Abstract
Background
Personality traits such as pathological engagement in approach behaviors, high levels of impulsivity and heightened negative affect are consistently observed in substance dependent individuals (SDI). The clinical course of addiction has been shown to differ between sexes. For example, women increase their rates of consumption of some drugs of abuse more quickly than men. Despite the potential influence of personality and sex on features of addiction, few studies have investigated the interaction of these factors in substance dependence.
Methods
Fifty-one SDI (26 male, 25 female) and 66 controls (41 male, 25 female) completed the Behavioral Inhibition/Behavioral Activation System (BIS/BAS) Scales, the Barratt Impulsiveness Scale, and the Positive and Negative Affect Schedule (PANAS-X). Data were analyzed with 2×2 ANCOVAs testing for main effects of group, sex and group by sex interactions, adjusting for education level.
Results
Significant group by sex interactions were observed for BAS scores [F(1,116)=7.03, p<.01] and Barratt Motor Impulsiveness [F(1,116)=6.11, p<.02] with female SDI showing the highest approach tendencies and impulsivity followed by male SDI, male controls, and finally female controls. SDI scored higher on negative affect [F(1,116)=25.23, p<.001] than controls. Behavioral Inhibition System scores were higher in women than men [F(1,116)=14.03, p< .001].
Conclusion
Higher BAS and motor impulsivity in SDI women relative to SDI men and control women suggest that personality traits that have been previously associated with drug use may be modulated by sex. These factors may contribute to differences in the disease course observed in male compared to female drug users.
Keywords: Substance dependence, sex, impulsivity, approach systems, affect
1. INTRODUCTION
While substance use disorders more frequently afflict men than women on the order of two or three times, evidence suggests that women with these disorders exhibit an accelerated clinical course (Greenfield et al., 2007). Women appear to advance more quickly from initial substance experimentation to regular use and dependence (Hernandez-Avila et al., 2004; Johnson et al., 2005; Randall et al., 1999). Though sex has not consistently been shown to predict treatment outcomes (Greenfield et al., 2007), studies find that women exhibit longer periods of use following relapse (Gallop et al., 2007), increase their rates of consumption of some drugs of abuse more quickly (Brady and Randall, 1999; Hernandez-Avila et al., 2004), and relapse at similar rates following abstinence in spite of better utilization of treatment options, e.g., more frequent participation in group counseling (Fiorentine et al., 1997; Gallop et al., 2007). Thus far, neuroendocrine investigations related to these clinically observed sex differences in SDI have provided insight into these phenomena. However, relatively few studies of sex differences in drug dependence have directly investigated multiple dimensions of personality related to addiction. Our objective was to investigate behavioral approach/inhibition systems, impulsivity, and affect in male and female substance dependent individuals (SDI) and control groups to examine whether group differences in personality constructs were modulated by sex.
Personality traits, such as heightened impulsivity, may play an integral role in the development and disease course of substance dependence. Increased impulsivity can be defined as having decreased inhibitory control over responses to rewarding or distracting stimuli (Ersche et al., 2010). Preclinical models of addiction support the idea that this type of dysregulation can lead to uncontrolled substance use and drug seeking behaviors (Everitt et al., 2008). Studies indicate that SDI samples are more impulsive than controls (Moeller and Dougherty, 2002; Nielsen et al., 2012) and such increases in impulsivity appear to contribute to substance use severity and vulnerability to dependence, (Lejuez et al., 2010; Moeller et al., 2001; Verdejo-Garcia et al., 2008), but these studies do not indicate whether sex differences influence these relationships highlighting a need to understand possible sex differences in SDI on this trait.
Most studies utilizing self-report measures of trait impulsivity in SDI samples do not report on findings related to sex (Bjork et al., 2004; Ersche et al., 2011; Lane et al., 2007), have not sought group by sex interactions, or were limited by small or single sex samples. At least one prior study found SDI women were both more impulsive (Barratt Impulsiveness Scale) and more often dependent on psychostimulants than men (Lejuez et al., 2007). However, this study did not include a control group and therefore could not evaluate group by sex interactions. Another study comparing SDI and controls on measures of impulsivity reported no main effect of sex and no group by sex interactions (Allen et al., 1998), but results are limited as drug categories used by this SDI sample were not reported and abstinence was not monitored. The current investigation includes a large sample of controls, detailed characterization of substance use, and verifiable abstinence among SDI.
