Abstract
Objectives
To develop RNA profiles that could serve as novel biomarkers for the response to aspirin.
Background
Aspirin reduces death and myocardial infarction (MI) suggesting that aspirin interacts with biological pathways that may underlie these events.
Methods
We administered aspirin, followed by whole blood RNA microarray profiling, in a discovery cohort of healthy volunteers (HV1,n=50), and two validation cohorts of volunteers (HV2,n=53) or outpatient cardiology patients (OPC, n=25). Platelet function was assessed by platelet function score (PFS; HV1/HV2) or VerifyNow Aspirin (OPC). Bayesian sparse factor analysis identified sets of coexpressed transcripts, which were examined for association with PFS in HV1 and validated in HV2 and OPC. Proteomic analysis confirmed the association of validated transcripts in platelet proteins. Validated gene sets were tested for association with death/MI in two patient cohorts (n=587, total) from RNA samples collected at cardiac catheterization.
Results
A set of 60 co-expressed genes named the “aspirin response signature” (ARS) was associated with PFS in HV1 (r = −0.31, p = 0.03), HV2 (r = −0.34, Bonferroni p = 0.03), and OPC (p = 0.046). Corresponding proteins for 17 ARS genes were identified in the platelet proteome, of which, six were associated with PFS. The ARS was associated with death/MI in both patient cohorts (odds ratio = 1.2, p = 0.01 and hazard ratio = 1.5, p = 0.001), independent of cardiovascular risk factors. Compared with traditional risk factors, reclassification (net reclassification index = 31 - 37%, p ≤ 0.0002) was improved by including the ARS or one of its genes, ITGA2B.
Conclusions
RNA profiles of platelet-specific genes are novel biomarkers for identifying those do not response adequately to aspirin and who are at risk for death/MI.
Keywords: aspirin, platelets, genes, myocardial infarction, biomarkers
Introduction
Identification of novel biomarkers for individuals at risk for CAD mortality, primarily due to platelet-mediated cardiovascular events such as MI, is a priority for reducing the burden of cardiovascular disease. Although genome-wide surveys of genomic variation and gene expression can identify loci associated with CAD (1-3), few can serve as biomarkers for cardiovascular events(4).
Aspirin is prescribed for the prevention of cardiovascular events, suggesting that aspirin interacts with biological pathways that may underlie these events. Platelet function assays are a surrogate biomarker for the effects of aspirin and are associated with cardiovascular events.(5) However, platelet function testing is not widely available primarily due to technical complexity. In contrast, whole blood RNA profiling using PCR-based assays is currently a widely available diagnostic testing platform. (6,7) Therefore, we hypothesized that aspirin could be used as a probe in conjunction with whole blood RNA profiling to elucidate novel biomarkers for platelet function in response to aspirin and for cardiovascular outcomes.
Methods
Platelet Function Outcomes in Healthy Volunteers Cohorts at Duke University Medical Center (DUMC)
We previously described (8) discovery and validation healthy volunteer cohorts (HV1 and HV2, Supplemental Methods, Figure 1) and the platelet function score (PFS) - a composite metric of the following platelet function assays: PFA100 (collagen/epinephrine) closure time and the areas under the optical aggregometry curve induced by adenosine diphosphate (10, 5, 1uM), epinephrine (10, 1, 0,5 uM), and collagen (5, 2 mg/ml). We measured the PFS and mean platelet volume (MPV) in HV1 (n = 50) after 2 weeks of dosing with 325 mg/day non-enteric coated, immediate release aspirin and HV2 (n = 53) after 4 weeks of dosing with 325 mg/day aspirin. In both cohorts whole blood RNA was collected into PAXgene® Blood RNA tubes (Becton, Dickinson, NJ, USA) before after aspirin exposure and stored at −80 C until microarray profiling. Platelet count was measured in platelet rich plasma in HV1.
Because three subjects in HV2 had participated in HV1, these were dropped from HV2, leaving 50 unique HV2 subjects. DUMC IRB approved the study protocols.
Platelet Function Outcomes in Patients At Risk For Cardiovascular Events At George Washington University (GWU)
We previously described (9) an outpatient cardiology cohort (OPC, Supplemental Methods, Figure 1) treated with 81mg/day aspirin assessed with the VerifyNow Aspirin device and whole blood RNA microarray analysis.
Clinical Outcomes in DUMC Patients
CATHGEN biorepository
The Catheterization Genetics (CATHGEN) biorepository has banked, whole blood RNA in PAXgene® tubes from DUMC patients from the time of cardiac catheterization, baseline medical history, and follow up for all-cause death and MI.(10,11) Two cohorts had available microarray data (Supplemental Methods, Figure 2):
Observational cohort
224 sequential samples were selected for RNA analysis, of which, 191 had sufficient RNA for microarray analysis.
Case:control cohort
A nested case:control cohort of participants who had experienced death or MI (n = 250) after their index catheterization and age-, sex-, and race-matched controls (n = 250) who were free of death/MI > 2 years after cardiac catheterization was identified.(12) 447 had sufficient RNA for microarray analysis; 44 overlapped with the observational cohort and were dropped, leaving 403 subjects for analysis.
Follow-up for death/MI was ascertained in both cohorts in October 2011; the median follow-up was 3.8 years. Patients with incomplete follow-up were censored at the time of last contact. Patients who had a history of cardiac transplantation at the time of catheterization (n =5), died within seven days (n =1), or failed quality control (n =1) were excluded. The remaining datasets left 190 samples in the observational cohort (48 death/MI events) and 397 (202 death/MI events) in the case-control cohort.
RNA extraction, labeling, microarray hybridization, quality control, and normalization
See Supplementary Methods for full details. Two microarray platforms were utilized: Affymetrix U133A2 array (HV-1, pre-aspirin) and U133 plus 2.0 array (all others). The Robust Multichip Average (RMA) method was used for normalization.
Real-Time PCR
See Supplementary Methods. Forty-five transcripts were selected for verification in the original RNA samples based on two criteria: 1) the strength of correlation of the probe set with PFS and 2) the strength of membership between the probe set and the set of co-expressed genes of interest.
