Abstract
Aims
The prevalence of hepatic steatosis may differ between post-menopausal African-Americans and non-Hispanic white women and by sex hormone binding globulin level. We examined prevalence of hepatic steatosis by race/ethnicity and associations with sex hormone binding globulin.
Methods
Participants included post-menopausal women who underwent hepatic ultrasound (n = 345) at the Michigan site of the Study of Women’s Health Across the Nation, a population-based study. We examined hepatic steatosis prevalence by race/ethnicity and used logistic regression models to calculate the odds of hepatic steatosis with race/ethnicity and sex hormone binding globulin, after adjustment for age, alcohol use, waist circumference, HDL cholesterol, triglycerides, systolic blood pressure and use of medications reported to lower intrahepatic fat.
Results
Fewer African-Americans than non-Hispanic white women had hepatic steatosis (23 vs. 36%, P = 0.01). African-Americans had lower triglyceride and LDL cholesterol levels, but higher blood pressure and follicle-stimulating hormone levels (P < 0.05). In the optimal-fitting multivariable models, women in the highest tertile of sex hormone binding globulin (60.2–220.3 nmol/l) had a lower odds of hepatic steatosis (odds ratio 0.43, 95% CI 0.20–0.93) compared with women in the lowest tertile of sex hormone binding globulin (10.5–40.3 nmol/l). There was an interaction between race/ethnicity and medication use whereby non-Hispanic white women using medications had three times higher odds of hepatic steatosis compared with African-Americans not using medications (odds ratio 3.36, 95% CI 1.07–10.58). Interactions between race/ethnicity and other variables, including sex hormone levels, were not significant.
Conclusions
Hepatic steatosis on ultrasound may be more common in post-menopausal non-Hispanic white women than African-Americans and was associated with lower levels of sex hormone binding globulin.
Introduction
The relationship between non-alcoholic fatty liver disease, also known as non-alcoholic hepatic steatosis, and glucose tolerance is complex. Insulin resistance is a well-recognized risk factor for hepatic steatosis [1], yet hepatic steatosis may also be a risk factor for incident diabetes, independent of traditional diabetes risk factors including age, body shape and size and insulin levels [2]. One potential mechanism relating hepatic steatosis and peripheral glucose is sex hormone binding globulin (SHBG), a glycoprotein manufactured primarily by the liver, whose production may be impaired in the presence of hepatic steatosis [3]. SHBG might lead to decreased hepatic gluconeogenesis apart from hepatic steatosis, as SHBG and fasting glucose are still significantly associated after adjustment for liver fat [3], although a study in men did not find this [4].
No population-based studies have examined the relationship between SHBG and the presence of hepatic steatosis in post-menopausal women. Post-menopausal status compared with pre-menopausal status has been associated with an almost twofold increase in odds, after adjustment for age [5,6], and, in Koreans, the prevalence of hepatic steatosis in post-menopausal Asian women is 24–28% compared with 6–8% in pre-menopausal women [5]. The menopausal transition is characterized by declines in SHBG and oestradiol and increases in relative androgenicity [7], suggesting that SHBG could contribute to the higher prevalence in post-menopausal women. Moreover, the menopausal transition has been associated with a worsening profile of the components of the metabolic syndrome, particularly lipid profiles [8], which are also risk factors for hepatic steatosis [9,10]. Among women, it is also possible that the relationship between sex hormones and hepatic steatosis differs between non-Hispanic white women and African-Americans. Studies conflict regarding racial/ethnic differences in sex hormone profiles [11–14], as well as regarding the prevalence of hepatic steatosis in non-Hispanic white subjects and African-Americans [15,16]
Taken together, these studies suggest that SHBG and the sex steroids that it binds might contribute both to hepatic steatosis and to racial/ethnic differences in hepatic steatosis among women. The Study of Women’s Health Across the Nation (SWAN) is an ongoing population-based cohort study designed to characterize biological and symptomatic changes that occur during and after menopause among women of different racial/ethnic backgrounds [17]. The Michigan Study of Women’s Health Across the Nation site ascertained hepatic steatosis with ultrasound, the most common imaging technique in clinical practice, at the 2010 annual follow-up visit, when participants were post-menopausal. Thus, we were able to examine the prevalence of hepatic steatosis in post-menopausal African-Americans and non-Hispanic white women and evaluate the contribution of sex hormone profile to hepatic steatosis. We hypothesized that non-Hispanic white women would more frequently have hepatic steatosis than African-Americans. We also hypothesized that lower SHBG and would be associated with increased prevalence of hepatic steatosis.
