smoking, t(22)=3.10, p=.005. There was no difference in responding for high preference music between the 0+2 puffs and no smoking conditions, t(22)=.26, p=.80. Responding for high preference music reward was increased by the 8+2 vs. 0+2 puff condition in 16 of the 23 subjects (70%), and by 8+2 vs. no smoking in 18 of the 23 (78%). Responding for high preference reward due to 8+2 puffs was correlated 0.48, p<.02, with such responding due to 0+2 puffs, and .70, p<.001, with responding for high preference music reward during no smoking, showing reasonably high consistency.
Fig. 1.
Mean±SE total number of responses for reinforcers per trial, by music reward level and smoking condition (N=23). * p<.05, ** p<.01 for the difference in responding from smoking 8 puffs upon arrival after overnight abstinence in within-subjects paired comparisons.
Finally, as expected, mean (SE) withdrawal at the end of the session was higher after the no smoking condition, 36.3±4.7, than the smoking conditions of 8+2 puffs, 19.1±3.1, t(22)=5.87, p<..01, and 0+2 puffs, 19.3±3.3, t(22)=4.23, p<.001, which did not differ from each other, t(22)=.05.
4. DISCUSSION
Overall responding for the sensory reward of music was enhanced by acute smoking. After overnight abstinence, modest smoke intake from about one full cigarette, but not from a half cigarette or less, increased reinforced responding for music reward relative to responding while not smoking (i.e., maintaining continued abstinence). These findings are consistent with results from our very recent test of acute smoking of nicotine versus denicotinized cigarettes, showing that nicotine intake per se enhances reinforcement from the sensory reward of high preference music but not from other types of rewards (Perkins and Karelitz, in press). They confirm that just one cigarette after abstinence is sufficient for these reinforcement enhancing effects of nicotine and that the threshold of nicotine intake for such enhancement is likely more than a half cigarette. Thus, it is conceivable that a brief smoking lapse after a quit attempt may enhance reinforcement from sensory reward stimuli in the environment, perhaps increasing enjoyment of the lapse cigarette and fostering greater risk of subsequent relapse (e.g., Shadel et al., 2011). Our results are also consistent with recent research suggesting no association of withdrawal relief with nicotine’s enhancement of reinforced responding (Perkins and Karelitz, in press; Perkins et al., in press), as withdrawal was similar after these modest smoking conditions despite the difference in responding for music reward.
Consistent with our prior study showing nicotine effects in enhancing high preference music reward (Perkins and Karelitz, in press), the current analyses showed that smoking 8+2 puffs, compared to smoking 0+2 puffs or no smoking, enhanced reinforcement due to the high preference reward but not due to the less preferred music reward levels (moderate, low). Therefore, smoking may particularly increase reinforcement from higher level sensory rewards, and the magnitude of a sensory reward’s reinforcing efficacy may influence degree of reinforcement enhancement from nicotine. Clarification of these parameters, and the mechanisms responsible, may require more complete understanding of the neuropharmacological substrates for nicotine’s reinforcement enhancing effects (Paterson, 2009).
Aside from the statistical power and controls provided by the within-subjects design, strengths of this study include the manipulation and validation of levels of reinforcing efficacy via differing preferences for the sensory reward, including their own highly preferred music, which was specifically enhanced by smoking. A second strength was use of subjects’ nicotine-containing preferred cigarette brand for all smoking during sessions to standardize palatability and minimize potential variability in non-nicotine factors that may influence responding. Both of these strengths maximize subject familiarity with the key independent variables, sensory reinforcing efficacy and acute smoke intake amount, and thereby increase the generalizability of these results to the real world. Among other strengths were the counter-balancing of the smoking conditions and of the trials varying in music reward preference, careful control over smoke intake between the two smoking sessions, and biochemical verification of overnight abstinence prior to each session.
On the other hand, use of subjects’ preferred brand in this manner prevented blinding them to brand during acute smoking, although this would be unlikely to explain the difference in responding for the high preference music reward between the two smoking sessions of this study. Use of smoking to administer differing amounts of nicotine required manipulation of puff amounts, from which subjects could not be kept blind. However, the only difference between these two smoking sessions was the intake of 8 or 0 puffs upon arrival; the modest number of puffs prior to each task trial was the same. It is also possible that smoking enhanced the high preference music reward because of its high familiarity (i.e., since it was provided directly by participants), compared to the moderate and low preference music types, which were not as familiar. Yet, matching these different types of music preference by level of familiarity would be quite challenging and perhaps impossible, if frequency of listening corresponds to one’s preference for the music.
Future research should determine the influence of a broader range of dosing with nicotine per se, separate from smoking, on enhancement of sensory reinforcement. The modest nicotine exposure here, and prior findings that these nicotine effects do not require the presence of dependence (Perkins and Karelitz, in press), suggests the onset of these reinforcement enhancing effects during smoking initiation may be rapid (Weaver et al., 2012). Such research also should assess effects of nicotine on other types of common reinforcers in the environment, sensory or non-sensory in nature (Raiff et al., 2012; van Gucht et al., 2010), to gauge generalizability of the notion that nicotine enhances reinforcement as a function of reward type. Studies should continue examining potential mechanisms, such as relating changes in brain activation due to acute nicotine to its reinforcement enhancing effects (e.g., Klinkenberg et al. 2013; Paterson, 2009; Wise, 1998). Finally, reinforcement enhancing effects of other drugs of abuse warrant study as they may very well be similar to those observed here with nicotine via smoking (e.g., Lloyd et al., 2012; Sheppard et al., 2012).
