Abstract
Background
Tramadol is an atypical analgesic with monoamine and modest mu opioid agonist activity. The purpose of this study was to evaluate: 1) the efficacy of extended-release (ER) tramadol in treating prescription opioid withdrawal and 2) whether cessation of ER tramadol produces opioid withdrawal.
Methods
Prescription opioid users with current opioid dependence and observed withdrawal participated in this inpatient, two-phase double blind, randomized placebo-controlled trial. In Phase 1 (days 1-7), participants were randomly assigned to matched oral placebo or ER tramadol (200 or 600 mg daily). In Phase 2 (days 8-13), all participants underwent double blind crossover to placebo. Breakthrough withdrawal medications were available for all subjects. Enrollment continued until 12 completers/group was achieved.
Results
Use of breakthrough withdrawal medication differed significantly (p<0.05) among groups in both phases; the 200 mg group received the least amount in Phase 1, and the 600 mg group received the most in both phases. In Phase 1, tramadol 200 mg produced significantly lower peak ratings than placebo on ratings of insomnia, lacrimation, muscular tension, and sneezing. Only tramadol 600 mg produced miosis in Phase 1. In Phase 2, tramadol 600 mg produced higher peak ratings of rhinorrhea, irritable, depressed, heavy/sluggish, and hot/cold flashes than placebo. There were no serious adverse events and no signal of abuse liability for tramadol.
Conclusions
ER tramadol 200 mg modestly attenuated opioid withdrawal. Mild opioid withdrawal occurred after cessation of treatment with 600 mg tramadol. These data support the continued investigation of tramadol as a treatment for opioid withdrawal.
Keywords: opioid withdrawal, prescription opioid dependence, tramadol, treatment, randomized clinical trial, efficacy
1. Introduction
The prevalence of prescription opioid abuse and dependence in the United States (US) has risen in the last decade, increasing from 1.5 to 1.9 million persons from 2002 to 2010 (SAMHSA, 2011; Compton and Volkow, 2006). As opioid analgesics are widely available and opioid dependence is a chronic relapsing medical disorder, the need for opioid dependence treatment will remain high (McLellan et al., 2000). Currently, there are only three approved medications (methadone, burprenorphine and naltrexone) available in the US for treatment of opioid dependence in contrast to the more than twenty medications available for other chronic medical disorders (e.g., hypertension, diabetes).
Tramadol, an atypical analgesic, is currently approved to treat moderate-to-severe pain. It provides analgesia through a dual mechanism of action; the racemic parent compound acts primarily to block reuptake of serotonin and norepinephrine while its hepatic metabolite, (+) o-desmethyltramadol (M1) acts as a mu opioid receptor agonist with modest intrinsic efficacy (Desmeules et al., 1996; Enggaard et al., 2006; Gillen et al., 2000; Hennies et al., 1988; Lai et al., 1996; Raffa et al., 1992). While tramadol clearly possesses some opioid agonist activity and abuse liability (e.g., drug liking), particularly by the oral route (parenteral dosing bypasses first-pass metabolism minimizing M1 production), it appears to have less abuse potential than prototypic mu opioid agonists and remains currently unscheduled under the US Controlled Substances Act (Epstein et al., 2006; Jasinski et al., 1993; Preston et al., 1991; Zacny, 2005). Post-marketing surveillance in the US also indicates that tramadol has lower rates of abuse and diversion than other widely abused opioid analgesics such as oxycodone (Cicero et al., 1999; Cicero et al., 2005; Inciardi et al., 2006; Woody et al., 2003).
Tramadol may be an efficacious treatment for opioid withdrawal. For example, two retrospective chart reviews of inpatients undergoing opioid detoxification evaluated the effects of immediate release (IR) tramadol (i.e., 600 mg-day 1, 400 mg-day 2, 200-300 mg-days 3-4) on opioid withdrawal symptoms. One retrospective study compared tramadol to subcutaneous buprenorphine (sublingual tablets were not yet available) and reported no significant group differences in treatment retention, withdrawal scores or clonidine use (Tamaskar et al., 2003). In a second retrospective study, retention was superior and scores on a non-standardized assessment of opioid withdrawal were reduced among patients treated with tramadol compared to clonidine (Sobey et al., 2003). More recently, a randomized double blind trial in Iran compared daily IR tramadol (600 mg) to daily methadone (60 mg for 3 days followed by dose taper) for treatment of heroin withdrawal. It showed no difference in objective opioid withdrawal scores (OOWS) between groups; however, retention was not reported and pre-randomization withdrawal scores were low (i.e., mean OOWS < 4) (Zarghami et al., 2012). Lastly, a prospective, randomized, controlled inpatient study showed that single doses of IR tramadol (200 and 400 mg) significantly reduced opioid withdrawal compared to placebo among morphine-maintained adults (60 mg SC daily; Lofwall et al., 2007).
To date, there has been no evaluation of repeated dosing of tramadol that has definitively and systematically evaluated its: 1) efficacy in relieving opioid withdrawal; and 2) capacity to produce opioid withdrawal after dosing cessation using a well-controlled, randomized study design. The current two-phase study evaluates both of these aims, and additionally, employs the extended-release formulation, which is FDA-approved for once daily dosing for analgesia (the IR product recommends dosing 4 times/day) and has not yet been evaluated for the indication of opioid withdrawal.
The study also evaluated ER tramadol among adults dependent on prescription opioids because prescription opioid dependent persons may have a lower level of physical dependence that could be amenable to treatment with a medication with more modest opioid agonist effects, like tramadol. For instance, prescription opioid dependent patients have lower Addiction Severity Index opioid subscale scores than heroin dependent patients (Sigmon, 2006) and are less likely to use opioids by the intravenous route (Brands et al., 2004; Moore et al., 2007; Rosenblum et al., 2007; Sigmon, 2006), which has been associated with more favorable treatment outcomes (Simpson et al., 1997). A more recent study also demonstrated that prescription opioid dependent persons had a greater number of opioid-negative urine specimens than heroin dependent persons after buprenorphine taper (Nielsen, et al., 2013).
2. METHODS
2.1 Participants
Opioid dependent individuals age 18-55 years reporting at least 21 days of non-medical use of a short-acting prescription opioid in the last 30 days and willing to undergo inpatient opioid detoxification for 14 days were recruited. Other inclusion criterion included: 1) diagnosis of current opioid dependence with physical dependence on a short-acting prescription opioid as determined by the Structured Clinical Interview for DSM-IV (Spitzer et al., 1992); 2) having an observed urine drug screen positive for a short-acting opioid; 3) healthy as determined by medical history and physical examination, screening laboratories and 12-lead electrocardiogram; and 4) displaying objective signs of opioid withdrawal at time of randomization. Exclusion criteria included other current physiologic drug dependence requiring medical treatment, seizure disorder, uncontrolled medical problems (e.g., asthma), current psychiatric illness (e.g., schizophrenia), recent use of medications with serotonergic action or inhibition or induction of P450 2D6, current pregnancy/lactation, buprenorphine or methadone positive urine drug screen, and self-report of heroin as current primary opioid used (i.e., in last 30 days). Outpatient screening occurred at the University of Kentucky (UK) Straus laboratory; the study was conducted on the UK inpatient research unit. Screening measures included the Beck Depression Inventory (BDI; Beck and Steer, 1984), Symptom Checklist-90 (Derogatis et al., 1976), Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991), Addiction Severity Index (ASI; McLellan et al., 1985), locally-generated time line follow back (TLFB) questionnaire of the last 30 days of opioid use (i.e., name of opioid, route, mg used), short form of the McGill Pain Questionnaire (MPQ) and NEO Personality Inventory (Melzack, 1987).