Similar to impulsivity, heightened sensitivity of the behavioral approach or activation system (BAS) as measured by the BIS/BAS scales (Carver and White, 1994) may also play an important role in the development and disease course of substance dependence. The BAS is said to motivate behaviors that would approach or move in a direction towards an appetitive outcome, goal, or reward and to generate positive affective states in this context (Gray, 1981). Gray (1994) views individuals higher in BAS sensitivity as predisposed to substance use disorders via their propensity for pathological engagement in approach behaviors. Previous studies have supported Gray’s theory that the BAS has clinical significance to substance dependence. For example, BAS sensitivity appears to be elevated in SDI samples (Ersche et al., 2011; Franken et al., 2006; Johnson et al., 2003) and the BAS has been directly correlated with substance use severity (Franken and Muris, 2006; Knyazev, 2004) and craving (Franken, 2002). Previous studies that have reported higher BAS scores in SDI did not find that BAS was modulated by sex but few studies comparing SDI to controls have employed direct measures of the BAS (Franken et al., 2006).
As another potential driving force in substance dependence, affect might underlie differences in clinical course modulated by sex. Negative affect, especially in concert with increased impulsivity, may motivate substance use (Verdejo-Garcia et al., 2007) and SDI groups generally demonstrate higher levels of negative affect compared with controls (Jackson and Sher, 2003; Watson and Clark, 1994). Given that depressive symptoms have long been recognized as more prevalent in women (Weissman and Klerman, 1977), investigation of group by sex interactions on affect seems warranted. Such interactions found on this dimension of personality could help account for the accelerated clinical course characteristic of female substance dependence.
The goal of this study was to compare SDI and controls on personality traits thought to be instrumental to substance dependence and to further examine modulation of these traits according to sex. Taken together, findings of increased behavioral approach, impulsivity, and negative affect specifically related to drug addicted women could further account for the clinical differences seen between male and female SDI groups.
2. MATERIALS AND METHODS
2.1 Participants
One-hundred seventeen participants were included in this study (n=41 male control, n=26 male SDI, n=25 female control, n=25 female SDI). SDI participants were in a sex specific long term residential treatment program at University of Colorado School of Medicine’s Addiction Research and Treatment Services (ARTS) and were abstinent from all drugs of abuse excluding tobacco for a minimum of 60 days and closely monitored for drug use while in treatment. All SDI met DSM-IV criteria for dependence upon psychostimulants (methamphetamine and/or cocaine). Control participants were recruited from the community and excluded for dependence upon alcohol or other drugs aside from tobacco. All participants received structured diagnostic interviews to screen DSM-IV criteria related to substance use and relevant mental disorders. Participants were excluded if they met diagnostic criteria for current depression (within last two months), schizophrenia, and bipolar disorder. All participants provided written informed consent approved by the Colorado Multiple Institution Review Board.
2.2. Screening Assessment
2.2.1 Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM)
This computerized structured interview (Cottler et al., 1989) assesses substance diagnoses and symptom counts for alcohol, tobacco, and other drugs of abuse (stimulants, cocaine, marijuana, hallucinogens, opioids, inhalants, sedatives, club drugs, and PCP) using DSM IV criteria. All participants were administered this detailed interview to verify a diagnosis of cocaine and/or methamphetamine dependence in our substance dependent group as well as to rule out dependence on alcohol or other drugs excluding tobacco in control participants.
2.2.2 Diagnostic Interview Schedule-version IV (CDIS-IV)
This computerized structured interview assesses psychiatric diagnoses using DSM IV criteria (Robins et al., 1995) and was administered to all participants and utilized to exclude those with a positive history of schizophrenia, bipolar disorder, or current major depression (within last two months).
2.2.3 IQ: Wechsler Abbreviated scale of Intelligence (WASI)
The WASI 2-subtest (Vocabulary and Matrix Reasoning) was administered to all participants to assess general intelligence (Psychological Corporation, 1999). Participants were excluded for IQ ≤ 80.
