Abstract
BACKGROUND
Topiramate increases GABAergic activity and antagonizes the AMPA/kainate subtype of glutamate receptors. Through these mechanisms of action, topiramate may reduce alcohol and cocaine reward and may reduce alcohol and cocaine craving. Topiramate has been shown to reduce drinking in persons with alcohol dependence, and reduce relapse in stimulant-dependent patients. The current trial was intended to test the ability of topiramate to promote cocaine and alcohol abstinence among patients addicted to both drugs.
METHODS
The study was a double-blind, placebo-controlled, 13-week trial involving 170 cocaine and alcohol dependent subjects. After achieving a period of cocaine and alcohol abstinence, subjects were randomized to topiramate, 300 mg daily, or identical placebo capsules. In addition, subjects received weekly individual psychotherapy. Primary outcome measures included self-reported alcohol and cocaine use, and thrice weekly urine drug screens. Secondary outcome measures included cocaine and alcohol craving, Addiction Severity Index results, cocaine withdrawal symptoms, and clinical global improvement ratings.
RESULTS
Topiramate was not better than placebo in reducing cocaine use on the a priori primary outcome measure, or in reducing alcohol use. Topiramate was not better than placebo in reducing cocaine craving. Topiramate-treated subjects, compared to placebo-treated subjects, were more likely to be retained in treatment and more likely to be abstinent from cocaine during the last three weeks of the trial. Subjects who entered treatment with more severe cocaine withdrawal symptoms responded better to topiramate.
DISCUSSION
Topiramate plus cognitive behavioral therapy may reduce cocaine use for some patients with comorbid cocaine and alcohol dependence.
Keywords: topiramate, cocaine, alcohol, clinical trial, placebo
1. INTRODUCTION
The co-occurrence of cocaine and alcohol dependence is common and patients suffering from both disorders are extremely difficult to treat. Studies have shown that 60–80% of cocaine dependent patients are also alcohol dependent (Carroll et al., 1993; Regier et al., 1990). Patients with both cocaine and alcohol dependence tend to have more psychosocial problems and worse treatment outcomes compared to patients addicted to either cocaine or alcohol alone (Brady et al., 1995; Carroll et al., 1993; Heil et al., 2001; Walsh et al., 1991). The combined use of alcohol and cocaine is fostered by a variety of factors including both conditioning (Wallace, 1989) and pharmacodynamic interactions between cocaine and alcohol (McCance-Katz et al., 1993). Thus, treatments aimed specifically at reducing either cocaine use or alcohol use alone may be inadequate and the best strategy for treating cocaine and alcohol dependent patients may be one that targets both addictions simultaneously.
Topiramate is a promising medication for either alcohol or stimulant dependence. Topiramate is an anticonvulsant medication with several different mechanisms of action. Topiramate increases cerebral levels of GABA and facilitates GABA neurotransmission through a non-benzodiazepine-associated binding site on the GABA A receptor (Kuzniecky et al., 1998; Petroff et al., 1999; White et al., 1997). By increasing GABAergic activity in the nucleus accumbens, topiramate may reduce the dopamine release associated with cocaine or alcohol use and reduce the reinforcing effects of cocaine and alcohol. In addition, topiramate inhibits glutamate neurotransmission through a blockade of AMPA/kainate receptors (Gibbs et al., 2000). This may reduce craving for alcohol and cocaine associated with exposure to conditioned cues. In animal models of cocaine relapse, topiramate’s blockade of AMPA receptors in the nucleus accumbens prevented reinstatement of cocaine self-administration (Cornish and Kalivas, 2000). Cocaine-dependent patients who experience cocaine withdrawal symptoms report a greater “high” from experimentally-administered cocaine (Newton et al., 2003; Sofuoglu et al., 2003; Uslaner et al., 1999). Therefore, a reduction in euphoria produced by topiramate may be particularly helpful in cocaine and alcohol dependent patients with more severe cocaine withdrawal symptoms.
