Abstract
A number of studies have implicated disruptions in prepulse inhibition (PPI) of the startle response in both schizophrenia patients and animal models of this disorder. These disruptions are believed to reflect deficits in sensorimotor gating and are ascribed to aberrant filtering of sensory inputs leading to sensory overload and enhanced “noise” in neural structures. Here we examined auditory evoked potentials in a rodent model of schizophrenia (MAM-GD17) during an auditory PPI paradigm to better understand this phenomenon. MAM rats exhibited reductions in specific components of auditory evoked potentials in the orbtiofrontal cortex and an abolition of the graded response to stimuli of differing intensities indicating deficient intensity processing in the orbitofrontal cortex. These data indicate that aberrant sensory information processing, rather than being attributable to enhanced noise in neural structures, may be better attributed to diminished evoked amplitudes resulting in a reduction in the “signal-to-noise” ratio. Therefore, the ability for sensory input to modulate the ongoing background activity may be severely disrupted in schizophrenia yielding an internal state which is insufficiently responsive to external input.
Keywords: MAM, schizophrenia, prepulse inhibition, auditory evoked potentials, intensity processing
Introduction
Among the multiple alterations observed in schizophrenia patients, deficits in prepulse inhibition of the startle response (PPI) has long been reported and evidence suggests that PPI abnormalities may correlate with the thought disorder associated with this disease (Braff et al., 2001). PPI refers to a sensorimotor gating phenomenon in which the prior presentation of a weak stimulus (pre-pulse) attenuates the response (startle reflex) induced by a sudden, intense stimulus and represents a particularly useful behavioural measure since the phenomenon has been reported across a large number of species (Kumari et al., 2003; Li et al., 2009; Swerdlow et al., 2001). These disruptions are believed to reflect deficits in sensorimotor gating and are ascribed to aberrant filtering of sensory inputs leading to sensory overload and enhanced “noise” in neural structures. PPI deficits have been reported across all commonly measured sensory modalities in humans although auditory paradigms dominate in rodent research. In addition to significant behavioral disturbances, schizophrenia patients also exhibit significant disruptions in the generation (Buchsbaum, 1977; Boutros et al., 1993; Ford et al., 1994) and gating (Freedman et al., 1991) of auditory evoked potentials. As such, schizophrenia is associated with both structural and functional disturbances at the level of the primary auditory cortex (Leitman et al., 2007) and it has been suggested that the hippocampus, also heavily implicated in the pathophysiology of schizophrenia (Malaspina et al., 1999), plays a crucial role in the generation of auditory potentials (Freedman et al., 1991; Bickford-Wimer et al., 1990). In addition, the ventral hippocampus is heavily implicated in the regulation of PPI, a regulation which appears to be independent of the ventral striatum (Wan et al., 1996). Thus there is great potential for overlap between the behavioral manifestation of PPI deficits and the impaired generation of auditory evoked potentials which remain unexplored.
Rats born to dams administered with the mitotoxin methylazoxymethanol acetate (MAM) during gestation (day 17) display behavioral and neural abnormalities consistent with those seen in schizophrenia, including structural (cortical thinning), oscillatory (diminished gamma rhythmicity) and behavioral deficits (PPI and latent inhibition) (Flagstad et al., 2004, 2005; Moore et al., 2006; Lodge et al., 2009; Hradetzky et al., 2012). We have previously reported diminished auditory evoked potential amplitudes during an inhibitory gating (IG) paradigm in which the amplitude of the second response is diminished (gated) with respect to the first during delivery of equal amplitude, paired auditory stimuli (Ewing and Grace, 2013). IG and PPI are believed to be subserved by different mechanisms (de Bruin et al., 1999, 2003). Thus, in the present study, we expand on our previous findings by assessing auditory evoked potentials to stimuli of different intensities during a standard PPI paradigm. The anatomical loci involved can be grouped into those which mediate PPI through brain stem nuclei; including the inferior and superior colliculus, pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus, substantia nigra pars reticular and caudal pontine reticular nucleus and those which regulate PPI through forebrain nuclei; including the prefrontal cortex, thalamus, hippocampus and ventral striatum (Kumari et al., 2003; Swerdlow et al., 2001). The regulatory circuit - striatum, hippocampus and thalamus - is commonly implicated in the pathophysiology of schizophrenia and is more greatly activated during PPI in healthy subjects than in schizophrenia patients (Kumari et al., 2003). Thus, we examined auditory evoked responses in brain regions involved in the regulation of the PPI response - those most commonly implicated in the pathophysiology of schizophrenia.