2.3 Personality and Affect Questionnaires
2.3.1 The Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) Scales
The BIS/BAS scales (Carver and White, 1994) consist of a 20 item self-report questionnaire used to measure the responsiveness of two basic motivational systems. The behavioral inhibition system (BIS) scale (7 items) assesses the tendency to respond with negative affect and behaviors that withdraw from aversive conditioned stimuli. The behavioral activation system scales (BAS, 13 items) assess the tendency to respond with positive affect and behaviors that approach appetitive stimuli. BAS is divided into 3 subscales: Drive (4 items) which relates to persistent pursuit of goals, Fun-Seeking (4 items) which involves the tendency to seek out novel rewards, and Reward Responsiveness (5 items) which assesses the tendency to experience positive affect in response to rewarding stimuli. Prior studies have demonstrated that the BIS/BAS scales possess adequate reliability (Carver and White, 1994; Leone et al., 2001).
2.3.2 Barratt Impulsiveness Scale- version 11 (Barratt)
This is a 30-item self-report questionnaire used to measure impulsivity (Patton et al., 1995). Participants rate whether phrases describing aspects of impulsivity pertain to themselves along a 4 point scale. The Barratt can be broken into 3 subscales assessing different facets of impulsivity. The Motor impulsivity subscale involves acting on the spur of the moment and includes statement such as, “I do things without thinking”. The Attentional impulsivity subscale assesses ability to maintain focused attention on a task and includes statements such as “I concentrate easily”. The nonplanning impulsivity subscale pertains to a lack of concern for the future and includes statements such as, “I plan tasks carefully”. Studies indicate that the Barratt and its subscales are reliable measures of impulsiveness (Patton et al., 1995; Stanford et al., 2009).
2.3.3. The Positive (+) and Negative (−) Affect Schedule-Expanded Form (PANAS-X)
PANAS-X assesses positive and negative affect through 60 words or phrases that refer to specific emotions (Watson and Clark, 1994). Participants are asked to self-rate the extent to which they have felt that emotion over the past few weeks on a 5 point scale (1= very slightly or not at all to 5=extremely). The PANAS-X has been shown to be a reliable measure of positive and negative affect (Watson and Clark, 1994).
2.4 Data Analysis
Distributions of all dependent variables were evaluated and found to be approximately normal; therefore, parametric statistical procedures were utilized. Pearson correlations assessed associations among demographic characteristics and outcomes across groups. Analyses of variance evaluated the group, sex, and group by sex interaction effects on demographic characteristics and outcomes. Both IQ and education were found to be significantly greater in SDI compared to controls, but because education level was also significantly greater in females compared to males, we adjusted for education in analyses of covariance (ANCOVAs) of all outcomes.
3. RESULTS
3.1. Participant Characteristics (demographics)
All SDI met DSM-IV criteria for dependence upon psychostimulants (methamphetamine and/or cocaine). Many SDI were dependent on tobacco (71%), alcohol (57%), cannabis (41%), opioids (22%), hallucinogens (10%), club drugs (8%), and PCP (2%). Eight controls (12%) were dependent on tobacco. No controls were dependent on drugs or alcohol.
Table 1 shows the correlations among demographic variables (age, education, and IQ) and personality variables (BIS/BAS, Barratt Impulsiveness, PANAS affect) across all subjects. Lower education and lower IQ were related to each other and to increased Barratt Impulsivity and PANAS (−) affect (p≤.01). Younger age was only correlated with BIS (p≤.05). Not surprisingly, significant intercorrelations were seen between BAS, Barratt Impulsiveness, and PANAS (−) affect (p≤.01). BIS was significantly correlated with PANAS (−) affect (p≤.01).
Table 1.