Patients and methods
Study population
The sample was drawn from the Michigan site of the Study of Women’s Health Across the Nation cohort. Recruitment procedures and the study design used have been described elsewhere [17–19] Briefly, in 1996–1997, women aged 40–55 years were screened from defined sampling frames at seven clinical sites throughout the USA. Eligible women were invited to participate in a longitudinal study of the natural history of the menopausal transition. To be eligible, women had to be between 42 and 52 years of age, have an intact uterus and at least one ovary, to report having had a menstrual period in the previous 3 months, to not report oestrogen therapy in the 3 months prior to recruitment, and not currently be pregnant or breastfeeding. All participants gave informed consent and all study procedures were approved by the University of Michigan institutional review board.
The Michigan site recruited women who self-identified as being African-American or non-Hispanic white. At the time of their 2010 follow-up visit, 345 (85%) of the 406 women from the study who participated underwent hepatic ultrasound. Women who did not undergo ultrasound had higher SHBG than women who underwent ultrasound (66.3 vs. 49.8 nM/l, P < 0.05). Women who did not have ultraounds were otherwise similar. Of the women with hepatic ultrasound measures, 14 women reported a history of cirrhosis or chronic liver disease attributable to viral hepatitis or hemachromatosis and were excluded, leaving a total analytic sample of 331 participants for this report.
Data collection
The Study of Women’s Health Across the Nation protocol includes annual questionnaires, anthropometrics, blood pressure assessments and serum measures. Women were defined as post-menopausal if they had no menses for 12 or more months. Information regarding use of medications reported to influence hepatic adiposity (including metformin, thiazolidinediones, orlistat or sibutramine) was obtained, as was exogenous oestrogen therapy. Oestrogen therapy was characterized as length of oestrogen therapy use and also as ‘ever use’ vs. ‘never use’. No women used sibutramine and only one woman used orlistat.
Phlebotomy was performed in the morning after an overnight fast, blood was refrigerated 1–2 h after phlebotomy and after centrifugation, and the serum was aliquotted and frozen. Serum was stored at −70 ºC. Insulin was measured in serum by solid phase radioimmunoassay (Coat-A-Count; Diagnostics Product Corp., Los Angeles, CA, USA) and glucose was measured using a hexokinase-coupled reaction (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN, USA). Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR), defined as [fasting insulin ‘ (fasting glucose/18.01)/22.5] [20]. Total cholesterol and triglycerides were analysed by enzymatic methods and HDL cholesterol was isolated after addition of heparin and 2 mol/l manganese(II) chloride (MnCl2). LDL cholesterol was calculated using the Friedewald equation. HbA1c was determined on a Tosoh G7 HPLC Analyzer (Tosoh Biosciences Inc., South San Francisco, CA, USA).
SHBG was a de novo two-site chemiluminescent assay (inter- and intra-assay coefficients of variation were 9.9 and 6.1%, respectively). Serum follicle stimulating hormone concentrations were measured with a two-site chemiluminometric immunoassay (inter- and intra-assay coefficients of variation were 12.0 and 6.0%, respectively). Oestradiol and testosterone assays were conducted in the University of Michigan Study of Women’s Health Across the Nation Endocrine Laboratory using the ACS-180 automated analyser (Bayer Diagnostics Corp., Norwood, MA, USA). Serum oestradiol concentrations were measured with a modified, off-line ACS:180 (oestradiol-6) immunoassay (inter- and intra-assay coefficients of variation were 10.6 and 6.4%, respectively). Testosterone concentrations were evaluated with the ACS:180 total testosterone assay, modified to increase precision in the low ranges (inter- and intra-assay coefficients of variation were 10.5 and 8.5%, respectively). Total testosterone was indexed to SHBG to calculate the free androgen index as the measure of relative androgenicity [free androgen index = 100 ‘ testosterone (ng/dl)/28.84 ‘ SHBG (nM)] [21].
All abdominal ultrasounds were performed by a single ultrasound technician unaware of the clinical and laboratory results of the participants, on a Sonoline Elegra Ultrasound Imaging System (Siemens Medical Systems Inc., Delaware, WA, USA), using a 3.5-MHz transducer, a phantom (411 LE 0.5; Gammex RMI Ltd., Nottingham, UK) and also read by a radiologist who was blinded to participant profile. Ultrasound studies were performed and classified according to the protocol of the Edinburgh Type 2 Diabetes Study [22]. The liver was graded for markers of hepatic steatosis including bright hepatic echo pattern compared with the echo response of the right kidney, attenuation of the echo beam and presence of focal fatty sparing. In the Edinburgh cohort [22], validation in a subset with 1H magnetic resonance spectroscopy noted that moderate and severe hepatic steatosis on ultrasound was associated with magnetic resonance spectroscopy hepatic fat fraction ≥ 6.1% in all cases, while less severe hepatic steatosis overlapped significantly with absence of hepatic steatosis with respect to magnetic resonance spectroscopy fat fraction. We performed preliminary analyses in our population and found that women with no hepatic steatosis and mild hepatic steatosis did not differ regarding the variables noted in Table 1, therefore in this report we compare women with no/mild hepatic steatosis with women with moderate/severe hepatic steatosis.