Platelet purification, Protein Sample Preparation, and Proteomics Analysis by LC-MS/MS
See Supplemental Methods.
Statistical Analysis
The raw and normalized microarray data are available in the Gene Expression Omnibus for the OPC cohort (GSE38511). The data for the HV1, HV2, and CATHGEN cohorts is available through the database of Genotypes and Phenotypes (phs000548.v1.p1 and phs000551.v1.p1). Unless stated otherwise, all tests were two-sided and were performed in R (2.10.0) or Matlab (R2010b); a p-value of < 0.05 was considered significant.
Discovery of coexpressed gene sets associated with PFS - Factor Modeling
The HV1, post-aspirin RMA normalized data were nonspecifically filtered (i.e., without regard to PFS) to remove probes with mean expression less than 2.0 (i.e., the gene was not expressed in whole blood) or with variance less than 0.25 (i.e., the gene was homogenously expressed), resulting in 2,929 probe sets for subsequent analysis. To discover “Factors” or sets of coexpressed genes representative of biological pathways, we used Bayesian factor regression modeling (BFRM, http://www.isds.duke.edu/research/software/west/bfrm/)(13,14) in an unsupervised fashion (i.e., without regard to PFS). Each of the probe sets used to estimate a particular Factor can be interpreted as a measurement of the activity of some (potentially unknown) biological pathway. Each sample can then be assigned a “Factor score”, which represents the aggregate expression of the transcripts within a Factor. The Factor scores can then be used for association with the phenotype of interest in subsequent analyses.
Factor projection, Gene membership within a Factor, Comparison of factor gene lists with selected gene sets, and Co-expression of transcripts represented by a Factor before and after aspirin exposure
Correlations between factor scores and platelet function
Pearson correlation was used to test for association between a Factor and PFS in HV1 and HV2. In the second validation cohort, OPC, we chose a one-sided t-test because we hypothesized a lower factor score in the aspirin-resistant vs. aspirin-sensitive groups.
Linear regression was used to assess the independent association of Factor scores and PFS after accounting for log-transformed MPV and/or platelet count.
Correction for multiple hypotheses testing
As HV1 was a hypothesis-generating pilot study we did not adjust p-values. In the first validation cohort, HV2, we adjusted p-values using Bonferonni correction. In the second validation cohort, we performed only one hypothesis test.
Analyses of RT-PCR data
The expression of each selected transcript relative to the three reference genes was expressed as Δ Cq (or “deltaCq”, See Supplemental Methods) and correlated with the corresponding microarray probe set or platelet function score using Pearson tests of correlation.
Platelet proteomic dataset analysis
See Supplemental Methods.
Analyses of CATHGEN cohorts
Logistic or Cox proportional hazards regression models were created in the case:control or observational cohorts, respectively, to test for association between the Factor and death/MI. Each model tested the Factor alone as well as after controlling for baseline variables (Supplemental Data, Table 6) associated with the Factor of interest. The assumption of proportional hazards for each Cox model was met. Odds (or hazards) ratios, 95% confidence intervals, and p-values are reported.
To assess the independent association between the Factor and death/MI, logistic regression models were built on the combined CATHGEN cohorts by forcing Framingham risk factors (age, sex, smoking, diabetes, hypertension, hyperlipidemia), African-American [AA] race, cohort, platelet count, and the presence of CAD (defined as a CAD index(15) >32 or history of coronary artery bypass surgery/MI/percutaneous coronary intervention), into the model and adding the Factor score or individual probe set gene expression. To assess the incremental prognostic value of gene expression we compared the performance of competing models (risk factors ± Factor/probe set expression), using the areas under the receiver operating characteristics curve (ROC) (16), the net reclassification index (NRI, using risk categories of < 10%, 10-20%, or > 20%(17) or category-free NRI (18), and the integrated discrimination improvement [IDI] (17)).
Results
Discovery and validation of a set of co-expressed genes in whole blood that correlate with platelet function on aspirin
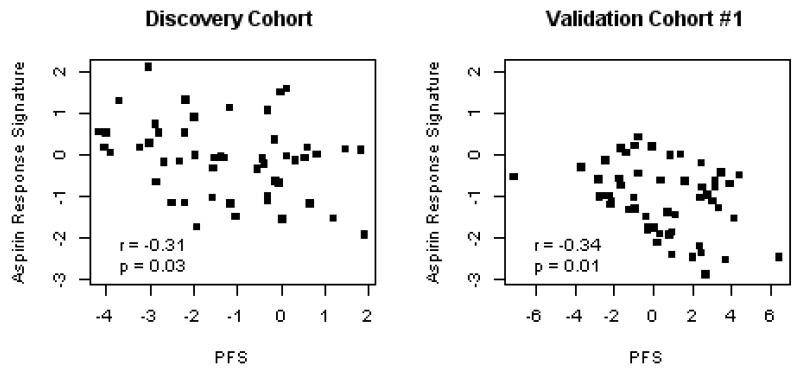
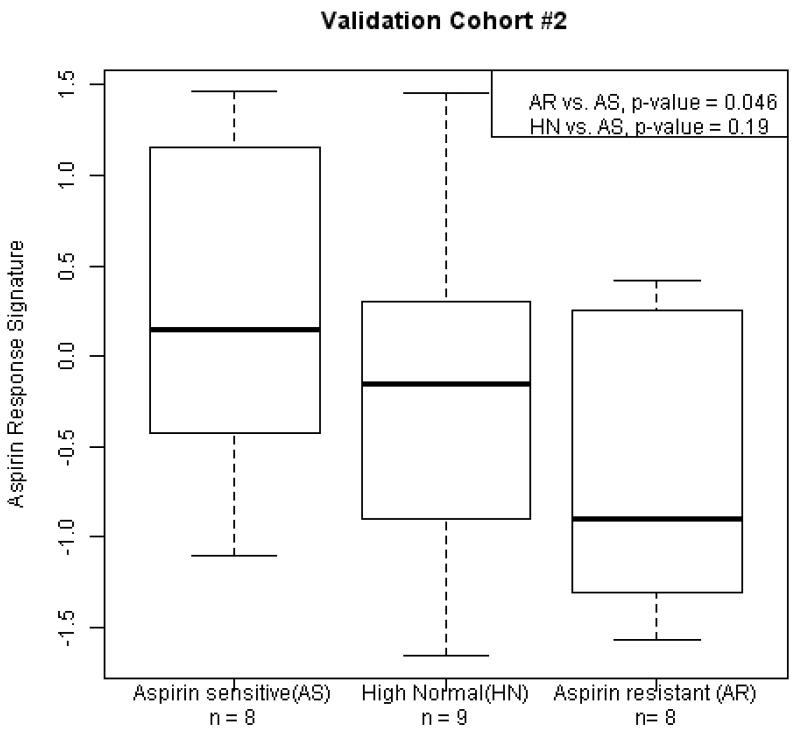
In the discovery cohort (HV1) we identified 20 Factors (numbered 1 - 20, Supplemental Data, Table 1) representing sets of highly correlated, co-expressed genes. To test the hypothesis that one or more of these gene sets were associated with PFS on aspirin, we correlated each set with PFS in HV1 and identified “Factor 14” (Figure 1A) and “Factor 3” (r = 0.27, p-value = 0.05). In the first validation cohort (HV2), we found a significant association between Factor 14 and PFS, with the same strength and direction as observed in HV1 (Figure 1B, Bonferroni adjusted p-value = 0.03), thus validating this association, however Factor 3 was not associated with PFS in HV2. We further validated Factor 14 with VerifyNow test results in the OPC cohort (Figure 2). Thus Factor 14, which we named the “aspirin response signature” (ARS), was validated in two independent cohorts as a set of co-expressed genes associated with platelet function on aspirin.