Correlation Matrix of demographic and personality variables
| ALL Groups | Edu | IQ | Barratt | PANAS+ | PANAS− | BIS | BAS |
|---|---|---|---|---|---|---|---|
| Age | −.16 | −.15 | .02 | .08 | −.10 | −.20 a | −.13 |
| Educ | .53 c | −.47 c | .01 | −.31 b | .10 | −.17 | |
| IQ | −.36 c | .03 | −.24 b | .09 | .06 | ||
| Barratt | −.22 a | .51 c | .09 | .30 c | |||
| PANAS+ | −.19 a | −.23 a | −.02 | ||||
| PANAS− | .25 b | .30 c | |||||
| BIS | −.02 |
≤.05
≤.01
≤.001
Table 2 shows that compared to controls, SDI were older (mean(SD), 35.45 (7.8) vs. 32.15 (9.2), [F(1,116)=5.00, p=.03]), less educated (12.5(1.6) years vs. 14.5(1.7) years, [F(1,116)=127.20, p<.001], and had a lower IQ (102.8(10.2) vs. 110.5 (11.0), [F(1,114)=15.05, p<.001]. Age and IQ did not differ significantly by sex but women had 0.6 more years of education than men (14.0 (1.9) vs. 13.4 (1.9), [F(1,116)=6.44, p=.01]. IQ and education level were intercorrelated but only education was related to both sex and group. Education level was therefore the only covariate included in the below ANCOVAs of personality variables.
TABLE 2.
Demographic and personality data. Scores are raw mean (SD). p-values are adjusted for education for all analyses (Group, Sex, Group by Sex interaction (GxS).
| Male Control |
Female Control |
Male SDI |
Female SDI |
Group | Sex | GxS | |
|---|---|---|---|---|---|---|---|
| Demographics | p values | ||||||
| N | 41 | 25 | 26 | 25 | |||
| Age a | 33.10(9.8) | 30.60(8.0) | 36.96(7.7) | 33.88(7.8) | 0.03 | 0.09 | 0.86 |
| Education a b | 14.05(1.7) | 15.24(1.4) | 12.35(1.8) | 12.68(1.3) | <0.001 | 0.01 | 0.16 |
| IQ a | 109.63(11.3) | 111.96(10.7) | 102.69(10.2) | 102.96(10.5) | <0.001 | 0.63 | 0.65 |
| Barratt a | 59.32(8.2) | 55.04(7.2) | 68.62(10.6) | 72.40(12.2) | <0.001 | 0.64 | 0.06 |
| Motor a c | 23.76(3.0) | 21.80(2.7) | 26.04(4.1) | 27.76(4.7) | <0.001 | 0.89 | 0.02 |
| Attention a | 14.00(3.4) | 13.60(2.7) | 16.31(3.4) | 17.52(4.2) | <0.001 | 0.30 | 0.33 |
| Non-Planning a | 21.56(4.2) | 19.64(4.7) | 26.27(4.9) | 27.12(5.4) | <0.001 | 0.97 | 0.23 |
| PANAS(+) | 36.00(6.2) | 35.28(7.7) | 35.81(6.6) | 36.16(6.9) | 0.68 | 0.83 | 0.65 |
| PANAS(−) a | 15.15(4.1) | 13.96(3.4) | 20.46(8.0) | 22.60(7.2) | <0.001 | 0.60 | 0.15 |
| BIS b | 18.34(3.2) | 21.68(3.3) | 19.65(2.2) | 21.16(3.7) | 0.32 | <0.001 | 0.16 |
| BAS ac | 39.93(4.3) | 37.52(4.5) | 42.00(4.9) | 44.40(5.4) | <0.001 | 0.87 | 0.01 |
| Drive a | 11.07(1.9) | 9.88(2.4) | 12.46(2.3) | 12.68(2.6) | <0.001 | 0.27 | 0.11 |
| Fun-Seeking ac | 11.61(2.4) | 10.68(2.0) | 12.77(1.9) | 13.48(2.5) | <0.001 | 0.59 | 0.04 |
| Reward Resp c | 17.24(1.5) | 16.96(1.6) | 16.77(2.2) | 18.24(1.8) | 0.26 | 0.10 | 0.01 |
Main effect of Group
Main effect of Sex
Group by sex Interaction
3.2. Behavioral Activation System (BAS)
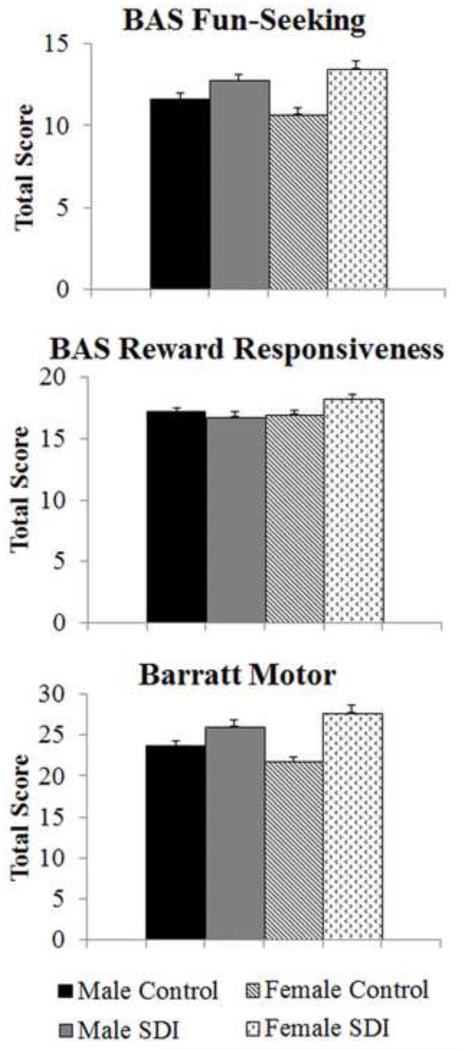
