Abstract
Background
The benefits of integrating substance abuse and psychiatric care may be limited by poor service utilization. This randomized clinical trial evaluated the efficacy of using contingency management to improve utilization of psychiatric services co-located and integrated within a community-based methadone maintenance treatment program.
Methods
Opioid-dependent outpatients (n = 125) with any current psychiatric disorder were randomly assigned to: 1) reinforced on-site integrated care (ROIC), with vouchers (worth $25.00) contingent on full adherence to each week of scheduled psychiatric services; or 2) standard on-site integrated care (SOIC). All participants received access to the same schedule of psychiatrist and mental health counseling sessions for 12-weeks.
Results
ROIC participants attended more overall psychiatric sessions at month 1 (M = 7.53 vs. 3.97, p < .001, month 2 (M = 6.31 vs. 2.81, p < .001, and month 3 (M = 5.71 vs. 2.44, p < .001). Both conditions evidenced reductions in psychiatric distress (p < .001) and similar rates of drug-positive urine samples. No differences in study retention were observed.
Conclusions
These findings suggest that contingency management can improve utilization of psychiatric services scheduled within an on-site and integrated treatment model. Delivering evidenced-based mental health counseling, or modifying the contingency plan to include illicit drug use, may be required to facilitate greater changes in psychiatric and substance abuse outcomes.
Keywords: methadone maintenance, psychiatric comorbidity, treatment adherence, contingency management
1. INTRODUCTION
The high prevalence of psychiatric disorders in people with substance use disorder (Brooner et al., 1997; McGovern et al., 2006), and the correspondingly low rates of psychiatric service utilization in this population (McGovern et al., 2006; Pringle et al., 2006), has facilitated study of on-site integrated treatment models (Donald et al., 2005; Sacks et al., 2008a). While integrated care models can improve access to enhanced and specialized psychiatric services by delivering them in a single treatment setting, available research has produced mostly equivocal results, with only some studies demonstrating even modest effects on psychiatric or drug use outcomes (see Donald et al., 2005, for a review). A potential explanation for these disappointing findings is the limited scope and utilization of on-site psychiatric services reported in the literature, which may limit the potential effectiveness of integrated care approaches (Donald et al., 2005; Sacks et al., 2008b).
Poor service utilization, which has been implicated as a primary reason for partial and poor response to a wide range of medical and psychiatric interventions (Sabate, 2003), is also apparent in the small number of published studies offering integrated services within substance abuse treatment settings (e.g., Bowen et al., 2000; Randall et al., 2001). Lydecker et al. (2010), for example, recently evaluated an ambitious 6-month protocol combining cognitive-behavioral group therapy and medication management for alcohol dependent individuals with comorbid depression. These authors found that group sessions were attended considerably less often than medication management appointments, and that both integrated and non-integrated protocols demonstrated reductions in depression symptoms. Utilization of scheduled services was positively associated with reduced depressive symptoms and drug use, suggesting that improving attendance rates in integrated care models might have good collateral effects on both psychiatric and substance use outcomes.
Engaging opioid dependent individuals in psychiatric care may be particularly challenging because they are often poorly adherent to even routine substance abuse services (see Kidorf et al., 2006, for a review). Brooner et al. (2004), for example, showed that opioid-dependent individuals randomly assigned to a control group that was offered both routine and intensified substance abuse care attended less than half of all scheduled services, with adherence considerably worse for any sessions offered supplemental to routine individual counseling. Low rates of utilization is also commonly found in studies evaluating the efficacy of specialized individual or group therapies (e.g., cognitive-behavioral or 12-step facilitation therapies) added to routine methadone maintenance practices (Carroll et al., 2012; Rawson et al., 2002).
Service delivery strategies that result in opioid abusers receiving a higher dose of prescribed services are likely to produce substantially better outcomes. One potential mechanism for improving adherence to scheduled services is contingency management, a motivational intervention that uses behavioral reinforcement principles to encourage behavior change (Higgins et al., 2004). Contingency management has been used most frequently within substance-dependent populations to reduce drug use (Lussier et al., 2006), and a growing literature supports its use for increasing treatment participation (e.g., attendance behavior). Voucher-based incentives as a delivery platform for behavioral reinforcement have shown efficacy in improving rates of substance abuse treatment enrollment (Kidorf et al., 2009), attendance to individual and group therapy (Helmus et al., 2003; Jones et al., 2001), and adherence to medication regimens (Sorensen et al., 2007).
The present study provides data from the first known randomized clinical trial evaluating the efficacy of voucher-based reinforcement to improve attendance to specialized psychiatric services integrated within an outpatient program for opioid-dependent patients. Opioid-dependent participants receiving methadone maintenance in a community-based program were provided co-located and integrated psychiatric care that included psychiatrist appointments, individual and group mental health counseling sessions, and good access to prescribed psychiatric medications for 12-weeks. Psychiatric care was integrated primarily by having substance abuse clinical staff deliver the psychiatric treatment protocol. One half of the participants received voucher reinforcement (i.e., $25.00) for each week they attended all scheduled psychiatric treatment services. The reinforced attendance condition was expected to attend more of their scheduled counseling and psychiatrist appointments, achieve greater reductions in psychiatric distress, and submit lower proportions of drug-positive urine samples.
