Abstract
Background
Dependent drug users show a diminished neural response to punishment, in both limbic and cortical regions, though it remains unclear how such changes influence cognitive processes critical to addiction. To assess this relationship, we examined the influence of monetary punishment on inhibitory control and adaptive post-error behaviour in abstinent cocaine dependent (CD) participants.
Methods
15 abstinent CD and 15 matched control participants performed a Go/No-go response inhibition task, which administered monetary fines for failed response inhibition, during collection of fMRI data.
Results
CD participants showed reduced inhibitory control and significantly less adaptive post-error slowing in response to punishment, when compared to controls. The diminished behavioural punishment sensitivity shown by CD participants was associated with significant hypoactive error-related BOLD responses in the dorsal anterior cingulate cortex (ACC), right insula and right prefrontal regions. Specifically, CD participants’ error-related response in these regions was not modulated by the presence of punishment, whereas control participants’ response showed a significant BOLD increase during punished errors.
Conclusions
CD participants showed a blunted response to failed control (errors) that was not modulated by punishment. Consistent with previous findings of reduced sensitivity to monetary loss in cocaine users, we further demonstrate that such insensitivity is associated with an inability to increase cognitive control in the face of negative consequences, a core symptom of addiction. The pattern of deficits in the CD group may have implications for interventions that attempt to improve cognitive control in drug dependent groups via positive/negative incentives.
Keywords: Performance monitoring, error-related, drug dependence, cocaine, cognitive control
1. INTRODUCTION
An abnormally high sensitivity to the rewarding properties of drug taking and cognitive control dysfunction are evident across substance-use dependent (SUD) populations and are predictive of poor treatment outcomes (especially relapse during abstinence; Goldstein and Volkow, 2011; Koob and Volkow, 2010). There is also evidence to suggest that this hypersensitivity extends to non-drug rewards (e.g., money) in drug-dependent populations and drug-naïve children who have familial ‘risk’ for SUD (Hommer et al., 2011), however, the findings are qualified by the use of paradigms that may be confounded by the requirement for temporal discounting and or risk taking (MacKillop et al., 2011). While contemporary neurobiological models highlight the importance of reward sensitivity and cognitive control in SUD (Jentsch and Taylor, 1999; Naqvi and Bechara, 2009; Paulus, 2007), it is unclear how these two features, abnormal reward sensitivity and cognitive control dysfunction, interact. One example of this interaction and the focus of the current study is a diminished ability to exert impulse control, and adapt behaviour, in response to negative feedback (punishment).
Previous research examining the processing of non-drug rewards in SUD samples has typically focused on positive (e.g., monetary reward) rather than negative outcomes (e.g., monetary punishment; Bjork et al., 2008b; Buhler et al., 2010; Monterosso et al., 2007; Reuter et al., 2005), revealing significant differences in functional activity within the reward network, when compared to healthy controls. The changes to non-drug reward processing in addiction have been argued to result from the transient increases in dopamine induced by drugs generating overly positive reward prediction errors (Schultz, 2011). In combination with increased reward sensitivity, drug addicted individuals show a reduced sensitivity to punishment in their behavioural performance (Bechara et al., 2002). Functional MRI studies of addicted drug users have also shown a diminished neural response to monetary loss (Beck et al., 2009; Bjork et al., 2008a; Wrase et al., 2007), in both sub-cortical limbic regions such as the striatum and cortical regions such as the anterior cingulate and insula cortices. These studies have typically not examined the consequences on behaviour of this reduced response to loss.
Cocaine addiction, and addiction more generally, is associated with significant cognitive control dysfunction (Bolla et al., 1999; Garavan and Hester, 2007; Goldstein et al., 2001; Li et al., 2008). Such dysfunction is thought to play a role in addiction because of the critical role cognitive control plays in inhibiting the immediate pursuit of rewarding stimuli and the development of maladaptive patterns of behaviour (Kalivas and Volkow, 2005). For example, drug addicted individuals will consistently choose smaller immediate rewards in preference to larger, but more delayed, rewards (irrespective of whether the reward is hypothetical or real; Kirby and Petry, 2004; Petry, 2001). Given that increased sensitivity to reward and a blunted sensitivity to punishment appear to reduce drug users’ control over rewarding stimuli, it is of interest whether this group shows the same punishment-related improvement in cognitive control observed in healthy controls (Simoes-Franklin et al., 2010). The use of punishment to shape appropriate behaviour is also a key element of clinical (and criminal law) interventions for drug addiction, and its reduced effectiveness with drug abusers has widespread ramifications.
