Abstract
Objective
To test the hypothesis that levels of adropin, a recently discovered peptide that displays important metabolic and cardiovascular functions, are lower in obstructive sleep apnea (OSA), especially when associated with endothelial dysfunction.
Study design
Age-, sex- and ethnicity-matched children (mean age: 7.2±1.4 years) were included into one of three groups based on the presence of OSA in an overnight sleep study, and on the time to post-occlusive maximal reperfusion (Tmax>45 sec) with a modified hyperemic test. Plasma adropin levels were assayed using a commercial ELISA kit.
Results
Among controls, mean morning adropin levels were 7.4 ng/ml (95% confidence intervals: 5.2–16.3 ng/ml) OSA children with abnormal endothelial function (OSA+/EF+) had significantly lower adropin levels (2.7±1.1 ng/ml, n=35) compared with matched controls (7.6±1.4 ng/ml; n=35; p<0.001), and to children with OSA and normal endothelial function (OSA+/EF−: 5.8±1.5 ng/ml, n=47; p<0.001). Plasma adropin <4.2 ng/ml reliably predicted endothelial functional status, but individual adropin levels were not significantly correlated with age, BMI-z score, obstructive apnea-hypopnea index (AHI) or nadir SpO2. Adropin levels were assessed after adenotonsillectomy in a subset of children with OSA (n=22), and showed increases in OSA+/EF+ (2.5±1.4 ng/ml to 6.4±1.9 ng/ml, n=14; p<0.01) but remained unchanged in OSA+EF− (5.7±1.3 ng/ml to 6.4±1.1 ng/ml, n=8; p>0.05).
Conclusion
Plasma adropin levels are reduced in pediatric OSA when endothelial dysfunction is present, and return to within normal values after adenotonsillectomy. Assessment of adropin circulating levels may provide a reliable indicator of vascular injury in the context of OSA on children.
Keywords: Obstructive sleep apnea, adropin, Obesity, nitric oxide, Endothelial function
Obstructive sleep apnea (OSA) is characterized by repetitive events of either partial or complete upper airway obstruction during sleep that lead to disruption of normal ventilation, hypoxemia, and sleep fragmentation. In recent years, OSA has emerged as a chronic low-grade inflammatory disease (1–5), and increasing evidence supports a causative link between pediatric OSA and cardiovascular diseases (CVD), including hypertension, endothelial dysfunction, and atherosclerosis (6–15).
Adropin is a recently described peptide hormone that is encoded by the energy homeostasis-associated (ENHO) gene (16). The gene produces a highly conserved across mammalian species small peptide that is abundant in liver and secreted into the circulation (16). Circulating adropin concentrations are highly regulated by energy intake as well as being involved in cardiovascular function, particularly in endothelial function (16–19). We therefore hypothesized that adropin levels would be lower in children with OSA, particularly among those children with evidence of endothelial dysfunction (ED), and as such provide a reliable marker for ED in the context of pediatric OSA.
Methods
The study was approved by the University of Louisville (protocol #474.99),and by the University of Chicago (protocol #10-708A) Human Research Committees, and informed consent was obtained from the legal caregiver of each participant. Inclusion criteria were the presence of OSA according to polysomnographic criteria, and age between 5 and 10 years. Furthermore, age- , sex-, and ethnicity-matched healthy non-snoring children without OSA who underwent overnight polysomnography were also invited to participate in the study. Children were excluded if they had known diabetes or pre-diabetes, any defined genetic abnormality or underlying systemic disease, or if they had acute infections.
Children were weighed on a calibrated scale to the nearest 0.1 kg and height (to nearest 0.1cm) was measured with a stadiometer (Holtain, Crymych, UK). Body mass index (BMI) was calculated and BMI z-score was computed using CDC 2000 growth standards (www.cdc.gov/growthcharts) and online software (www.cdc.gov/epiinfo). A BMI z-score >1.65 (>95th percentile) was considered as fulfilling criteria for obesity.
Overnight polysomnography (PSG) was conducted and consistently scored as previously described (20–22). Central, obstructive, and mixed apneic events were counted. An obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for a duration of at least 2 breaths. Hypopneas were defined as a decrease in oronasal flow of ≥50% with a corresponding decrease in SpO2 of ≥4% or an arousal. The obstructive apnea hypopnea index (AHI) was defined as the number of obstructive apneas and hypopneas per hour of total sleep time (TST). Arousals were identified as defined by the American Sleep Disorders Association Task Force report (23, 24). The oxyhemoglobin desaturation index (ODI) was calculated as the number of SpO2 >3%drops per hour of TST.