Figure 1. The aggregate expression of a set of coexpressed, whole blood genes correlates with platelet function in response to aspirin.
Two independent cohorts of healthy volunteers were exposed to 325mg/day aspirin, followed by whole blood microarray profiling. Platelet function was assessed by the platelet function score (PFS(8)). The aggregate expression of a set of coexpressed genes (aspirin response signature [ARS], x-axis), is plotted against the PFS (y-axis) after aspirin exposure. Pearson correlation coefficients and p-values are reported.
Figure 2. Aspirin response signature (ARS) is associated with platelet function in patients at risk for cardiovascular disease.
Patients treated with 81mg/day aspirin were assessed with the VerifyNow Aspirin device.(9) Three categories of individuals were profiled by microarray based on their aspirin response units (ARU): Aspirin resistant (AR, ARU > 550); High normal (HN, 500 < ARU < 550); Aspirin sensitive (AS, ARU < 550). ARS values are for each group are plotted and compared using two-sample t-tests. P-values are one-sided.
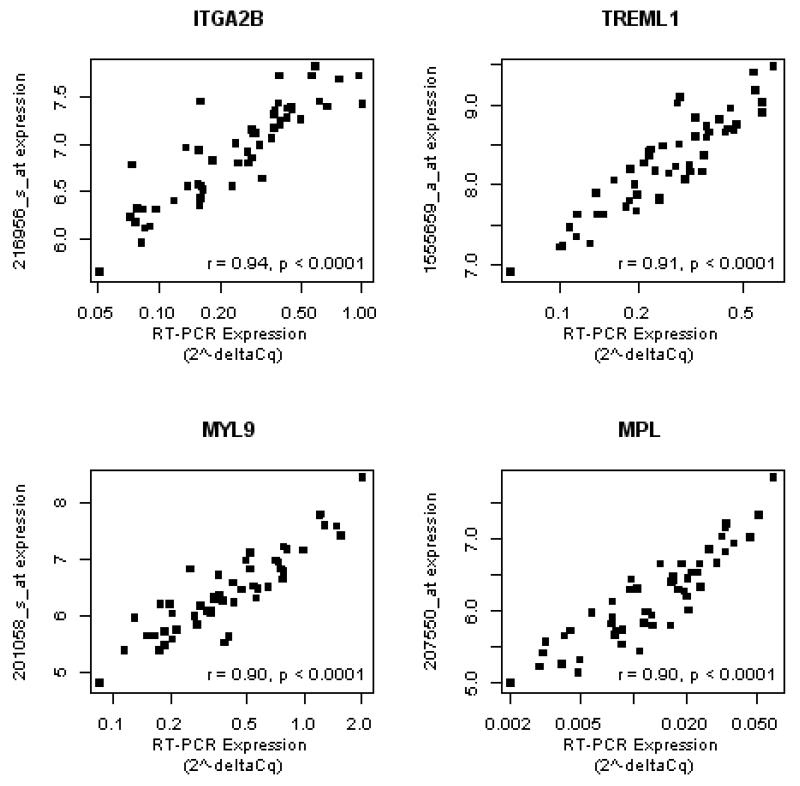
To verify the microarray-based expression of the ARS transcripts, we selected 45 of the 60 genes (see Methods for selection criteria) for verification in whole blood RNA from the HV2 cohort. Using RT-PCR, 42/45 transcripts significantly correlated with their microarray-based expression with 16/42 transcripts, including ITGA2B, TREML1, MYL9, and MPL, strongly (r > 0.80) correlating with microarray based gene expression (Figure 3 and Supplemental Data, Table 2). For the majority of transcripts there was concordance between both the RT-PCR and microarray correlations with PFS (Supplemental Data, Table 3 and Figure 1). Therefore, RT-PCR assays validate the microarray-based expression associations with PFS for most ARS transcripts.
Figure 3. PCR-based assays verify the microarray-based gene expression values for aspirin response signature genes.
Real-time PCR assays were designed to verify selected transcripts represented by the aspirin response signature (ARS) in the HV2 cohort. The deltaCq for each assay was correlated with the RMA normalized, probe set expression for the corresponding ARS gene using Pearson correlation (see Supplementary Data, Table 2). For the four genes with the highest PCR vs. microarray-based correlation (ITGA2B, MYL9, TREML1, and MPL), we plot the relative quantity (2−deltaCq, x-axis, log-scale) vs. the corresponding probe set expression (y-axis), correlation coefficient, and p-value.