In two placebo-controlled clinical trials, topiramate promoted abstinence from alcohol by reducing heavy drinking days among alcohol-dependent individuals (Johnson et al., 2003, 2007b). Topiramate was also efficacious in promoting stimulant abstinence in three placebo-controlled trials of stimulant dependence treatment. First, in a 13-week pilot trial, topiramate reduced relapse to cocaine use in 40 cocaine dependent patients (Kampman et al., 2004). Levin and colleagues showed that the combination of topiramate and mixed amphetamine salts was more effective in promoting cocaine abstinence in patients with more severe cocaine dependence, defined as having more cocaine use days in the month prior to entering the trial (Mariani et al., 2012). Finally, Elkashef and colleagues found that topiramate reduced methamphetamine use and reduced the relapse rate in methamphetamine-dependent patients who attained a period of abstinence prior to starting topiramate (Elkashef et al.,2012).
2. METHODS
2.1. Subjects
The subjects were 170 DSM-IV cocaine dependent men and women drawn from treatment-seeking cocaine users between the ages of 18 and 70. Drug dependence diagnoses were obtained using the Structured Clinical Interview for DSM IV (SCID-IV) (First, 1996). Other psychiatric diagnoses were obtained using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). In the 30 days prior to study entry, subjects used no less than $200-worth of cocaine and met the following drinking criteria as measured by the Timeline Followback (TLFB; Sobell, 1995): a. drank within 30 days of intake day, b. reported a minimum of 48 standard alcoholic drinks (avg. 12 drinks/wk) for women and 60 standard drinks (15 drinks per week) for men in a consecutive 30-day period over the 90-day period prior to starting intake, and c. had 2 or more days of heavy drinking (defined as 5 or more drinks per day in males and 4 or more drinks per day in females) in this same pre-treatment period.
Medical screening included a complete medical history and physical examination conducted by a certified nurse practitioner. Baseline laboratory testing included a chemistry screen, complete blood count, urinalysis, and a 12 lead EKG. Women received urinary pregnancy testing prior to starting medications, and at monthly intervals throughout the study. Chemistry screening, CBC, urinalysis and EKG were repeated at the end of the trial. Liver function tests and carbon dioxide levels were obtained monthly during the trial.
Subjects with current dependence (DSM-IV criteria) on any additional drug except nicotine and cannabis were excluded. Psychiatric exclusion criteria included psychosis, dementia, and the use of other psychotropic medications. Medical exclusion criteria included unstable medical illnesses, impaired renal function, and a history of hypersensitivity to topiramate. Subjects with a history of kidney stones or subjects taking carbonic anhydrase inhibitors or any other antiepileptic drug were excluded from the study.
2.2. Procedures
Subjects were treatment-seeking cocaine and alcohol users recruited at the University of Pennsylvania Treatment Research Center (TRC). The TRC recruits through advertisement in the local media as well as through professional referrals. All subjects signed informed consent prior to participation in the trial, after an investigator explained to them the study procedures. The study was reviewed and approved by the Institutional Review Board (IRB) of the University of Pennsylvania. Subjects were reimbursed $5.00 at each visit for completing all research procedures; at the last visit of the medication phase of the trial and at the follow up visit they received $25.00 because of the greater number of research procedures done at those two visits. Subjects received an additional $5.00 each week for returning the previous week’s medication package in order to facilitate the pill-count compliance check. If needed, two transit tokens were provided at each visit.
Eligible subjects entered a baseline phase during which all pretreatment measures were obtained and subjects began psychosocial treatment. The baseline phase could last up to three weeks during which time subjects were required to attain baseline abstinence from cocaine and alcohol. This was defined as three consecutive days of abstinence from cocaine and alcohol, determined by self-reports and confirmed by negative urine drug screens, a negative breathalyzer tests, and collateral report, a Clinical Institute Withdrawal Scale for Alcohol (CIWA-AR; Sullivan and Sellers, 1989) score below eight. Eligible subjects were then randomized to receive either topiramate or placebo. The dose was titrated to 300 mg daily following the schedule shown in Table 1. Subjects attended the clinic three days per week.
Table 1.