Materials & Methods
Animals and surgery
Timed pregnant female Sprague-Dawley rats were obtained at gestational day 15 and housed individually with free access to food and water. A DNA methylating agent (methylazoxymethanol (MAM) in saline) or vehicle (saline) was administered (20mg/kg, 1ml/kg, i.p.) on gestational day 17. Male pups were weaned on post-natal day 21 and housed in groups of two or three with free access to food and water. Experiments occurred no earlier than 12 weeks of age. All animals were handled according to the guidelines outlined in the USPHS Care and Use of Laboratory Animals and all protocols approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Under isoflurane anaesthesia (induction: 5%, maintenance: 2% in oxygen), rats weighing between 560–610g (n(MAM) = 10, n(SAL) = 8) were anesthetized, mounted in a sterotaxic frame, a rostral-caudal incision made, the skull exposed and bregma identified. Burr holes were drilled and wire electrodes (125μm, polyimide insulated, stainless steel (Plastics 1)) implanted bilaterally into the prelimbic (RC2: +3.4, ML: ±0.7mm, DV: −4.0mm), infralimbic (RC: +3.4, ML: ±0.7mm, DV: −5.0mm) and orbitofrontal cortices (RC: +3.2, ML: ±3.4mm, DV: −5.0mm, PrL, IL and OFC respectively), the core (RC: +1.2, ML: ±2.0mm, DV: −7.2mm) and shell (RC: +1.2, ML: ±2.0mm, DV: −8.0mm) of the nucleus accumbens (AcbC, AcbSh respectively), the mediodorsal thalamic nucleus (RC: −3.3, ML: ±0.7mm, DV: −5.5mm, MD), the ventral tegmental area (RC: −5.3, ML: ±0.7mm, DV: −8.5mm, VTA) and ventral hippocampus (RC: −6.0, ML: ±4.5mm, DV: −8.2mm, vHipp). Two ground screw electrodes (Plastics 1) were affixed at either side of lambda and 6 additional stainless steel screws fixed in the skull. All electrodes and connectors (E363, Plastics 1) were secured with dental cement and the incision sutured tightly around the implant. Carprofen (Rimaydyl™) was administered for analgesia (5mg/kg s.c.) and body temperature maintained with a small heating pad during surgery (Fintronics Inc.). Animals were housed singly following surgery, given free access to rat chow softened with Children’s Tylenol for the first 2 days and allowed to recover for at least a week before beginning recordings.
Data acquisition
Animals were tethered to the recording system (RHA2000-EVAL board, Intan Technologies, LLC) via a custom-made 11 channel cable and commutator (Plastics 1). All recordings were made against a ground screw affixed above the cerebellum. Signals were amplified (gain = 800), high pass filtered at 1Hz, low pass filtered at 7.5kHz and sampled at 25kHz. Animals were habituated to the recording apparatus over 3 days before beginning data acquisition. Auditory evoked potential event markers were recorded via one of the auxiliary inputs on the RHA2000-EVAL board. Recordings were made while animals were loosely restrained within a 4l glass jar inside a sound attenuated chamber (SR lab, San Diego Instruments) and auditory stimuli presented via a speaker mounted in the roof of the chamber.
Prepulse inhibition
Animals were presented with auditory stimuli identical to those presented during a typical prepulse inhibition paradigm (Egerton et al., 2008). Once loosely restrained within the startle chamber, animals were given 5 minutes to acclimate to a 60dB background of white noise. Three blocks of trials followed in which the inter-trial interval varied between 8 and 23 seconds. The first and last blocks consisted of 6 startle-stimulus (120dB white noise presented for 40ms) alone trials. The central block consisted of 52 trials presented in a pseudorandom order. Of the 52 trials 12 were startle-stimulus alone trials and 10 were trials in which no-stimulus was presented. In the remaining trials the startle-stimulus was preceded by a “pre-pulse” of white noise, 20ms in duration, presented 100ms before the startle-stimulus. The pre-pulse amplitudes were 4, 8 and 16dB louder than the background volume with 10 trials at each pre-pulse intensity.