A significant group by sex interaction was found for BAS total [F(1, 116)=7.03, p<.01]. Approach scores were highest for SDI females, followed by SDI males, controls males, and control females. There was also a group by sex interaction for 2 of the BAS subscales as shown in Figure 1a,b for BAS Fun-Seeking [F(1,116)=4.45, p<.04] and BAS Reward Responsiveness [F(1,116)=6.77, p=.01]. ANCOVA showed a main effect of group on the BAS Drive subscale with SDI scoring higher than controls (12.6(2.4) vs. 10.62 (2.2)) [F(1,116)=16.71, p<.001]. There was no main effect of sex on BAS total or subscales.
Figure 1.
Mean subscale scores with standard error bars are shown. There was a significant group by sex interaction on BAS Fun-Seeking [F(1,116)=4.45, p<.04], BAS Reward Responsiveness [F(1,116)=6.77, p=.01], and Barratt Motor [F(1,116)=6.11, p<.02] subscales.
3.3. Behavioral Inhibition System (BIS)
There was no group by sex interaction or main effect of group on BIS scores. ANCOVA showed a main effect of sex and, consistent with the literature (Carver and White, 1994; Leone et al., 2001), women scored higher than men (21.42 (3.5) vs.18.85 (2.9) [F(1,116)=14.03, p<.001]).
3.4. Barratt Impulsiveness Scale-Version 11
ANCOVA showed a main effect of group with SDI scoring higher than controls on Barratt Total (70.47(11.5) vs. 57.7(8.1), [F(1,116)=23.45, p<.001]. A trend towards a group by sex interaction was found for Barratt total score [F(1,116)=3.68, p=.06]. ANCOVA revealed a significant group by sex interaction for the Barratt Motor subscale [F(1,116)=6.11, p<.02] with female SDI much more motor impulsive 27.76(4.7), mean(SD) than female controls 21.80(2.6) while male SDI were mildly more impulsive 26.04(4.1) than male controls 23.76(3.0) (Figure 1c). A main effect of group was found for Barratt Attention [F(1,116)=8.7, p<.01] and Non-planning [F(1,116)=18.4, p<.001] subscales with SDI scoring significantly higher on these impulsivity measures than controls.
3.5. PANAS (−)
ANCOVA showed a main effect of group [F(1,116)=25.23, p<.001] with SDI having higher PANAS (−) affect (mean=21.51, SD=7.6) compared with controls (mean=14.70, SD=3.9). There was no main effect of sex and no group by sex interaction.
3.6. PANAS (+)
ANCOVA showed no main effect of group, no main effect of sex, and no group by sex interaction.
4. DISCUSSION
This investigation of abstinent substance dependent men and women examined if sex modulates group differences in personality traits associated with drug abuse. Our results indicate that SDI show significantly greater impulsivity, behavioral approach, and negative affect than controls; this combination of pathological engagement in approach behaviors (elevated BAS sensitivity), heightened impulsivity, and increased negative affect may signify a clinical phenotype common to substance use disorders. Consistent with prior investigations of BIS/BAS (Ersche et al., 2011; Franken et al., 2006) the SDI group displayed significantly elevated BAS sensitivity driven primarily by the BAS Drive and Fun-Seeking subscales. Unlike prior investigations, we found a significant group by sex interaction such that female SDI were far higher on BAS than female controls and male SDI, while male SDI had higher BAS compared to male controls, but the differences were not nearly as large. It is noteworthy that this group by sex interaction was most prominent on the Reward Responsiveness subscale.