Dose titration schedule
| Wk. | A.M. Dose | P.M. Dose | Total |
|---|---|---|---|
| 1 | 0 | 25 mg | 25 mg |
| 2 | 0 | 50 mg | 50 mg |
| 3 | 0 | 75 mg | 75 mg |
| 4 | 25 | 75 mg | 100 mg |
| 5 | 50 mg | 100 mg | 150 mg |
| 6 | 100 mg | 100mg | 200 mg |
| 7 | 100 mg | 150 mg | 250 mg |
| 8 | 150 mg | 150 mg | 300 mg |
| 9 | 150 mg | 150 mg | 300 mg |
| 10 | 150 mg | 150 mg | 300 mg |
| 11 | 150 mg | 150 mg | 300 mg |
| 12 | 150 mg | 150 mg | 300 mg |
The Investigational Drug Service of the University of Pennsylvania prepared study medication by overencapsulating topiramate tablets and preparing identical appearing placebo capsules. Capsules were placed in blister packs with each day’s dose clearly marked. Medications were dispensed by a nurse practitioner each week and the previous week’s blister pack was collected. Compliance was measured by pill count. None of the dose ingestions were observed in the clinic.
In addition to medication or placebo, subjects received weekly individual cognitive-behavioral relapse prevention therapy utilizing a Cognitive-Behavioral Coping Skills Therapy (CBT) manual. The CBT therapy manual and supporting materials were developed for the National Institute on Alcohol Abuse and Alcoholism Project MATCH (Kadden, 1992). The basic format was accepted, although specific procedures were adapted for treatment of comorbid cocaine dependence by our group. Master’s level therapists with additional training in CBT provided therapy.
2.3. Measures
Self-reported alcohol and cocaine use were measured using the Timeline Followback (Sobell, 1995). Self-reported cocaine use was verified by qualitative urine benzoylecgonine tests (UBT) obtained thrice weekly. Urine collection was monitored by temperature checks. Samples less than 90 degrees, or greater than 100 degrees Fahrenheit were considered invalid and were not accepted. Samples were analyzed for benzoylecgonine by fluorescent polarization assay. Samples containing equal to or greater than 300 ng/ml of benzoylecgonine were considered to be positive.
Treatment retention was determined by attendance at research visits. Subjects were considered dropouts if they stopped attending research visits and did not return within the 14 weeks of the trial. Severity of addiction-related problems was measured by the Addiction Severity Index (ASI; McLellan, 1992) administered at baseline and three more times during the trial. The study nurse practitioner rated overall improvement weekly using the Clinical Global Impression Scale (CGI; Guy, 1976). The Minnesota Cocaine Craving Scale (MCCS) was used to measure cocaine craving intensity (MCCS-I), cocaine craving frequency (MCCS-F) and cocaine craving duration (MCCS-D) (Halikas et al., 1991). Alcohol craving was measured weekly using the Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999). Cocaine withdrawal symptoms were measured weekly using the Cocaine Selective Severity Assessment (CSSA) (Kampman et al., 1998). Safety measures included adverse events, which were monitored at each visit.
2.4. Statistical analysis
Subjects were first compared on a variety of baseline characteristics to assess randomization balance across the two treatment groups, using chi-square tests for categorical characteristics and t tests for continuous characteristics. The primary analyses did not include additional covariates; characteristics that showed significant imbalance across the groups were examined as covariates in supplementary analyses.
Generalized estimating equation (GEE) models (Diggle et al., 2002) were used to compare the groups on weekly cocaine use, as measured by a combination of three UBT measures, together with self-report based on the TLFB. This was the planned primary outcome measure for cocaine use. Each study week was coded as abstinent or not abstinent based on the following definition: a study week was coded as an abstinent week if the participant reported no cocaine use during the study week, and provided at least two negative and no positive UBT samples during the study week. If the participant reported use, or if they provided at least one positive UBT during the study week, then that week was coded as a use week; otherwise the week was coded as missing. To assess the influence of missing UBT measures, GEE analyses of the UBT measures were performed with missing weeks ignored, and also with missing tests imputed as positive, and finally using pattern mixture models (Molenberghs and Verbeke, 2005) based on the number of available weeks as an indicator of missing data.