The diagnosis of OSA was defined by the presence of snoring during the sleep study, an obstructive apnea-hypopnea index (AHI) ≥ 2 / hour of total sleep time (TST), a nadir saturation of <92%, and a respiratory arousal index of ≥ 2 / hour TST. Control children were nonsnoring children with an AHI < 1 / hour of total sleep time.
Endothelial function was assessed with a modified hyperemic test after cuff-induced occlusion of the radial and ulnar arteries by placing the cuff over the wrist as previously reported (6, 8, 9). Briefly, a laser Doppler sensor (Perimed AB, Periflux 5000 System integrated with the PF 5050 pressure unit, Järfälla, Sweden) was applied over the volar aspect of the hand at the second-finger distal metacarpal surface, and the hand was gently immobilized. Once cutaneous blood flow over the area was stable, the pressure within an inflatable cuff placed distal to the elbow and connected to a computer-controlled manometer was raised to 160 to 180mm Hg for 60 seconds, during which blood flow was reduced to undetectable levels. To enable consistent deflation times, the cuff was deflated under computer control, and post-occlusive hyperemic responses were assessed. Time to peak blood flow following relief of occlusion (Tmax) was considered as representative of the post-occlusion hyperemic response. Based on extensive previous experience, we defined a Tmax of >45 sec as abnormal and indicative of altered endothelial function (6, 8, 9). Accordingly, children with OSA were subdivided into 2 groups, namely those with normal (OSA+EF−) and abnormal endothelial function tests (OSA+EF+).
Fasting blood samples were drawn by venipuncture in the morning after the sleep study. Blood samples were immediately centrifuged and frozen at −80 °C until assay. Plasma adropin concentration was measured in duplicate using a commercially available ELISA (Peninsula Laboratories, Bachem, San Carlos, CA). The assay detects immunoreactivity in both mouse and human plasma, and there is 100% homology between both peptides. We also spiked human plasma with synthetic adropin 34–76 (catalog # 032-35; Phoenix Pharmaceuticals, Inc., Burlingame, CA) using a wide range of concentrations (0.1–30.0 ng/ml), and observed a linear rate of recovery within this range (r2: 0.98) between observed and predicted concentrations of adropin. The lowest detection limit was 0.25 ng/ml; the intra-assay coefficient of variation (CV) determined using quality-control human plasma samples with adropin values ranging from 0.5–5 ng/ml was 10.4%, and the inter-assay CV was 22%.
Statistical Analyses
Data were expressed by mean±SD. Significant differences between groups were analyzed using either unpaired Student t tests or ANOVA for continuous variables and chi-square tests for categorical variables. Bonferroni corrections were applied for multiple comparisons. If the data were not normally distributed, they were logarithmically transformed. Pearson’s correlation analyses were performed to examine association between adropin levels and anthropometric, Tmax, and polysomnographic variables. Statistical analyses were performed using SPSS software (version 19.0; SPPS Inc., Chicago, IL.). In addition, receiver operator curves (ROC) were calculated for prediction of endothelial dysfunction based on adropin levels, and the area under the curve (AUC), sensitivity and specificity of the optimal cut-off adropin plasma concentration are reported. All p-values reported are 2-tailed with statistical significance set at <0.05.
Results
Because there are no normative data regarding adropin plasma concentrations in children, we initially assessed adropin levels in a cohort of 71 healthy children who were nonsnorers and whose overnight sleep studies were within normal limits. Mean morning adropin levels were 7.4 ng/ml (95% confidence intervals: 5.2–16.3 ng/ml). Among the 71 children, endothelial function was assessed in 35 children who served as controls for the OSA groups.
Children with OSA with and without endothelial dysfunction did not differ in their demographic or anthropometric characteristics, and were also similar to controls (Table I). Furthermore, the 2 OSA groups displayed similar disturbances in the polysomnographic measures, which were significantly different from controls (Table II). However, in OSA children with abnormal endothelial function (OSA+/EF+), significantly lower adropin levels were present compared with age-, sex-, ethnicity-, and BMI-z score matched controls (Table I). Furthermore, OSA+/EF+ also exhibited significantly lower adropin plasma concentrations when compared with children with OSA and normal endothelial function (Table I). Plasma adropin levels were not significantly correlated with age, BMI-z score, or the obstructive apnea-hypopnea index (AHI), nadir SpO2 or respiratory arousal index.