All participants provided written informed consent and were paid for participation. They received $25 for each screening and follow-up visit ($15 for a single assessment; e.g., vitals). For the inpatient stay, subjects received $35/night regardless of completion status. Those completing the study received a $30/night bonus in order to reinforce study completion. Subjects were informed that they would receive either ER tramadol or placebo and that this could change at any time during the study. The study was approved by the UK Institutional Review Board in accordance with the Declaration of Helsinki and conducted under an FDA investigator-initiated New Drug Application (69,214). A Certificate of Confidentiality was obtained from the National Institutes of Health. The trial was registered at www.clinicaltrials.gov (NCT00980044).
2.2 Trial design and randomization
This two-week inpatient study had two phases. Phase 1 (days 1-7) utilized a double blind, placebo-controlled parallel group design that randomized subjects to one of three groups [twice daily oral placebo or ER tramadol (200 or 600 mg daily; given in two divided doses)]. The 200 mg dose was chosen because it alleviated opioid withdrawal in a previous study (Lofwall et al., 2007). The 600 mg dose was chosen because it was twice the maximum recommended ER tramadol dose for pain and treatment of opioid withdrawal with medications with opioid agonist activity (i.e., methadone and buprenorphine) frequently requires higher doses than treatment for pain. On the morning after admission (at 0730 hrs, a mean of 18 hrs 49 min after admission), urn randomization was used to stratify the groups on gender, primary route of opioid use (intravenous versus oral/intranasal use), and observer-rated opioid adjectives withdrawal score (three levels: 1-5, 6-10, and >10 based on the range of scores from a previous study (Lofwall et al., 2007). In Phase 2 (days 8-13), participants assigned to tramadol in Phase 1 underwent double blind crossover to placebo while the Phase 1 placebo group remained on blinded placebo. Enrollment continued until the a priori target of 12 subjects/group completed the study (based upon power analyses of two randomized studies evaluating efficacy of medication effects on opioid withdrawal (Lofwall et al., 2007; Sigmon et al., 2004).
2.3 Intervention
After meeting eligibility criteria, subjects were scheduled for inpatient admission. Medication administration began at 0900 the day after admission to ensure that, at the time of randomization, signs of opioid withdrawal were evident (a study outcome and eligibility criterion). Blinded study drug was administered at 0900 and 2100 on days 1-13, during which four oral medications were available as needed to treat breakthrough withdrawal symptoms. These included: 1) alumina, magnesia and simethicone 30 ml every 4 hrs for dyspepsia/nausea; 2) bismuth subsalicylate 30 ml every 4 hrs for diarrhea (≤ 6 doses/24hrs); 3) acetaminophen 650 mg every 6 hrs for headache, muscle aches, or other discomfort (no more than 4 doses/24hrs); and 4) zolpidem 5 mg for insomnia between 2300 and 0500 hrs (two doses maximum/evening). Discharge occurred on day 14. Regardless of completion status, an outpatient visit four weeks after discharge was scheduled to resolve ongoing adverse events and to assess ongoing drug use.
While inpatient, subjects received a caffeine-free diet and were allowed to smoke ad lib under supervision between data collection intervals. Urine samples were collected and tested for the presence of amphetamine, barbiturate, benzodiazepine, cocaine, methamphetamine, opiate, methadone, buprenorphine, oxycodone, and marijuana (American Screening Corporation, Shreveport, LA) daily and for pregnancy weekly.
2.4 Outcome measures
The two a priori defined primary outcome measures were number of breakthrough withdrawal doses received and total subject-rated opioid withdrawal adjective score [based upon two earlier studies; (Lofwall et al., 2007; Sigmon et al., 2004)]. Other standardized withdrawal measures, pupil diameter (VIP-200 Pupillometer, Neuroptics, Inc.) and a set of visual analog scale items (VAS; rated 0-100) were collected eight times per day [at 0800 (on day 1 this represents baseline), 1000, 1200, 1400, 1600, 1800, 2000, and 2200 hrs]. Withdrawal measures included the following rating instruments: a) 21-item subject- and observer-rated opioid adjective antagonist (i.e., withdrawal) rating questionnaire whereby each item is scored from 0 (not at all) to 4 (extremely) (Lofwall et al., 2007; Strain et al., 1995); b) 10-item short opiate withdrawal scale [SOWS; each item scored from 0 (none) to 3 (severe)] (Gossop, 1990); c) 13-item objective opiate withdrawal scale [OOWS; each item scored 0 (absent) or 1 (present)] (Handelsman et al., 1987); and d) the 7-item modified Himmelsbach scale with each item scored 0 to 2 (Eissenberg et al., 1996). Total withdrawal scores for each measure were calculated by summing the individual item scores. VAS items assessed opiate craving as well as “drug effect,” “high,” “liking,” “bad effects,” “good effects,” “side effects,” and “feel sick” from the blinded study medication, how much subjects “crave” the blinded study medication, and how much it was “helping” their opioid withdrawal.
Immediately prior to each scheduled dose, participants were asked, in reference to the last dose, to rate its street value ($) and identify whether it was tramadol or placebo. Vital signs (blood pressure, pulse, respiratory rate, and temperature) were collected before each dose and at 1400 hrs. Once daily, the digit symbol substitution task (McLeod et al., 1982), MPQ, BDI, Minnesota Nicotine Withdrawal Scale (Hughes, 1992; Toll et al., 2007), and Brief Questionnaire of Smoking Urges were administered (Tiffany and Drobes, 1991). Smoking measures were part of a secondary study (results reported elsewhere).
2.5 Blinded Medication
ER tramadol (Ultram ER®) 100 mg and 300 mg tablets (Cardinal Health, Knoxville, TN) were used for the twice-daily 100 mg and 300 mg doses, respectively. Placebo was cornstarch powder (Spectrum Chemical Mfg. Corp, Gardena, CA). All doses were over-encapsulated with size 0 gelatin capsules (Health Care Logistics, Circleville, OH) and loose-filled with cornstarch to maintain the blind.
2.6 Analyses
Participant characteristics and baseline measures were compared between groups using a 1-factor model and chi-square tests. Cox proportional hazards model analyses evaluated trial retention by group for 1) Phase 1 for all subjects randomized (data censored at day 7) and 2) Phase 2 (among only those entering Phase 2) with data censored at day 14 (Cox, 1972). Primary analyses for all outcomes were evaluated using repeated measures models with an AR(1) covariance structure with three factors (drug, day, and time) separately for each phase. Day was defined as the 24-hr period starting at 0900 hr (first daily blinded dose administration). Change-from-baseline values were analyzed for heart rate because of significant baseline group differences. Secondary analyses included 1-factor (group) model evaluating peak maximum values (nadir for pupils and respirations) over days 1-3, the period when short-acting opioid withdrawal is expected to peak and days 8-13. Dunnett tests were conducted to compare each tramadol condition to the placebo reference condition. Only significant Dunnett tests and main effects of group and interactions involving group are reported for secondary outcomes. All models were run with Proc Mixed in SAS 9.3 (SAS Institute, Inc., Cary, NC). Data are presented as means (standard error) unless otherwise indicated. Statistical significance was set at p<0.05.
3. RESULTS
3.1 Participants and baseline measures
Table 1 shows participant characteristics. There were no significant group differences on baseline characteristics with the exception of baseline heart rate (placebo: 73.1 [3.1]; tramadol 200 mg: 75.3 [2.4]; tramadol 600 mg: 83.6 [2.8]). Figure 1 shows participant flow through the study, which was conducted from September 21, 2009 to March 8, 2012. Survival analyses revealed no group differences in Phase 1 (Cox analysis X2=0.004; p=.95; Hazard Ratio 0.98 [CI 0.50-1.91]) or Phase 2 (Cox analysis X2=.000; p=.99; Hazard Ratio 0.99 [CI 0.304-3.228]) retention. Supplementary Tables 1 and 21 further describe the frequency, amount (mg) and types of prescription opioids used.