Table 1.
Characteristics of 331 women who underwent hepatic ultrasound at 2010 follow-up visit by race/ethnicity
| African-Americans (n = 201) | Non-Hispanic white women (n = 130) | P-value | |
|---|---|---|---|
| Hepatic steatosis | 47 (23.4%) | 47 (36.2%) | 0.01 |
| Demographic characteristics and medical history | |||
| Age (years) | 59.4 (0.2) | 59.9 (0.2) | 0.10 |
| Years since final menstrual period | 7.9 (0.3) | 7.8 (0.3) | 0.77 |
| Number of alcoholic beverages/day | |||
| < 1 | 137 (68.2%) | 77 (59.2%) | 0.17 |
| 1 | 56 (27.9%) | 49 (37.7%) | |
| 2 or more | 8 (4.0%) | 4 (3.1%) | |
| Diagnosed diabetes | 50 (24.9%) | 27 (20.8%) | 0.39 |
| Medication use | 31 (15.4%) | 21 (16.2%) | 0.86 |
| Oestrogen therapy (ever/never) | 16 (8.0%) | 14 (10.8%) | 0.39 |
| Cardiometabolic biomarkers | |||
| Waist circumference (cm) | 101.1 (1.2) | 101.3 (1.5) | 0.93 |
| BMI (kg/m2) | 34.3 (0.6) | 34.0 (0.8) | 0.70 |
| Fasting glucose (mmol/l) | 5.71 (0.16) | 6.09 (0.30) | 0.22 |
| Fasting insulin (pmol/l) | 124.3 (11.8) | 116.7 (11.8) | 0.65 |
| Homeostasis model assessment of insulin resistance | 4.9 (0.47) | 4.9 (0.67) | 0.97 |
| Triglycerides (mmol/l) | 1.26 (0.04) | 1.55 (0.08) | < 0.01 |
| HDL cholesterol (mmol/l) | 1.44 (0.03) | 1.49 (0.03) | 0.34 |
| LDL cholesterol (mmol/l) | 2.96 (0.06) | 3.17 (0.08) | 0.045 |
| Systolic blood pressure (mmHg) | 133.6 (1.4) | 123.9 (1.6) | < 0.01 |
| Diastolic blood pressure (mmHg) | 75.5 (0.8) | 70.6 (0.8) | < 0.01 |
| Sex hormones | |||
| Sex hormone binding globulin (nM/l) | |||
| Tertile 1: 10.5–40.3 (nM/l) | 69 (34.2%) | 48 (36.9%) | 0.29 |
| Tertile 2: 40.5–59.5 (nM/l) | 72 (35.8%) | 60 (46.2%) | |
| Tertile 3: 60.2–220.3 (nM/l) | 60 (29.9%) | 46 (35.4%) | |
| Testosterone (nmol/l) | 1.85 (0.05) | 1.79 (0.07) | 0.43 |
| Oestradiol (pmol/l) | 92.5 (5.5) | 107.9 (16.2) | 0.32 |
| Follicle stimulating hormone (IU/l) | 52.4 (1.9) | 58.6 (2.4) | 0.04 |
| Free androgen index | 122.6 (6.0) | 120.3 (7.2) | 0.81 |
Means (SE) or n (%) shown.
Statistical analysis
First, we examined race/ethnic differences in demographic, health history, cardiometabolic biomarkers and sex hormones using t-tests for continuous variables and χ2-tests for categorical variables. Log-transformations were employed for continuous variables with skewed distributions, i.e. insulin, triglyceride and sex hormones; the log-transformed levels were compared and back-transformed for presentation. SHBG was examined as a continuous variable and as categorical tertiles in the absence of established cut points for SHBG. Next, we examined the unadjusted associations between each variable with the presence of hepatic steatosis for the overall study population and by race/ethnicity. Because of previous reports noting that hepatic steatosis differed between African-Americans and non-Hispanic white subjects [15], interactions were evaluated between race/ethnicity and other variables including sex hormones and metabolic markers; interactions were only significant for race/ethnicity and medication use and thus was evaluated for inclusion in multivariate models.