To date, the strongest explanation for the accelerated clinical course in female compared to male SDI draws upon neuroendocrine findings. Estradiol treatments administered to rats potentiates the sensitization of locomotor activity induced by amphetamine and cocaine (Peris et al., 1991) and the acute positive reinforcing effects of stimulants in humans appear to be enhanced during the luteal phase (rising estradiol in the presence of low progesterone) of the menstrual cycle (Justice and de Wit, 1999, 2000). It is possible that a corresponding increase in BAS reward responsiveness in our psychostimulant dependent female participants could reflect underlying neuroendocrine difference between sexes. There also is widespread theorization that BAS sensitivity reflects the reactivity of dopaminergic activity in mesocorticolimbic areas in response to reward (Beaver et al., 2006; Pickering and Gray, 2001). Our findings suggest that this heightened reactivity may be particularly pronounced in SDI women compared with men.
Our group by sex interaction on BAS in SDI has not been described previously. This absence of similar findings may derive from samples with an inadequate number of women (Ersche et al., 2011), disparate substance use disorders between studies (Franken et al., 2006; van Toor et al., 2011), and the lack of a control group thus prohibiting such comparisons (Franken, 2002; Franken and Muris, 2006; Knyazev, 2004). We observed a main effect of sex on BIS with women having higher Behavioral inhibition overall consistent with prior research (Carver and White, 1994; Knyazev, 2004; Leone et al., 2001). The relationship between the BIS and substance use is unclear though a large normative sample of 4501 Russian individuals aged 14-25 (Knyazev, 2004) found that BIS sensitivity may protect women from substance use while the reverse may be true in men. Such findings indicate that BIS/BAS may interact differentially with substance dependence depending on sex.
Barratt Impulsivity did not show a significant group by sex interaction overall, though there was a trend towards such an interaction. We did find a significant group by sex interaction on the Barratt Motor Impulsiveness subscale and similar to our BAS result, female SDI were much more Motor impulsive than their control counterparts while male SDI were less elevated in impulsivity compared to control males. This result coincides with Lejuez et al., 2007 which found elevated Barratt Impulsiveness in female SDI compared with their male counterparts (Lejuez et al., 2007). Several studies, however, have failed to find such differences (Stanford et al., 2009). Possible explanations for inconsistent results may be small sample sizes (Bjork et al., 2004; Lane et al., 2007), disparate impulsivity assessments, e.g., behavioral or self-report and imbalanced male to female ratios (Stanford et al., 2009). Our finding of a group by sex interaction on Barratt Motor may indicate that female SDI tend to initiate drug use at a younger age, use greater quantities of drug, experience stronger cravings, and more withdrawal symptoms, as elevated scores on this particular subscale have been significantly correlated with each of these disease indices in cocaine users (Moeller et al., 2001; Prisciandaro et al., 2012). Consistent with prior investigations (Stanford et al., 2009) our SDI group also scored as highly impulsive compared to controls.
We found a main effect of group on negative affect with SDI scoring much higher PANAS (−) compared to controls consistent with previous studies (Jackson and Sher, 2003; Watson and Clark, 1994). It has been theorized that substance dependence may be driven at least in part by an attempt to avoid or self-regulate aversive internal states (Koob and Le Moal, 1997) and SDI tend to self report negative affect as the prime mover in their decision to use drugs (Baker et al., 2004). However, we were surprised at not finding a group by sex interaction on negative affect considering that substance dependent females are known to suffer more mood disturbances (Brooner et al., 1997; Griffin et al., 1989; Weiss et al., 2003) than men. While Lejuez et al., (2007) found significantly higher levels of negative emotionality on the Multi-Dimensional Personality Questionnaire (MPQ; Tellegen and Waller, 2008) in female SDI compared with male SDI other past investigations have largely failed to find such differences (McGue et al., 1997; Verdejo-Garcia et al., 2007). These disparate results may in part stem from the exclusion of participants endorsing major depressive symptoms. Unlike Lejuez et al., (2007), the present study excluded participants meeting DSM-IV criteria for a major depressive episode within the past two months. Similarly, participants were excluded from McGue et al. (1997) and Verdejo-Garcia et al. (2007) for any past history of major depression. In line with the literature we did not find a main effect of sex on our PANAS-X measures (Crawford and Henry, 2004; Thompson, 2007; Watson and Clark, 1994).