Electrode localization, exclusions and statistical analysis
Electrode locations were marked, under anaesthesia, by driving a 200μA constant current through each electrode for 10s. Brains were removed and fixed in 8% paraformaldehyde with added potassium ferrocyanide to aid in electrode localization. Animals with incorrectly placed electrodes were excluded on a region by region basis leaving final group sizes of >7 per region, per treatment.
The amplitude and latency of auditory evoked potentials were seen to be volume dependent with earlier occuring peak amplitudes occurring at 22, 26 and 30ms (29, 34 and 39ms for the ventral hippocampus only) and later occuring peak amplitudes occuring at 46, 57 and 68ms for prepulse intensities of +4dB, +8dB and +16dB respectively (Fig. 1). The effect of prepulse intensity and treatment were tested via a two-way ANOVA with LSD post-hoc tests where appropriate over these peak amplitudes for each brain region for potentials evoked to the prepulse stimuli. The effect of prepulse intensity and treatment were tested via a two-way ANOVA for potentials evoked to the startle-stimulus for peak amplitudes occuring at 22ms following presentation of the startle-stimulus. The significance level was corrected using an FDR method (false discovery rate (Benjamini and Yekutieli, 2012)) for the 16 brain regions yielding a significance level of 0.01479. All analyses were performed using GNU Octave and all values are reported to either 3 significant figures or 3 decimal places as appropriate.
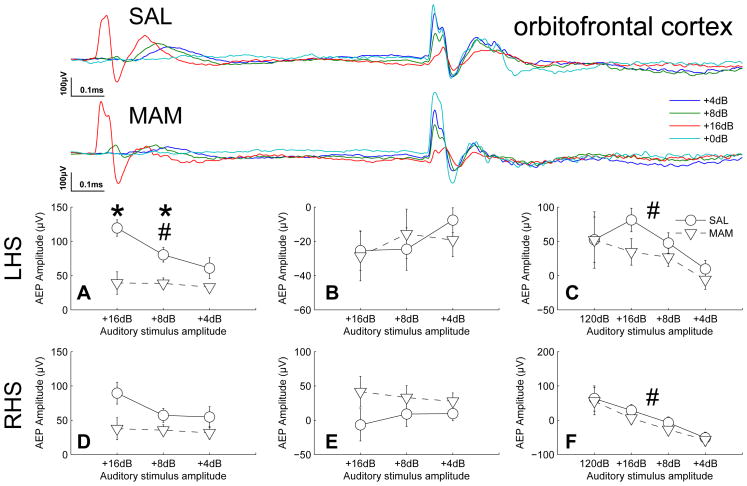
Figure 1.
MAM treated animals exhibited deficits in auditory information processing in the orbitofrontal cortex. Specifically, the graded response to graded sound intensities (16,8 and 4dB above background) seen in SAL treated animals was abolished in MAM animals. This reduction in gradient was driven by significantly reduced amplitudes of the potentials evoked to prepulse intensites of +8dB and +16db. (Top traces of each subfigure) Grand averaged AEPs for SAL and MAM treated animals respectively. (A,D) Earlier occuring maximum evoked potential amplitudes and (B,E) later occuring maximum evoked potential amplitudes for the prepulse intensities. (C,F) Evoked potential amplitudes of the P20 component of the potentials evoked to the startle-stimulus during both prepulse and startle alone trials (+0dB). * indicates a significant effect of treatment between SAL and MAM animals. # indicates a significant effect of prepulse intensity. Data are plotted as the mean ± SEM.