Table 1.
Subject demographic and drug use characteristics
| Placebo (n=12) |
T 200 mg (n=12) |
T 600 mg (n=12) |
|
|---|---|---|---|
| Age (years) | 32.0 (2.2) | 26.8 (0.9) | 31.6 (1.5) |
| Education (years completed) | 11.8 (0.5) | 10.4 (0.5) | 11.1 (0.6) |
| Male gender, n (%)* | 8 (66.7) | 7 (58.3) | 7 (58.3) |
| Race n (%): | |||
| Caucasian | 10 (83.3) | 12 (100.0) | 12 (100.0) |
| African American | 2 (16.7) | 0 (0.0) | 0 (0.0) |
| Weight (kg) | 73.4 (4.3) | 70.4 (2.5) | 77.6 (3.7) |
| Unemployed, n (%) | 9 (75.0) | 8 (66.7) | 10 (83.3) |
| # days in last 30 use of: | |||
| Alcohol | 1.4 (0.5) | 1.8 (1.1) | 0.8 (0.4) |
| Cannabis | 9.4 (3.7) | 8.1 (3.8) | 6.1 (2.9) |
| Cocaine | 0.7 (0.4) | 0.3 (0.1) | 0.1 (0.1) |
| Heroin | 0.3 (0.2) | 0.1 (0.1) | 0.1 (0.1) |
| Methadone | 0.2 (0.1) | 0.0 (0.0) | 0.2 (0.2) |
| Sedative/Hypnotics | 0.8 (0.4) | 3.1 (1.5) | 0.7 (0.4) |
| Short-acting prescription (rx) opioids | 29.0 (0.6) | 29.3 (0.4) | 28.9 (0.4) |
| Suboxone | 0.3 (0.2) | 0.1 (0.1) | 0.9 (0.8) |
| Tramadol | 0.0 (0.0) | 0.0 (0.0) | 0.3 (0.2) |
| # cigarettes smoked/day | 17.1 (1.8) | 21.3 (2.6) | 21.9 (2.1) |
| Years of regular (>2-3x/week) non-medical rx opioid use | 7.5 (1.4) | 6.0 (1.2) | 6.5 (1.5) |
| ASI composite scores: | |||
| Drug | 0.32 (0.03) | 0.30 (0.02) | 0.25 (0.03) |
| Alcohol | 0.04 (0.01) | 0.04 (0.02) | 0.01 (0.01) |
| Primary route of rx opioid use in last 30 days, n (%): | |||
| Intravenous* | 3 (25.0) | 4 (33.3) | 3 (25.0) |
| Intranasal | 9 (75.0) | 7 (58.3) | 8 (66.7) |
| Oral | 0 (0.0) | 1 (8.3) | 1 (8.3) |
Values are mean (SE) unless otherwise indicated. T = extended release tramadol. There were no significant differences between groups on any measure.
indicates a stratification variable.
Figure 1.
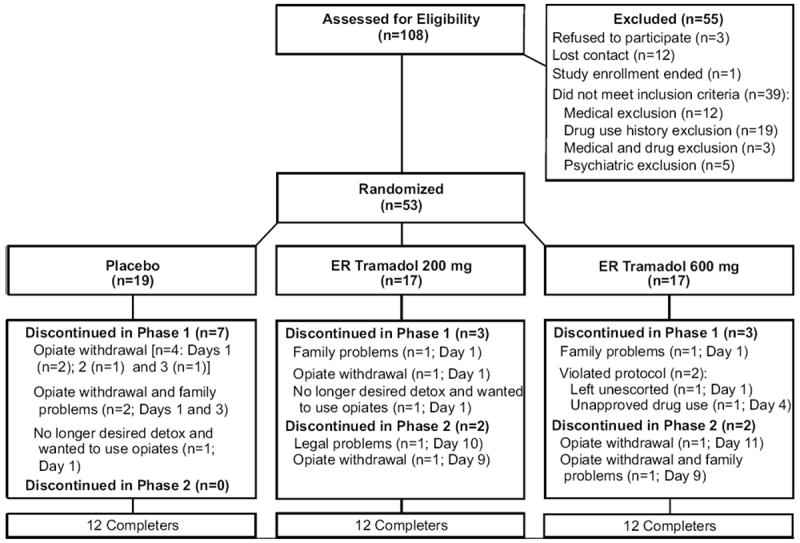
Participant flow through the study. ER = extended-release.
3.2 Phase 1 primary outcomes
Breakthrough withdrawal medication administered by day is displayed in Figure 2. Time course analyses of the amount of breakthrough withdrawal medications received (top panel) showed a significant main effect of group [F(4.320); p=0.022] and day [F(3.010); p=0.008)]. The fewest average doses per day were taken by the tramadol 200 mg group [1.8(0.2)] followed by the placebo [2.5(0.2)] and tramadol 600 mg [3.1(0.2)] groups. The placebo and tramadol 200 mg groups requested fewer breakthrough doses over time. In contrast, the daily number of breakthrough doses remained relatively steady for tramadol 600 mg. Time course analyses of each individual medication revealed a similar pattern of effects for acetaminophen [Group: F(4.96); p=0.013 and Day: F(3.510); p=0.003]. Specifically, subjects receiving tramadol 200 mg requested significantly fewer acetaminophen doses/day than subjects receiving placebo (Dunnett p=0.044). There were significant group effects also for alumina, magnesia, and simethicone [F(5.230); p=0.011]; more doses/day were given in the tramadol 600 mg condition compared to placebo (Dunnett p=0.02). For bismuth subsalicylate, there was a significant group × day interaction [F(2.440); p=0.006] with more doses given on day 2 in the tramadol 200 mg group than placebo group [Dunnett p<0.01), although the mean number of doses was low (<1.0) in both groups. There were no significant results for zolpidem.
Figure 2.
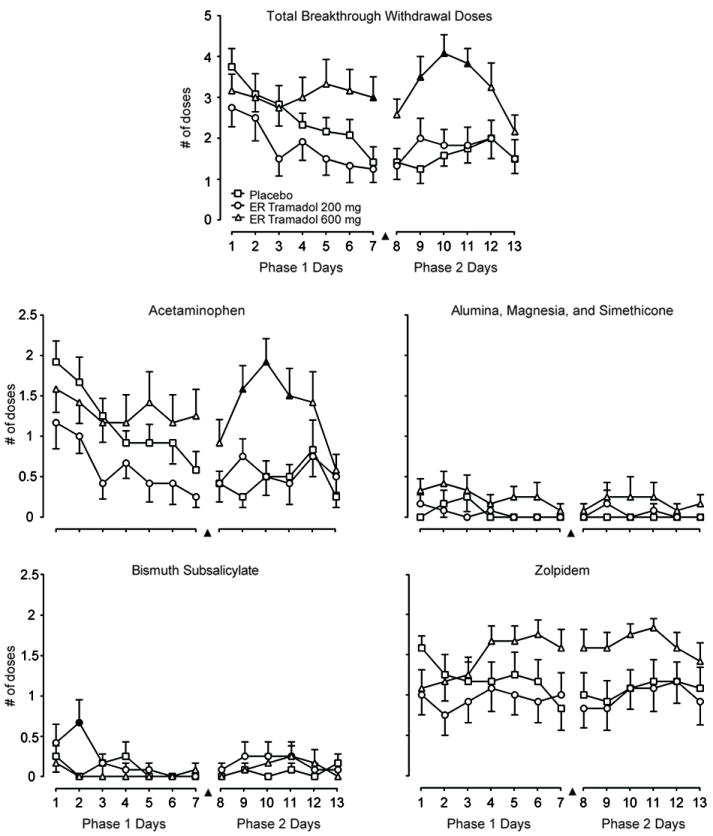
Breakthrough withdrawal medication doses by group and day within each phase. ER = extended-release. ▲indicates when both tramadol groups underwent double blind crossover to placebo; placebo group remained on placebo. Data are means with S.E. bars in one direction. There were significant Phase 1 group effects for the number of doses of all breakthrough withdrawal medications (top panel) and for each individual medication except zolpidem. During Phase 2, there were significant group effects for total doses, acetaminophen, and zolpidem. A filled symbol indicates a significant post-hoc difference compared to placebo at that time point.