Aspirin response signature transcripts are primarily of platelet origin
We observed that the transcripts with the strongest correlation with PFS (Table 1) mapped to several well-known platelet transcripts: ITGA2B, CLU, IGF2BP3, GP1BB, and SPARC. Based on this observation we hypothesized that transcripts represented by the ARS were of platelet origin. To test this hypothesis, we examined the overlap and enrichment of the 60 genes represented by the ARS with pre-defined gene sets specific to various peripheral blood cell types. Up to 24 of the 60 ARS genes significantly overlapped with platelet- or megakaryocyte-specific genes, whereas none overlapped with non-platelet peripheral blood cell type genes (Supplemental Data, Tables 4 and 5). Further, in the CATHGEN cohorts, we found the strongest correlation between expression of the ARS and platelet count (r = 0.41, p < 2e−16) with no strong, positive correlations with any other peripheral blood cell type counts: white blood cells (r = −0.01, p = 0.87), lymphocytes (r = −0.25, p = 1.2e-05), neutrophils (r = 0.16, p = 0.01), or monocytes (r = 0.06, p = 0.27).
Table 1. Genes represented by the ARS and their correlation with platelet function with aspirin*.
| Affymetrix Probe ID |
Gene Symbol |
Gene Description | Combined PFS beta coefficient * |
Combined P-value |
|---|---|---|---|---|
| Factor 14 | n/a | n/a | −0.76088 | 0.0017 |
| Individual Factor 14 transcripts | ||||
| 208782_at | FSTL1 | follistatin-like 1 | −1.6579 | 0.0003 |
| 201059_at | CTTN | cortactin | −1.2817 | 0.0015 |
| 201906_s_at | CTDSPL | CTD (carboxy-terminal domain, RNA polymerase II, polypeptide A) small phosphatase-like |
−1.3795 | 0.0025 |
| 1555659_a_at | TREML1 | triggering receptor expressed on myeloid cells-like 1 |
−1.0767 | 0.0034 |
| 212667_at | SPARC | secreted protein, acidic, cysteine-rich (osteonectin) | −1.214 | 0.0048 |
| 216956_s_at | ITGA2B | integrin, alpha 2b (platelet glycoprotein IIb of IIb/IIIa complex, antigen CD41) |
−1.0689 | 0.0048 |
| 230942_at | CMTM5 | CKLF-like MARVEL transmembrane domain containing 5 |
−1.1641 | 0.0061 |
| 57588_at | SLC24A3 | solute carrier family 24 (sodium/potassium/calcium exchanger), member 3 |
−1.3053 | 0.0063 |
| 207550_at | MPL | myeloproliferative leukemia virus oncogene | −0.931 | 0.0066 |
| 219090_at | SLC24A3 | solute carrier family 24 (sodium/potassium/calcium exchanger), member 3 |
−1.1123 | 0.0080 |
| 208791_at | CLU | clusterin | −0.9584 | 0.0085 |
| 206494_s_at | ITGA2B | integrin, alpha 2b (platelet glycoprotein IIb of IIb/IIIa complex, antigen CD41) |
−0.7279 | 0.0087 |
| 227189_at | CPNE5 | copine V | −1.2062 | 0.0088 |
| 220496_at | CLEC1B | C-type lectin domain family 1, member B | −1.2077 | 0.0090 |
| 206493_at | ITGA2B | integrin, alpha 2b (platelet glycoprotein IIb of IIb/IIIa complex, antigen CD41) |
−0.8966 | 0.0094 |
| 206049_at | SELP | selectin P (granule membrane protein 140kDa, antigen CD62) |
−1.1642 | 0.0104 |
| 203819_s_at | IGF2BP3 | insulin-like growth factor 2 mRNA binding protein 3 |
−1.2947 | 0.0123 |
| 225354_s_at | SH3BGRL2 | SH3 domain binding glutamic acid-rich protein like 2 |
−1.0895 | 0.0146 |
| 207808_s_at | PROS1 | protein S (alpha) | −1.1049 | 0.0174 |
| 207206_s_at | ALOX12 | arachidonate 12-lipoxygenase | −0.8756 | 0.0207 |
| 212813_at | JAM3 | junctional adhesion molecule 3 | −1.0454 | 0.0215 |
| 1560262_at | LRRC32 | leucine rich repeat containing 32 | −0.9376 | 0.0226 |
| 204628_s_at | ITGB3 | integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) |
−0.968 | 0.0242 |
| 214146_s_at | PPBP | pro-platelet basic protein (chemokine (C-X-C motif) ligand 7) |
−0.713 | 0.0243 |
| 211026_s_at | MGLL | monoglyceride lipase | −1.0027 | 0.0249 |
| 208792_s_at | CLU | clusterin | −0.8122 | 0.0266 |
| 201108_s_at | THBS1 | thrombospondin 1 | −0.9169 | 0.0276 |
| 201058_s_at | MYL9 | myosin, light chain 9, regulatory | −0.5909 | 0.0287 |
| 206390_x_at | PF4 | platelet factor 4 (chemokine (C-X-C motif) ligand 4) |
−0.9017 | 0.0296 |
| 206655_s_at | GP1BB | glycoprotein Ib (platelet), beta polypeptide | −0.8934 | 0.0317 |
| 209651_at | TGFB1I1 | transforming growth factor beta 1 induced transcript 1 |
−0.7618 | 0.0326 |
| 207414_s_at | PCSK6 | proprotein convertase subtilisin/kexin type 6 | −0.8566 | 0.0351 |
| 200665_s_at | SPARC | secreted protein, acidic, cysteine-rich (osteonectin) | −0.8261 | 0.0410 |
| 212077_at | CALD1 | caldesmon 1 | −0.5688 | 0.0505 |
| 203817_at | GUCY1B3 | guanylate cyclase 1, soluble, beta 3 | −0.8511 | 0.0546 |
| 227088_at | PDE5A | phosphodiesterase 5A, cGMP-specific | −0.918 | 0.0571 |
| 226152_at | TTC7B | tetratricopeptide repeat domain 7B | −0.7986 | 0.0594 |
| 206167_s_at | ARHGAP6 | Rho GTPase activating protein 6 | −0.8437 | 0.0677 |
| 37966_at | PARVB | parvin, beta | −0.7708 | 0.0717 |
| 208601_s_at | TUBB1 | tubulin, beta 1 | −0.5959 | 0.0736 |
