Abstract
Background
Anti-smoking public service announcements (PSAs) often include smoking-related cues; however, visual drug cues can trigger acute cravings that may impede cognitive processing of the anti-smoking message. This experiment evaluated effects of smoking cues in PSAs on smoking urges, immediate smoking behavior, and persuasion measures in daily smokers.
Methods
Three-hundred eighteen non-treatment seeking smokers completed a single laboratory session during which they viewed sets of PSAs differentiated by presence of smoking cues (central to the PSA’s argument, peripheral, or no cues) and argument strength (high versus low). After viewing the PSAs, participants completed self-report measures of smoking urges, attitudes toward quitting, self-efficacy, and intentions to quit smoking. Smoking behavior was recorded during a one-hour ad-libitum smoking period immediately following PSA viewing and assessment.
Results
There was a significant positive effect of argument strength on attitudes toward quitting smoking (p = 0.012). There were no main effects of smoking cues or smoking cue by argument strength interactions on any of the outcome measures.
Conclusions
Visual smoking cues in PSAs do not increase urges to smoke, nor is there evidence that the inclusion of such cues impede the recall or persuasive effects of anti-smoking arguments.
Keywords: smoking, tobacco, public service announcements, smoking cues, health communication
1. INTRODUCTION
Mass media campaigns employing anti-smoking public service announcements (PSAs) have shown promise in reducing smoking prevalence (Emery et al., 2012; Hu et al., 1995), although not all campaigns are successful (Durkin et al., 2012). Anti-smoking PSAs often include smoking-related cues in order to illustrate the negative consequences of smoking. However, visual drug cues can trigger cravings to smoke (Carter and Tiffany, 1999) and may play a role in relapse (Shiffman et al., 2002). Indeed, preliminary work suggests that smoking cues in anti-tobacco PSAs increase smoking urges if the central argument is weak (Kang et al., 2009). Furthermore, smokers display attentional biases to smoking cues (Bradley et al., 2004; Waters et al., 2003) that may affect cognitive processing of the PSA. By distracting smokers from the central message and providing a clear motivator to continue smoking (i.e., increased urge to smoke), the presence of smoking cues in anti-smoking PSAs could be counter-productive to the goal of reducing smoking prevalence.
We examined effects of smoking cues in PSAs on smoking urges, cognitive measures (e.g., attitudes, self-efficacy, intentions, and recall), and smoking behavior in a sample of 318 daily smokers. PSAs were coded by independent raters for the presence of smoking cues, including whether cues were central or peripheral to the PSA’s central argument. Based on prior research (Kang et al., 2009), we included argument strength (AS, low versus high) as a factor, resulting in six PSA conditions (all between-subject). We hypothesized that 1) PSAs containing smoking cues, particularly peripheral cues, would increase smoking urges (primary outcome), have a negative influence on cognitions about quitting smoking, recall of PSA arguments, and increase post-viewing smoking behavior (secondary outcomes); and 2) the negative effects of smoking cues on these measures would be more pronounced for PSAs with weaker arguments (cues by argument strength interaction). An exploratory analysis utilized eye-tracking to examine whether time spent viewing cues predicted primary or secondary outcomes.
2. METHODS
2.1. PSA Selection
A selection of 99 PSAs coded for argument strength (AS; Strasser et al., 2009; Zhao et al., 2011) were evaluated by both well-trained and naïve raters for the presence of smoking cues. Argument strength was assessed for each PSA in this study following procedures detailed in Zhao et al. (2011). Argument strength is an aggregate rating averaged across independent samples of smokers. The ratings were obtained as a part of coding work on a large collection of anti-smoking PSAs (for example, see Zhao et al., 2011; Strasser et al., 2009; Lee et al., 2013). Argument strength raw scores were normalized by conversion into z-scores for each sample of raters and arguments tested. PSAs were selected for the current study based on high and low z score values, as reported in Table S11. PSAs were classified as having “central cues” if cues were directly part of the message, “peripheral cues” if cues were present but not directly related to the message, and “no cues” if no cues were seen in the PSA. The correlations between pairs of expert raters averaged 0.71 and the internal reliabilities for sets of naïve raters averaged 0.86. Sets of four PSAs were chosen to represent each of the following AS x cue conditions: (1) low AS, no cue; (2) low AS, peripheral cue; (3) low AS, central cue; (4) high AS, no cue; (5) high AS, peripheral cue; and (6) high AS, central cue (Table S1).