Results
Frontal Cortices
In the left orbitofrontal cortex a significant reduction in the graded response to pre-pulse stimuli of different intensities was observed in MAM animals when compared with saline-treated controls, indicated by the significant effects of prepulse intensity (16, 8 and 4dB above background, F2,28 = 6.32, p = 0.005), treatment (MAM vs. SAL, F1,28 = 11.8, p = 0.004) and the interaction between them (F2,28 = 4.04, p = 0.010, Fig. 1). Significant reductions in the maximum amplitude of the evoked response to prepulse stimuli were seen at intensities of 8dB (p = 0.012, t = 2.9, Fig. 1) and 16dB (p = 0.0015, t = 3.9, Fig. 1). Presentation of a prepulse had a significant, intensity-dependent effect on the amplitude of the P20 component of the potential evoked in response to the startle-stimulus, indicated by the significant effect of prepulse intensity (F3,28 = 8.61, p = 0.001). However, no differences due to treatment were noted for this effect (F1,28 = 2.89, p = 0.111, Fig. 1). Despite seemingly apparent similarites in the right orbitofrontal cortex (Fig. 2) comparable effects during responses to the prepulse were not found to be statistically significant (intensity, F2,28 = 2.02, p = 0.151; treatment, F1,28 = 5.87, p = 0.030; interaction, F2,28 = 2.40, p = 0.109, Fig. 1). However, presentation of a prepulse had a significant, intensity-dependent effect on the amplitude of the P20 component of the potential evoked in response to the startle-stimulus, indicated by the significant effect of prepulse intensity (F3,28 = 7.07, p < 0.001, Fig. 1).
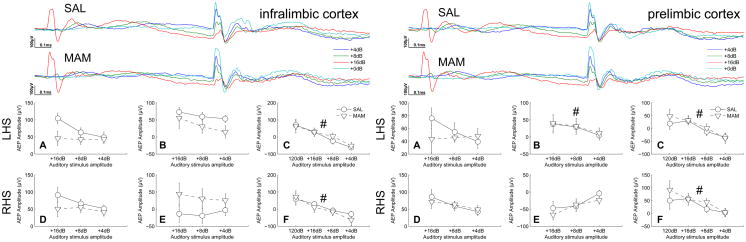
Figure 2.
Deficits in auditory information processing seen in the orbitofrontal cortex of MAM rats was not seen in the prelimbic and infralimbic cortices. However, the prelimbic cortex appeared to play a role in intensity processing at a greater latency than that seen in the orbitofrontal cortex. Both the prelimbic and infralimbic cortices display a prepulse intensity-dependent modulation of the response to the startle stimulus. (Top traces of each subfigure) Grand averaged AEPs for SAL and MAM treated animals respectively. (A,D) Earlier occuring maximum evoked potential amplitudes and (B,E) later occuring maximum evoked potential amplitudes for the prepulse intensities. (C,F) Evoked potential amplitudes of the P20 component of the potentials evoked to the startle-stimulus during both prepulse and startle alone trials (+0dB). * indicates a significant effect of treatment between SAL and MAM animals. # indicates a significant effect of prepulse intensity. Data are plotted as the mean ± SEM.
In the prelimbic cortices intensity-dependent effects were observed during later components of the prepulse evoked potentials (46, 57 and 68ms for intensities of 16, 8 and 4dB above background respectively) being statistical significant in the left hemisphere (F2,28 = 9.66, p < 0.001; right hemisphere, F2,28 = 3.65, p = 0.039, Fig. 2). Similarly, presentation of a prepulse had a significant, intensity-dependent effect on the amplitude of the P20 component of the startle-evoked potentials, indicated by the significant effect of prepulse intensity (left, F3,28 = 6.74, p < 0.001; right, F3,28 = 15.3, p ≪ 0.001, Fig. 2). No differences were attributable to treatment during responses evoked to the prepulse or the startle-stimulus.
In the infralimbic cortex presentation of a prepulse had a significant, intensity-dependent effect on the amplitude of the P20 component of the potential evoked in response to the startle-stimulus, indicated by the significant effect of prepulse intensity (right, F3,28 = 15.5, p ≪ 0.001; left, F3,28 = 7.87, p < 0.001, Fig. 2). However, no differences due to treatment were observed during responses evoked to the prepulse or the startle-stimulus nor were any intensity-dependent effects detected in response to the prepulse itself.
In summary, MAM-treated rats exhibited a deficit in the generation of a graded response to auditory stimuli of differing intensities in the orbitofrontal cortex during early phases of the evoked potentials. The prelimbic cortex also appears to be involved in amplitude processing at greater latencies although no differences were detected as a consequence of treatment. The orbitofrontal, prelimbic and infralimbic cortex all demonstrate a graded reduction in the amplitude of the potential evoked by the startling stimulus although again no differences were detected as a consequence of treatment.