2. METHOD
2.1 Participants
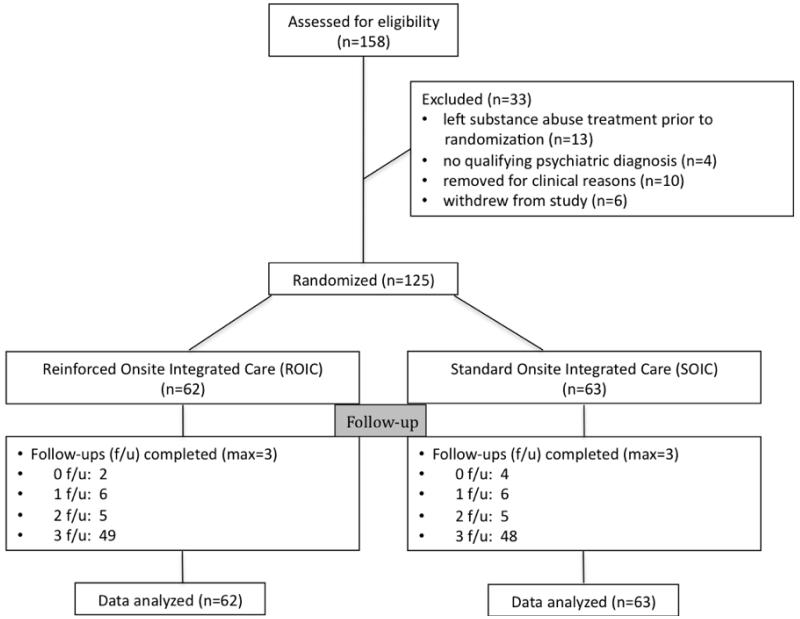
Study participants were 125 opioid-dependent outpatients enrolled in a community-based opioid-agonist clinic (see Consort Diagram, Figure 1). Participants were recruited from 12/15/09 to 4/30/12. Forty percent (n = 50) of participants were new admissions; the remainder (n = 73) had been in the program for at least 60-days. Patients were study eligible if they met DSM-IV-R criteria for a current psychiatric diagnosis and expressed interest in receiving psychiatric treatment offered within the program. Patients were not eligible for the study if they were pregnant, had an acute medical problem that required immediate and intense medical management, or had cognitive impairment that interfered with comprehension of study procedures. The study was approved by the Johns Hopkins University Institutional Review Board.
Figure 1.
Consort Diagram
Table 1 shows that the most participants were Caucasian and female, and the mean age at study enrollment was 39.1 years. Major depression was the most prevalent DSM-IV Axis I psychiatric disorder, followed by Post Traumatic Stress Disorder, and Panic Disorder. Over 40% of the sample was diagnosed with Antisocial Personality Disorder, and 44% of the urine samples collected during the one-month baseline tested positive for at least one illicit drug. Comparisons of randomized (n = 125) and non-randomized (n = 33) enrollees on demographic variables found only one significant difference -- fewer randomized participants were classified as new admissions (40% vs. 61%; χ2 = 4.49, p = 0.03).
Table 1.
Baseline demographics, psychiatric and substance use disorders1, urinalysis results, and prescribed medications2
| Characteristic | Overall (n=125) |
ROIC3 (n=62) |
SOIC3 (n=63) |
χ2 or t-test (df) | p-value |
|---|---|---|---|---|---|
|
| |||||
| M (SD) or % | M (SD) or % | M (SD) or % | |||
|
| |||||
| Demographics | |||||
|
| |||||
| Gender (%) | |||||
| Male | 46.4% | 50.0% | 42.9% | χ2=0.64 | 0.42 |
| Female | 53.6% | 50.0% | 57.1% | ||
|
| |||||
| Race (%) | |||||
| White | 64.8% | 64.5% | 65.1% | χ2=1.61 | 0.65 |
| Minority4 | 35.2% | 35.5% | 34.9% | ||
|
| |||||
| Age (years) | 39.1 (10.2) | 39.4 (10.4) | 38.9 (10.1) | t=0.32 | 0.74 |
|
| |||||
| Education (highest grade completed) | 11.5 (2.2) | 11.5 (2.4) | 11.5 (1.9) | t= 0.14 | 0.88 |
|
| |||||
| Married (%) | 24.8% | 22.6% | 27.0% | χ2=0.32 | 0.56 |
|
| |||||
| Employed (%) | 20.0% | 14.5% | 25.4% | χ2=2.31 | 0.12 |
|
| |||||
| New admission (< 60 days in treatment) | 40.0% | 35.5% | 44.4% | χ2=1.05 | 0.31 |
|
| |||||
| Psychiatric Disorders 1 | |||||
|
| |||||
| Current Axis I disorder | 100.0% | 100.0% | 100.0% | -- | -- |
|
| |||||
| Current psychotic disorder | 2.4% | 4.8% | 0% | * | * |
| Schizophrenia | -- | -- | 0% | * | * |
| Schizoaffective disorder | 2.4% | 4.8% | 0% | * | * |
|
| |||||
| Current mood disorder | 72.8% | 71.0% | 74.6% | χ2=0.20 | 0.64 |
| Major Depression | 36.0% | 33.3% | 40.3% | χ2=1.09 | 0.29 |
| Bipolar I | 17.6% | 24.2% | 11.1% | χ2=3.68 | 0.05 |
| Dysthymia | 14.4% | 14.5% | 14.3% | χ2=0.00 | 0.97 |
|
| |||||
| Current anxiety disorder | 59.2% | 61.3% | 57.1% | χ2=0.22 | 0.64 |
| Posttraumatic stress disorder | 29.6% | 25.8% | 33.3% | χ2=0.64 | 0.42 |
| Panic disorder | 17.6% | 17.7% | 17.5% | χ2=0.00 | 0.96 |
| Generalized anxiety disorder | 10.4% | 12.9% | 7.9% | χ2=0.82 | 0.36 |
|
| |||||
| Axis II disorder | 63.2% | 62.9% | 63.5% | χ2=0.01 | 0.95 |
| Antisocial personality disorder (APD) | 41.6% | 41.9% | 41.3% | χ2=0.00 | 0.93 |
| Other Axis II (not including APD) | 44.8% | 38.7% | 50.8% | χ2=1.84 | 0.17 |
|
| |||||
| Substance Use Disorders 1 | |||||