Drug addicted participants have also been shown to have diminished feedback-related activity during cognitive errors, principally in the anterior cingulate and insula cortices (Franken et al., 2007; Hester et al., 2009b; London et al., 2005). Error-related activity in these regions is known to be critical to post-error processes such as conscious error detection and post-error adaptation of performance (Hester et al., 2009a; Kerns et al., 2004), with the diminished error-related activity in addiction linked to poor error awareness (Hester et al., 2009b; Moeller et al., 2010). Previous studies have not manipulated punishment to examine how this influences the level of error-related hypoactivity, or the potential consequence of diminished responsivity to punishment on adaptation of performance
The aim of the current study was to examine how these two features – abnormal punishment sensitivity and cognitive control dysfunction, interact via the administration of Go/No-go response inhibition task that indexes the ability to exert impulse control, and adapt behaviour, in response to negative feedback (punishment). Response inhibition performance was assessed during differing levels of monetary feedback (neutral and punishment) for inhibition failures, and the association of this response to subsequent behavioural adaptations and cognitive control performance. We hypothesized that CD participants would show significantly poorer inhibitory control performance when compared to control participants, particularly under conditions of monetary punishment (relative to neutral). And, further, that the poorer performance under punishment conditions would be associated with a hypoactive error-related response in CD participants, particularly in regions critical to post-error adaptive behavior such as the dACC. The rationale for recruiting abstinent cocaine users was to assess neurocognition in this domain without the acute influence of recent cocaine use.
2. METHODS AND MATERIALS
2.1 Subjects
Fifteen abstinent cocaine dependent (CD) participants (2 female, mean age = 38.2, range = 24–51) were recruited from in-patient and outpatient addiction treatment centres located in New York State. 15 matched control participants (2 female, mean age 42.7, range: 23–55) were recruited from the Volunteer Recruitment Pool at Nathan S. Kline Institute for Psychiatric Research. Groups were also matched for educational attainment (Cocaine: 13.1 years, Control: 13.0) and Wide Ranging Achievement Test (WRAT) estimated IQ (CD: 98.9, Control: 102.8). All 15 patients received a primary Axis I diagnosis of Cocaine Dependence and from the onset of treatment were closely monitored for continued abstinence with random urine toxicology testing for multiple substances at least two times a week for at least the 4 weeks prior to participating in the study. Patients would also meet at least once a week with a personal counsellor who was accredited through the state of New York as an alcoholism and substance abuse counsellor. The duration of abstinence, as assessed through negative biweekly random urine screens for the durations noted, was confirmed with the counsellors at the addiction treatment centres. Exclusion criteria are provided in the supplementary materials1.
The average time since last use of cocaine was self-reported at 335 days (range 30–1825 days), and participants reported using cocaine for an average of 5.12 years (range = 1 to 16 years). The duration of lifetime use and self-reported abstinence duration were not significantly related (r = −.19, p = .53). The duration of cocaine use and period of abstinence were not significantly related to the other demographic variables (i.e., age, education, WRAT IQ).
2.2. Inhibition Punishment Task
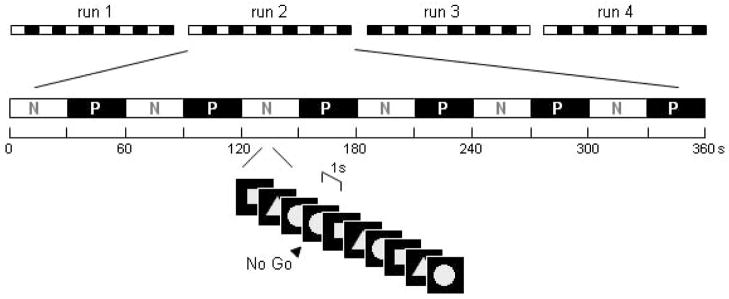
We examined response inhibition performance (see Figure 1; Simoes-Franklin et al., 2010), via a motor Go/No-go response inhibition task that alternates between neutral and punishment conditions. The Punishment Go/No-go (PGNG) task presents a serial stream of cycling shapes (square, circle, triangle), each presented for 900ms followed by a 100ms inter-stimulus interval. Participants were trained to respond to each of the stimuli with a single ‘Go trial’ button press, and withhold this response whenever a shape repeated on consecutive trials. The task alternated between neutral and punishment conditions every 30 trials. In the neutral condition the symbols were presented in white and participants were instructed to perform the task as accurately as possible. In the punishment condition stimuli were presented in red and participants were instructed that they would lose 15c (from an initial amount of $20) for each commission error during a No-go trial. Four blocks of 360 trials, divided into 12 alternating runs of neutral and punishment conditions (30 trials per run), were administered to participants. The blocks included 144 No-go trials (72 per condition).