Ventral Striatum
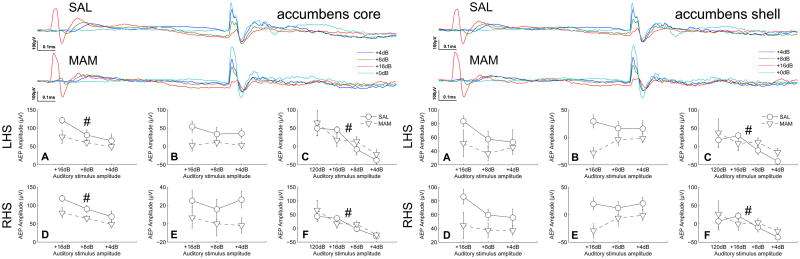
In the core of the nucleus accumbens intensity-dependent effects were observed during early components of the prepulse evoked potentials (22, 26 and 30ms for intensities of 16, 8 and 4dB above background respectively) being statistical significance in both hemispheres (left, F2,28 = 7.62, p = 0.002; right, F2,28 = 9.24, p = 0.001, Fig. 3). Similarly, presentation of a prepulse had a significant, intensity-dependent effect on the amplitude of the P20 component of the startle-evoked potentials, indicated by the significant effect of prepulse intensity (left, F3,28 = 15.1, p ≪ 0.001; right, F3,28 = 11.6, p < 0.001, Fig. 3). No differences were attributable to treatment during responses evoked to the prepulse or the startle-stimulus.
Figure 3.
No deficits in auditory information processing were seen in the ventral striatum of MAM rats when compared with SAL treated controls. However, the core, a major recipient of cortical afferents, appeared to play a role in intensity processing at a a similar latency to that seen in the orbitofrontal cortex. Both the core and the shell display an prepulse intensity-dependent modulation of the response to the startle stimulus. (Top traces of each subfigure) Grand averaged AEPs for SAL and MAM treated animals respectively. (A,D) Earlier occuring maximum evoked potential amplitudes and (B,E) later occuring maximum evoked potential amplitudes for the prepulse intensities. (C,F) Evoked potential amplitudes of the P20 component of the potentials evoked to the startle-stimulus during both prepulse and startle alone trials (+0dB). * indicates a significant effect of treatment between SAL and MAM animals. # indicates a significant effect of prepulse intensity. Data are plotted as the mean ± SEM.
In contrast with the core no significant effects of either treatment or prepulse intensity were observed in the left or right accumbens shell in response to the prepulse (left, F2,28 = 1.64, p = 0.21; right, F2,28 = 1.51, p = 0.23, Fig. 3). However, presentation of a prepulse yielded a simlar intensity-dependent reduction in the amplitude of the P20 component of the startle evoked potentials (left, F3,28 = 7.29, p < 0.001; right, F3,28 = 5.07, p = 0.004, Fig. 3). No differences were attributable to treatment during responses evoked to the prepulse or the startle-stimulus.
The accumbens core, a major recipient of cortical afferents, appears to be involved similarly to the orbitofrontal cortex during early auditory intensity processing. However, in contrast with the orbitofrontal cortex the accumbens core displayed no deficits in generating responses to the startle stimulus in MAM-treated rats.
Thalamus
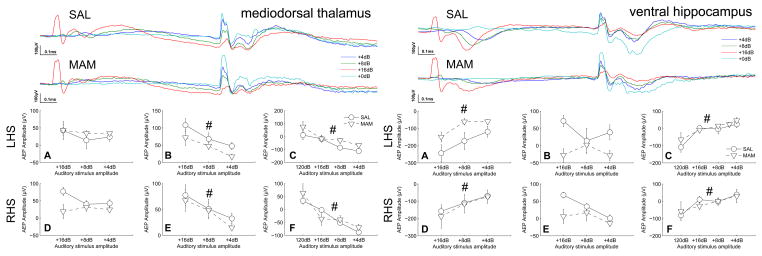
In the mediodorsal thalamic nucleus intensity-dependent effects were observed during later components of the prepulse evoked potentials (46, 57 and 68ms for intensities of 16, 8 and 4dB above background respectively) being statistical significant in both hemispheres (left, F2,28 = 12.5, p < 0.001; right, F2,28 = 15.6, p ≪ 0.001, Fig. 4). Similarly, presentation of a prepulse had a significant, intensity-dependent effect on the amplitude of the P20 component of the startle-evoked potentials, indicated by the significant effect of prepulse intensity (left, F3,28 = 6.87, p < 0.001; right, F3,28 = 9.16, p ≪ 0.001, Fig. 4). No differences were attributable to treatment during responses evoked to the prepulse or the startle-stimulus.