Subject-rated opioid adjective withdrawal ratings are displayed by day (collapsed across time) in Figure 3 (top panel). There were only main effects of day [F(7.600); p<0.0001) and time [F(3.540); p=0.001], whereby scores decreased over the 7-day period in all conditions.
Figure 3.
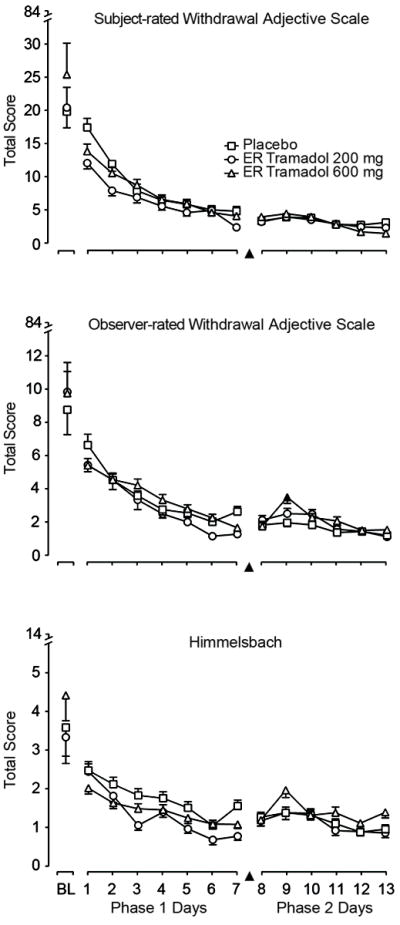
Opioid withdrawal ratings by group and day for each phase. ER = extended-release. Data are means with S.E. bars in one direction. There were no significant effects for the subject-rated withdrawal adjectives in either phase; however, there were significant group effects for both observer-rated withdrawal measures (e.g., the middle and lower panels) during Phase 1. During Phase 2, there was a significant group × time interaction on the observer withdrawal adjectives only. A filled symbol indicates a significant post-hoc difference compared to placebo at that time point.
3.3 Phase 1 secondary outcomes
The observer-rated withdrawal adjective scale (middle panel) and Himmelsbach (bottom panel) results are shown in Figure 3. The observer-rated adjective withdrawal scale showed that tramadol 200 mg generally had the lowest scores over time, and there was a significant group × time interaction [F(2.540; p=0.002)]. There was a main effect of group on the Himmelsbach [F(6.020); p=0.006] with post-hoc tests showing that, compared to placebo, both tramadol conditions had lower ratings (200 mg p=0.004; 600 mg p=0.037). The OOWS and SOWS showed no significant group effects (data not shown).
Time course analyses showed no significant group effects on VAS items, BDI, MPQ and the DSST. There were significant drug effects on heart rate [F(31.590; p<0.0001)], diastolic blood pressure [F(3.320; p=0.048)], temperature [F(9.060; p=0.001)] and respiration [F(3.470; p=0.043)] whereby placebo always had the highest mean values; but, while statistically significant, these were not clinically significant. Only tramadol 600 mg produced significant miosis compared to placebo on time course analyses [see Figure 4: group F(21.560); p<0.0001].
Figure 4.
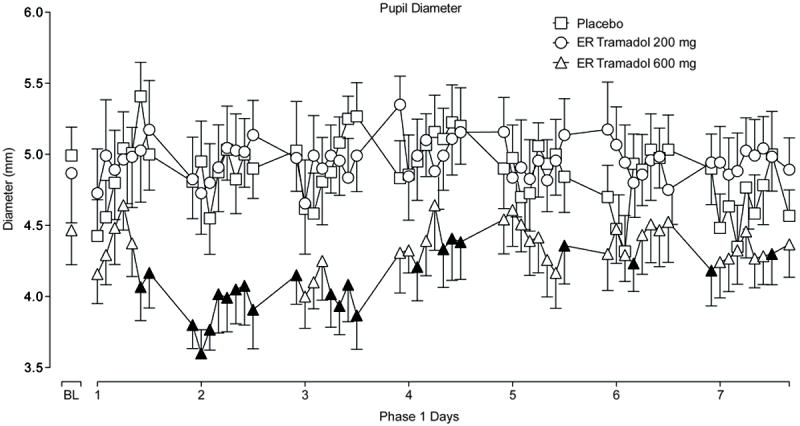
Pupil diameter over time by group in Phase 1. ER = extended-release. Data are means with S.E. bars in one direction. There were significant main effects of group on pupil diameter during Phase 1, whereby pupil diameter was smaller among those receiving ER tramadol 600 mg daily compared to placebo (p<0.05). A filled symbol indicates a significant post-hoc difference compared to placebo at that time point.
Peak scores over days 1-3 are displayed in Table 2 with individual item values displayed if there were statistically significant drug effects. For example, compared to placebo, both tramadol doses produced lower peak ratings on sneezing, but only tramadol 200 mg produced lower peak ratings on lacrimation, muscular tension, and insomnia. Pupil diameter was smallest and heart rate increased the least in the tramadol 600 mg condition. Peak scores over days 1-7 also were conducted on withdrawal measures and the MPQ because the time course data for breakthrough withdrawal medications suggested there may group differences emerging after day 3; there were no additional significant results (data not shown).
Table 2.