|
| |||||
| Alcohol | 6.4% | 4.8% | 7.8% | * | * |
| Sedative | 15.2% | 12.9% | 17.5% | χ2=0.45 | 0.50 |
| Cocaine | 16.8% | 21.0% | 12.7% | χ2=1.52 | 0.21 |
|
| |||||
| Urinalysis Results | |||||
|
| |||||
| Opioid-positive (%) | 16% (0.27) | 15% (0.26) | 16% (0.30) | t= 0.27 | 0.78 |
| Cocaine-positive (%) | 18% (0.32) | 20% (0.32) | 16% (0.31) | t=0.73 | 0.46 |
| Sedative-positive (%) | 16% (0.29) | 15% (0.28) | 16% (0.31) | t=0.26 | 0.79 |
| Any-positive (%) | 43% (0.41) | 45% (0.41 | 42% (0.42) | t=0.40 | 0.69 |
|
| |||||
| Prescribed Medications | |||||
|
| |||||
| Substance abuse | |||||
| Methadone | 100.0% | 100.0% | 100.0% | -- | -- |
| Methadone dose | 84.6 (23.3) | 89.2 (23.7) | 80.0 (22.2) | t=2.23 | 0.03 |
|
| |||||
| Psychiatric | |||||
| Any psychiatric medication | 93.6% | 98.4% | 88.9% | * | * |
| Heterocyclic antidepressants | 38.4% | 38.7% | 38.1% | χ2=0.00 | 0.94 |
| SSI antidepressants | 52.0% | 54.8% | 49.2% | χ2=0.39 | 0.52 |
| Unspecified antidepressants | 31.2% | 30.6% | 31.7% | χ2=0.01 | 0.89 |
| Mood stabilizers | 18.4% | 22.6% | 14.3% | χ2=1.43 | 0.23 |
| Anti-anxiety medications | 16.0% | 17.7% | 14.3% | χ2=0.27 | 0.59 |
| Typical antipsychotics | 8.8% | 11.3% | 6.3% | χ2=0.95 | 0.33 |
| Atypical antipsychotics | 14.4% | 17.7% | 11.1% | χ2=1.11 | 0.29 |
Diagnoses determined using the Structured Clinical Interview for the DSM-IV. With the exception of psychotic and alcohol use disorders, only psychiatric disorders prevalent in at least 10% of the sample are included.
Substance abuse and psychiatric medications. Participants were often prescribed more than one psychiatric medication.
ROIC: Reinforced Onsite Integrated Care; SOIC: Standard Integrated Onsite Care
Most minority participants (n = 44) were African American (n = 32; 73%) or Hispanic (n = 5; 11%).
Due to low cell counts, chi square results would be invalid
2.2 Assessments
Participants completed the Structured Clinical Interview for the DSM-IV (SCID-I and SCID-II; First et al., 1995) during the second week of baseline. The SCID-I is a structured interview that uses a decision-tree approach for determining diagnoses of many DSM-IV Axis I psychiatric disorders; the SCID-II was used for making diagnoses of Axis II personality disorders. The SCID can be used to distinguish independent and substance-induced psychiatric disorders, though in the present study only one participant was diagnosed with a substance-induced disorder (i.e., major depression). Participants receiving a psychiatric diagnosis were clinically reappraised by one of the study investigators, who also screened participants for suicidal ideation, thought disorder, delusions, and hallucinations. The Addiction Severity Index (ASI; McLellan et al., 1992, 2006) was administered at baseline and monthly to assess problem severity in seven areas commonly affected by substance use. The Hopkins Symptom Checklist - Revised (SCL-90-R; Derogatis, 1983; Derogatis and Cleary, 1977) was also administered at baseline and monthly to measure self-reported psychiatric distress (using a 0-4 Likert Scale) across 90-items and 9-subscales (e.g., depression, anxiety). The present study used the Global Severity Index (GSI) score, which is the average rating given to all 90 items and correlates highly to the individual scales. Finally, the Self-Report Measure of Medication Adherence (SMMA; Morisky et al., 1986) was administered monthly to assess adherence to prescribed psychiatric medications. The SMMA uses a 4-point Likert Scale, with lower scores indicating better adherence. Study interviewers completed an intensive training protocol to establish and sustain good inter-rater reliability (Kidorf et al., 2004).