Figure 4.
No deficits in auditory information processing were seen in the mediodorsal thalamic nucleus or ventral hippocampus of MAM rats when compared with SAL treated controls. However, the mediodorsal thalamic nucleus, a major origin of cortical efferents, appeared to play a role in intensity processing at a similar latency to that seen in the prelimbic cortex. The ventral hippocampus also appeared to play a role in intensity processing. Both the mediodorsal thalamus and the ventral hippocampus display an amplitude dependent modulation of the response to the startle-stimulus. (Top traces of each subfigure) Grand averaged AEPs for SAL and MAM treated animals respectively. (A,D) Earlier occuring maximum evoked potential amplitudes and (B,E) later occuring maximum evoked potential amplitudes for the prepulse intensities. (C,F) Evoked potential amplitudes of the P20 component of the potentials evoked to the startle-stimulus during both prepulse and startle alone trials (+0dB). * indicates a significant effect of treatment between SAL and MAM animals. # indicates a significant effect of prepulse intensity. Data are plotted as the mean ± SEM.
The mediodorsal thalamic nucleus, a major recipient of basal ganglial output and major input to the frontal cortices, appears to be involved similarly to the prelimbic cortex during later stages of auditory intensity processing.
Hippocampus & ventral tegmental area
In the ventral hippocampus intensity dependent effects were observed during the early components of the prepulse evoked potentials (42, 26 and 30ms for intensities of 16, 8 and 4dB above background respectively) being statistical significant in both hemispheres (left, F2,28 = 34.3, p ≪ 0.001; right, F2,28 = 16.6, p ≪ 0.001, Fig. 4). Similarly, presentation of a prepulse had a significant, intensity-dependent effect on the amplitude of the P20 component of the startle evoked potentials, indicated by the significant effect of prepulse intensity (left, F3,28 = 12.7, p ≪ 0.001; right, F3,28 = 5.2, p = 0.004, Fig. 4). No differences were attributable to treatment during responses evoked to the prepulse or the startle stimulus. No significant differences were observed in the ventral tegmental area (data not shown).
The ventral hippocampus, which projects heavily to the frontal cortices and ventral striatum, appears to be involved in auditory intensity processing on a timescale which bisects the processing latencies seen in the orbitofrontal cortex/ventral striatum and the prelimbic cortex/medial thalamus.
Discussion
Alterations in the generation of evoked potentials in response to auditory stimuli in a common PPI paradigm were observed in the MAM-model of schizophrenia when compared with saline treated controls. Further, we have provided evidence for impaired sound intensity processing in the orbitofrontal cortex. In rats exposed to MAM during gestation, there were deficits in auditory intensity processing when compared with saline-treated controls during a standard PPI paradigm. These deficits were brain region specific, with deficits in intensity processing in the orbitofrontal cortex occuring at latencies of 22–30ms. These deficits in the processing of the prepulse do not appear to induce further deficits in the processing of the subsequent startle stimulus in the orbitofrontal cortex, ventral striatum, mediodorsal thalamus and ventral hippocampus.
The ventral hippocampus is implicated in the regulation of PPI, a mediation that has been suggested to be independent of the ventral striatum (Wan et al., 1996). Our results indicate different roles for these loci in PPI responses. Striatal involvement in PPI regulation occurs earlier than does the peak response of the ventral hippocampus (Fig. 3 and 4). Further, the peak response of the mediodorsal thalamic nucleus appears to occur later still suggestive of a specific processing route through this circuit. The orbitofrontal cortex appears to be heavily involved in both the processing of the startle-stimulus and in the processing of intensity information in the pre-pulse stimuli (Fig. 1) with peak responses occuring at latencies similar to those observed in the striatum and it appears that it is dysfunction within the orbitofrontal cortex that may underpin aberrant intensity processing in MAM-treated rats.