Peak values calculated during Phase 1 Over Days 1-3
| Peak maximum values: | Placebo (n=12) |
T 200 mg (n=12) |
T 600 mg (n=12) |
p value |
|---|---|---|---|---|
| Withdrawal measures: | ||||
| Subject-rated adjectives total | 23.1 (4.3) | 19.2 (2.4) | 24.5 (3.5) | - |
| Observer-rated adjectives total | 13.3 (2.1) | 13.6 (3.3) | 12.4 (1.2) | - |
| -Sneezing | 1.2 (0.3) | 0.3 (0.2) | 0.0 (0.0) | 0.003 |
| SOWS total | 10.2 (1.9) | 8.1 (1.0) | 10.8 (1.3) | - |
| -Muscular Tension | 1.3 (0.3) | 0.6 (0.2) | 1.3 (0.3) | 0.048 |
| -Insomnia | 1.8 (0.3) | 1.0 (0.3) | 2.1 (0.2) | 0.036 |
| OOWS total | 4.9 (0.6) | 5.4 (0.8) | 4.8 (0.4) | - |
| -Lacrimation | 0.7 (0.1) | 0.2 (0.1) | 0.3 (0.1) | 0.037 |
| Himmelsbach total | 5.5 (0.6) | 5.5 (0.6) | 4.8 (0.3) | - |
| -Lacrimation | 1.1 (0.2) | 0.3 (0.2) | 0.5 (0.2) | 0.028 |
| VAS medication items: | ||||
| Helping withdrawal | 36.6 (8.7) | 46.9 (9.5) | 46.1 (11.7) | - |
| Side effects | 5.9 (3.2) | 6.2 (4.9) | 9.5 (7.5) | - |
| Any drug effect | 28.8 (7.3) | 36.9 (9.3) | 42.2 (11.5) | - |
| High | 0.7 (0.5) | 0.0 (0.0) | 6.5 (5.8) | - |
| Good effects | 37.8 (8.5) | 39.4 (9.4) | 43.8 (12.0) | - |
| Like | 30.5 (9.2) | 39.6 (9.5) | 39.5 (11.9) | - |
| Bad effects | 8.6 (3.9) | 7.3 (5.7) | 10.2 (7.1) | - |
| Feel sick | 9.3 (8.3) | 6.2 (5.6) | 5.5 (5.4) | - |
| Crave | 8.4 (5.7) | 6.7 (6.7) | 5.8 (5.7) | - |
| MPQ Total | 16.9 (3.9) | 15.6 (4.0) | 13.4 (2.6) | - |
| BDI Total | 11.8 (1.4) | 11.0 (2.5) | 8.3 (2.1) | - |
| VAS Desire opiates | 71.7 (8.6) | 82.5 (5.9) | 89.3 (5.0) | - |
| Street value ($) | 2.2 (0.9) | 1.8 (1.0) | 1.6 (0.6) | - |
| Physiologic measures: | ||||
| SBP (mm Hg) | 142.6 (4.5) | 130.9 (4.3) | 137.9 (4.1) | - |
| DBP (mm Hg) | 84.7 (2.5) | 79.3 (2.9) | 78.8 (2.4) | - |
| Heart Rate (bpm)* | 19.3 (3.5) | 14.6 (3.5) | 3.7 (1.4) | 0.002 |
| Temperature (F) | 98.8 (0.2) | 98.6 (0.2) | 98.3 (0.2) | - |
| Respiratory rate nadir | 13.6 (0.2) | 12.7 (0.4) | 13.7 (0.4) | - |
| Pupil diameter max (mm) | 5.8 (0.2) | 6.0 (0.2) | 5.2 (0.2) | 0.032 |
| Pupil diameter nadir (mm) | 3.7 (0.2) | 4.2 (0.3) | 3.1 (0.1) | 0.003 |
| DSST accuracy nadir (%) | 88.5 (2.4) | 85.6 (3.2) | 88.8 (2.3) | - |
Data are peak max means (SE) unless otherwise indicated. Doses listed are extended release tramadol total daily doses.
indicates peak change from baseline. Bolded value indicates significant Dunnett compared to placebo. - indicates insignificant ANOVA group p value.
The percentage of correctly identified blinded medication doses was 64% for the tramadol 200 mg group, 52% for the tramadol 600 mg group, and 61% for the placebo group. Mean street values of placebo and both tramadol doses were never greater than $2.00 or $1.50, respectively, on any day at any time. Adverse events were rated as mild to moderate in severity. In the tramadol 600 mg group, one volunteer with a sore throat was diagnosed with group B strep on day 4, and one volunteer reported onset of “unsteady urine flow” without dysuria on day 3 that resolved on day 11; urinalysis was normal. One subject on tramadol 200 mg complained of intermittent “sharp” chest pain lasting 4-5 seconds on days 1 and 4; an echocardiogram and ECG were within normal limits. A subject on tramadol 200 mg complained of left-sided flank pain, and a subject on placebo complained of abdominal pain; both had unremarkable medical work-ups and symptoms resolved without intervention.
3.4 Phase 2 primary outcomes
Time course analyses showed significant main effects of group and day for total doses of all breakthrough withdrawal medications [group F(11.310); p<0.0001 and day [F(3.050); p=0.012] and acetaminophen [group F(9.970); p<0.0001 and day F(4.050); p=0.020]. Specifically, as displayed in Figure 2, subjects transferring to placebo from tramadol 600 mg requested more rescue doses, particularly on days 9 through 11, compared to those remaining on placebo from Phase 1 (total rescue doses Dunnett p<0.0001; acetaminophen Dunnett p=0.001). Zolpidem showed a similar pattern of effects with a significant group effect [F(3.810); p=0.033]. There were no significant effects for bismuth subsalicylate or alumina, magnesia, and simethicone, which were administered infrequently. There were no significant group differences on the subject-rated opioid adjectives withdrawal scale; mean scores for each group were low (<5) in this phase (see Figure 3 top panel).
3.5 Phase 2 secondary outcomes
Figure 3 (middle panel) shows the observer adjectives, which had a significant group × time interaction [F(1.870); p=0.030]. Mean withdrawal scores for tramadol 600 mg increased from days 8 to 9 such that on day 9, tramadol 600 mg had significantly higher total withdrawal scores compared to placebo. After day 9, withdrawal scores for tramadol 600 mg decreased to values similar to tramadol 200 mg and placebo. Scores for tramadol 200 mg and placebo remained relatively flat over time. There were no significant time course results on other withdrawal measure total scores.
Peak results from days 8-13 are shown in Table 3. There were significant differences in peak effects between the tramadol 600 mg compared to placebo on several individual withdrawal items. Specifically, the tramadol 600 mg group had higher peak ratings on observer-rated withdrawal items including heavy/sluggish, irritable, depressed, and rhinorrhea, and subjectively, they reported higher peak ratings of hot/cold flashes. Time course and peak analyses showed no significant group effect on VAS items, vitals, MPQ, BDI or DSST. There was a significant group × time [F(1.920); p=0.025] effect on pupils, but Dunnett tests were not significant and visual inspection also showed no evidence of mydriasis for those transferring from either tramadol group to placebo.
Table 3.
Peak values calculated during Phase 2 over Days 8-13
| Peak maximum values: | Placebo (n=12) |
T 200 mg (n=12) |
T 600 mg (n=12) |
p value |
|---|---|---|---|---|
| Withdrawal measures: | ||||
| Subject-rated adjectives total | 7.4 (2.2) | 7.5 (1.9) | 10.5 (1.8) | - |
| -Hot/cold | 0.0 (0.0) | 0.3 (0.1) | 0.5 (0.2) | - |
| Observer-rated adjectives total | 7.2 (0.8) | 7.8 (1.5) | 9.7 (1.3) | - |
| -Heavy/sluggish | 1.2 (0.3) | 1.3 (0.1) | 2.1 (0.2) | 0.014 |
| -Irritable | 0.6 (0.2) | 0.3 (0.1) | 1.6 (0.4) | 0.003 |
| -Depressed | 0.6 (0.2) | 0.9 (0.3) | 1.4 (0.2) | - |
| SOWS total | 3.4 (1.1) | 3.0 (1.0) | 4.8 (0.9) | - |
| Himmelsbach total | 4.8 (0.5) | 4.7 (0.7) | 5.3 (0.5) | - |
| -Rhinorrhea | 1.1 (0.2) | 1.4 (0.2) | 1.7 (0.1) | - |
| VAS medication items: | ||||
| Helping withdrawal | 23.1 (10.9) | 28.4 (10.6) | 28.7 (9.8) | - |
| Side effects | 8.9 (8.3) | 0.0 (0.0) | 1.6 (1.3) | - |
| Any drug effect | 24.5 (10.6) | 30.4 (10.4) | 27.8 (9.3) | - |
| High | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | - |
| Good effects | 26.3 (10.5) | 30.4 (10.6) | 28.1 (9.2) | - |
| Like | 25.5 (10.6) | 24.3 (8.7) | 23.8 (9.2) | - |
| Bad effects | 8.8 (8.3) | 0.4 (0.4) | 0.8 (0.6) | - |
| Feel sick | 1.5 (1.5) | 0.1 (0.1) | 0.0 (0.0) | - |
| Crave | 1.3 (1.3) | 9.7 (5.9) | 4.8 (4.8) | - |
| MPQ total | 6.3 (2.3) | 8.3 (2.6) | 9.8 (3.0) | - |
| BDI total | 7.4 (1.4) | 7.8 (2.1) | 6.8 (2.1) | - |
| VAS desire opiates | 52.6 (10.5) | 37.6 (10.1) | 58.8 (11.1) | - |
| Street value ($) | 1.4 (0.6) | 1.8 (1.1) | 1.1 (0.6) | - |
| Physiologic measures: | ||||
| SBP (mm Hg) | 140.9 (3.5) | 138.5 (4.2) | 140.8 (3.8) | - |
| DBP (mm Hg) | 82.6 (2.3) | 84.0 (2.1) | 84.8 (2.0) | - |
| Heart rate (bpm) | 99.5 (2.7) | 98.3 (2.3) | 101.8 (1.6) | - |
| Temperature (F) | 98.8 (0.1) | 98.8 (0.1) | 99.0 (0.1) | - |
| Respiratory rate nadir | 13.3 (0.3) | 13.2 (0.3) | 13.0 (0.3) | - |
| Pupil diameter max (mm) | 5.7 (0.2) | 5.9 (0.2) | 5.6 (0.1) | - |
| Pupil diameter nadir (mm) | 3.5 (0.2) | 3.6 (0.2) | 3.2 (0.2) | - |
| DSST accuracy nadir (%) | 90.3 (1.6) | 87.2 (2.5) | 89.0 (1.9) | - |
Data are peak max means (SE) unless otherwise indicated. Doses listed are Phase 1 extended release tramadol total daily doses before crossing over to placebo. - indicates insignificant ANOVA group effect p value. Bolded value indicates significant Dunnett comparison to placebo.