Variables that were associated with hepatic steatosis in the literature or in the bivariate analyses were considered for inclusion in multivariable models. Variables known to be collinear (i.e. diabetes status, insulin, glucose, use of metformin and thiazolidinediones) were not considered in the same model. We examined use of metformin and thiazolidinediones, as reports have noted that these medications are associated with lower hepatic steatosis [23,24], as opposed to other hypoglycaemic medications such as sulphonylureas or insulin [24]. Although we only had a single measure of hepatic steatosis obtained at the 12th year of follow-up, we attempted to capture prospective relationships and specifically risk factors obtained before the menopausal transition by examining baseline levels of covariates as well as covariates at year 12. We created separate models containing baseline and year 12 levels as these values were highly collinear. Year 12 variables had stronger associations with hepatic steatosis than baseline levels for all risk factors, so year 12 data are presented. Beause of limited sample size, the best fit in the model was selected based on the Akaike information criterion (AIC), whereby the model with the smallest Akaike information criterion was selected [25]. In a sensitivity analysis, we excluded the few women who reported two or more alcoholic beverages per day, but the pattern of results was similar, so associations are reported for all of the Study of Women’s Health Across the Nation women who underwent ultrasound. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Thirty-six-per cent (n = 47) of non-Hispanic white women and 23% (n = 47) of African-Americans had hepatic steatosis. Table 1 shows the distribution of risk factors among non-Hispanic white women and African-Americans who underwent ultrasound. Only eight non-Hispanic white women and four African-Americans reported two or more alcoholic beverages per day. Non-Hispanic white women had higher triglycerides and LDL cholesterol than African-Americans, while African-Americans had higher systolic and diastolic blood pressure than non-Hispanic white women. For the sex hormones, only follicle stimulating hormone differed by race/ethnicity. Table 2 shows the distribution of risk factors by hepatic steatosis status. Non-Hispanic white race/ethnicity, alcohol intake, use of medications and oestrogen therapy were associated with hepatic steatosis. Greater waist circumference, BMI, fasting glucose, insulin, HOMA-IR, triglyceride and lower HDL were also associated with hepatic steatosis, but blood pressure was not. Lower SHBG and follicle stimulating hormone and greater free androgen index were associated with hepatic steatosis, but oestradiol and testosterone were not. Seventeen per cent of women used metformin (n = 56) and 2% used pioglitazone (n = 7); all of the pioglitazone users were metformin users also.
Table 2.
Characteristics of 331 women who underwent hepatic ultrasound at 2010 follow-up visit by hepatic steatosis status
| No hepatic steatosis (n = 237) | Hepatic steatosis (n = 94) | P-value | |
|---|---|---|---|
| Demographic characteristics and medical history | |||
| Non-Hispanic white women | 83 (35.0%) | 47 (50.0%) | 0.01 |
| Age (years) | 59.4 (0.2) | 60.0 (0.3) | 0.07 |
| Years since final menstrual period | 7.6 (0.3) | 8.5 (0.4) | 0.08 |
| Number of alcoholic beverages/day | |||
| < 1 | 143 (60.3%) | 71 (75.5%) | 0.04 |
| 1 | 84 (35.4%) | 21 (22.34%) | |
| 2 or more | 10 (4.2%) | 2 (2.1%) | |
| Diagnosed diabetes | 49 (20.7%) | 28 (29.8%) | 0.08 |
| Medication use (metformin, thiazolidinediones, orlistat) | 31 (13.1%) | 21 (22.3%) | 0.04 |
| Oestrogen therapy (ever/never) | 27 (11.4%) | 3 (3.2%) | 0.03 |
| Cardiometabolic biomarkers | |||
| Waist circumference (cm) | 97.3 (1.1) | 110.8 (1.5) | < 0.01 |
| BMI (kg/m2) | 32.7 (0.5) | 37.8 (0.8) | < 0.01 |
| Fasting glucose (mmol/l) | 5.38 (0.11) | 7.06 (0.42) | < 0.01 |
| Fasting insulin (pmol/l) | 94.5 (9.03) | 188.2 (18.8) | < 0.01 |
| Homeostasis model assessment of insulin resistance | 3.6 (0.4) | 8.4 (0.9) | < 0.01 |
| Triglycerides (mmol/l) | 1.25 (0.04) | 1.68 (0.10) | < 0.01 |
| HDL cholesterol (mmol/l) | 1.52 (0.03) | 1.31 (0.04) | < 0.01 |
| LDL cholesterol (mmol/l) | 3.05 (0.06) | 3.01 (0.09) | 0.67 |
| Systolic blood pressure (mmHg) | 128.9 (1.2) | 131.9 (2.1) | 0.21 |
| Diastolic blood pressure (mmHg) | 73.9 (0.7) | 72.9 (1.1) | 0.46 |
| Sex hormones | |||
| Sex hormone binding globulin (nM/l) | |||
| Tertile 1: 10.5–40.3 (nM/l) | 67 (28.3%) | 50 (53.2%) | < 0.01 |
| Tertile 2: 40.5–59.5 (nM/l) | 79 (33.3%) | 29 (30.9%) | |
| Tertile 3: 60.2–220.3(nM/l) | 91 (38.4%) | 15 (16.0%) | |
| Testosterone (mmol/l) | 1.79 (0.05) | 1.92 (0.07) | 0.15 |
| Oestradiol (pmol/l) | 97.6 (9.54) | 99.9 (5.5) | 0.88 |
| Follicle stimulating hormone (IU/l) | 58.4 (1.8) | 45.9 (2.2) | < 0.01 |
| Free androgen index | 107.9 (4.6) | 155.8 (10.5) | < 0.01 |
Means (SE) or n (%) shown.