Our primary models included terms for treatment groups and for polynomial time effects. We also examined whether group-by-time effects improved the fit of the model. In fitting these models to the data, terms significant at the 5% level were included in the GEE models, as were lower order effects contained in a significant interaction. Empirical standard errors (Wald and Score statistics) were used to assess significance.
Similar models were also used to analyze other repeated outcomes (TLFB, PACS, CSSA, ASI-Drug, ASI-Alcohol, ASI-Days Cocaine Use, CGI-O, CGI-S, MCCS-I, MCSS-F, MCCS-D). Weekly differences in percent days drinking and percent days heavy drinking were the planned primary outcome measures for alcohol use. Retention in study was compared using Cox proportional hazards models, and the binary response of being abstinent for the final three weeks of the study was compared using a logistic regression model.
3. RESULTS
3.1. Baseline demographic and drug use
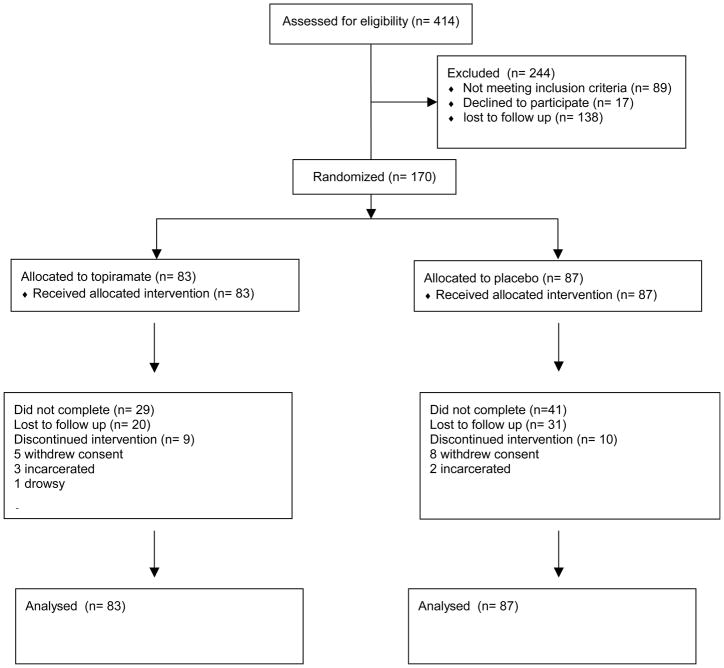
Subjects entered into the trial were drawn from an original group of 414 cocaine users who were screened for the trial. From this original group of 414, 138 were lost to follow up, 89 did not meet inclusion/exclusion criteria and 17 withdrew consent. On the whole, the two medication groups, topiramate and placebo, were very similar in demographics and baseline drug use characteristics (see Table 2). The average age of the subjects was about 45. Most were African American men and most smoked crack cocaine. On average, subjects had used cocaine about 12–13 days in the month prior to treatment and spent around $500 on drugs in the month prior to treatment. They used alcohol 14–16 days in the month prior to treatment, spending about $150 on alcohol during that time. There were no significant differences between the two groups in any baseline demographic or drug use variable.
Table 2.