Subjects transferring from Phase 1 tramadol 200 and 600 mg groups correctly identified their blinded doses as placebo 47% and 60% of the time, respectively, while those remaining on placebo correctly identified receiving placebo 72% of the time (p>0.05). Adverse events in this phase were of mild-to-moderate severity and occurred only among subjects crossing over from tramadol 600 mg. Three subjects complained of pain in their teeth with onset on day 11 or 12, one subject had onset of a non-pruritic painful rash on day 12 that resolved with topical hydrocortisone, and one subject had an asymptomatic transient elevated heart rate of 108 in the context of being upset about an interpersonal problem.
3.6 4-week follow-up outcomes
Twenty-five subjects (all completers) returned for follow-up 4 weeks after inpatient discharge. Fourteen tested positive on urine drug screen for an opioid, most commonly oxycodone. Their ASI Drug Composite score was 0.10 (0.02).
4. DISCUSSION
The present two-phase inpatient clinical trial represents the first randomized double blind, placebo-controlled evaluation of ER tramadol in prescription opioid dependent adults willing to undergo opioid detoxification. Phase 1 evaluated the efficacy of ER tramadol given for 7 days for treating opioid withdrawal, and Phase 2 evaluated whether acute dosing cessation of ER tramadol produced evidence of opioid withdrawal over a 6-day period. Results showed significant differences among treatment groups during both study phases on the amount of breakthrough withdrawal medication administered, a primary outcome. Specifically, in Phase 1, the tramadol 200 mg group required the least number of breakthrough doses, followed by placebo, and then tramadol 600 mg. The 200 mg dose group also had lower peak ratings on several withdrawal items (e.g., insomnia). In Phase 2, subjects transferring to placebo from tramadol 600 mg received the most breakthrough medication and had significantly (p<0.05) higher total ratings of opioid withdrawal on two observer-rated measures (p<0.05) and higher peak ratings on several withdrawal signs (e.g., rhinorrhea). The study doses were generally well-tolerated, safe and did not produce signals of abuse liability.
In Phase 1, there were statistically significant group differences on total scores on observer-rated withdrawal measures (see Figure 3). Compared to placebo, subjects receiving tramadol 200 mg reported lower peak ratings of insomnia and muscular tension and observers rated this group as having lower peak ratings of lacrimation and sneezing (see Table 2). This attenuation of peak ratings of signs and symptoms of withdrawal was generally modest in overall magnitude. The lack of more robust group differences in opioid withdrawal measures is not due to a lack of baseline opioid withdrawal. Mean baseline objective and subjective withdrawal adjective scores were similar to those among persons maintained on 60 mg of parenteral morphine daily (Lofwall et al. 2007). Rather, it is likely due, in part, to having breakthrough withdrawal medications available. Thus, the placebo group was not a “no treatment” group, consistent with the result that there were no differences among groups on treatment retention. This highlights the importance, as well as strength and sensitivity, of using amount of breakthrough medication as an additional primary outcome measure, similar to other clinical trials of opioid withdrawal where it is ethically questionable to offer “no treatment” (Sigmon et al., 2004). These results provide a positive signal of clinical efficacy; however, it is not clear whether these differences will be substantial enough to be clinically meaningful and effective. It is encouraging, however, to note that a randomized placebo-controlled study of depot buprenorphine microcapsules (58 mg) versus placebo for treatment of opioid withdrawal (primarily heroin users) found no differences in measures of withdrawal between groups but did report differences in use of adjunctive breakthrough withdrawal medications. A study of tramadol effectiveness for treatment of opioid withdrawal is now warranted.
A strength of the study is that it evaluated both a therapeutic (200 mg daily) and a supra-therapeutic dose (600 mg daily) of ER tramadol given for 7 days. The lower tramadol dose was superior to the higher tramadol dose and placebo, producing decreased use of breakthrough withdrawal medications that was evident by day 3 (see Figure 2 top panel). This delay is not surprising because: 1) the time to peak concentration of the parent compound and M1, the mu-opioid agonist metabolite, are 12 and 15 hours, respectively; and 2) it takes approximately 2 days to reach steady-state based on terminal elimination half-lives (7.9 hours for tramadol and 8.8 hours for M1) (Hair et al., 2006; Ortho-McNeil, 2006). However, it was surprising that tramadol 200 mg was superior to the 600 mg dose, particularly when the 600 mg dose was the one that produced clear evidence of M1 opioid agonist action (i.e., pupillary miosis) (Fliegert et al., 2005). However, the lack of miosis with the 200 mg dose does not necessarily indicate that this dose was lacking in opioid agonist activity. Pretreatment with naltrexone blocks tramadol-induced miosis and produces mydriasis, which is postulated to be due to the noradrenergic action of tramadol (Stoops et al., 2012). It is possible that the monoamine action of tramadol suppressed the manifestation of opioid agonist action on the pupil at this dose. However, medications with more opioid agonist activity would be expected to be more efficacious in suppressing withdrawal, which was not the case here. There are two possible explanations for this finding.
First, it is notable that the reason the 200 mg group used the least amount of total breakthrough withdrawal medication was primarily because they used less acetaminophen and less zolpidem. Body aches and insomnia are symptoms of opioid withdrawal, and while there were no drug effects on MPQ ratings, tramadol 200 mg produced the lowest peak ratings on insomnia and muscular tension in the SOWS. As tramadol produces its analgesia through both monoaminergic and opioid mechanisms (Raffa et al., 1992), perhaps it is tramadol’s dual mechanism of analgesic action, and not solely its opioid action, that accounts for its efficacy in attenuating insomnia and muscular tension. As pain is a common problem among opioid dependent patients, and a large number (44-98%) of opioid dependent patients complain of pain as a primary reason to use prescription opioids (Brands et al., 2004; Rosenblum et al., 2003), tramadol may be particularly useful as a withdrawal treatment for a dually diagnosed opioid dependent and chronic non-malignant pain population.