Figure 1. The Punishment Go/No-go Task.
The PGNG task presents a serial stream of cycling shapes (square, circle, triangle) each presented for 900ms followed by a 100ms inter-stimulus interval. Participants were trained to respond to each of the stimuli with a single ‘Go trial’ button press, and withhold this response whenever a shape repeated on consecutive trials. The task alternated between neutral and punishment conditions every 30 trials. In the neutral condition the symbols were presented in white and participants were instructed to perform the task as accurately as possible. In the punishment condition stimuli were presented in red and participants were instructed that they would lose 15c (from an initial amount of $20) for each commission error during a No-go trial. Four blocks of 360 trials, divided into 12 alternating runs of neutral and punishment conditions (30 trials per run), were administered to participants. The blocks included 144 No-go trials (72 per condition).
2.3. Data Analysis
The fMRI data acquisition and pre-processing analysis is detailed in the supplementary materials2. A mixed regression group fMRI analysis was employed comprising five regressors. A square-wave regressor, convolved with a standard hemodynamic response function, coded for the neutral-punishment pattern in a block design manner, using the Neutral condition as a baseline and the punishment condition as the On period (block regressor). Group activation maps for event-type (stops, errors) and the punishment block were determined with one-sample t-tests against the null hypothesis of zero event-related activation changes (i.e., no change relative to baseline). Significant voxels passed a voxelwise statistical threshold (t = 4.31, p ≤ .001) and were required to be part of a larger 142μl cluster of contiguous significant voxels.
The primary comparison of interest was group differences in activation between the punishment and neutral conditions, for both stops and errors. For instance, the activation clusters from the whole-brain analyses of stops from each group and each condition were used to create a map for the purposes of a functionally-defined ROI analysis. This map includes the voxels of activation indicated as significant in any of the constituent group maps. The mean activation for each cluster in the combined stops map was calculated and the mean activation levels for punishment and neutral stops were compared using repeated measures ANOVA (group x condition), corrected via a modified Bonferroni procedure for multiple comparisons (Keppel, 1991). These steps were repeated to compare activity levels for errors in the neutral and punishment conditions.
3. RESULTS
3.1. Behavioural Results
Performance indices for both control and CD participants are presented in Table 1. Control participants’ inhibitory control, as measured by No-go accuracy, was significantly better than CD participants, F(1,28)=4.38, p<.05. A main effect of condition was also present, with performance in the punishment condition significantly better than the neutral condition, F(1,28)=40.6, p<.01. The improvement in performance during the punishment condition relative to the neutral condition was greater for controls when compared to CD participants, but the interaction effect failed to reach significance, F(1,28) = 3.01, p = .09. Go-trial omission error rates were below 1% and did not differ across groups or condition (p > .05).
Table 1.
Mean accuracy (% successful inhibitions), Go trial and No-go error reaction time and standard error of measurement scores for Cocaine Dependent (CD) (n = 15) and Control (n = 15) groups on the Punishment Go/No-go task.
| Category | CD | Controls | p | ||
|---|---|---|---|---|---|
|
| |||||
| M | SEM | M | SEM | ||
| No-go Accuracy | |||||
| Neutral | 39.6 | 5.3 | 52.8 | 5.7 | * |
| Punishment | 48.6 | 5.6 | 68.4 | 5.8 | * |
| Go RT | |||||
| Neutral | 323.8 | 17.5 | 353.8 | 17.5 | |
| Punishment | 327.6 | 17.1 | 367.2 | 17.1 | |
| Error RT | |||||
| Neutral | 326.4 | 22.8 | 355.9 | 22.6 | |
| Punishment | 338.0 | 18.7 | 344.2 | 19.3 | |
| Post-Error RT difference | |||||
| Neutral | −20.1 | 18.4 | −14.8 | 19.4 | |
| Punishment | −17.1 | 19.6 | 63.1 | 18.6 | * |
Significant difference between cocaine and control groups (p < .05)
Control participants’ Go trial response time (RT) was not significantly different to the CD participants, F(1,28) = 2.03, p =.16. Go RTs were significantly slower during the punishment condition when compared to the neutral, F(1,28) = 6.67, p = .01, however, the magnitude of this effect for control participants (14ms) was not significantly larger than for CD participants (4ms), F(1,28) = 2.05, p = .16. Response times during failed attempts to withhold during No-go trials did not demonstrate significant main effects of group, F(1,28) = 0.41, p > .05, or condition, F(1,28) = .01, p > .05, or an interaction between these factors, F(1,28) = 0.85, p > .05.