| 204115_at | GNG11 | guanine nucleotide binding protein (G protein), gamma 11 |
−0.5622 | 0.1229 |
| 241133_at | PRSS1 | protease, serine, 1 (trypsin 1) | −0.4814 | 0.1243 |
| 203680_at | PRKAR2B | protein kinase, cAMP-dependent, regulatory, type II, beta |
−0.5049 | 0.1365 |
| 205442_at | MFAP3L | microfibrillar-associated protein 3-like | −0.4724 | 0.1385 |
| 212151_at | PBX1 | pre-B-cell leukemia transcription factor 1 | −0.6059 | 0.1729 |
| 212573_at | ENDOD1 | endonuclease domain containing 1 | −0.7276 | 0.1735 |
| 230690_at | TUBB1 | tubulin, beta 1 | −0.578 | 0.1864 |
| 230645_at | FRMD3 | FERM domain containing 3 | −0.6391 | 0.2102 |
| 225974_at | TMEM64 | transmembrane protein 64 | 0.38321 | 0.2227 |
| 1553842_at | BEND2 | chromosome × open reading frame 20 | −0.5657 | 0.2258 |
| 228708_at | RAB27B | RAB27B, member RAS oncogene family | −0.4836 | 0.2512 |
| 227180_at | ELOVL7 | ELOVL family member 7, elongation of long chain fatty acids (yeast) |
−0.3943 | 0.2823 |
| 212148_at | PBX1 | pre-B-cell leukemia transcription factor 1 | −0.3139 | 0.2970 |
| 203414_at | MMD | monocyte to macrophage differentiation-associated | −0.4287 | 0.3236 |
| 1552773_at | CLEC4D | C-type lectin domain family 4, member D | 0.37545 | 0.3543 |
| 222717_at | SDPR | serum deprivation response (phosphatidylserine binding protein) |
−0.3009 | 0.3830 |
| 224823_at | MYLK | myosin, light chain kinase | −0.2911 | 0.4644 |
| 214974_x_at | CXCL5 | chemokine (C-X-C motif) ligand 5 | −0.1621 | 0.5011 |
| 229778_at | C12ORF39 | chromosome 12 open reading frame 39 | −0.2032 | 0.5020 |
| 235331_x_at | PCGF5 | polycomb group ring finger 5 | 0.22781 | 0.5470 |
| 212651_at | RHOBTB1 | Rho-related BTB domain containing 1 | −0.2395 | 0.5755 |
| 206110_at | HIST1H3H | histone cluster 1, H3h | −0.2021 | 0.5827 |
| 215779_s_at | HIST1H2BG | histone cluster 1, H2bg | −0.2623 | 0.5896 |
| 207815_at | PF4V1 | platelet factor 4 variant 1 | −0.0927 | 0.6139 |
| 226188_at | LGALSL | lectin, galactoside-binding-like− | 0.23728 | 0.6142 |
| 221556_at | CDC14B | CDC14 cell division cycle 14 homolog B (S. cerevisiae) |
−0.2127 | 0.6530 |
| 207156_at | HIST1H2AG | histone cluster 1, H2ag | −0.1534 | 0.6882 |
| 210387_at | HIST1H2BG | histone cluster 1, H2bg | −0.1494 | 0.6906 |
| 225166_at | ARHGAP18 | Rho GTPase activating protein 18 | 0.15541 | 0.7353 |
| 206272_at | RAB4A | RAB4A, member RAS oncogene family | 0.09962 | 0.7967 |
| 210986_s_at | TPM1 | tropomyosin 1 (alpha) | 0.08749 | 0.8404 |
| 227451_s_at | C6ORF79 | chromosome 6 open reading frame 79 | −0.0134 | 0.9791 |
= the beta coefficient for the expression of either the aggregate expression of the ARS or each probe set represented by the ARS using the combined HV1 and HV2 datasets from a regression model containing gene expression and cohort (HV1 vs. HV2) with corresponding p-value; PFS = platelet function score.
To confirm the platelet origin of the ARS genes, we analyzed purified platelet lysates by label-free proteomics in the HV2 cohort. We identified 17 proteins from the ARS gene set in the proteomics dataset, of which, six were associated with PFS including ITGA2B, ITGB3, and MYL9 (Table 2), all in the same direction their corresponding transcripts. Therefore, from these data we conclude that a large number of ARS transcripts originate in platelets and are thus reporting on a coexpressed pathway of platelet transcripts and proteins associated with platelet function on aspirin.
Table 2. Aspirin response signature proteins identified in platelet protein and their correlations with PFS on aspirin.
| Protein Name | Correlation with PFS | p-value |
|---|---|---|
| TBB1 | −0.32 | 0.02 |
| GP1BB | −0.29 | 0.03 |
| ITA2B | −0.29 | 0.03 |
| ITB3 | −0.28 | 0.04 |
| MYL9 | −0.27 | 0.05 |
| RB27B | −0.26 | 0.06 |
| LEGL | −0.24 | 0.08 |
| TSP1 | −0.24 | 0.08 |
| CALD1 | −0.22 | 0.11 |
| SRC8 | −0.21 | 0.12 |
| SH3L2 | −0.20 | 0.16 |
| CXCL7 | −0.20 | 0.15 |
| SDPR | −0.18 | 0.19 |
| PLF4 | −0.18 | 0.20 |
| SPRC | −0.14 | 0.31 |
| PDE5A | −0.09 | 0.49 |
| CLUS | 0.06 | 0.67 |
Because mean platelet volume (MPV) is associated with platelet function (19) and the platelet origin of ARS transcripts, we assessed the extent to which the association between ARS and PFS was confounded by platelet volume or count. After controlling for MPV, the ARS remained significantly (adjusted regression coefficient for ARS = −0.5, standard error = 0.2, and p-value = 0.05 for HV1; and −0.87, 0.4, and p-value = 0.03 for HV2) associated with PFS. Further, in HV1, where platelet count and volume were both measured, the ARS remained significantly (−0.5 ± 0.2, p = 0.04) associated with PFS after their inclusion. Therefore, the association between ARS and platelet function is independent of other readily available platelet parameters such as count and MPV.