Second, tramadol produces dose-related inhibition of the serotonin and norepinephrine transporter (Barann et al., 2006; Raffa et al., 1992; Raffa et al., 1993), such that increased blockade with the 600 mg dose may have produced unpleasant effects. This could explain why this group received more breakthrough withdrawal medication than even the placebo group. Peak “bad effects” (9.5) and “side effects” (10.2) ratings were highest in the 600 mg group, although these were not statistically significant. Other work with non-dependent opioid abusers and opioid dependent adults in withdrawal has shown dose-related increases in “bad effects” after administration of single doses of IR tramadol. These unpleasant effects with escalating doses have been hypothesized to be secondary to excess monoaminergic activity (Babalonis et al., 2013; Lofwall et al., 2007; Stoops et al., 2012). Overall, it is possible that both explanations contributed to the findings here, which then suggests that optimal repeated dosing of tramadol for opioid withdrawal treatment may lie within the analgesic dosing range (maximum recommended ER daily dose is 300 mg) or if above it, less than 600 mg daily.
Importantly, tramadol was safely administered and produced few adverse events. One subject on tramadol 600 mg reported symptoms of urinary retention that resolved after the placebo crossover. This has been previously reported and is thought to be secondary to tramadol’s opioid agonist activity, which can increase bladder sphincter tone (Meyboom et al., 1999).
There was no difference between tramadol and placebo on ratings of positive subjective effects, such as craving the medication, liking and good effects that are indicative of abuse liability. These findings are consistent with other behavioral pharmacology and post-marketing surveillance studies evaluating IR tramadol reporting reduced abuse liability compared to other prototypic mu-opioid analgesics (Cicero et al., 1999, 2005; Epstein et al., 2006; Jasinski et al., 1993; Preston et al., 1991; Stoops et al., 2012; Woody et al., 2003). However, a recent study demonstrated that non-dependent prescription opioid abusers would work on a progressive ratio schedule to receive IR tramadol 400 mg to a similar degree as oxycodone 40 mg (Babalonis et al., 2013). The reinforcing efficacy of ER tramadol in an opioid dependent sample is not yet known but is relevant as tramadol continues to be considered as a potential treatment for opioid withdrawal and is now within the top 25 most commonly prescribed drugs in the US (IMS Health, 2012).
Acute cessation of ER tramadol dosing after one week of 600 mg daily, but not 200 mg daily, produced evidence of mild opioid withdrawal. This finding is internally consistent with the result that only the 600 mg dose produced evidence of opioid agonist activity (i.e., miosis) although mydriasis was not present during Phase 2. It is also concordant with case reports of opioid withdrawal after tramadol dosing cessation and laboratory findings of prominent naloxone-precipitated opioid withdrawal among adults maintained on 800 mg of IR tramadol, but less robust precipitated withdrawal while maintained on 200 mg (Barsotti et al., 2003; Freye and Levy, 2000; Lanier et al., 2010; Leo et al., 2000; Rodriguez Villamanan et al., 2000; Tjaderborn et al., 2009).
A study limitation is that it excluded persons dependent primarily on heroin. A recent large multi-site clinical trial of prescription opioid dependent adults undergoing detoxification with buprenorphine showed high relapse rates, not unlike those with heroin dependence (Weiss et al., 2011). Future work with tramadol should include heroin dependent patients. In addition, this was an inpatient study whereby frequent evaluation of withdrawal was possible in order to evaluate the efficacy of ER tramadol; the results here are not generalizable to an outpatient setting.
In summary, the study results show that the lower ER tramadol dose of 200 mg daily was more efficacious than placebo and the 600 mg dose in treating withdrawal from short-acting prescription opioids. In fact, the 600 mg dose appeared to worsen opioid withdrawal as evidenced by more use of breakthrough withdrawal medications. Both doses were safe and well-tolerated; however, the 600 mg dose also produced evidence of opioid withdrawal. Taken together, these results support the further investigation of ER tramadol for treating opioid withdrawal with ER doses less than 600 mg daily in a larger outpatient study.
Supplementary Material
Acknowledgments
We express our gratitude to the UK Investigational Drug Pharmacy, the UK Center on Drug and Alcohol Research staff, and the nursing staff at the UK Center for Clinical and Translational Science (CCTS) that all worked diligently on this study.
Role of Funding Source This study was supported by the National Institute on Drug Abuse (NIDA) R01 DA027068 (MRL) and T32 DA007304 and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health grant UL1RR033173. NIDA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors Dr. Lofwall designed the study, wrote the protocol and the first draft of the manuscript, and provided primary study oversight. Dr. Babalonis assisted with recruitment, screening, and management of the study. Dr. Walsh helped design the study, provided study oversight and interpretation of the data. Dr. Siegel conducted physical exams and provided medical coverage. Dr. Campbell read study ECG’s and provided medical consultation. Mr. Nuzzo conducted the randomizations, assisted with study oversight and statistical analyses. All authors contributed to and have approved the final manuscript.
Conflict of Interest Dr. Lofwall has served as a consultant for Orexo Pharmaceuticals, received honorarium for serving on an advisory board and giving educational talks on opioid dependence from PCM Scientific, and received an investigator-initiated research grant from Reckitt Benckiser. Sharon Walsh has received speaking honoraria and travel reimbursement from Reckitt Benckiser, consulting fees from Cephalon Inc., and honoraria for expert safety panel service from Meda Pharmaceuticals (all manufacturers of opioid products). Mr. Nuzzo was a statistical consultant and project coordinator for the NIDA Clinical Trials Network (CTN), NIDA Clinical Coordinating Center, for Yaupon Therapeutics, Inc., and for the Behavioral Pharmacology Research Unit, The Johns Hopkins University. Dr. Siegel has received honorarium for giving education talks about antipsychotic medications for Janssen Pharmaceuticals. Dr. Campbell reports no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2010 National Survey on Drug Use and Health (NSDUH): Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658; Rockville, MD: 2011. [Google Scholar]
- Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 2013;129:116–24. doi: 10.1016/j.drugalcdep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barann M, Urban B, Stamer U, Dorner Z, Bonisch H, Bruss M. Effects of tramadol and O-demethyl-tramadol on human 5-HT reuptake carriers and human 5-HT3A receptors: a possible mechanism for tramadol-induced early emesis. Eur J Pharmacol. 2006;531:54–58. doi: 10.1016/j.ejphar.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Barsotti CE, Mycyk MB, Reyes J. Withdrawal syndrome from tramadol hydrochloride. Am J Emerg Med. 2003;21:87–88. doi: 10.1053/ajem.2003.50039. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Benziger DP, Kaiko RF, Miotto JB, Fitzmartin RD, Reder RF, Chasin M. Differential effects of food on the bioavailability of controlled-release oxycodone tablets and immediate-release oxycodone solution. J Pharm Sci. 1996;85:407–410. doi: 10.1021/js950403a. [DOI] [PubMed] [Google Scholar]
- Benziger DP, Miotto J, Grandy RP, Thomas GB, Swanton RE, Fitzmartin RD. A pharmacokinetic/pharmacodynamic study of controlled-release oxycodone. J Pain Symptom Manage. 1997;13:75–82. doi: 10.1016/s0885-3924(96)00300-4. [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Senay EC, Woody GE. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999;57:7–22. doi: 10.1016/s0376-8716(99)00041-1. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, Munoz A. Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994-2004. Pharmacoepidemiol Drug Saf. 2005;14:851–859. doi: 10.1002/pds.1113. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–289. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41:7–12. doi: 10.1111/j.1365-2125.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML. Buprenorphine’s physical dependence potential: antagonist-precipitated withdrawal in humans. J Pharmacol Exp Ther. 1996;276:449–459. [PubMed] [Google Scholar]