Participants submitted urine samples for testing once per week using a modified random schedule (Monday, Wednesday, or Friday). Urine samples were obtained under direct observation (through a one-way mirror) and tested at a certified laboratory that employed TLC and EMIT testing for the presence of opioids, cocaine, and benzodiazepines. Most participants (78%; n = 97) completed all three monthly assessment follow-ups. No condition differences were observed in mean number of follow-ups completed (ROIC: M = 2.62 (SD = .79) vs. SOIC: M = 2.53 (SD = .91); t = .58, p = .56). Participants were paid $40.00 for completion of the baseline assessment battery, and $15.00 for completing each follow-up assessment.
2.3 Procedure
Participants were randomly assigned (using a computerized randomization program) to one of two study conditions for 12-weeks: reinforced on-site integrated care (ROIC) versus standard on-site integrated care (SOIC). ROIC participants received voucher-based incentives ($25.00 per week) for each week they attended all of their scheduled psychiatric sessions. The maximum amount of voucher incentives that a participant could earn was $300.00. These monetary-based vouchers could be exchanged any time during the study for goods and services in the community and were purchased by a research assistant. SOIC participants were not provided the opportunity to earn voucher incentives.
Participants in each condition were offered an identical set and schedule of psychiatric services in the program, integrated within the substance abuse treatment by having the same staff treating their substance use disorder providing psychiatric care. The psychiatric service schedule included individual psychiatrist appointments (usually scheduled once every 2 weeks), individual mental health counseling sessions (once per week), and group mental health education and support sessions (once per week). The research assistant helped participants in each condition schedule their first psychiatrist appointment and individual and group mental health counseling sessions.
Board-certified psychiatrists in the ATS program used the first session to confirm the SCID diagnosis and formulate the initial care plan. Individual mental health counseling was conducted by the participant’s primary substance abuse counselor, who was state certified as a Professional Counselor. These sessions lasted about 50 minutes and were scheduled weekly in addition to the participant’s routine substance abuse counseling schedule. Counselors used a case management approach that employed empathic and psycho-educational strategies to help participants reach goals established in the treatment plan, monitor psychiatric symptoms, discuss possible interactions between their psychiatric and substance use diagnoses, and encourage adherence to prescribed psychiatric medications (Center for Substance Abuse Treatment, 2005). The weekly mental health education and support group was led by doctoral staff and lasted 60 minutes. The group sessions provided education about the psychiatric diagnoses and their symptom expression and interactions with substance use symptoms and disorder, monitored and discussed patient expectations for psychiatric medications, response to the medications and overall adherence with their prescribed use, and provided both cognitive behavioral and supportive interventions to help participants manage symptoms and maximize functioning.
2.4 Access to prescribed psychiatric medications
All participants had good access to prescribed psychiatric medications. The study paid for psychiatric medications for those participants without insurance coverage for their medications. Most participants (94%) were started on at least one psychiatric medicine (see Table 1).
2.5 Staff training and fidelity to psychiatric treatment protocol
Substance abuse staff attended seminars on the treatment of substance users with co-occurring disorders, led by study investigators (MK, RKB, VK) and other faculty members. Senior staff provided routine clinical supervision using standards published by the Center of Substance Abuse Treatment (CSAT, 2005). Psychiatrists and counselors completed forms each week to document all treatment contacts. These forms were given to research support staff that monitored adherence to the treatment protocol on a weekly basis. Urinalysis results for participants were distributed weekly to all treatment staff.
T-tests were used to compare ratios of scheduled psychiatric sessions per month to assess fidelity to delivery of the psychiatric treatment protocol. ROIC and SOIC participants were scheduled to attend a similar number of individual (ROIC: M = 3.66; SD = 0.99 vs. SOIC: M = 3.60; SD = 1.09; t = .54, p = .58), group (ROIC: M = 3.70; SD = .97 vs. SOIC: M = 3.66; SD = 1.04; t = .41, p = .67), and psychiatrist (ROIC: M = 2.41; SE = 1.12 vs. SOIC: M = 2.34; SD = 1.30, t = .56, p = .57) sessions during the study.
2.6 Routine substance abuse treatment
All participants received the same scope and schedule of routine substance abuse treatment in the program, which included daily methadone administration and an adaptive stepped care counseling approach that varied the number of weekly substance abuse counseling sessions based on substance abuse treatment response (Brooner et al., 2004). No condition differences were observed in scheduled (M = 9.03; SD = 9.10) or attended (M = 5.87; SD = 6.36) substance abuse counseling sessions during the study.