Subject characteristics, expressed as percents or means (standard deviation)
| Variable | Topiramate N=83 | Placebo N=87 |
|---|---|---|
| Age | 45 (7.0) | 43 (8.0) |
| % Male | 78% | 80% |
| Race | ||
| African American | 86% | 80% |
| Caucasian | 14% | 20% |
| Years of education | 12.7 (2.0) | 12.7 (2.1) |
| Days of alcohol use in past 30 days | 14.8 (7.6) | 17.01 (8.0) |
| Days of cocaine use in past 30 days | 12.2 (7.1) | 13.2 (7.9) |
| $ spent for drugs | $561 (859) | $523 (493) |
| $ spent for alcohol | $129 (173) | $153 (197) |
| Route of cocaine use | ||
| Oral | 0% | 2% |
| Intranasal | 20% | 22% |
| Smoked | 80% | 75% |
| Injected | 0% | 1% |
| Years of cocaine use, lifetime | 14.2 (7.0) | 13.6 (8.0) |
| Years of alcohol use, lifetime | 21.7 (8.7) | 21.5 (9.5) |
| Percent days drinking baseline | 59.9 (27.3) | 62.2 (27.3) |
| Percent days Heavy drinking baseline | 48.4 (28.4) | 50.3 (29.6) |
| Drinks per drinking day baseline | 12.9 (8.0) | 13.8 (11.7) |
| ASI Composite Drug Score | .240 (.070) | .260 (.080) |
| ASI Composite Alcohol Score | .494 (.200) | .531 (.184) |
| ASI Composite Employment Score | .721 (.278) | .712 (.285) |
| ASI Composite Legal Score | .040(.127) | .052 (.122) |
| ASI Composite Family/Social Score | .165 (.208 ) | .192 (.232) |
| ASI Composite Psychiatric Score | .126 (.187) | .152 (.202) |
| ASI Composite Medical Score | .260 (.354) | .210 (.318) |
3.2. Treatment retention
Fifty-nine percent of the subjects completed the trial, 46/87 (53%) in the placebo group and 54/83 (65%) in the topiramate group. Survival analysis of time to dropout showed a significant difference between the groups (Log rank test = 22.32, p <.001). Treatment retention, measured by the number of visits attended favored the topiramate group. On average, topiramate-treated subjects attended 25.5 visits and placebo-treated subjects attended 21.6 visits (Kruskal-Wallis χ2(1)=4.24, p=0.04)
3.3. UBT Results
The primary a priori outcome for cocaine use was weekly self-reported abstinence from the TLFB supported by UBT results. For the combined UBT/TLFB weekly response, placebo-treated subjects had an average of 10.14 (SD=3.67) non-missing treatment weeks, and topiramate-treated subjects averaged 10.60 (SD=3.61), with no significant difference between the groups (Kruskal-Wallis χ2(1)=2.01, p=0.16). For the analysis with missing weeks ignored, the group by time interaction was not significant (χ2(1)=1.25, p=0.26). The estimated odds ratio in favor of abstinence for topiramate versus placebo was 1.53 (95% CI = 0.94, 2.48), which was not significant (χ2(1)=2.93, p=0.08). When missing weeks were imputed as non-abstinent weeks, the group by time interaction was not significant (χ2(1)=1.53, p=0.22) and the estimated odds ratio was 1.40 (95% CI = (0.87, 2.25)), which was again not-significant (χ2(1)=1.86, p=0.17). The models showed non-significant decreases in use across time (p=0.09 when missing weeks were ignored, and p=0.16 when missing weeks were imputed as non-abstinence).
A pattern mixture analysis showed no interaction between treatment group and the number of missed weeks (χ2(1)=1.72, p=0.19). Repeating all of the above analyses using mixed effects models, which have weaker assumptions on missing data but stronger assumptions on repeated measures structure, yielded essentially the same pattern of results. The general agreement across the GEE, pattern mixture, and mixed effects models suggest that the missing data do not have much effect on our main comparisons.
A comparison of the percent of subjects in each group who were abstinent from cocaine for the last three weeks of the trial, based on self-reported cocaine use supported by UBT results showed a significant difference favoring the topiramate treated subjects, 20% vs. 7%, χ2(1)= 6.7 p=0.01 when missing weeks are regarded as use, and 27% vs. 10%, χ2(1)= 6.23 p=0.01 when missing data are ignored.
Subjects with CSSA scores in the highest tertile (corresponding to patients with CSSA score above 18) on the day of randomization appeared to exhibit larger topiramate effects than subjects with lower scores. Among subjects with CSSA scores greater than 18 (n=59), topiramate-treated subjects, on average, submitted significantly more cocaine negative UBT samples during the trial compared to placebo-treated subjects (17.6 vs. 8.8, t= 2.9, p=.01). In the GEE models with missing data ignored, the interaction between a binary indicator of high CSSA and medication group was significant (χ2(1)=4.37, p=0.04), with a significant effect in favor of abstinence for topiramate-treated subjects in the high CSSA group (OR=2.45, 95% CI = (1.16, 2.65), p=0.02), and no effect of topiramate in the low CSSA group (OR=1.17, 95% CI = (0.61, 2.24), p=0.63). Similar results were found when missing weeks were imputed as nonabstinent. The interaction between a binary indicator of high CSSA and medication group was significant, χ2(1)=4.66, p=0.03, with a significant effect in favor of abstinence for topiramate-treated subjects in the high CSSA group and no effect of topiramate in the low CSSA group.