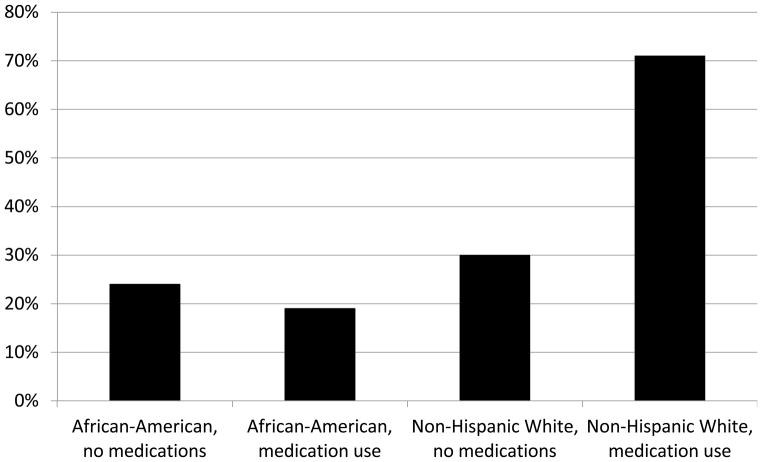
The pattern of associations between risk factors shown in Table 2 and hepatic steatosis was generally similar among African-Americans and non-Hispanic white women, with one notable exception. Use of metformin and thiazolidinediones was associated with increased hepatic steatosis in non-Hispanic white women, but not among African-Americans; African-Americans who used metformin and thiazolidinediones had the lowest prevalence of hepatic steatosis (19%) and non-Hispanic white women who used medications had the highest (71%) (Fig. 1). HbA1c values were highest among non-Hispanic white women using medications [64 ± 1 mmol/mol (8.0 ± 2.2%)] compared with non-Hispanic white women not using medications [40 ± 1 mmol/mol (5.8 ± 0.8%)], African-Americans using medications [53 ± 1 mmol/mol (7.0 ± 1.2%)] and African-Americans not using medications [42 ± 13 mmol/mol (6.0 ± 1.0%)].
Figure 1.
Unadjusted prevalence of hepatic steatosis in non-Hispanic white women and African-Americans by use of medications reported to decrease hepatic adiposity.
In the optimal-fitting multivariable model (Table 3), higher SHBG values were associated with decreased odds of hepatic steatosis; women in the highest SHBG tertile (60.2–220.3 nM/l) had nearly a 60% reduction in their odds of hepatic steatosis compared with women in the lowest tertile (10.5–40.3 nM/l). There was a statistically significant decreasing trend in odds of hepatic steatosis corresponding to increasing SHBG tertiles. Greater waist circumference was associated with increased odds of hepatic steatosis (odds ratio 1.03, 95% CI 1.01–1.05). Women with a waist circumference at the 75th percentile had 70% increased odds of hepatic steatosis as compared with women with a waist circumference at the 25th percentile. Of note, we also evaluated models containing other variables that were significant in bivariate analyses, including oestrogen therapy and the free androgen index. These models had poorer fit using Akaike information criterion criteria than those including the variables in Table 3. Compared with African-Americans not using medications, non-Hispanic white women using medications had more than three times greater odds of hepatic steatosis (odds ratio 3.36, 95% CI 1.07–10.58), whereas no differences were observed for non-Hispanic white women not using medications or African-Americans using medications (Table 3). We also examined how the relationship between SHBG and hepatic steatosis was altered by other risk factors for hepatic steatosis (BMI, waist circumference, lipid subfractions, HOMA-IR) and whether this relationship differed by race/ethnicity, diabetes status or use of diabetic medication. In all of these subgroups, higher SHBG levels were still significantly and strongly associated with hepatic steatosis and were not markedly reduced by consideration of BMI or waist circumference (results not shown). We also examined the subgroups of women without diabetes, women using metformin and thiazolidinediones, and women with diabetes but not using metformin or thiazoldinediones, and found a similar pattern of associations. (results not shown).
Table 3.