2.7 Data analyses
Chi-square and t-tests were used to compare ROIC and SOIC conditions on demographics, SCID-based current psychiatric and substance use disorder diagnoses, and baseline urinalysis results. A t-test was used to compare conditions on number of days participants remained in treatment post-randomization, and a chi-square test was used to compare conditions on study completion. T-tests were used to compare conditions on scheduled and attended mental health and substance use sessions, and SRMS scores. Mixed models were used to compare conditions on SCL-90 GSI scores and urinalysis results; significant main effects were followed by post-hoc Tukey-Kramer tests. Sensitivity analyses (not reported in the manuscript) using the baseline variables of pre-study treatment days, methadone dose, SCL-90 GSI scores, and ASI Psychiatric scores as covariates yielded no differences in the study results. Finally, a post-hoc multiple regression model was used to evaluate the relationship between attendance to mental health sessions and GSI scores at each time point, followed by a mixed-model analysis controlling for baseline methadone dose and baseline GSI scores.
3. RESULTS
3.1 Baseline characteristics
No condition differences were observed for demographics, new admission status, prevalence of substance use disorders, or urinalysis results. ROIC participants were more likely diagnosed with Bipolar Disorder, and had a higher mean methadone dose.
3.2 Study retention
ROIC and SOIC participants remained in psychiatric treatment for a similar number of days (ROIC: M = 77.1; SD = 16.3 vs. SOIC: M = 77.3; SD = 16.6; t = .07, p = .94); 82.2% of ROIC participants and 82.5% of SOIC participants completed the 12-week randomized study (χ2 = .001, p = .96).
3.3 Mental health service utilization
As shown in Table 2, ROIC participants attended more individual and group mental health sessions each study month. ROIC participants attended more psychiatrist sessions during Months 1 and 2, but not Month 3. ROIC participants earned a mean of $166.53 (SD = 90.76) during the study for adherence to the psychiatric protocol; 94% (n = 58) earned at least one voucher.
Table 2.
Mean number of individual, group, and psychiatrist sessions attended at Months 1, 2, and 3
| Sessions | ROIC1 (n = 62) |
SOIC1 (n = 63) |
t | p |
|---|---|---|---|---|
|
| ||||
| M (SD) | M( SD) | |||
|
| ||||
| Individual | ||||
| Month 1 | 2.4 (1.2) | 1.4 (1.4) | 4.52 | <.001 |
| Month 2 | 2.3 (1.3) | 1.2 (1.5) | 4.38 | <.001 |
| Month 3 | 2.2 (1.5) | 1.0 (1.3) | 4.94 | <.001 |
|
| ||||
| Group | ||||
| Month 1 | 2.5 (1.3) | 0.4 (0.7) | 11.00 | <.001 |
| Month 2 | 2.2 (1.4) | 0.3 (0.8) | 9.28 | <.001 |
| Month 3 | 2.2 (1.7) | 0.2 (0.6) | 8.91 | <.001 |
|
| ||||
| Psychiatrist | ||||
| Month 1 | 2.6 (0.9) | 2.2 (1.1) | 2.48 | 0.01 |
| Month 2 | 1.9 (1.1) | 1.4 (1.1) | 2.57 | 0.01 |
| Month 3 | 1.4 (1.0) | 1.3 (1.2) | 0.34 | 0.73 |
|
| ||||
| Overall | ||||
| Month 1 | 7.5 (2.7) | 4.0 (2.4) | 7.80 | <.001 |
| Month 2 | 6.3 (3.1) | 2.8 (2.5) | 6.96 | <.001 |
| Month 3 | 5.7 (3.7) | 2.4 (2.5) | 5.83 | <.001 |
ROIC: Reinforced Onsite Integrated Care; SOIC: Standard Onsite Integrated Care
3.4 Medication administration and adherence
Most participants were prescribed psychiatric medications; no condition differences were observed in the class of medications administered (see Table 1). Participants in both conditions reported good adherence to psychiatric medications. However, ROIC participants reported lower SRMS scores (i.e., more adherence with psychiatric medications) at Month 1 (ROIC: M = .55, SD = .61 vs. SOIC: M = .89, SD = .92; t = 2.22, p = .02) and Month 3 (ROIC: M = .50, SD = .72 vs. SOIC: M = .94, SD = .88; t = 2.52, p = .01), but not at Month 2 (ROIC: M = .54, SD = .66 vs. SOIC: M = .82, SD = .95; t = 1.66, p = .09).
3.5 Psychiatric distress
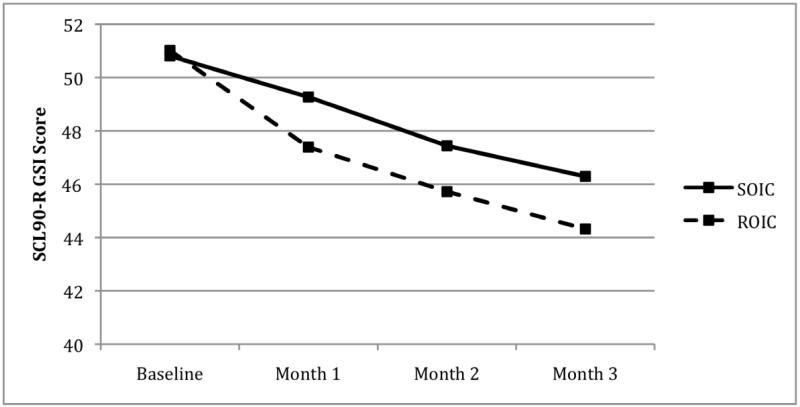
As shown in Figure 2, participants in both studies showed reductions in SCL-90-R GSI scores over time (F(3, 317) = 15.54, p < .001). Tukey-Kramer post-hoc tests showed that GSI scores at all three time points were significantly lower than baseline, and Month 3 GSI scores were significantly lower than Month 1 GSI scores. No condition differences (F(1, 317) = .56, p = .45) or condition × time interactions (F(3, 317) = 1.10, p = .39) were observed. Post-hoc multiple regression analyses showed non-significant relationships between mental health session attendance and GSI scores at month 1 (F = .00, p = .97), month 2 (F = .04, p = .84, and month 3 (F = 1.31, p = .25). A mixed model analysis, controlling for the effect of baseline methadone dose and GSI scores, was also not significant (F = .00, p = .97).