3.4 Alcohol Use Results
Alcohol use was not significantly different between the two groups. Topiramate treated subjects drank on 16% of days compared to 20% among placebo treated subjects. GEE analysis of percent days drinking over the course of the 13 weeks of the medication phase of the trial showed no difference between the two groups (GEE Z= −1.30, p=0.19). Likewise there was no difference between the two groups in heavy drinking days, defined as more than 4 standard drinks for men and 3 standard drinks for women, which was 10% for topiramate and 14 % for placebo (GEE Z= −0.81, p=0.42). Finally, the mean numbers of drinks per drinking day were 5.21 (se=0.58) for topiramate and 6.10 (se=0.61) for placebo participants, with no significant difference between the groups (GEE Z=−0.76, p=0.45).
3.5 Results from Secondary Outcome Measures
We examined the “previous 30 day” measures of days of cocaine use, days of alcohol use, days of alcohol intoxication, and dollars spent on drugs, and the seven composite scores from the ASI obtained at baseline, midway through the trials and at the end of the trial. There were no significant group by time interactions (all p-values > 0.19).
Cocaine craving and alcohol craving were measured weekly using the MCCS and the PACS respectively. Cocaine craving measured by the MCCS declined significantly during the trial in both groups and there were no between group differences. Alcohol craving measured by PACS scores declined significantly over time during the trial (Z= 7.41, p <.001) and scores were significantly lower among topiramate treated subjects (Z= 1.99, P<.05).
Improvement was rated by the nurse practitioner weekly using the CGI. Improvement was rated on a scale from very much improved (0) to much worse (6). The improvement scores from CGI were evaluated as dichotomized variables (very much improved and much improved vs. all other scores). GEE models showed a significant trend toward improved/very much improved over time (Z =7.48, P<.001), but no significant difference in change over time between the two groups (Z= 1.31, p=0.19).
The CSSA measures overall cocaine withdrawal symptoms. GEE models showed a significant decline in the CSSA composite score over the trial for both groups (Z= 5.97, P<.001). There was no significant difference found between the two groups for CSSA composite scores (Z = 0.99, p=0.32).
3.6 Medication adherence
Medication adherence was measured by pill count. The percentage of pills taken was calculated by subtracting the number of pills returned each week from the number of pills dispensed. Both groups showed good adherence. On average, subjects in the placebo group took 73% of prescribed pills, and subjects in the topiramate group took 78% of their prescribed pills (t = −1.36, df =133, p = .177).
3.7 Safety analyses
Adverse events were assessed at each visit. Topiramate was well tolerated. Adverse events were mainly mild and generally evenly distributed between the topiramate and placebo groups (see Table 3). The most commonly reported adverse events included sedation, paresthesias, headache, and dry mouth. The only adverse event noted to occur more frequently among topiramate treated subjects was paresthesias (20% vs. 3%).
Table 3.
Adverse events reported by 10% or more of subjects.
| Adverse event | Topiramate n = 83 | Placebo n= 87 |
|---|---|---|
| Sedation | 20% | 17% |
| Paresthesias* | 20% | 3% |
| Headache | 7% | 3% |
| Dry mouth | 7% | 3% |
significantly different (p<.05)
4. DISCUSSION
The efficacy of topiramate was evaluated in subjects with both cocaine and alcohol dependence, using a double-blind, placebo-controlled trial. This trial was designed to test topiramate’s efficacy as a relapse prevention medication. Therefore, subjects included in this trial were asked to be abstinent from cocaine and alcohol for three days just before being randomized to study medication. While topiramate was not significantly better than placebo in preventing relapse in the majority of subjects, as evidenced by GEE analyses (the planned primary cocaine outcome), there were significantly more topiramate than placebo-treated subjects who achieved three weeks of continuous abstinence from cocaine at the end of the trial (20% vs. 7%). Subgroup analyses revealed that topiramate appeared to be more effective in subjects who entered the trial with more severe cocaine withdrawal symptoms.