Intensity perception in healthy subjects allows for a greater response to louder sounds and its failure suggests that MAM rats may, as suggested in schizophrenia patients, fail to extract meaning from intensity information (Bach et al., 2011). Here we have shown that rats exposed to MAM during gestation fail to generate the graded response to prepulse sounds of different intensities. The amplitude and latency of auditory evoked potentials was seen to be intensity-dependent with louder sounds occurring sooner and evoking a larger amplitude response (Fig. 1). This may suggest that louder sounds may be perceived as being closer and more relevant (Bach et al., 2011). The latency of the peak amplitudes for each prepulse intensity were not found to differ between MAM- and SAL- treated rats which may indicate that the perception of the sound intensity remains intact in MAM animals. However the graded amplitude of responses evoked to these differing intensities is abolished in MAM-rats indicating that, although the sound may have been perceived as louder (or closer), it is then not assigned due relevance via the generation of a sufficiently large response. This may relate to MAM rats having impairment in utilizing this information and ascribing relevance to sounds of different intensities. It is unlikely that this deficit reflects a simple dysfunction in hearing since no differences were detected in any brain region during startle-alone trials and intensity perception (defined by the latency of peak response) remained intact.
It has been argued that a deficit in the extraction of meaning from sensory information in schizophrenia may be underpinned by enhanced noise in neural structures (Bach et al., 2011). Despite the noise-like frequency distribution (1/fn) of neural oscillations (Bédard et al., 2006; Bédard and Destexhe, 2009) the notion of “noise” is easily misinterpreted. Spontaneous activity is by far the greatest contributor to ongoing neural activity: activity evoked by sensory inputs is minor by comparison, often being barely detectable above ongoing background activity which has lead some to suggest that “wakefulness is a dream modulated by the senses” (Llinás and Paré, 1991) a notion that is supported by contemporary electrophysiological evidence (Destexhe, 2011). Thus, although neural oscillations appear noise-like, they are anything but, instead reflecting continuous internal processing (Destexhe, 2011). We have found no evidence for an increased amplitude of this ongoing activity (Ewing and Grace, 2013) but significant evidence for a reduction in the amplitude of sensory driven oscillations. Thus aberrant sensory information processing, rather than being attributable to enhanced noise in neural structures, may be better attributed to diminished evoked amplitudes resulting in an reduction in the signal-to-”noise” ratio. Therefore, the ability for sensory input to modulate the ongoing “dream” of wakefulness may be severely disrupted in schizophrenia yielding an internal state which is insufficiently responsive to external input.
Conclusions
It has been suggested that schizophrenic patients, while capable of discriminating between sounds of different intensities, are impaired in utilizing this information in an adaptive manner (Bach et al., 2011). Here we identify remarkably analagous deficits in the MAM model of schizophrenia. These animals appear to be capable of discriminating between sounds of different intensities (latency to peak response) but fail to evoke graded amplitude potentials in response to the different stimulus intensities. This may relate to MAM-rats having an impairment in utilizing this information and ascribing relevance to sounds of different intensities. PPI is thought to be a result of diminished attention to the startle stimulus due to the ongoing processing of the prepulse. Thus PPI deficits in MAM animals may occur as a consequence of this failure to accurately process the prepulse. It has been suggested that an ability to appropriately filter sensory input may be a core deficit in schizophrenia. Here we propose an alternative view whereby the activity evoked by sensory input, rather than being poorly filtered, is simply insufficient to modulate the processing of ongoing background activity. Thus, while both hypotheses propose a diminished signal-to-noise ratio in neural circuits we suggest that this is a consequence of a reduction in the “signal” rather than by an increase in the “noise”.