Figure 2.
Changes in SCL-90-R Global Severity Index (GSI) scores over time in the Standard Onsite Integrated Condition (SOIC) and Reinforced Onsite Integrated Condition (ROIC).
3.6 Substance use outcomes
No condition (F(1, 345) = 0.00, p = .96), time (F(3, 345) = 0.79, p = .50), or condition × time (F(3, 345) = 0.23, p = .88) effects were observed for opioid-positive urine samples. No condition (F(1, 345) = 2.05, p = 0.15), time (F(3, 345) = 2.53, p = .06), or condition × time (F (3, 345) = 0.77, p = 0.51) effects were observed for cocaine-positive urine samples. While no condition (F(1, 345) = 0.05, p = 0.82) or condition × time (F(3, 345) = 0.25, p = .86) effects were observed for sedative-positive urine samples, a time effect (F(3, 345) = 2.96, p = 0.03) showed that the proportion of sedative positive samples increased somewhat from Month 1 (M = .16) to Month 3 (M = .18). No main effects or interactions were observed for any of the ASI composite scores.
4. DISCUSSION
This is the first known randomized trial evaluating the efficacy of contingency management for improving the delivery of onsite and integrated psychiatric services in a sample of opioid-dependent outpatients with comorbid psychiatric disorder. A $25/week voucher incentive was associated with almost a 2-fold increase in overall rates of attendance to scheduled psychiatric treatment sessions. The efficacy of contingency management was revealed most powerfully in utilization of individual and group counseling, interventions that are routinely poorly attended by substance users with or without psychiatric comorbidity (Kidorf et al., 2006), but which can support pharmacological interventions by enhancing medication adherence and facilitating longer-term treatment gains (Pampallona et al., 2004).
Nevertheless, enhanced utilization of counseling services in the ROIC condition did not translate to greater reductions in psychiatric distress. Both conditions evidenced strong reductions in psychiatric distress, and had good overall rates of psychiatrist session attendance and medication adherence. This finding supports studies showing that opioid abusers can respond positively to psychiatric treatment (Nunes et al., 1998), though it is qualified due to the absence of a no-treatment comparison condition.
That the ROIC condition failed to facilitate better psychiatric outcomes than the SOIC condition has many potential explanations. Condition differences in service utilization, which totaled about three counseling sessions and one psychiatrist appointment over the 3-month study, may have been insufficient to facilitate differences in psychiatric distress. While it is possible that a higher dose of counseling in the ROIC condition may have improved outcomes, post-hoc analyses conducted across conditions showed no relationship between service utilization and psychiatric distress. Lydecker et al. (2010) demonstrated that service utilization was positively associated with better psychiatric outcomes in both integrated and parallel care models, though that study was conducted with a different population of substance users who were offered evidenced-based psychosocial treatments (i.e., cognitive-behavioral or 12-step facilitation therapy) over a longer (i.e., 6-months) trial.
In the present study, counselors were trained and supervised to deliver a case management mental health counseling strategy advocated by the Center for Substance Abuse Treatment (CSAT, 2005) and easily adaptable to community-based treatment programs. It is possible that higher intensities of a more specialized approach like CBT, which has demonstrated good effects with substance using and depressed samples (e.g., Carroll et al., 1994; Wampold et al., 2002), may have had a stronger impact on psychiatric distress outcomes in the ROIC condition. Similarly, alternative group therapy models, including those using CBT principles (Rawson et al., 2002) or significant other supports (Kidorf et al., 2005), may have been more efficacious at higher doses than a general support and education group.
Reductions in psychiatric distress had no apparent effect on drug use, and modest increases in sedative use were observed across conditions. Psychiatric and substance use disorders are two separate conditions, and the treatment of one often has little bearing on the other (Drake et al., 2008). In addition, psychiatric distress is only one of many factors affecting drug use in opioid abusers. Perhaps directing the contingency management intervention toward reducing drug use may have added to the efficacy of the ROIC condition. Studies that have reinforced both attendance and drug use have facilitated improvements in both of these outcomes in methadone maintenance settings (Brooner et al., 2007; Petry et al., 2005).