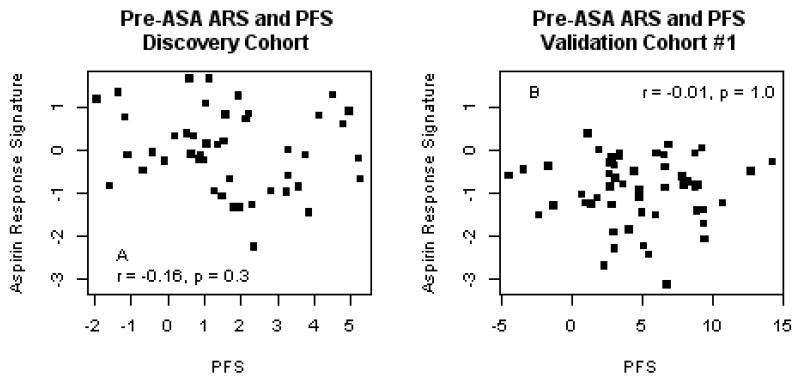
Prior to the administration of aspirin, the aspirin response signature is not associated with platelet function
Because pre-aspirin platelet function is a strong predictor of post-aspirin platelet function(8), we tested the hypothesis that the aggregate expression of the ARS genes was correlated with native, pre-aspirin PFS. In neither HV1 nor HV2 did we observe a correlation between the ARS and pre-aspirin PFS (Figure 3). Despite the absence of a correlation with PFS prior to aspirin, the ARS genes were similarly co-expressed before and after aspirin exposure (Supplemental Data, Figure 2). Therefore, although the set of ARS genes are highly correlated with one another prior to aspirin exposure, their aggregate expression does not appear to contribute to native, pre-aspirin platelet function. Instead, the expression of the ARS genes specifically reflects platelet function on aspirin.
The aspirin response signature is an independent prognostic biomarker for cardiovascular events
Because of the association of the ARS with platelet function on aspirin and aspirin’s role in preventing cardiovascular events, we tested the hypothesis that the ARS was associated with the risk of death/MI in two independent patient cohorts. In both case-control and observational cohorts, the ARS was significantly associated with death/MI in univariate analyses (odds ratio [OR] =1.2, 95% confidence interval [CI] =1.04-1.4, p =0.04 and hazard ratio [HR] =1.4, [CI] = 1.1-1.7, p =0.002, respectively). The majority of the individual transcripts represented by the ARS were also associated with death/MI in both cohorts. (Supplemental Data, Table 7)
To determine the extent to which the ARS or an individual probe set for ITGA2B was an independent prognostic biomarker for events, we combined the CATHGEN cohorts and found that the ARS (OR =1.3, CI = [1.1, 1.5], p =0.001) or the microarray-based expression of ITGA2B (probe set = 206494_s_at, OR = 1.5, CI = [1.2, 1.8], p = 0.0001) were independently associated with death/MI after adjustment for Framingham risk factors(20), race, platelet count, and presence of angiographic CAD.
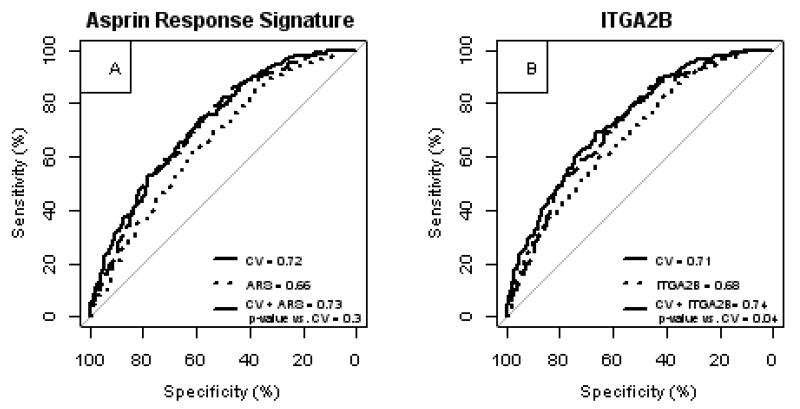
To further assess the potential use of the ARS as a risk biomarker we tested the hypothesis that the ARS or ITGA2B probe set expression would improve measures of discrimination. Compared with a model using clinical risk factors alone, the inclusion of the ARS improved most measures of risk discrimination (Table 3, Figure 5A). Inclusion of ITGA2B probe set expression significantly improved all measures of discrimination (Table 3, Figure 5B). Thus, the ARS or the expression of an individual ARS transcript such as ITGA2B were independent prognostic biomarkers for risk of death/MI.
Table 3. Measures of discrimination with and without inclusion of gene expression profiles.
| Measure | Traditional Risk Factors |
Traditional Risk Factors + ARS |
Traditional Risk Factors + 216956_s_at** (ITGA2B) |
|---|---|---|---|
|
Area under ROC curve 95% confidence interval [CI] p-value* |
0.72 [0.68-0.76] n/a |
0.73 [0.69-0.77] 0.3 |
0.74 [0.70-0.78] 0.04 |
|
Net reclassification index (<10%, 10-20%, > 20%) CI p-value |
- | 0.06 [0.02 – 0.10] 0.005 |
0.12 [0.07 - 0.17] < 1e-05 |
|
Net reclassification index (category-free) CI p-value |
- | 0.31 [0.15 – 0.47] 2e-04 |
0.37 [0.21 – 0.54] 8.7e-06 |
|
Integrated discrimination improvement CI p-value |
- | 0.01 [0.002 – 0.02] 0.006 |
0.03 [0.02 – 0.05] 2e-05 |
all p-values are for comparisons with ‘Traditional Risk Factors’ model which includes: age, sex,African-American race, smoking, diabetes, hypertension, hyperlipidemia, cohort, and the presence of coronary artery disease;
The 216956_s_at probe set represents ITGA2B gene expression on the Affymetrix microarray; ARS = aspirin response signature; ROC = receiver operating characteristic
Figure 5. Peripheral blood gene expression adds additional prognostic information for death or myocardial infarction.