It is possible that topiramate supported cocaine abstinence due to its anxiolytic properties. It is also possible that topiramate promoted cocaine abstinence by reducing the euphoric effects of cocaine. Topiramate was shown to reduce the reinforcing effects of high doses of cocaine in a human laboratory trial (Johnson et al., 2012). This effect of topiramate may be particularly useful in cocaine users with more severe cocaine withdrawal symptoms who have been shown to have higher levels of cocaine euphoria (Sofuoglu et al., 2003).
Although topiramate was superior to placebo in reducing alcohol craving, topiramate-treated subjects did not appear to drink less than those on placebo, as measured by the percent days of drinking, percent days of heavy drinking or drinks per drinking day recorded on a TLFB. The fact that topiramate did not reduce alcohol use better than placebo in this trial is puzzling. In trials of topiramate for alcohol dependence, the main effect of topiramate was seen in reduction of heavy drinking days. Although subjects included in the current trial met DSM IV criteria for both cocaine and alcohol dependence, alcohol use among these subjects was not as severe as is often seen in trials of solely alcohol dependent subjects. The percent days of heavy drinking among subjects during this trial was about 12%, which is much less than the percent days of heavy drinking recorded in a recent trial of quetiapine for alcohol dependence (about 30%; Litten et al., 2012) and much less than the roughly 48% heavy drinking days recorded in a multicenter trial of topiramate for alcohol dependence (Johnson et al., 2007a). It is possible that a floor effect on heavy drinking days made it impossible to detect an effect in heavy drinking.
This trial has several limitations. The total number of subjects was relatively small and thus the results should be considered preliminary. The study drop-out rate was formidable, though it was comparable with most trials involving cocaine-dependent subjects. The subjects included in this trial were mainly African American residents of a large city who smoked crack cocaine. Therefore, the results of this trial may not generalize to other populations of cocaine and alcohol dependent patients. The subjects included in this trial had to achieve enough cocaine abstinence to submit a BE negative UBT on the day of randomization. Therefore, more severe cocaine-dependent patients, who could not achieve some initial cocaine abstinence, might not respond to topiramate or might need inpatient stabilization prior to starting topiramate.
Despite its weaknesses the results of this trial suggest that topiramate may be beneficial for the treatment of comorbid cocaine and alcohol dependence. More subjects treated with topiramate in this trial achieved a period of stable abstinence from cocaine at the end of the trial. Subjects with more severe cocaine withdrawal symptoms appeared to have benefited the most from topiramate.
Figure 1.
Flow chart of study participants
Acknowledgments
Grant support was provided by from the National Institute on Drug Abuse P60-DA-05186-17, P50 DA012756, and T32 MH065218.
Role of Funding Source The National Institute on Drug Abuse (NIDA) provided funding for this trial through the following grants: P60-DA-05186-17, P50 DA012756, and T32 MH065218. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper.
Footnotes
Contributors Drs. Kampman, Pettinati, Lynch and O’Brien designed the study and wrote the protocol. Dr. Lynch and Mr. Wierzbicki analyzed the data. Dr. Spratt participated in patient selection and medical monitoring of the trial. All the authors contributed to and approved the final manuscript.