Multivariable adjusted model for hepatic steatosis among participants in the Michigan Study of Women’s Health Across the Nation
| Characteristic | Adjusted odds ratio, 95% confidence interval |
|---|---|
| Age (years) | 1.12 (1.01–1.23) |
| African-American with no metformin/thiazolidinedione use | Reference |
| African-American with metformin/thiazolidinedione use | 0.41 (0.14–1.15) |
| Non-Hispanic white women with no metformin/thiazolidinedione use | 1.41 (0.76–2.64) |
| Non-Hispanic white women with metformin/thiazolidinedione use | 3.36 (1.07–10.58) |
| Waist circumference (cm) | 1.03 (1.01–1.05) |
| HDL cholesterol (mmol/l) | 0.99 (0.96–1.01) |
| Triglycerides (mmol/l) | 1.00 (0.99–1.01) |
| Alcoholic drinks per day | |
| < 1 | Reference |
| 1 or more | 0.58 (0.31–1.07) |
| Sex hormone binding globulin (nM/l) | |
| Tertile 1: 10.5–40.3 (nM/l) | Reference |
| Tertile 2: 40.5–59.5 (nM/l) | 0.64 (0.34–1.22) |
| Tertile 3: 60.2–220.3 (nM/l) | 0.42 (0.19–0.91) |
Discussion
In a community-based cohort of post-menopausal women, we found that hepatic steatosis, a strong risk factor for diabetes, was common on ultrasound. Nearly one quarter of African-Americans and just over one third of non-Hispanic white women were affected. Increased SHBG, the primary binding protein of sex hormones and a risk factor for diabetes, was strongly associated with decreased odds of hepatic steatosis in both African-Americans and non-Hispanic white women.
One explanation for these findings is that SHBG is manufactured by the liver, and it is possible that steatotic livers produce less SHBG, and that SHBG and hepatic steatosis have a common antecedent such as visceral adiposity or insulin resistance [3]. Among pre-menopausal women with polycystic ovarian syndrome, SHBG has been associated with mesenteric fat thickness [26] and mesenteric fat (which drains into the portal circulation) and was more strongly associated with fatty liver than anthropometric measures of fat or preperitoneal fat [26]. Also among women with polycystic ovarian syndrome, SHBG is associated with hepatic steatosis even after adjustment for visceral steatosis [27]. Among approximately 155 pre- and post-menopausal Korean women, lower SHBG was associated with hepatic steatosis on ultrasound after adjustment for waist circumference, hypoglycaemic medications and fasting glucose and insulin [28]. However, a report in men did not find that SHBG correlated with intrahepatic fat or hepatokines (fetuin A and FGF21) [4], even as SHBG correlated with peripheral glucose. Therefore, it is also possible that SHBG is associated with hepatic steatosis, particularly in the setting of elevated glucose, and a significant proportion of women in our study had adverse metabolic profiles, or it is also possible that the association between SHBG and hepatic steatosis differs between women and men.
Two other population-based studies of hepatic steatosis prevalence among women conflict regarding racial/ethnic differences. The Dallas Heart Study reported that non-Hispanic white women and African-Americans had a similar prevalence of hepatic steatosis [29]. In contrast, the National Heart, Lung, and Blood Institute (NHLBI) Family Heart Study reported that non-Hispanic white women had a greater prevalence of hepatic steatosis than African-Americans [16]. Our participants were approximately 60 years of age, NHLBI Family Heart Study participants were approximately 56 years of age and Dallas Heart Study participants were approximately 47 years of age. Therefore it is possible that greater racial/ethnic differences in hepatic steatosis exist primarily among older, post-menopausal women. Although we had also hypothesized that racial/ethnic differences might be at least partially explained by racial/ethnic differences in sex steroids post-menopause, adjustment for SHBG and also adjusting for oestrogen therapy did not explain the association between race/ethnicity and hepatic steatosis (data not shown) and the inclusion of oestrogen therapy and sex steroids in multivariate models did not improve model fit.
As in other population-based reports [16,29], African-Americans in the Michigan Study of Women’s Health Across the Nation had lower triglycerides than non-Hispanic white women, consistent with racial/ethnic differences in fatty acid metabolism and hepatic triglyceride deposition [30]. A genetic variant of PNPLA3 [rs6006460(T)] has been associated with lower hepatic fat among African-Americans, while the PNPLA3 rs738409 G susceptibility allele has been associated with hepatic steatosis among non-Hispanic white women [31,32]. Unlike other variants, PNPLA3 does not affect any other metabolic traits, but may increase hepatic steatosis by preventing hepatic breakdown of triglycerides [32]. While the association is strong, not all persons with the susceptibility alleles have hepatic steatosis, despite the additional presence of adverse risk factors such as obesity [32]. To our knowledge, interactions between these polymorphisms and medication effectiveness have not been examined. Medications previously reported to reduce hepatic adiposity, particularly the hypoglycaemic medications of metformin and thiazolidinediones, were associated with increased odds of hepatic steatosis in non-Hispanic white women, but not in African-Americans. Medication use may have been a proxy for greater insulin resistance, as HbA1c values were higher among women using medications than among women not using medications. Medications, combined with other factors in the model such as SHBG, waist circumference, and race/ethnicity, captured a greater proportion of variance than models with insulin resistance, perhaps because medication prescription may also capture other steatosis risk factors such as inflammation [33]. However, it is unclear why the association between medication use and greater prevalence of hepatic steatosis would be stronger in non-Hispanic white women than in African-Americans. Although we did not find racial/ethnic differences in self-reported use of medications, medications may interact with other risk factors that do differ by race/ethnicity, such as triglycerides or these polymorphisms [34].