Patients in the case:control and observational cohorts were combined and analyzed with respect to death/myocardial infarction (MI) outcomes. The receiver operating characteristics curves were plotted for predictive models containing cardiovascular risk factors, platelet count, presence of coronary artery disease, cohort (collectively, CV) and gene expression, or both were compared. ARS = aspirin response signature. The probe set, 216956_s_at represents ITGA2B gene expression.
Discussion
We used aspirin as a probe to identify novel genes and biomarkers associated with platelet function and cardiovascular events. We hypothesized that administering aspirin while simultaneously assaying the blood transcriptome might identify sets of genes that are related to aspirin’s cardioprotective effect. We identified a set of platelet-enriched, co-expressed genes and proteins, the “ARS”, that was reproducibly associated with platelet function in response to aspirin. When tested as a prognostic biomarker, the ARS or an individual ARS transcript (e.g., ITGA2B), independently and incrementally predicted the risk of death/MI compared with traditional risk factors. Our data shows that 1) the genomic response to a pharmacologic “challenge” with aspirin can reveal genes that underlie platelet function on aspirin and mechanisms responsible for death/MI and 2) that whole blood RNA profiling may identify novel biomarkers that discriminate individuals at heightened risk for death/MI.
Transcripts associated with platelet function on aspirin are associated with cardiovascular events
We found neither association between the ARS and the presence of CAD, nor overlap between ARS genes those previously associated with CAD.(1,6) Instead, we found that the ARS was associated with death/MI after controlling for CAD and CAD risk markers. These findings highlight a unique and novel role that the biologic pathway represented by ARS genes have in the development of cardiovascular events, independent of CAD. We conclude that the biology of aspirin is complex and involves additional mechanisms beyond inhibiting platelet COX-1 and some of these mechanisms underlie risk for cardiovascular events.
A novel and translatable biomarker of platelet function in response to aspirin and the risk for cardiovascular events
Clinicians currently need a readily available biomarker for the response to aspirin. Despite the availability of platelet function assays, their widespread use is severely constrained by the need for specialized equipment and trained personnel. Point-of-care tests are available, but require testing to be completed within hours of phlebotomy; thus, they are out of reach for the vast majority of outpatients on aspirin. Further, most patients taking aspirin for chronic prevention are outpatients where results at the point-of-care are not required. Instead, testing in central laboratories, as is common for LDLc for statins, would be sufficient for determining aspirin response in the outpatient setting. Because of the coexpressed nature of the ARS genes, several individual transcripts (Table 1) correlated best with platelet function. We demonstrated that PCR for individual transcripts could be used in lieu of microarrays (Figure 3 and Supplemental Data, Table 2) for many ARS genes, thus demonstrating the feasibility of a blood-based diagnostic test.
Whole blood RNA testing is a well-established testing diagnostic testing platform. For cardiac allograft rejection and CAD diagnosis, whole blood microarray analyses were both transitioned to a PCR-based platform(6,7): AlloMap® and Corus® CAD, respectively. AlloMap® has been approved by the FDA and both are covered by major insurances. Therefore, there is a feasible path for blood-based RNA biomarkers to clinical adoption, FDA approval and insurance coverage.
Peripheral blood gene expression profiling reveals co-expressed transcripts of platelet origin associated with platelet function in response to aspirin
The genes underlying variable platelet function on aspirin have been difficult to identify(21) or explain a small portion of the observed variability(22). We hypothesized that whole blood RNA profiling, which de facto contains platelet transcripts, would yield biological pathways important for the response to aspirin. We demonstrated that the transcripts represented by the ARS are likely of platelet origin (Supplemental Data, Tables 4 and 5). When we analyzed platelet-enriched protein, we not only confirmed the well-known roles of ITGA2B and ITGB3, but also and ascribe new roles many other platelet genes: MYL9, CLU, PPKAR2B, TREML1, and CTTN with respect to platelet function on aspirin and cardiovascular events. Additionally, recent genome wide association studies identified a PEAR1 polymorphism associated with platelet PEAR1 levels and platelet function on aspirin.(22) We excluded the probe set (228618_at) mapping to PEAR1 because its variance (0.21) fell below our variance criteria (0.25, see Methods). However, in a post hoc analysis, PEAR1 expression strongly correlated (r = 0.9) with ARS levels. Therefore, our approach identified previously known and novel platelet genes associated with platelet function in response to aspirin.
We observed an association between ARS and platelet function only after the administration of aspirin, suggesting that the latent effect of ARS genes on platelet function is unmasked in response to aspirin. Consistent with these findings, when we stratified the CATHGEN cohort by aspirin use, we observed that the association between the ARS and death/MI was higher in those using aspirin at the time of catheterization (OR = 1.4 vs. 1.1 in aspirin users vs. nonusers). We hypothesize that the molecular mechanisms represented by the ARS contributes minimally to native platelet function in the absence of aspirin. In contrast, when platelet COX-1, a protein not represented by the ARS, is suppressed by 325mg/day aspirin dosing(23), the effects of these platelet enriched genes is revealed such that the resulting level of platelet function is then determined by the ARS. Alternatively, aspirin exposure may alter the genomic and protein content of circulating platelets. The precise mechanism by which platelet function on aspirin is related to the expression of the ARS genes and proteins on aspirin is the subject of ongoing work.
Limitations
Several limitations deserve consideration. Neither platelet function nor mean platelet volume (MPV) were measured in CATHGEN. Therefore, we cannot know whether heightened ARS levels altered platelet function or volumes in addition to an increased risk of death/MI. To our knowledge, large cohorts with platelet function, banked RNA, longitudinal follow up, and a sufficient number of events are not available. Further, in our discovery and validation cohorts, the association of the ARS with PFS was independent of platelet count and MPV, suggesting the ARS provides an independent parameter of platelet function that underlies cardiovascular events. Second, although there was no association between the ARS and modifiable risk factors (e.g. diabetes, hyperlipidemia, or hypertension), because we did not assess the degree to which these risk factors were controlled we do not know if addressing these risk factors could modulate ARS levels. Finally, the comparison of the ARS gene set with that of platelets, megakaryocytes, and platelet proteomics analyses demonstrate that the top ARS genes correlative of platelet function on aspirin were of platelet origin. However, some ARS genes (e.g. TTC7B and FSTL1) are also expressed in non-platelet cell types, suggesting that mechanism(s) represented by ARS genes may involve more than just platelets.