2.2. Participants
Participants were screened for eligibility via telephone; eligible participants were 21 to 65 years old, reported smoking at least 5 cigarettes per day (CPD) for at least the past 6 months, and were not currently seeking smoking cessation treatment. Exclusion criteria included current use of nicotine replacement therapy or other smoking cessation treatment; self-reported history of substance use disorders (not including nicotine); physical or visual impairment that would prevent the participant from viewing the computer monitor, responding on a keyboard, or prevent successful eye-tracking (i.e., glasses); and current or planned pregnancy.
2.3. Study Design and Procedures
The study utilized a 3 (no cue, peripheral cue, central cue) × 2 (high vs. low argument strength) factorial design. During a 2.5 hour session, participants provided written informed consent followed by a breath alcohol reading (>0.01 exclusionary) and breath carbon monoxide (CO) reading (<5 ppm exclusionary). In order to standardize time since last cigarette, they smoked one of their own cigarettes and provided a second CO breath sample before completing measures on demographics and smoking history. Participants were seated in a comfortable chair approximately one meter away from the computer monitor. Eye-tracking was calibrated for each participant as described elsewhere (Strasser et al., 2012).
As described above, participants were stratified by nicotine dependence (FTND < 4 vs. FNTD ≥ 4) and then randomly assigned to view 4 PSAs within one of the 6 conditions. After viewing, participants completed a series of assessments (see Measures section below) and moved to a ventilated smoking research room equipped with a sofa and television monitor, where they were asked to sit for a 1-hour period as the experimenter reviewed their data. Participants were informed that smoking was permitted in this room. Using well-validated procedures for ad libitum smoking assessment (Distefan et al., 1999), a research technician observed the participant and recorded time (in minutes/seconds) to first cigarette puff and the total number of puffs taken during this period. At the end of the session, participants completed an additional measure of smoking urges and provided cigarette ratings.
2.4. Measures
Covariates
Participants completed standardized questionnaires on demographics and smoking history. The Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) assessed nicotine dependence, and the Questionnaire of Smoking Urges - brief version (QSU-Brief; Cox et al., 2001) assessed baseline smoking urges.
Outcome Measures
The primary outcome was smoking urges assessed using the QSU-Brief (Cox et al. 2001). Secondary outcomes assessed persuasive effects of the PSAs: Intention to quit smoking in the next 3 months was assessed using two items “how likely to attempt...” and “how likely to succeed” rated on a 4-point Likert scale (1 = “I definitely will not”, 4 = “I definitely will” (Cronbach’s alpha = 0.89; (Norman et al., 1999). Attitudes toward quitting were assessed using 7-point differential scales asking participants to rate “quitting smoking completely and permanently in the next 3 months” as: bad/good, unenjoyable/enjoyable, unpleasant/pleasant, foolish/wise, and harmful/beneficial (Cronbach’s alpha = 0.73; Strasser et al., 2009; Yzer et al., 2003). Perceived self-efficacy was assessed using 10 items asking participants to rate on a scale of 1 (“not at all sure”) to 4 (“completely sure”) their ability to “Avoid smoking again after an initial quit attempt,” etc. (Cronbach’s alpha = 0.92; Strasser et al., 2009; Yzer et al., 2003). Finally, participants answered four true/false statements assessing recall for each PSA. Correct answers counted as one point; incorrect answers or an answer of “I don’t know” counted for zero points. An overall recall index was created by summing scores across all four PSAs (maximum possible score = 16).
2.5. Statistical Analysis
Descriptive statistics were obtained for all variables. The primary behavioral outcome (pre- to post-PSA change in smoking urges) was examined using a 3 by 2 ANOVA (including CPD as a covariate). Secondary outcomes (cognitive and smoking behavior measures) were examined using similar models. An exploratory analysis utilized linear regression models to estimate the influence of time spent looking at smoking cues on smoking urges (eye-tracking measure), intent to quit smoking, attitudes about quitting, perceived self-efficacy, and recall (AS, cue condition, and CPD were included as covariates). Our sample size of 318 participants provided >80% power to detect a moderate effect size with α = 0.05.