Table 1.
Characteristics of children with OSA with and without endothelial dysfunction and matched controls.
| OSA+EF+ (n=35) |
OSA+EF− (n=47) |
Control (n=35) |
|
|---|---|---|---|
| Age (years) | 7.2±1.4 | 7.2±1.6 | 7.2±1.4 |
| Sex (male, %) | 60.0 | 59.5 | 60.0 |
| Ethnicity (Caucasian, %) | 40.0 | 48.9 | 40.0 |
| BMI-z score | 1.48±0.75 | 1.52±0.91 | 1.25±0.68 |
| Obese (%) | 31.4 | 31.9 | 28.6 |
| Tmax (sec) | 53.8±6.9§,& | 36.2±7.1& | 33.6±23.2§ |
| Adropin levels (ng/ml) | 2.7±1.1§,& | 5.8±1.5& | 7.6±1.4§ |
All data are expressed as mean±SD.
P<0.001, Controls vs. OSA+EF+ group.
P<0.001, OSA+EF+ group vs. OSA+EF- group.
Table 2.
Polysomnographic data among children with OSA with and without endothelial dysfunction and matched controls.
| OSA+EF+ (n=35) |
OSA+EF− (n=47) |
Control (n=35) |
|
|---|---|---|---|
| Stage 1 (%) | 7.2±3.4 | 6.9±3.2 | 4.2±3.1 |
| Stage 2 (%) | 51.9±6.7 | 49.2±7.5 | 37.9±8.7 |
| Stage 3 (%) | 8.6±4.1 | 8.4±3.6 | 12.9±3.8 |
| Stage 4 (%) | 17.7±8.5 | 19.0±8.7 | 24.5±7.6 |
| REM sleep (%) | 15.5±6.4 | 15.9±7.5 | 21.1±8.6 |
| Sleep latency (min) | 18.6±17.2 | 21.2±19.9 | 29.7±12.3 |
| REM latency (min) | 128.7±41.0 | 131.3±45.1 | 147.4±45.2 |
|
Respiratory Arousal Index (events /hour TST) |
5.1±2.7§ | 3.05±2.11# | 0.02±0.01§, # |
|
Apnea Hypopnea Index (events/ hour TST) |
17.9±6.5§ | 18.2±7.9# | 0.36±0.21§, # |
|
SpO2 Nadir (%) ODI (events/hour) |
81.2±3.3§ 14.4±5.5§ |
83.4±3.5# 15.3±6.1# |
93.9±1.0§, # 0.1±0.0§, # |
All data are expressed as mean±SD. ODI – oxyhemoglobin desaturation index
P<0.001, Controls vs. OSA+EF+ group.
P<0.001, Controls vs. OSA+EF− group.
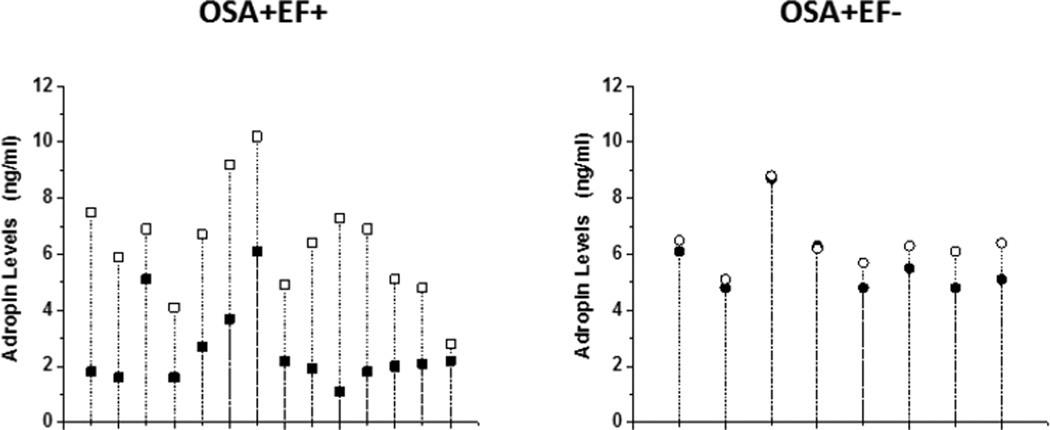
Plasma adropin levels were assessed after adenotonsillectomy in a subset of children with OSA (n=22), and showed increases in OSA+/EF+ (2.5±1.4 ng/ml to 6.4±1.9 ng/ml, n=14; p<0.01) but remained unchanged in OSA+EF− (5.7±1.3 ng/ml to 6.4±1.1 ng/ml, n=8; p>0.05; Figure 1). Of note, the rates of normalization of the sleep study after surgery were 75.0% and 78.5% in the 2 groups (p not significant)
Figure 1.