The maximum cost of attendance incentives used in this study (i.e., M = $165.00) might raise questions about feasibility in community treatment settings. It is worth noting that the significantly higher frequency of missed counseling sessions in the control condition would cost providers considerably more in billing losses than the per case cost associated with using the attendance reinforcement, in addition to the negative impact that missed sessions have on the benefits of the sessions that were received but perhaps not measured in this study. Nevertheless, a formal cost analysis is required to further advance this research. Alternative low-cost strategies to reinforce attendance to routine and intensified substance abuse counseling sessions have been used successfully in methadone maintenance programs (e.g., take-home medication and access to methadone), and these approaches might work with specialized psychiatric services (Brooner et al., 2004; Kidorf et al., 2005).
The 3-month duration of this trial is a notable study limitation. A longer trial could have evaluated the efficacy of reinforced attendance over a longer course (and higher dose) of psychiatric care and evaluated the effects of removing incentives on subsequent attendance and outcomes. The benefits of some verbal therapies in substance users are often not revealed until after the intervention is completed (Carroll et al., 1994; Rawson et al., 2002). It is also possible that psychiatric care modified behaviors or cognitions (e.g., motivation; treatment readiness) that were not measured explicitly in the present study. That about one-quarter of the enrolled sample left the study prior to randomization limits the external validity of these findings.
The study controlled for scope and frequency of psychiatric services, but neither therapy content nor prescription practices, which may have introduced unknown error variance across participants and conditions. Similarly, the use of evidenced-based psychosocial therapies may have improved the integrated care model. Finally, the present study added psychiatric care to intensive substance abuse treatment protocol to avoid dilution of services in patients with severe substance use problems. Other strategies for integrating care (e.g., Mueser et al., 2013; Watkins et al., 2011; Weiss et al., 2007) may have provided stronger reductions of psychiatric distress if offered at higher doses.
In sum, improving health care outcomes while reducing its overall cost is a challenging goal for any service provider. The health care field is responding in myriad ways to address this mounting pressure, and improving patient adherence is one potential pathway. The present findings show that a monetary-based attendance incentive resulted in patients receiving about 70% of the dose of treatment prescribed for them, though this intervention was not sufficiently powerful to improve psychiatric or substance abuse outcomes compared to a non-reinforced integrated care model. Future studies might evaluate alternative strategies of integrating contingency management and psychiatric interventions to facilitate better outcomes for this population of patients with co-occurring opioid-dependence and psychiatric disorders.
Acknowledgements
This study was supported by research grant DA028154-02 (P.I., Dr. Kidorf) from the National Institute of Health - National Institute on Drug Abuse (NIH-NIDA). NIH-NIDA had no other role other than financial support. We gratefully acknowledge the research support staff whose effort and diligence were instrumental to both the quality and integrity of the study, especially Kori Kindbom, M.A. (Research Coordinator), Michael Sklar, M.A., Rachel Burns, B.A., Jennifer Mucha, M.A., and Mark Levinson, M.A.
Role of Funding Source. Funding for this study was provided by NIH Grant DA028154-02. The NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR DISCLOSURES
Contributors. Drs. Kidorf, Brooner, King, and Peirce designed the study. Dr. Ghazarian undertook the statistical analysis. Dr. Kidorf wrote the first draft of the manuscript. Drs. Brooner, Gandotra, Antoine, King, and Peirce contributed to the implementation and conduct of the study, and edited earlier versions of this manuscript.
Conflict of Interest. No author reported a conflict of interest.
REFERENCES
- Bowen RC, D’Arcy C, Keegan D, Senthilselvan A. A controlled trial of cognitive behavioral treatment of panic in alcoholic inpatients with comorbid panic disorder. Addict Behav. 2000;25:593–597. doi: 10.1016/s0306-4603(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Brooner RK, Kidorf MS, King VL, Stoller KB, Neufeld K, Kolodner K. Comparing adaptive stepped care and monetary voucher interventions in the treatment of opioid dependent outpatients. Drug Alcohol Depend. 2007;88S:S14–S23. doi: 10.1016/j.drugalcdep.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooner RK, Kidorf MS, King VL, Stoller KB, Peirce JM, Bigelow GE, Kolodner K. Behavioral contingencies improve counseling attendance in an adaptive treatment model. J. Subst. Abuse Treat. 2004;27:223–232. doi: 10.1016/j.jsat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, Schmidt CW, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch. Gen. Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Shi J, Eagan D, Ball SA. Efficacy of disulfiram and twelve step facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo-controlled trial. Drug Alcohol Depend. 2012;126:224–231. doi: 10.1016/j.drugalcdep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin FH. One year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: delayed emergence of psychotherapy effects. Arch. Gen. Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment . Substance Abuse Treatment for Persons with Co-occurring Disorders. Rockville, MD: 2005. TIP series 42 No. DHHS Publication No. (SMA) 05-3922) [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R. Administration, Scoring, and Procedures Manual II. Clinical Psychometric Research; Baltimore, MD: 1983. [Google Scholar]