Our study has several strengths. We examined a well-characterized population-based cohort of women using a measure of hepatic steatosis that is commonly used in clinical practice and may be applied in large-scale epidemiologic studies. Unlike other epidemiologic studies of hepatic steatosis among diverse samples of women, the African-Americans and non-Hispanic white women in our population were similar with respect to known risk factors for hepatic steatosis, thereby reducing the likelihood of confounding by these measures. The Michigan Study of Women’s Health Across the Nation population includes excellent ascertainment of known risk factors, including health history, cardiometabolic biomarkers and sex hormone values not available in other studies. Limitations of our study include the lack of biopsy for confirmation, as this is logistically challenging for epidemiologic studies, and lack of transaminases as a peripheral marker of inflammation. We note that the spectrum of hepatic steatosis includes non-alcoholic steatotic hepatitis, but also includes more benign fatty liver disease, and thus we cannot comment about the severity of inflammation or scarring. While models examining risk factors measured from a decade previously did not reveal a different pattern of associations, we only had a single measure of hepatic steatosis. Thus, our analysis was cross-sectional and we could not determine causality. We did not have measures of visceral adiposity or insulin sensitivity based on clamp studies. Finally, the greater prevalence of hepatic steatosis in the women in the Michigan Study of Women’s Health Across the Nation as compared with other cohort studies could reflect unique characteristics of the geographical area. Of note, the Dallas study recruited from Dallas County, Texas and the NHLBI study enrolled participants from the Framingham Heart Study cohort as well as the Atherosclerosis Risk in Communities Study (Framingham, MA; Minneapolis, MN; and Forsythe County, NC).
In summary, we found that hepatic steatosis, a strong risk factor for diabetes, is common in post-menopausal women, and non-Hispanic white women had a higher prevalence than African-Americans upon ultrasound. Risk factors in African-Americans are similar to those in non-Hispanic white women and consist of greater age and adiposity as well as lower HDL cholesterol. Lower SHBG is a marker for hepatic steatosis apart from waist circumference, metformin and thiazolidinedione use. Further studies regarding the natural history of hepatic steatosis in these two groups and the impact of therapies to reduce hepatic steatosis by race/ethnicity are needed. Finally, further investigation regarding the roles of SHBG and hepatic steatosis in mediating glucose tolerance are needed in populations that are racially and ethnically diverse.
What’s new?
Although hepatic steatosis is common in post-menopausal women, previous studies have not examined risk factors in this population, particularly sex steroids and sex hormone binding globulin.
We report that increased sex hormone binding globulin, the primary binding protein of sex hormones and a risk factor for diabetes, was strongly associated with decreased odds of hepatic steatosis in both race/ethnicities.
Acknowledgments
Funding sources
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). This work used the Michigan Diabetes Research and Training Center funded by grant number P60DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases. This work was also supported by K23DK071552 and R0DK083297.
Clinical Centres: University of Michigan, Ann Arbor—Siobán Harlow, principal investigator (PI) 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009—present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011—present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Programme Office: National Institute on Aging, Bethesda, MD—Sherry Sherman 1994–present; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Centre: University of Pittsburgh, Pittsburgh, PA—Kim Sutton-Tyrrell, PI 2001–present; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
The authors thank the study staff at each site. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. This work was presented in part at the Endocrine Society Annual Meeting in Houston, Texas, June 2012.
Abbreviations
- SHBG
sex hormone binding globulin
Footnotes
Competing interests
None declared.