Drop plots showing individual changes in adropin levels before (closed symbols) and after adenotonsillectomy (open symbols) in 14 children with OSA and endothelial dysfunction (square symbols) and in 8 children with OSA and no evidence of endothelial dysfunction (circle symbols).
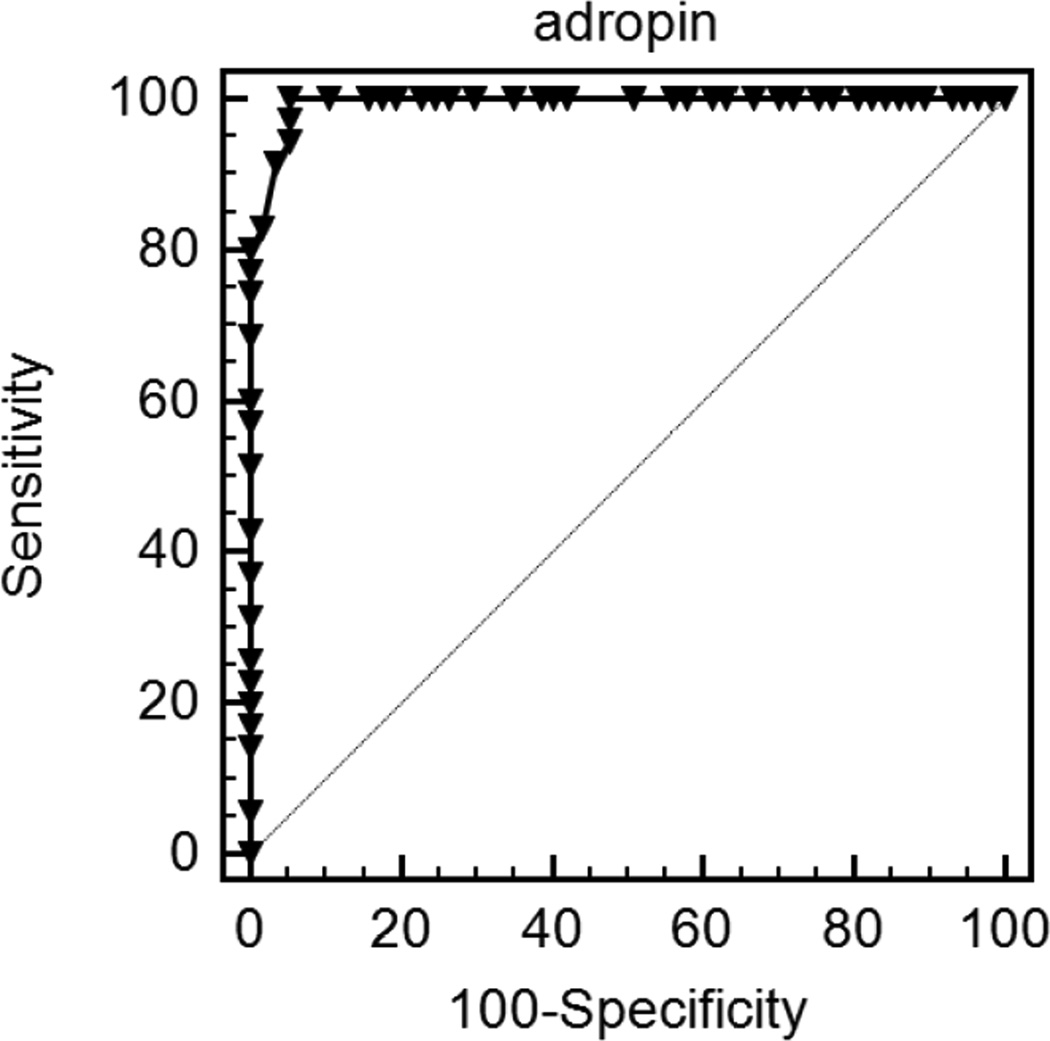
To examine the predictive value of adropin levels in the context of endothelial functional status, ROC revealed that a cut-off value of adropin <4.2 ng/ml provided a 100% sensitivity, 94% specificity, and a AUC of 0.993 (p<0.0001; Figure 2).