Post-error slowing, calculated as the difference in reaction time between the Go Trial RT immediately prior and following a failed No-go response (i.e., positive scores indicate post-error slowing), demonstrated a a significant effect of condition, F(1,28) = 8.57, p = .007, wherein post-error slowing was greater for the punishment than neutral condition (the neutral condition showed virtually no post-error change). The main effect of group was significant, F(1,28) = 4.23, p = .04. The significant interaction between condition and group, F(1,28) = 4.34, p = .04, highlighted the substantially greater post-error slowing of control participants in the punishment condition when compared to CD participants (55ms versus 8ms), whereas no difference was present during the neutral condition (7ms versus 0.1ms). The same pattern of significant effects is observed when post-error slowing is calculated using the difference in reaction time between the failed No-go response and the Go Trial immediately following it. These results demonstrated a significant effect of condition, F(1,28) = 4.74, p < .05, wherein post-error slowing was greater for the punishment than neutral condition (the neutral condition showed post-error speeding). The main effect of group was significant, F(1,28) = 4.83, p < .05. The significant interaction between condition and group, F(1,28) = 4.08, p < .05, highlighted the substantially greater post-error slowing from control participants in the punishment condition when compared to CD participants (63ms versus −17ms), F(1,28)=9.35, p < .05, whereas no difference was present during the neutral condition.
Individual differences in the magnitude of post-error slowing during the punishment condition correlated with response inhibition accuracy during the punishment condition (r = .37, p = .04), whereas the equivalent measures in the neutral condition did not significantly relate (r = −.19, p > .05). Examination of the relationship between self-report measures of cocaine use (years of use, period of abstinence) and task performance revealed no significant correlations.
3.2. Imaging Data
3.2.1 Tonic Activity
The block analysis identified 18 regions that were tonically more active during the punishment condition relative to the neutral condition (see Table 2), which are largely consistent with a previous study examining a different sample of healthy controls (Simoes-Franklin et al., 2010). Of these regions, several demonstrated a significant group effect, including bilateral middle frontal, dorsal anterior cingulate, left putamen and left inferior frontal gyrus. All regions showed significantly greater activity for CD participants in comparison to controls, and no region showed the opposite pattern. Control group activity in four of the regions showing a group difference (right middle frontal, left inferior frontal and right superior parietal cortices) did not significantly exceed baseline (t-test versus zero activity). This finding would suggest that CD participants were tonically activating regions not typically activated by control participants.
Table 2.
Regions showing greater tonic activity in the punishment block, relative to neutral. Regions also demonstrating a significant effect of group (C = Control, CD = Cocaine Dependent) are indicated in the Group column.
| Brain region | Volume (μl) | MNI coordinates
|
Group | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L Middle Frontal | 4879 | −24 | 8 | 41 | CD>C |
| L Putamen | 4869 | −25 | 8 | 6 | CD>C |
| R Putamen | 3287 | 19 | 5 | 11 | |
| R Middle Frontal | 1465 | 36 | 0 | 44 | CD>C |
| L Inferior Parietal | 1368 | −28 | −55 | 45 | |
| L Postcentral Gyrus | 868 | −38 | −31 | 42 | |
| L Thalamus | 561 | −16 | −12 | 15 | |
| R Middle Frontal | 441 | 25 | −15 | 36 | CD>C |
| R Anterior Cingulate | 414 | 4 | 16 | 33 | CD>C |
| R Inferior Frontal | 359 | 47 | 12 | 7 | |
| R Insula Lobe | 322 | 37 | 12 | 10 | |
| L Inferior Frontal | 310 | −32 | 34 | 8 | CD>C |
| R Cuneus | 219 | 9 | −69 | 21 | |
| L Middle Occipital | 211 | −40 | −72 | 8 | |
| L Postcentral | 210 | −35 | −41 | 50 | |
| R Middle Frontal | 181 | 26 | 13 | 50 | |
| R Superior Parietal | 180 | 27 | −53 | 57 | CD>C |
| L Inferior Frontal | 172 | −44 | 23 | 30 | |
Activity for the CD group did not significantly correlate with either duration of cocaine use or period of abstinence.