- Derogatis LR, Cleary PA. Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br. J. Soc. Clin. Psychol. 1977;16:347–356. doi: 10.1111/j.2044-8260.1977.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Donald M, Dower J, Kavanagh D. Integrated versus non-integrated management and care for clients with co-occurring mental health and substance use disorders: a qualitative systematic review of randomized controlled trials. Soc. Sci. Med. 2005;60:1371–1383. doi: 10.1016/j.socscimed.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Drake RE, O’Neal EL, Wallach MA. A systematic review of psychosocial research on psychosocial interventions for people with co-occurring severe mental and substance use disorders. J. Subst. Abuse Treat. 2008;34:123–138. doi: 10.1016/j.jsat.2007.01.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders - Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Helmus TC, Saules KK, Schoener EP, Roll JM. Reinforcement of counseling attendance and alcohol abstinence in a community-based dual-diagnosis treatment program: a feasibility study. Psychol. Addict. Behav. 2003;17:249–251. doi: 10.1037/0893-164X.17.3.249. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu. Rev. Psychol. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Jones HE, Haug N, Silverman K, Stitzer M, Svikis D. The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone-maintained pregnant women. Drug Alcohol Depend. 2001;61:297–306. doi: 10.1016/s0376-8716(00)00152-6. [DOI] [PubMed] [Google Scholar]
- Kidorf M, Disney ER, King VL, Neufeld K, Beilenson PL, Brooner RK. Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug Alcohol Depend. 2004;74:115–122. doi: 10.1016/j.drugalcdep.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Kidorf M, King VL, Brooner RK. Counseling and psychosocial services. In: Strain EC, Stitzer ML, editors. The Treatment of Opioid Dependence. Johns Hopkins University Press; Baltimore: 2006. pp. 119–150. [Google Scholar]
- Kidorf M, King VL, Neufeld K, Pierce J, Kolodner K, Brooner RK. Improving substance abuse treatment enrollment in community syringe exchangers. Addiction. 2009;104:786–795. doi: 10.1111/j.1360-0443.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- Kidorf M, King VL, Neufeld K, Stoller KB, Peirce J, Brooner RK. Involving significant others in the care of patients receiving methadone. J. Subst. Abuse Treat. 2005;29:19–27. doi: 10.1016/j.jsat.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Lydecker KP, Tate SR, Cummins KM, McQuaid J, Granholm E, Brown SA. Clinical outcomes of an integrated treatment for depression and substance use disorders. Psychol. Addict. Behav. 2010;24:453–465. doi: 10.1037/a0019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern MP, Xie H, Segal SR, Siembab L, Drake RE. Addiction treatment services and co-occurring disorders. J. Subst. Abuse Treat. 2006;31:267–275. doi: 10.1016/j.jsat.2006.05.003. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am. J. Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med. Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Deavers T, Penn DL, Classisi JE. Psychosocial treatments for schizophrenia. Annu. Rev. Clin. Psychol. 2013;9:465–497. doi: 10.1146/annurev-clinpsy-050212-185620. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Quitkin FM, Donovan SJ, Deliyannides D, McGrath PJ, Woody G. Imipramine treatment of opiate dependent patients with depressive disorders. Arch. Gen. Psychiatry. 1998;55:153–160. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C. Combined pharmacotherapy and psychological treatment for depression: a systematic review. Arch. Gen. Psychiatry. 2004;61:714–719. doi: 10.1001/archpsyc.61.7.714. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin BM, Simcic F. Prize reinforcement contingency management for cocaine dependence: integration with group therapy in a methadone clinic. J. Consult. Clin. Psychol. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Pringle JL, Emptage NP, Hubbard RL. Unmet needs for comprehensive services in outpatient addiction treatment. J. Subst. Abuse Treat. 2006;30:183–189. doi: 10.1016/j.jsat.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Randall C, Thomas S, Thevos AK. Concurrent alcoholism and social anxiety disorder: a first step toward developing effective treatments. Alcohol. Clin. Exp. Res. 2001;25:210–220. [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabbee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch. Gen. Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Sabate E, editor. Adherence to Long-term Therapies: Evidence for Action. World Health Organization; Geneva: 2003. http://whqlibdoc.who.int/publications/2003/9241545992.pdf. [Google Scholar]
- Sacks S, Chandler R, Gonzales J. Responding to the challenge of co-occurring disorders: suggestions for future research. J. Subst. Abuse Treat. 2008a;34:139–146. doi: 10.1016/j.jsat.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks S, McKendrick K, Sacks J, Banks S, Harle M. Enhanced outpatient treatment for co-occurring disorders: main outcomes. J. Subst. Abuse Treat. 2008b;34:48–60. doi: 10.1016/j.jsat.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL, Tulsky JP, Barnett P, Hall S. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampold BE, Minami T, Baskin TW, Callen TS. A meta-(re)analysis of the effects of cognitive therapy versus “other therapies” for depression. J. Affect. Disord. 2002;68:159–165. doi: 10.1016/s0165-0327(00)00287-1. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Hunter SB, Hepner KA, Paddock SM, de la Cruz E, Zhou AJ, Gilmore J. An effectiveness trial of group cognitive behavioral therapy for patients with persistent depressive symptoms in substance abuse treatment. Arch. Gen. Psychiatry. 2011;68:577–584. doi: 10.1001/archgenpsychiatry.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Kolodziej ME, Greenfield SF, Najavits LM, Daley DC, Doreau HR, Hennen JA. A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. Am. J. Psychiatry. 2007;164:100–107. doi: 10.1176/ajp.2007.164.1.100. [DOI] [PubMed] [Google Scholar]