Acknowledgements
The authors thank Michael Eddy for his help in conducting this study.
Role of Funding Source
This research was supported by NIDA Grant DA31218 to KAP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author KAP designed the study, oversaw protocol development, planned statistical analyses, and wrote the first draft of the manuscript. Author JLK managed participant recruitment, data collection, and performed statistical analyses. Both authors contributed to and have approved the final manuscript.
Conflict of Interest
Neither author has any potential conflicts of interest to report.
REFERENCES
- American Psychiatric Association (APA) Diagnostic and Statistical Manual-IV. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. DSM-IIIR nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi: 10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier M, Liu X, Chaudhri N, Sved A. The role of nicotine in smoking: a dual-reinforcement model. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use, Nebraska Symposium on Motivation. vol 55. New York: Springer-Verlag; 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol. Biochem. Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Social Sciences. 2nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Fowler H. Implications of sensory reinforcement. In: Glaser R, editor. The Nature of Reinforcement. New York: Academic Press; 1971. pp. 151–195. [Google Scholar]
- Hatsukami DK, Morgan SR, Pickens RW, Champagne SE. Situational factors in cigarette smoking. Addict. Behav. 1990;15:1–12. doi: 10.1016/0306-4603(90)90002-f. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Instructions for use of the Minnesota Withdrawal Scale-Revised. 2007 Retrieved from www/uvm.edu/~hbpl. [Google Scholar]
- Huitema B. Analysis of Covariance and Alternatives. New York: John Wiley & Sons; 1980. [Google Scholar]
- Klinkenberg I, Blokland A, Riedel AJ, Sambeth A. Cholinergic modulation of auditory processing, sensory gating and novelty detection in human participants. Psychopharmacol. 2013;225:903–921. doi: 10.1007/s00213-012-2872-0. [DOI] [PubMed] [Google Scholar]
- Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nic. Tobacco Res. 2003;5:673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Kausch MA, Gancarz AM, Beyley LJ, Richards JB. Effects of novelty and methamphetamine on conditioned and sensory reinforcement. Behav. Brain Res. 2012;234:312–322. doi: 10.1016/j.bbr.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman WD, Jongerius JL. Apple Picker: computer software for studying human responding on concurrent and multiple schedules. Behav. Res. Meth. Instr. Comput. 1985;17:222–225. [Google Scholar]
- Palmatier MI, O’Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology. 2012;219:1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Paterson NE. The neuropharmacological substrates of nicotine reward: reinforcing versus reinforcement-enhancing effects of nicotine. Behav. Pharmacol. 2009;20:211–225. doi: 10.1097/FBP.0b013e32832c7083. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grottenthaler A, Wilson AS. Lack of reinforcement-enhancing effects of nicotine in non-dependent smokers. Psychopharmacol. 2009;205:635–645. doi: 10.1007/s00213-009-1574-8. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacol. (Berl.) 2013 doi: 10.1007/s00213-013-3054-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect measure and situation, but not on nicotine. Biol. Psychiatry. 2010;67:707–714. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nic. Tobacco Res. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC, Stratton E. Possible reinforcement enhancing effects of bupropion during initial smoking abstinence. Nic. Tobacco Res. 2013;15:1141–1145. doi: 10.1093/ntr/nts224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacol. 2008;201:305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Jarvis BP, Rapoza D. Prevalence of video game use, cigarette smoking, and acceptability of a video-game based smoking cessation intervention among online adults. Nic. Tobacco Res. 2012;14:63–77. doi: 10.1093/ntr/nts079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Martino SC, Setodji C, Cervone D, Witkiewitz K, Beckjord EG, Scharf D, Shih R. Lapse-induced surges in craving influence relapse in adult smokers: an experimental investigation. Health Psychol. 2011;30:588–596. doi: 10.1037/a0023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AB, Gross SC, Pavelka SA, Hall MJ, Palmatier MI. Caffeine increases the motivation to obtain non-drug reinforcers in rats. Drug Alcohol Depend. 2012;124:216–222. doi: 10.1016/j.drugalcdep.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee. Biochemical verification of tobacco use and cessation. Nic. Tobacco Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Van den Bergh O, Beckers T, Vansteenwagen D. Smoking behavior in context: where and when do people smoke? J. Behav. Ther. Exp. Psychiatry. 2010;41:172–177. doi: 10.1016/j.jbtep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Weaver MT, Geier CF, Levin ME, Caggiula AR, Sved AF, Donny EC. Adolescent exposure to nicotine results in reinforcement enhancement but does not affect adult responding in rats. Drug Alcohol Depend. 2012;125:307–312. doi: 10.1016/j.drugalcdep.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Stitzer ML. Effect of puff size instructions on puff volume. Addict. Behav. 1986;11:17–23. doi: 10.1016/0306-4603(86)90004-3. [DOI] [PubMed] [Google Scholar]