Footnotes
abbrev. RC, rostrocaudal; ML, mediolateral; DV, dorsoventral
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach DR, Buxtorf K, Strik WK, Neuhoff JG, Seifritz E. Evidence for impaired sound intensity processing in schizophrenia. Schizophrenia bulletin. 2011 Mar;37 (2):426–31. doi: 10.1093/schbul/sbp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard C, Destexhe A. Macroscopic models of local field potentials and the apparent 1/f noise in brain activity. Biophysical journal. 2009 Apr;96 (7):2589–603. doi: 10.1016/j.bpj.2008.12.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard C, Kröger H, Destexhe A. Does the 1/f Frequency Scaling of Brain Signals Reflect Self-Organized Critical States? Physical Review Letters. 2006 Sep;97 (11):1–4. doi: 10.1103/PhysRevLett.97.118102. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics. 2012;29 (4):1165–1188. [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biological psychiatry. 1990 Jan;27 (2):183–92. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Boutros N, Zouridakis G, Rustin T, Peabody C. The P50 component of the auditory evoked potential and subtypes of schizophrenia. Psychiatry research. 1993;47:243–254. doi: 10.1016/0165-1781(93)90082-r. [DOI] [PubMed] [Google Scholar]
- Braff D, Geyer M, Swerdlow N. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001 Jul;156 (2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS. The middle evoked response components and schizophrenia. Schizophrenia Bulletin. 1977;3 (1):93–104. doi: 10.1093/schbul/3.1.93. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Ellenbroek Ba, Cools aR, Coenen aM, van Luijtelaar EL. Differential effects of ketamine on gating of auditory evoked potentials and prepulse inhibition in rats. Psychopharmacology. 1999 Feb;142 (1):9–17. doi: 10.1007/s002130050856. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, van Luijtelaar EL, Cools AR, Ellenbroek BA. Filtering Disturbances in Schizophrenic Patients. Gating of Auditory Evoked Potentials and Prepulse Inhibition of the Acoustic Startle Response Compared. Emphasis on the Role of Dopamine. Current Neuropharmacology. 2003;1 (1):47–87. [Google Scholar]
- Destexhe A. Intracellular and computational evidence for a dominant role of internal network activity in cortical computations. Current opinion in neurobiology. 2011 Oct;21 (5):717–25. doi: 10.1016/j.conb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt Ja. Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology. 2008 May;198 (1):37–49. doi: 10.1007/s00213-008-1071-5. [DOI] [PubMed] [Google Scholar]
- Ewing S, Grace A. Deep brain stimulation of the ventral hippocampus restores deficits in processing of auditory evoked potentials in a rodent developmental disruption model of schizophrenia. Schizophrenia Research. 2013;143:2013. doi: 10.1016/j.schres.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagstad P, AMr, Glenthø j B, van Beek J, Michael-Titus A, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29 (11):2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Glenthø j BY, Didriksen M. Cognitive deficits caused by late gestational disruption of neurogenesis in rats: a preclinical model of schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005 Feb;30 (2):250–60. doi: 10.1038/sj.npp.1300625. [DOI] [PubMed] [Google Scholar]
- Ford J, White P, Lim K. Schizophrenics have fewer and smaller P300s: A single-trial analysis. Biological Psychiatry. 1994 Jan;35 (2):96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunctions in schizophrenia. Schizophrenia research. 1991;4 (2):233–43. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Hradetzky E, Sanderson TM, Tsang TM, Sherwood JL, Fitzjohn SM, Lakics V, Malik N, Schoeffmann S, O’Neill MJ, Cheng TM, Harris LW, Rahmoune H, Guest PC, Sher E, Collingridge GL, Holmes E, Tricklebank MD, Bahn S. The Methylazoxymethanol Acetate (MAM-E17) Rat Model: Molecular and Functional Effects in the Hippocampus. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012 Sep;37:364–377. doi: 10.1038/npp.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Gray Ja, Geyer Ma, Ffytche D, Soni W, Mitter-schiffthaler MT, Vythelingum GN, Simmons A, Williams SC, Sharma T. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Research: Neuroimaging. 2003 Feb;122 (2):99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Hoptman MJ, Foxe JJ, Saccente E, Wylie GR, Nierenberg J, Jalbrzikowski M, Lim KO, Javitt DC. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. The American journal of psychiatry. 2007 Mar;164 (3):474–82. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neuroscience and biobehavioral reviews. 2009 Sep;33 (8):1157–67. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Llinás R, Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44 (3):521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace Aa. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009 Feb;29 (8):2344–54. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman a, Lignelli a, Gorman J, Van Heertum R. SPECT study of visual fixation in schizophrenia and comparison subjects. Biological psychiatry. 1999 Jul;46 (1):89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer Ma, Grace Aa. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biological psychiatry. 2006 Aug;60 (3):253–64. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer Ma, Braff D. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001 Jul;156 (2–3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Wan F, Caine S, Swerdlow N. The ventral subiculum modulation of prepulse inhibition is not mediated via dopamine D 2 or nucleus accumbens non-NMDA glutamate receptor activity. European journal of pharmacology. 1996;314:9–18. doi: 10.1016/s0014-2999(96)00535-3. [DOI] [PubMed] [Google Scholar]