- Enggaard TP, Poulsen L, Arendt-Nielsen L, Brosen K, Ossig J, Sindrup SH. The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6. Anesth Analg. 2006;102:146–150. doi: 10.1213/01.ane.0000189613.61910.32. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Jasinski DR. Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol Psychol. 2006;73:90–99. doi: 10.1016/j.biopsycho.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegert F, Kurth B, Gohler K. The effects of tramadol on static and dynamic pupillometry in healthy subjects--the relationship between pharmacodynamics, pharmacokinetics and CYP2D6 metaboliser status. Eur J Clin Pharmacol. 2005;61:257–266. doi: 10.1007/s00228-005-0920-y. [DOI] [PubMed] [Google Scholar]
- Freye E, Levy J. Acute abstinence syndrome following abrupt cessation of long-term use of tramadol (Ultram): a case study. Eur J Pain. 2000;4:307–311. doi: 10.1053/eujp.2000.0187. [DOI] [PubMed] [Google Scholar]
- Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:116–121. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- Gossop M. The development of a Short Opiate Withdrawal Scale (SOWS) Addict Behav. 1990;15:487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- Gutstein, Akil . Chapter 21 Opioid Analgesics. In: Brunton LL, Lazo JS, Parker K, editors. Goodman and Gillman’s The Pharmacologic Basis of Therapeutics. Eleventh Edition. McGraw Hill; New York: 2006. [Google Scholar]
- Hair PI, Curran MP, Keam SJ. Tramadol extended-release tablets. Drugs. 2006;66:2017–2027. doi: 10.2165/00003495-200666150-00014. discussion 2028-2030. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- IMS Health. National Prescription AuditTM, Top 25 Pharmaceutical Products by Dispensed Prescriptions in the US Market. 2012 http://www.imshealth.com.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung. 1988;38:877–880. [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Inciardi JA, Cicero TJ, Munoz A, Adams EH, Geller A, Senay EC, Woody GE. The diversion of ultram, ultracet, and generic tramadol HCL. J Addict Dis. 2006;25:53–58. doi: 10.1300/J069v25n02_08. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Preston KL, Sullivan JT, Testa M. Abuse potential of oral tramadol. NIDA Res Monogr. 1993;132:103. [PubMed] [Google Scholar]
- Lai J, Ma SW, Porreca F, Raffa RB. Tramadol, M1 metabolite and enantiomer affinities for cloned human opioid receptors expressed in transfected HN9.10 neuroblastoma cells. Eur J Pharmacol. 1996;316:369–372. doi: 10.1016/s0014-2999(96)00770-4. [DOI] [PubMed] [Google Scholar]
- Lanier RK, Lofwall MR, Mintzer MZ, Bigelow GE, Strain EC. Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology. 2010;211:457–66. doi: 10.1007/s00213-010-1919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo RJ, Narendran R, DeGuiseppe B. Methadone detoxification of tramadol dependence. J Subst Abuse Treat. 2000;19:297–299. doi: 10.1016/s0740-5472(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Bigelow GE, Strain EC. Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology. 2007;194:381–393. doi: 10.1007/s00213-007-0847-3. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Moody DE, Fang WB, Nuzzo PA, Walsh SL. Pharmacokinetics of intranasal crushed OxyContin and intravenous oxycodone in nondependent prescription opioid abusers. J Clin Pharmacol. 2012;52:600–606. doi: 10.1177/0091270011401620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrument. 1982;14:463–466. [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Meyboom RH, Brodie-Meijer CC, Diemont WL, van Puijenbroek EP. Bladder dysfunction during the use of tramadol. Pharmacoepidemiol Drug Saf. 1999;8(Suppl. 1):S63–64. doi: 10.1002/(sici)1099-1557(199904)8:1+<s63::aid-pds399>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Thomas C, Hasson A, Ling W. A comparison of buprenorphine taper outcomes between prescription opioid and heroin users. J Addict Med. 7:33–38. doi: 10.1097/ADM.0b013e318277e92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortho-McNeil I. Ultram ER (Tramadol HCl Extended Release Tablets) Package Insert LB0047-01 2006 [Google Scholar]
- Poyhia R, Seppala T, Olkkola KT, Kalso E. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol. 1992;33:617–621. doi: 10.1111/j.1365-2125.1992.tb04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Jasinski DR, Testa M. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27:7–17. doi: 10.1016/0376-8716(91)90081-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–285. [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, Jacoby HI, Selve N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther. 1993;267:331–340. [PubMed] [Google Scholar]
- Rodriguez Villamanan JC, Albaladejo Blanco C, Sanchez Sanchez A, Carvajal A, Martin Arias L, Garcia del Pozo J. Withdrawal syndrome after long-term treatment with tramadol. Br J Gen Pract. 2000;50:406. [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–2378. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Magura S, Haddox JD. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. Characterizing the emerging population of prescription opioid abusers. Am J Addict. 2006;15:208–212. doi: 10.1080/10550490600625624. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Wong CJ, Chausmer AL, Liebson IA, Bigelow GE. Evaluation of an injection depot formulation of buprenorphine: placebo comparison. Addiction. 2004;99:1439–1449. doi: 10.1111/j.1360-0443.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug Alcohol Depend. 1997;47:227–235. doi: 10.1016/s0376-8716(97)00099-9. [DOI] [PubMed] [Google Scholar]
- Sobey PW, Parran TV, Jr, Grey SF, Adelman CL, Yu J. The use of tramadol for acute heroin withdrawal: a comparison to clonidine. J Addict Dis. 2003;22:13–25. doi: 10.1300/j069v22n04_03. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Hatton KW, Lofwall MR, Nuzzo PA, Walsh SL. Intravenous oxycodone, hydrocodone, and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology. 2010;212:193–203. doi: 10.1007/s00213-010-1942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lofwall MR, Nuzzo PA, Craig LB, Siegel AJ, Walsh SL. Pharmacodynamic profile of tramadol in humans: influence of naltrexone pretreatment. Psychopharmacology. 2012;223:427–38. doi: 10.1007/s00213-012-2739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE. Buprenorphine effects in methadone-maintained volunteers: effects at two hours after methadone. J Pharmacol Exp Ther. 1995;272:628–638. [PubMed] [Google Scholar]
- Tamaskar R, Parran TV, Jr, Heggi A, Brateanu A, Rabb M, Yu J. Tramadol versus buprenorphine for the treatment of opiate withdrawal: a retrospective cohort control study. J Addict Dis. 2003;22:5–12. doi: 10.1300/j069v22n04_02. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tjaderborn M, Jonsson AK, Ahlner J, Hagg S. Tramadol dependence: a survey of spontaneously reported cases in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1192–1198. doi: 10.1002/pds.1838. [DOI] [PubMed] [Google Scholar]
- Toll BA, Schepis TS, O’Malley SS, McKee SA, Krishnan-Sarin S. Subjective reactivity to the first cigarette of the day as a predictor of smoking relapse: a preliminary study. Drug Alcohol Depend. 2007;89:302–305. doi: 10.1016/j.drugalcdep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Senay EC, Geller A, Adams EH, Inciardi JA, Schnoll S, Munoz A, Cicero TJ. An independent assessment of MEDWatch reporting for abuse/dependence and withdrawal from Ultram (tramadol hydrochloride) Drug Alcohol Depend. 2003;72:163–168. doi: 10.1016/s0376-8716(03)00198-4. [DOI] [PubMed] [Google Scholar]
- Zacny JP. Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend. 2005;80:273–278. doi: 10.1016/j.drugalcdep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Zarghami M, Masoum B, Shiran MR. Tramadol versus methadone for treatment of opiate withdrawal: a double-blind, randomized, clinical trial. J Addict Dis. 2012;31:112–117. doi: 10.1080/10550887.2012.665728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