3. RESULTS
3.1. Descriptive Statistics
Three hundred eighteen participants completed the study. Of these, 160 (50.3%) were female; the majority were African American (63.8%) and reported education beyond high school (69.8%). The mean age was 32.5 years (SD 9.9, range 20-61) and mean CPD was 13.9 (SD 5.8, range 5-30). There were no significant differences in age, sex, or CPD among PSA conditions.
3.2. Primary outcome: Smoking Urges
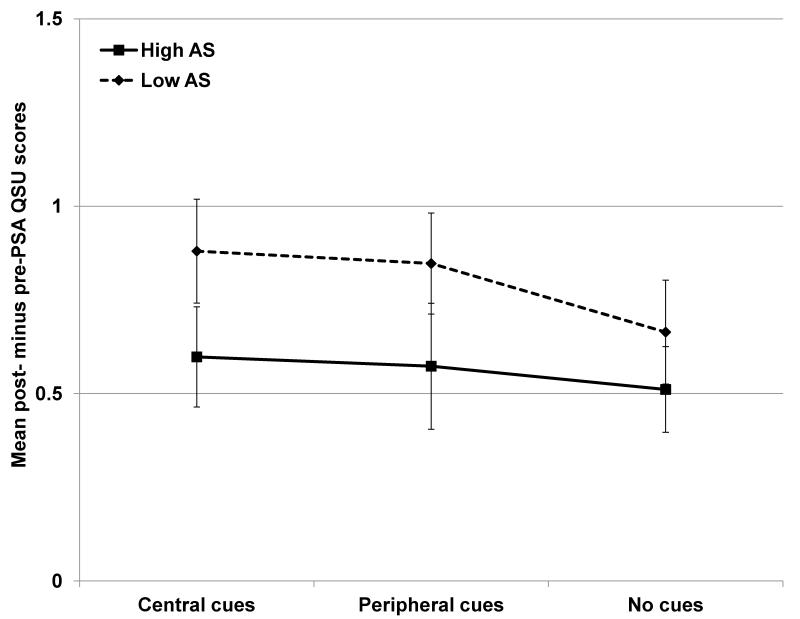
Across all conditions, there was a significant increase in reported smoking urges from baseline to the post-PSA assessment; the mean QSU-B score increased from 2.01 to 2.69 (standard error (SE) 0.07 and 0.08 respectively, p < 0.0001). There was no main effect of cue condition on change in smoking urge, and the AS by cue interaction was also non-significant (ps > 0.6; Figure 1). The main effect of AS on smoking urge was nonsignificant, although the trend suggested that low AS PSAs led to greater urge increases (p=0.063).
Figure 1.
Mean change in QSU scores (post-minus pre-PSA scores) plotted by condition. There was a significant increase in smoking urge from pre- to post-viewing across all conditions (p < 0.0001), with a trend toward greater increases in the low argument strength (AS) condition compared to high (F(317) = 3.49, p = 0.063). There were no main or interacting effects of cue condition on urges (ps > 0.6).
3.3. Secondary outcomes
Mean scores on the cognitive measures were as follows: attitudes toward quitting = 4.3 (SE 0.05); intention to quit = 2.3 (SE 0.04); efficacy = 1.8 (SE 0.04); recall = 10.9 (SE 0.6). Mean time to first cigarette was 481 sec (SE 39) and mean number of puffs taken was 18.0 (SE 0.6). Correlations between nicotine dependence measures and outcome measures may be found in Table S22. There were no effects of cue condition or cue by AS interactions on any of the cognitive measures or on time to first cigarette or total number of puffs taken during the ad-lib smoking period (ps > 0.2). There was a small but significant positive effect of AS on attitudes toward quitting (F(317) = 6.43, p = 0.012).
3.4. Exploratory Analysis
Despite participants spending more time viewing cues in the high AS condition (mean view time 14.0s versus 8.2s in low AS, p < 0.0001), and central cues more than peripheral cues (mean view time 12.1s versus 10.2s, p < 0.001), there were no associations between cue viewing time and any of the cognitive or behavioral measures.