Figure 2.
Receiver operator curves using adropin plasma concentration cut-off value of <4.2 ng/ml for prediction of endothelial dysfunction in children with OSA.
Discussion
This study shows that circulating levels of adropin in children are markedly reduced in the presence of OSA, but only when endothelial dysfunction is present. These findings support the notion that OSA will selectively and detrimentally affect the integrity of the endothelium in a subset of vulnerable children, and that adropin appears to provide a reliable surrogate marker of such important vulnerability.
The concept that the presence of delayed post-occlusive hyperemic responses represents disruption of endothelial integrity, and thus provides an early risk marker of cardiovascular disease has gained widespread acceptance. In OSA, the risk for emergence of endothelial dysfunction is greatly increased in both adult and pediatric patients with OSA (9, 25, 26), particularly when obesity is concurrently present (6, 27). The bioavailability of nitric oxide is a critical component underlying the adequacy of the post-occlusive hyperemic responses (28), and recent work would suggest that one of the roles of adropin is to enhance the expression of endothelial nitric oxide synthase (eNOS) in the endothelium via activation of vascular endothelial growth factor receptor (VEGFR2) 2-phosphatidylinositol 3-kinase-Akt and VEGFR2-extracellular signal regulated kinase 1/2 pathways (18). Thus, adropin plasma levels as reported in the children with OSA may in fact provide a reliable indicator to the presence or absence of reduced nitric oxide bioavailability in the endothelium, an important correlate of disrupted vascular integrity. Such assumption would, of course, be both technically and ethically difficult to demonstrate considering the obvious obstacles to accessing representative endothelial cell samples in children. For similar reasons, it is difficult to elucidate whether the reduced plasma levels of adropin originate from the effects of OSA on the liver or brain, where adropin is primarily generated (16), or whether OSA specifically alters the expression of adropin in endothelial cells where it is also synthesized (18). Furthermore, it remains unclear why some children exhibit reductions in adropin and in endothelial functions, and others do not, despite the presence of OSA of similar degree of severity. The recent identification of epigenetic changes in the eNOS gene promoter exclusively among children who also manifest reduced eNOS expression as well as altered microvascular hyperemic responses following occlusion, suggests the presence of complex interactions between OSA and the vasculature (29).
An interesting finding involves the higher overall levels of adropin in children when compared with those previously reported in adults (30). These findings agree with those of Butler et al who reported a decline in adropin concentration with aging (30). However, contrary to the reductions in adropin associated with increasing BMI, we did not observe any relationship between BMI-z score and adropin levels. It is possible that such associations may only be revealed through substantial expansion of the cohort size.
Treatment of OSA will generally lead to normalization of endothelial function (9). Here, we found that in a subset of children who were assessed after adenotonsillectomy, the majority of the children with low adropin levels, who incidentally also had abnormal endothelial function, showed marked increases in the levels of this peptide after treatment, and no substantial changes occurred after surgery in the group with normal endothelial function and normal pre-surgical adropin concentrations (Figure 1). These findings not only further support the potential value of measuring adropin levels as a reporter for vascular susceptibility in the context of pediatric OSA, but may further serve as indicators of therapeutic outcomes.
In summary, adropin levels are reduced in children with OSA who exhibit endothelial dysfunction. Treatment of OSA with adenotonsillectomy appears to lead to the return of plasma adropin levels to normal. Furthermore, assessment of adropin concentrations in children being evaluated for the presence of sleep-disordered breathing may help identify children at risk for endothelial dysfunction.