This study is strengthened by a relatively large sample size with severe and well characterized SDI who resided in a long term residential treatment where they were closely monitored for abstinence. A limitation is that our groups were not matched in education and IQ. Still, meta-analysis of 135 studies found little evidence to suggest that IQ is directly related to impulsivity (Ackerman and Heggestad, 1997) and our covariance of education in our analyses likely compensated for some of these imbalances. While we tried to control for differences in education we acknowledge that there may be other factors that could account for our result including socioeconomic differences or selection biases related to sex specific levels of severity required for admittance to drug treatment programs. As another limitation, a number of personality constructs were investigated and though multiple a priori predictions were in place, the potential role of Type 1 error cannot be ruled out, thus highlighting the need for further study of this area.
Few past studies have directly investigated group by sex interactions in SDI and control groups but future studies may benefit from investigating the effect drug of choice may play on these interactions and develop new treatment paradigms related to such findings. In particular, stimulant and other drug dependent samples are more consistently found to have high BAS sensitivity than are alcoholics (Franken et al., 2006; van Toor et al., 2011). This may reflect the tendency to approach the illegal and dangerous pursuits characteristic of cocaine and methamphetamine use. Treatment strategies have tended to focus on the compulsive aspects of drug use e.g. craving. Future replication of our results may suggest that female SDI might benefit more than male SDI from therapies targeting aspects of trait impulsivity and approach tendencies.
Acknowledgments
The authors would like to acknowledge the staff at Addiction Research Treatment Services (ARTS) and Debra Singel, RT.
Role of Funding Source
Funding for this study was provided by NIDA grants R01 DA024104 and DA027748; NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Perry: design, analysis, data interpretation, manuscript; Krmpotich: analysis, interpretation, manuscript; Thompson: data interpretation, manuscript; Mikulich-Gilbertson: data interpretation, manuscript; Banich: manuscript; Tanabe: design, analysis, interpretation manuscript
Conflict of Interest
All authors declared they have no conflicts of interest.
REFERENCES
- Ackerman PL, Heggestad ED. Intelligence, personality, and interests: evidence for overlapping traits. Psychol. Bull. 1997;121:219–245. doi: 10.1037/0033-2909.121.2.219. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Moeller FG, Rhoades HM, Cherek DR. Impulsivity and history of drug dependence. Drug Alcohol Depend. 1998;50:137–145. doi: 10.1016/s0376-8716(98)00023-4. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J. Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr. Clin. North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, Schmidt CW, Jr., Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch. Gen. Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS Scales. J. Person. Soc. Psychol. 1994;67:319–333. [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br. J. Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol. Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentine R, Anglin MD, Gil-Rivas V, Taylor E. Drug treatment: explaining the gender paradox. Subst. Use Misuse. 1997;32:653–678. doi: 10.3109/10826089709039369. [DOI] [PubMed] [Google Scholar]
- Franken IH. Behavioral approach system (BAS) sensitivity predicts alcohol craving. Person. Individ. Diff. 2002;32:349–355. [Google Scholar]
- Franken IH, Muris P. BIS/BAS personality characteristics and college students’ substance use. Person. Individ. Diff. 2006;40:1497–1503. [Google Scholar]
- Franken IH, Muris P, Georgieva I. Gray’s model of personality and addiction. Addict. Behav. 2006;31:399–403. doi: 10.1016/j.addbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Gallop RJ, Crits-Christoph P, Ten Have TR, Barber JP, Frank A, Griffin ML, Thase ME. Differential transitions between cocaine use and abstinence for men and women. J. Consult. Clin. Psychol. 2007;75:95–103. doi: 10.1037/0022-006X.75.1.95. [DOI] [PubMed] [Google Scholar]
- Gray JA. A critique of Eysenck’s theory of personality. In: Eysenck HJ, editor. A Model for Personality. Springer; New York: 1981. pp. 246–276. [Google Scholar]