4. DISCUSSION
Anti-smoking PSAs often include smoking-related cues to illustrate the harmful effects of smoking. The results of this large experimental analysis of smoking cue effects within these PSAs provides no evidence for untoward effects of cue presence or view time on smoking urges, cognitions about quitting smoking, recall of the PSA message, or immediate post-viewing smoking behavior.
Smoking cues presented in other settings have been shown to increase smoking urges. For example, smokers presented within in vivo smoking cues or visual cues exhibit increases in smoking urges compared to control conditions in various laboratory settings (Carter and Tiffany, 1999). Also, the presence of smoking cues in movies can increase smoking urge in viewers who smoke (Lochbuehler et al., 2009; Sargent et al., 2009). In contrast, the anti-smoking arguments within anti-tobacco PSAs may suppress cue-induced smoking urges, or suppress the reports of such urges; however, the lack of evidence for cue effects on any of the cognitive or smoking behavior measures suggests that these findings do not reflect under-reporting of urges. On the other hand, suppression of cue-induced urges by anti-smoking arguments may suggest a possible mechanism for positive associations between anti-smoking PSA exposure and reduced relapse among recent quitters (Wakefield et al., 2013). Future studies employing a similar design and incorporating a “no-argument” condition may offer more insight into the influence of anti-smoking arguments on smoking urges.
It should be noted that a previous study did report a trend toward greater increases in smoking urge following viewing of anti-tobacco PSAs with smoking cues present, when the PSAs were low in AS (Kang et al., 2009). Another study suggested that smoking cues may undermine PSAs persuasive effects in former smokers, although there was no effect on smoking urges (Lee et al., 2013). However, there are differences in study design between the current study and the prior studies that may account for differing results. For example, Kang et al. (2009) employed a within-subject design wherein the cue condition was always presented after the no-cue condition. Thus, cue presentation and time since last cigarette were confounded; however, it should be noted that increases in urges were observed only in the low argument strength condition. The current study employed a between-subject design for cue condition to minimize this confound, and utilized a larger sample size to provide sufficient power to detect moderate effects on smoking urges and behavioral outcomes; we detected no such effects. It is possible that smoking cues in PSAs produce a smaller effect on smoking urges that we could not detect; however, the clinical relevance of such an effect is uncertain.
This study is not without limitations. We report results based on a single exposure to four PSAs; repeated exposure to anti-smoking messages over time may be necessary to prompt changes in attitudes or behavior (Emery et al., 2012; Wakefield et al., 2008), regardless of smoking cues. Furthermore, the PSAs in this study were drawn from existing campaigns. Since no campaign would deliberately employ weak arguments as interventions, differences in AS across conditions (although validated; Zhao et al., 2011) were limited. Finally, although the lack of cue effects on smoking behavior was consistent with reported smoking urges and persuasion measures, our measurement of smoking behavior was conducted in a laboratory setting which may not fully reflect effects of cues in a more naturalistic setting.
Anti-smoking PSAs can be a powerful tool for tobacco control programs (Siegel, 1998; Wakefield et al., 2010), and identification of factors which impact the persuasiveness of a PSA will aid development of more effective programs. Our results indicate that, despite general associations between smoking cues and urge to smoke, the presence of smoking cues in PSAs does not increase smoking urges, prompt changes in immediate smoking behavior, or have untoward effects on cognitions about quitting smoking.
Supplementary Material
Acknowledgments
Role of funding source
This work was supported by the National Cancer Institute Center of Excellence in Cancer Communication Research (CECCR), P50-CA095856 and P20-CA095856-06 (Hornik). M Falcone is supported by NIH grant T32 GM008076. The funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
M Falcone was responsible for data analysis and manuscript writing; C Lerman and JN Capella were responsible for study design, interpretation and manuscript writing; C Jepson analyzed data and assisted in manuscript preparation; PM Sanborn assisted in data collection, scoring and manuscript preparation; and AA Strasser was responsible for study design, data analysis and manuscript writing. All authors have approved the final version of the manuscript.
Conflict of Interest
Dr. Lerman has served as a consultant and has received research funding from Pfizer that is unrelated to this project. The other authors declare no conflicts of interest.