Acknowledgments
D.G. is supported by National Institutes of Health (HL-65270, HL-086662, and HL-107160).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177(4):369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol. 2011;178(3):465–474. doi: 10.1016/j.resp.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9(3):254–259. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldbart AD, Levitas A, Greenberg-Dotan S, Ben Shimol S, Broides A, Puterman M, Tal A. B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest. 2010;138(3):528–535. doi: 10.1378/chest.10-0150. [DOI] [PubMed] [Google Scholar]
- 5.Chu L, Yao H, Wang B. Impact of adenotonsillectomy on high-sensitivity C-reactive protein levels in obese children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2012;147(3):538–543. doi: 10.1177/0194599812444419. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141(3):682–691. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kheirandish-Gozal L, Bhattacharjee R, Gozal D. Autonomic alterations and endothelial dysfunction in pediatric obstructive sleep apnea. Sleep Med. 2010;11(7):714–720. doi: 10.1016/j.sleep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010 Jul 1;182(1):92–97. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007 Nov 13;116(20):2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126(5):e1161–e1167. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- 11.Amin R, Somers VK, McConnell K, Willging P, Myer C, Sherman M, McPhail G, Morgenthal A, Fenchel M, Bean J, Kimball T, Daniels S. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51(1):84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 12.Crisalli JA, McConnell K, Vandyke RD, Fenchel MC, Somers VK, Shamszumann A, Chini B, Daniels SR, Amin RS. Baroreflex sensitivity after adenotonsillectomy in children with obstructive sleep apnea during wakefulness and sleep. Sleep. 2012;35(10):1335–1343. doi: 10.5665/sleep.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horne RS, Yang JS, Walter LM, Richardson HL, O'Driscoll DM, Foster AM, Wong S, Ng ML, Bashir F, Patterson R, Nixon GM, Jolley D, Walker AM, Anderson V, Trinder J, Davey MJ. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. 2011;128(1):e85–e92. doi: 10.1542/peds.2010-3431. [DOI] [PubMed] [Google Scholar]
- 14.Muzumdar HV, Sin S, Nikova M, Gates G, Kim D, Arens R. Changes in heart rate variability after adenotonsillectomy in children with obstructive sleep apnea. Chest. 2011;139(5):1050–1059. doi: 10.1378/chest.10-1555. [DOI] [PubMed] [Google Scholar]
- 15.Montesano M, Miano S, Paolino MC, Massolo AC, Ianniello F, Forlani M, Villa MP. Autonomic cardiovascular tests in children with obstructive sleep apnea syndrome. Sleep. 2010 Oct;33(10):1349–1355. doi: 10.1093/sleep/33.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA, Thearle M, Ferrante AW, Jr, Mynatt RL, Burris TP, Dong JZ, Halem HA, Culler MD, Heisler LK, Stephens JM, Butler AA. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian W, Gu X, Qin Y, Zheng X. Elevated plasma levels of adropin in heart failure patients. Intern Med. 2011;50:1523–1527. doi: 10.2169/internalmedicine.50.5163. [DOI] [PubMed] [Google Scholar]
- 18.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, Al-Omran M, Teoh H, Verma S. Adropin is a novel regulator of endothelial function. Circulation. 2010;122:S185–S192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 19.Kumar KG, Zhang J, Gao S, Rossi J, McGuinness OP, Halem HH, Culler MD, Mynatt RL, Butler AA. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 20:1394–1402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 21.Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153(2):866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 22.Rechstschaffen A KA. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Services/Brain Research Institute, University of California; 1968. [Google Scholar]
- 23.Schulz H. Phasic or transient? Comment on the terminology of the AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2007;3(7):752. [PMC free article] [PubMed] [Google Scholar]
- 24.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 25.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: An American Heart Association/American College Of Cardiology Foundation scientific statement from the American Heart Association Council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. In collaboration with the National Heart, Lung, And Blood Institute National Center On Sleep Disorders Research (National Institutes Of Health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 26.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharjee R, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in obese non-hypertensive children without evidence of sleep disordered breathing. BMC Pediatr. 2010;10:8. doi: 10.1186/1471-2431-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyke KE, Tschakovsky ME. The relationship between shear stress and flowmediated dilatation: Implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kheirandish-Gozal L, Khalyfa A, Gozal D, Bhattacharjee R, Wang Y. Endothelial Dysfunction In Children With Obstructive Sleep Apnea Is Associated With Epigenetic Changes in the eNOS Gene. Chest. 2013 doi: 10.1378/chest.12-2026. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler AA, Tam CS, Stanhope KL, Wolfe BM, Ali MR, O'Keeffe M, St-Onge MP, Ravussin E, Havel PJ. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J Clin Endocrinol Metab. 2012;97(10):3783–3791. doi: 10.1210/jc.2012-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]