3.2.2. Errors
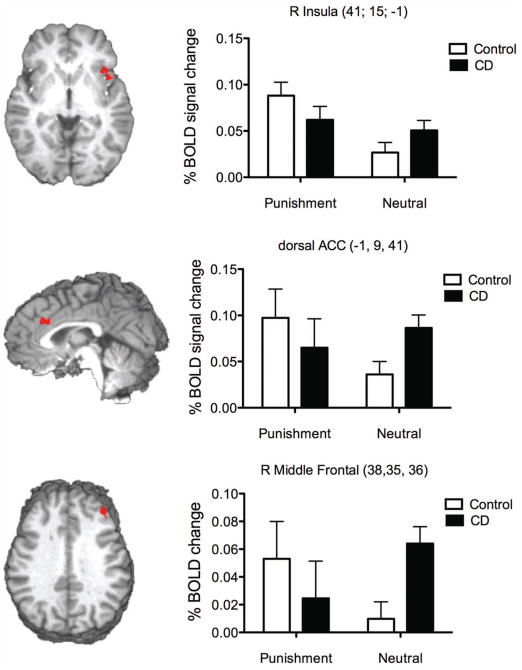
The analysis of event-related error activity examined the influence of condition (punishment, neutral), group (CD, control), and their interaction. A main effect of condition was observed in four regions (see Table 3), with a cluster in the right insula cortex demonstrating significantly greater activity during the punishment condition when compared to the neutral condition (see Figure 2). The opposite effect was seen in the right supramarginal, right angular and left inferior parietal gyri. There were no main effects of group but there were significant interactions between group and condition in four regions, with three clusters in the right insula, dorsal ACC and right middle frontal gyrus demonstrating a significant increase in activity during the punishment condition when compared to the neutral condition for control but not CD participants (Figure 2). In contrast, activity in the right angular gyrus was significantly greater in the neutral condition when compared to the punishment condition for CD participants, but not control participants.
Table 3.
Regions of event-related Error BOLD activity (combined across groups) demonstrating significant group (Cocaine Dependent (CD), control), condition (punishment, neutral), or interaction effects.
| Brain region | Volume (μl) | MNI coordinates | Event-Related | Tonic | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | Condition | Group x Cond | Condition | Group | ||
| Right Insula | 721 | 37 | 17 | 3 | P>N | * | P>N | |
| R Inferior Parietal | 454 | 55 | −48 | 45 | CD>C | |||
| L Anterior Cingulate | 380 | −3 | 22 | 34 | * | P>N | ||
| L Inferior Frontal | 308 | −38 | 18 | 6 | P>N | CD>C | ||
| R Superior Temporal | 293 | 56 | −26 | 3 | P>N | |||
| R Supramarginal | 332 | 61 | −35 | 26 | N>P | |||
| R Angular | 219 | 50 | −66 | 21 | N>P | * | ||
| L Temporal | 194 | −37 | 17 | −11 | CD>C | |||
| L Inferior Parietal | 163 | −46 | −51 | 32 | N>P | |||
| R Middle Frontal | 159 | 38 | 35 | 36 | * | P>N | ||
Figure 2. Error-related brain activity showing significant interaction (Condition x Group) effects.
Bar graphs represent mean BOLD % signal change (relative to baseline) for each group (Cocaine Dependent (CD), Control) during punishment and neutral condition errors. Error bars represent the standard error of the mean. The MNI coordinates for each region are listed in the title and the brain slices shown represent the view at the relevant x, y or z-coordinate (e.g., coronal slices relate to the y-coordinate).
The relationship between error-related activity, duration of cocaine use and period of abstinence were also examined using correlation analyses. Duration of use positively correlated with punishment condition error activity in the right middle frontal region (r = .54, p = .03), and days of abstinence positively correlated with neutral condition activity in left inferior frontal (r = .53, p = .04), dorsal ACC (r = .54, p = .04) and right insula (r = .54, p = .04) regions. These relationships did not survive correction for multiple comparisons.