Conflict of Interest All the authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brady KT, Sonne S, Randall CL, Adinoff B, Malcolm R. Features of cocaine dependence with concurrent alcohol abuse. Drug Alcohol Depend. 1995;39:69–71. doi: 10.1016/0376-8716(95)01128-l. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54:199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford University Press; Oxford: 2002. [Google Scholar]
- Elkashef A, Kahn R, Yu E, Iturriaga E, Li SH, Anderson A, Chiang N, Ait-Daoud N, Weiss D, McSherry F, Serpi T, Rawson R, Hrymoc M, Weis D, McCann M, Pham T, Stock C, Dickinson R, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Li MD, Johnson BA, Elkashef A, Kahn R, Yu E, Iturriaga E, Li SH, Anderson A, Chiang N, Ait-Daoud N, Weiss D, McSherry F, Serpi T, Rawson R, Hrymoc M, Weis D, McCann M, Pham T, Stock C, Dickinson R, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Li MD, Johnson BA. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297–1306. doi: 10.1111/j.1360-0443.2011.03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-IP, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–1295. [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl 1):S10–16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Guy W, editor. Assessment Manual for Psychopharmacology: Publication ADM 76–338. US Department of Health Education and Welfare; Washington, DC: 1976. [Google Scholar]
- Halikas JA, Kuhn KL, Crosby R, Carlson G, Crea F. The measurement of craving in cocaine patients using the Minnesota Cocaine Craving Scale. Comp Psychiatry. 1991;32:22–27. doi: 10.1016/0010-440x(91)90066-l. [DOI] [PubMed] [Google Scholar]
- Heil SH, Badger GJ, Higgins ST. Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. J Stud Alcohol. 2001;62:14–22. doi: 10.15288/jsa.2001.62.14. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ, Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Gunderson EW, Haughey HM, Wang X, Liu L. Topiramate’s effects on cocaine-induced subjective mood, craving and preference for money over drug taking. Addict Biol. 2012;18:405–416. doi: 10.1111/j.1369-1600.2012.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM Topiramate for Alcoholism Advisory B, Topiramate for Alcoholism Study G. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007a;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM, Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM Topiramate for Alcoholism Advisory B, Topiramate for Alcoholism Study G. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007b;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kadden R, Carroll KM, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Cognitive Behavioral Coping Skills Manual. U.S. Governmmet Printing Office; Washington, DC: 1992. [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP, Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinieb RM, D’Angelo LD, Epperson L. Reliability and validity of the cocaine selective severity assessment. Addict Behav. 1998;23:49–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Hetherington H, Ho S, Pan J, Martin R, Gilliam F, Hugg J, Faught E. Topiramate increases cerebral GABA in healthy humans. Neurology. 1998;51:627–629. doi: 10.1212/wnl.51.2.627. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson B, Pettinati H, Swift R, Afshar M, Brunette MF, Tiouririne NAD, Kampman K, Stout R, Group NS. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:406–416. doi: 10.1111/j.1530-0277.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani JJ, MP, AB, EVN, DJB, FRL Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology. 1993;111:39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. Springer; New York: 2005. [Google Scholar]
- Newton TF, Kalechstein AD, Tervo KE, Ling W, Newton TF, Kalechstein AD, Tervo KE, Ling W. Irritability following abstinence from cocaine predicts euphoric effects of cocaine administration. Addict Behav. 2003;28:817–821. doi: 10.1016/s0306-4603(01)00273-8. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Hyder F, Mattson RH, Rothman DL. Topiramate increases brain GABA, homocarnosine, and pyrrolidinone in patients with epilepsy. Neurology. 1999;52:473–478. doi: 10.1212/wnl.52.3.473. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Addiction Research Foundation; Toronto: 1995. [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Brown SB, Hatsukami DK, Sofuoglu M, Dudish-Poulsen S, Brown SB, Hatsukami DK. Association of cocaine withdrawal symptoms with more severe dependence and enhanced subjective response to cocaine. Drug Alcohol Depend. 2003;69:273–282. doi: 10.1016/s0376-8716(02)00328-9. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers E. Assessment of alcohol withdrawal: the revised clinical instiute withdrawal assessment for alcohol scale. Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Kalechstein A, Richter T, Ling W, Newton T. Association of depressive symptoms during abstinence with the subjective high produced by cocaine. Am J Psychiatry. 1999;156:1444–1446. doi: 10.1176/ajp.156.9.1444. [DOI] [PubMed] [Google Scholar]
- Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Walsh DC, Hingson RW, Merrigan DM, Cupples LA, Levenson SM, Coffman GA. Associations between alcohol and cocaine use in a sample of problem-drinking employees. J Stud Alcohol. 1991;52:17–25. doi: 10.15288/jsa.1991.52.17. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Res. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]