Conclusion
In summary, we used aspirin as a probe in conjunction with RNA profiling and identified novel biomarkers that identify individuals at highest risk for death/MI independent of clinical risk factors.
Supplementary Material
Figure 4. A set of coexpressed peripheral blood genes does not correlate with native, pre-aspirin platelet function.
The aggregate expression of coexpressed genes, is plotted against the platelet function before the administration of aspirin in the discovery cohort (HV1, A, n = 45) and validation cohort (HV2, B, n = 50) healthy volunteers. Pearson correlation coefficients and p-values are reported. ARS = aspirin response signature; PFS = platelet function score.
Acknowledgements
We would like to acknowledge Jason Rose, MD for collecting the platelet count data for the CATHGEN cohorts.
Funding: This study was funded by institutional funds provided by the Duke Institute for Genome Sciences & Policy, a National Institutes of Health (NIH) T32 Training grant (5T32HL007101 to DV), a grant (5UL1RR024128) from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research, a grant (5RC1GM091083 to GSG) from the National Institutes of General Medical Sciences, grant (5U01DD000014 to TLO) from the Centers for Disease Control and Prevention, and the David H Murdock Research Institute.
Glossary
Abbreviations
- PFS
Platelet Function Score
- ARS
Aspirin Response Signature
- CAD
Coronary Artery Disease
- MI
Myocardial Infarction
- RT-PCR
Real-time Polymerase Chain Reaction
- PCR
Polymerace Chain Reaction
- RNA
Ribonucleic Acid
- IRB
Institutional Review Board
- DNA
Deoxyribonucleic Acid
- LDLc
low density lipoprotein cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wingrove JA, Daniels SE, Sehnert AJ, et al. Correlation of Peripheral-Blood Gene Expression With the Extent of Coronary Artery Stenosis. Circ Cardiovasc Genet. 2008;1:31–38. doi: 10.1161/CIRCGENETICS.108.782730. [DOI] [PubMed] [Google Scholar]
- 2.Helgadottir A, Thorleifsson G, Manolescu A, et al. A Common Variant on Chromosome 9p21 Affects the Risk of Myocardial Infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 3.Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–53. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly MP, Li M, He J, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–92. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frelinger AL, III, Li Y, Linden MD, et al. Association of Cyclooxygenase-1-Dependent and -Independent Platelet Function Assays With Adverse Clinical Outcomes in Aspirin-Treated Patients Presenting for Cardiac Catheterization. Circulation. 2009;120:2586–2596. doi: 10.1161/CIRCULATIONAHA.109.900589. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg S, Elashoff MR, Beineke P, et al. Multicenter Validation of the Diagnostic Accuracy of a Blood-Based Gene Expression Test for Assessing Obstructive Coronary Artery Disease in Nondiabetic Patients. Annals of Internal Medicine. 2010;153:425–434. doi: 10.7326/0003-4819-153-7-201010050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-Expression Profiling for Rejection Surveillance after Cardiac Transplantation. New England Journal of Medicine. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 8.Voora D, Ortel TL, Lucas JE, Chi JT, Becker RC, Ginsburg GS. Time-dependent changes in non-COX-1-dependent platelet function with daily aspirin therapy. Journal of Thrombosis and Thrombolysis. 2012;33:246–57. doi: 10.1007/s11239-012-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallahi P, Katz R, Toma I, et al. Aspirin insensitive thrombophilia: Transcript profiling of blood identifies platelet abnormalities and HLA restriction. Gene. 2013 doi: 10.1016/j.gene.2013.02.032. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Hauser ER, Shah SH, et al. Peakwide mapping on chromosome 3q13 identifies the kalirin gene as a novel candidate gene for coronary artery disease. American journal of human genetics. 2007;80:650–63. doi: 10.1086/512981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosati RA, McNeer JF, Starmer CF, Mittler BS, Morris JJ, Jr., Wallace AG. A New Information System for Medical Practice. Arch Intern Med. 1975;135:1017–1024. [PubMed] [Google Scholar]
- 12.Shah SH, Granger CB, Hauser ER, et al. Reclassification of cardiovascular risk using integrated clinical and molecular biosignatures: Design of and rationale for the Measurement to Understand the Reclassification of Disease of Cabarrus and Kannapolis (MURDOCK) Horizon 1 Cardiovascular Disease Study. American Heart Journal. 2010;160:371–379 e2. doi: 10.1016/j.ahj.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Carvalho C, Lucas JE, West M. BFRM: Bayesian factor regression modeling. Bulletin of the International Society of Bayesian Analysis. 2007;14:4–5. [Google Scholar]
- 14.Carvalho CM, Chang J, Lucas JE, Nevins JR, Wang Q, West M. High-Dimensional Sparse Factor Modeling: Applications in Gene Expression Genomics. Journal of the American Statistical Association. 2008;103:1438–1456. doi: 10.1198/016214508000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–25. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 17.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in Medicine. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. British Journal of Haematology. 1982;50:509–19. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA: the journal of the American Medical Association. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 21.Mathias R, Kim Y, Sung H, et al. A combined genome-wide linkage and association approach to find susceptibility loci for platelet function phenotypes in European American and African American families with coronary artery disease. BMC Medical Genomics. 2010;3:22. doi: 10.1186/1755-8794-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faraday N, Yanek LR, Yang XP, et al. Identification of a specific intronic PEAR1 gene variant associated with greater platelet aggregability and protein expression. Blood. 2011;118:3367–3375. doi: 10.1182/blood-2010-11-320788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurbel PA, Bliden KP, DiChiara J, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115:3156–64. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.