References
- 1.Utzschneider K, Kahn S. The role of insulin resistance in non-alcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 2.Sung K, Jeong W, Wild S, Byrne C. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35:717–722. doi: 10.2337/dc11-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peter A, Kantartzis K, Machann J, Shick F, Staiger H, Machicao F, et al. Relationship of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59:3167–3173. doi: 10.2337/db10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet F, Cephise F, Gautier A, Dubois S, Massart C, Camara A, et al. Role of sex steroids, intra-hepatic fat and liver enzymes in the association between SHBG and metabolic features. Clin Endocrinol (Oxf) 2012;XX:XXX–XXX. doi: 10.1111/cen.12089. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Jeon W, Kim S, Kim H, Park D, Cho Y, et al. Prevalence and risk factors of non-alcholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 6.Volzke H, Schwarz S, Baumeister S, Wallaschofski H, Schwahn C, Grabe H, et al. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56:594–595. doi: 10.1136/gut.2006.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers M, Zheng H, McConnell D, Nan B, Karvonen-Gutierrez C, Randolph J., Jr Testosterone, sex hormone-binding globulin, and free-androgen index among adult women: chronological and ovarian aging. Hum Reprod. 2009;24:2276–2285. doi: 10.1093/humrep/dep209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen I, Powell L, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark J, Diehl A. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124:248–250. doi: 10.1053/gast.2003.50032. [DOI] [PubMed] [Google Scholar]
- 10.Wagenknecht L, Scherzinger A, Stamm E, Hanley A, Norris J, Chen Y, et al. Correlates and heritability of non-alcoholic fatty liver disease in a minority cohort. Obesity. 2009;17:1240–1246. doi: 10.1038/oby.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph J, Jr, Zheng H, Sowers M, Crandall C, Crawford S, Gold E, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96:746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setiawan V, Pike M, Kolonel L, Nomura A, Goodman M, Henderson B. Racial/ethnic differences in endometrial cancer risk: the Multiethnic Cohort Study. Am J Epidemiol. 2006;165:262–270. doi: 10.1093/aje/kwk010. [DOI] [PubMed] [Google Scholar]
- 13.McTiernan A, Wu L, Barnabei V, Chen C, Hendrix S, Modugno F, et al. Relation of demographic factors, menstrual history, reproduction and medication use to sex hormone levels in postmenpausal women. Breast Cancer Res. 2008;108:217–231. doi: 10.1007/s10549-007-9588-6. [DOI] [PubMed] [Google Scholar]
- 14.Golden S, Dobs A, Vaidya D, Szklo M, Gapstur S, Kopp P, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero R, Vega G, Grundy S, Browning J. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.North K, Graff M, Franceschini N, Reiner A, Feitosa M, Carr J, et al. Sex and race differences in the prevalence of fatty liver disease as measured by computed tomography liver attenuation in European-American and African-American participants of the NHLBI Family Heart Study. Eur J Gastroenterol Hepatol. 2012;24:9–16. doi: 10.1097/MEG.0b013e32834a94fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowers M, Derby C, Jannausch M, Torrens J, Pasternak R. Insulin resistance, hemostatic factors, and hormone interactions in pre- and perimenopausal women: SWAN. J Clin Endocrinol Metab. 2003;88:4904–4910. doi: 10.1210/jc.2003-030350. [DOI] [PubMed] [Google Scholar]
- 18.Sutton-Tyrrell K, Wildman R, Matthews K, Chae C, Lasley B, Brockwell S, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 19.Sowers M, McConnell D, Jannausch M, Randolph J, Brook R, Gold E, et al. Oestrogen metabolites in relation to isoprostanes as a measure of oxidative stress. Clin Endocrinol (Oxf) 2008;68:806–813. doi: 10.1111/j.1365-2265.2007.03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson R, Pratley R, Bogardus C, Narayan K, Roumain J, Imperatore G, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 21.Randolph J, Jr, Sowers M, Gold E, Mohr B, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 22.Price J, Reynolds R, Mitchell R, Williamson R, Fowkes F, Deary I, et al. The Edinburgh Type 2 Diabetes Study: study protocol. BMC Endocr Disord 2008. 2008 Dec 11;XX:XXX–XXX. doi: 10.1186/1472-6823-8-18. pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson R, Price J, Glancy S, Perry E, Nee L, Hayes P, et al. Prevalence of and risk factors for hepatic steatosis and non-alcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139–1144. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism, and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 25.Akaike H. A new look at the Bayes procedure. Biometrika. 1978;65:53–59. [Google Scholar]
- 26.Ma R, Liu K, Lam P, Cheung L, Tam W, Ko G, et al. Sonographic measurement of mesenteric fat predicts presence of fatty liver among subjects with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:799–807. doi: 10.1210/jc.2010-1608. [DOI] [PubMed] [Google Scholar]
- 27.Jones H, Sprung V, Pugh C, Daousi C, Irwin A, Aziz N, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to non-hyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 28.Shin J, Kim S, Lee M, Kim H, Ye B, Shin Y, et al. Serum sex hormone-binding globulin levels are independently associated with non-alcoholic fatty liver disease in people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:156–162. doi: 10.1016/j.diabres.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Browning J, Szczepaniak L, Dobbins R, Nuremberg P, Horton J, Cohen J, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 30.Brilakis E, Khera A, McGuire D, See R, Banerjee S, Murphy S, et al. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: observations from the Dallas Heart Study. Atherosclerosis. 2008;199:110–115. doi: 10.1016/j.atherosclerosis.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio L, et al. Genetic variation in PNPLA3 confers susceptibility to non-alcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speliotes E, Yerges-Armstrong L, Wu J, Hernaez R, Kim L, Palmer C, et al. Genome-wide association analysis identifies variants associated with non-alcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matafome P, Louro T, Rodrigues L, Crisostomo J, Nunes E, Amaral C, et al. Metformin and atorvastatin combination further protect the liver in type 2 diabetes with hyperlipidaemia. Diabetes Metab Res Rev. 2011;27:54–62. doi: 10.1002/dmrr.1157. [DOI] [PubMed] [Google Scholar]
- 34.Nestler J. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]