- Gray JA. Framework for a taxonomy of psychiatric disorder. In: van Goozen SHM, Van de Poll NE, editors. Emotions: Essays on Emotion Theory. Lawrence Erlbaum Associates; Mahwah, NJ: 1994. pp. 29–59. [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 2007;86:1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch. Gen. Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ. Alcohol use disorders and psychological distress: a prospective state-trait analysis. J. Abnorm. Psychol. 2003;112:599–613. doi: 10.1037/0021-843X.112.4.599. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Richter L, Kleber HD, McLellan AT, Carise D. Telescoping of drinking-related behaviors: gender, racial/ethnic, and age comparisons. Subst. Use Misuse. 2005;40:1139–1151. doi: 10.1081/JA-200042281. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ. BIS/BAS levels and psychiatric disorder: an epidemiological study. J. Psychopathol. Behav. Assess. 2003;25:25–36. [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacol. (Berl.) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71:51–59. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Behavioural activation as predictor of substance use: mediating and moderating role of attitudes and social relationships. Drug Alcohol Depend. 2004;75:309–321. doi: 10.1016/j.drugalcdep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am. J. Drug Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Reynolds EK, Daughters SB, Curtin JJ. Risk factors in the relationship between gender and crack/cocaine. Exp. Clin. Psychopharmacol. 2007;15:165–175. doi: 10.1037/1064-1297.15.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol. Clin. Exp. Res. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone L, Perugini M, Bagozzi RP, Pierro A, Mannetti L. Construct validity and generalizability of the Carver-White behavioural inhibition system/behavioural activation system scales. Eur. J. Person. 2001;15:373–390. [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. effects of gender and alcoholism subtype. Alcohol. Clin. Exp. Res. 1997;21:513–520. [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM. Impulsivity and substance abuse: what is the connection? Addict. Disord. Treat. 2002;1:3–10. [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J. Subst. Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Ho A, Bahl A, Varma P, Kellogg S, Borg L, Kreek MJ. Former heroin addicts with or without a history of cocaine dependence are more impulsive than controls. Drug Alcohol Depend. 2012;124:113–120. doi: 10.1016/j.drugalcdep.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566:255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- Pickering AD, Gray JA. Dopamine, appetitive reinforcement, and the neuropsychology of human learning: an individual differences approach. In: Eliasz A, Angleitner A, editors. Advances in Individual Differences Research. PABST Science Publishers; Lengerich, Germany: 2001. pp. 113–149. [Google Scholar]
- Prisciandaro JJ, Korte JE, McRae-Clark AL, Brady KT. Associations between behavioral disinhibition and cocaine use history in individuals with cocaine dependence. Addict. Behav. 2012;37:1185–1188. doi: 10.1016/j.addbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J. Stud. Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz K, Compton W. Diagnostic Interview Schedule— Version IV. Washington School of Medicine; St Louis: 1995. [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Person. Individ. Diff. 2009;47:385–395. [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: development of the Multidimensional Personality Questionnaire. In: Boyle GJ, Matthews G, Saklofske DH, editors. The Sage Handbook of Personality Theory and Assessment. Vol. 2. Sage Publishing; Thousand Oaks, CA: 2008. pp. 261–292. [Google Scholar]
- Thompson ER. Development and validation of an internationally reliable short-form of the positive and negative affect schedule (PANAS) J. Cross-Cult. Psychol. 2007;38:227–242. [Google Scholar]
- van Toor D, Roozen HG, Evans BE, Rombout L, Van de Wetering BJ, Vingerhoets AJ. The effects of psychiatric distress, inhibition, and impulsivity on decision making in patients with substance use disorders: a matched control study. J. Clin. Exp. Neuropsychol. 2011;33:161–168. doi: 10.1080/13803395.2010.493300. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007;91:213–219. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X Manual for the Positive and Negative Affect Schedule (Expanded Form) University of Iowa; 1994. [Google Scholar]
- Weiss SR, Kung HC, Pearson JL. Emerging issues in gender and ethnic differences in substance abuse and treatment. Curr. Womens Health Rep. 2003;3:245–253. [PubMed] [Google Scholar]
- Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch. Gen. Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]