REFERENCES
- Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav. Pharmacol. 2004;15:29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Distefan JM, Gilpin EA, Sargent JD, Pierce JP. Do movie stars encourage adolescents to start smoking? Evidence from California. Prev. Med. 1999;28:1–11. doi: 10.1006/pmed.1998.0409. [DOI] [PubMed] [Google Scholar]
- Durkin S, Brennan E, Wakefield M. Mass media campaigns to promote smoking cessation among adults: an integrative review. Tob. Control. 2012;21:127–138. doi: 10.1136/tobaccocontrol-2011-050345. [DOI] [PubMed] [Google Scholar]
- Emery S, Kim Y, Choi YK, Szczypka G, Wakefield M, Chaloupka FJ. The effects of smoking-related television advertising on smoking and intentions to quit among adults in the United States: 1999-2007. Am. J. Public Health. 2012;102:751, 757. doi: 10.2105/AJPH.2011.300443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hu TW, Sung HY, Keeler TE. Reducing cigarette consumption in California: tobacco taxes vs an anti-smoking media campaign. Am. J. Public Health. 1995;85:1218–1222. doi: 10.2105/ajph.85.9.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Cappella JN, Strasser AA, Lerman C. The effect of smoking cues in antismoking advertisements on smoking urge and psychophysiological reactions. Nicotine Tob. Res. 2009;11:254–261. doi: 10.1093/ntr/ntn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Cappella JN, Lerman C, Strasser AA. Effects of smoking cues and argument strength of antismoking advertisements on former smokers’ self-efficacy, attitude, and intention to refrain from smoking. Nicotine Tob. Res. 2013 doi: 10.1093/ntr/nts171. Epub ahead of print. doi:10.1093/ntr/nts171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochbuehler K, Engels RC, Scholte RH. Influence of smoking cues in movies on craving among smokers. Addiction. 2009;104:2102–2109. doi: 10.1111/j.1360-0443.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- Norman P, Conner M, Bell R. The theory of planned behavior and smoking cessation. Health Psychol. 1999;18:89–94. doi: 10.1037//0278-6133.18.1.89. [DOI] [PubMed] [Google Scholar]
- Sargent JD, Morgenstern M, Isensee B, Hanewinkel R. Movie smoking and urge to smoke among adult smokers. Nicotine Tob. Res. 2009;11:1042–1046. doi: 10.1093/ntr/ntp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J. Abnorm. Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Siegel M. Mass media antismoking campaigns: a powerful tool for health promotion. Ann. Intern. Med. 1998;129:128–132. doi: 10.7326/0003-4819-129-2-199807150-00013. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Cappella JN, Jepson C, Fishbein M, Tang KZ, Han E, Lerman C. Experimental evaluation of antitobacco PSAs: effects of message content and format on physiological and behavioral outcomes. Nicotine Tob. Res. 2009;11:293–302. doi: 10.1093/ntr/ntn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Tang KZ, Romer D, Jepson C, Cappella JN. Graphic warning labels in cigarette advertisements: recall and viewing patterns. Am. J. Prev. Med. 2012;43:41–47. doi: 10.1016/j.amepre.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield MA, Durkin S, Spittal MJ, Siahpush M, Scollo M, Simpson JA, Chapman S, White V, Hill D. Impact of tobacco control policies and mass media campaigns on monthly adult smoking prevalence. Am. J. Public Health. 2008;98:1443–1450. doi: 10.2105/AJPH.2007.128991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet. 2010;376:1261–1271. doi: 10.1016/S0140-6736(10)60809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield MA, Bowe SJ, Durkin SJ, Yong HH, Spittal MJ, Simpson JA, Borland R. Does tobacco-control mass media campaign exposure prevent relapse among recent quitters? Nicotine Tob. Res. 2013;15:385–392. doi: 10.1093/ntr/nts134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Yzer MC, Cappella JN, Fishbein M, Hornik R, Ahern RK. The effectiveness of gateway communications in anti-marijuana campaigns. J. Health Commun. 2003;8:129–143. doi: 10.1080/10810730305695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Strasser AA, Cappella JN, Lerman C, Fishbein M. A measure of perceived argument strength: reliability and validity in health communication contexts. Commun. Methods Measures. 2011;5:48–75. doi: 10.1080/19312458.2010.547822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.