The difference between tonic activity levels during the punishment and neutral conditions was calculated using the error-related regions of interest (ROI), indicating six regions with significantly greater tonic activity during the punishment condition: right insula, dorsal ACC, left inferior frontal gyrus, right superior temporal gyrus and the right middle frontal gyrus. Of these regions, the left inferior frontal cluster was the only ROI to show a significant group difference, with significantly greater activity for the CD participants in comparison to control group. Thus, the reduced event-related activity for punished errors observed in CD participants was not due to this group showing greater tonic activity in these same areas.
An examination of the relationship between post-error slowing and BOLD activity in the error-related clusters during the punishment condition revealed two significant relationships, in the right middle frontal gyrus (r = .64, p = .01), and dorsal ACC (r = .38, p = .03), with the relationships in the right insula (r = .35, p = .06) demonstrating a similar trend. The right MFG cluster relationship was consistent across groups (controls: r = .65; CD: r = .66), whereas the relationship in the right insula (controls: r = .40; CD: r = −.04) and dorsal ACC (controls: r = .37; CD: r = −.05) was greater for controls than for CD participants.
3.2.3. Stops
The analysis of event-related response inhibition-related activity identified a significant main effect of condition on clusters in right inferior frontal, dorsal ACC, left insula, posterior cingulate, bilateral putamen, right precuneus and right caudate (Table 4). All regions showed significantly reduced activity in the punishment condition compared to the neutral condition. CD participants showed significantly greater activity than control participants in nine clusters, including dorsal ACC, posterior cingulate, bilateral putamen, bilateral IFG, left insula and right superior frontal gyrus. A significant interaction between condition and group was seen in both the right caudate and posterior cingulate, wherein CD participants showed significantly greater activity during the neutral condition compared to punishment condition, whereas control participants showed no difference.
Table 4.
Regions of event-related Stop BOLD activity (combined across groups) demonstrating significant group (Cocaine Dependent (CD), control), condition (punishment, neutral), or interaction effects. Phasic effects relate to event-related activity during stops, whilst tonic activity is calculated as a block average across punishment periods
| Brain region | Volume (μl) | MNI coordinates | Phasic | Tonic | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| x | y | z | Condition | Group | Group x Cond | Condition | Group | ||
| R Anterior Cingulate | 1354 | 1 | 26 | 36 | N>P | CD>C | P>N | ||
| L Putamen | 722 | −20 | 7 | 6 | N>P | CD>C | P>N | CD>C | |
| L Insula | 418 | −47 | 6 | 6 | N>P | CD>C | P>N | ||
| L Inferior Frontal | 401 | −37 | 18 | −14 | N>P | CD>C | |||
| R Caudate | 336 | 11 | −1 | 16 | N>P | * | |||
| R Precuneus | 315 | 3 | −64 | 41 | N>P | ||||
| L Superior Temporal | 300 | −54 | −43 | 13 | N>P | ||||
| R Putamen | 278 | 24 | 4 | 15 | N>P | CD>C | P>N | ||
| L Middle Cingulate | 238 | −1 | −12 | 45 | N>P | CD>C | * | CD>C | |
| R Inferior Frontal | 156 | 29 | 30 | −6 | N>P | CD>C | |||
| L Cuneus | 155 | −3 | −73 | 25 | N>P | N>P | |||
| L Anterior Cingulate | 144 | −1 | 21 | 20 | CD>C | ||||
| R Superior Frontal | 142 | 24 | 55 | 13 | CD>C | ||||
Within stop-related regions, tonic activity was significantly greater for the punishment condition in several clusters, including bilateral putamen, dorsal ACC, left cuneus and left insula. CD participants showed significantly greater tonic punishment activity in the left putamen and posterior cingulate clusters.
The relationship between stop-related activity, duration of cocaine use and period of abstinence were also examined using correlation analyses. Duration of use positively correlated with both neutral and punishment condition stop activity in the right caudate (r = .54, p = .03; r = .55, p = .03), and during the punishment condition in the right putamen (r = .54, p = .03). The correlations did not survive correction for multiple comparisons.
4. DISCUSSION
Given the previous findings of reduced punishment sensitivity in CD participants, we hypothesized that, relative to matched controls, they would show poorer cognitive control and performance adaptation (post-error slowing) in response to the imposition of monetary penalties for response inhibition failures. Consistent with previous findings (Ersche et al., 2010; Fillmore and Rush, 2002; Goldstein et al., 2004; Hester and Garavan, 2004; Li et al., 2006; Verdejo-Garcia et al., 2007), CD participants had significantly impaired inhibitory control when compared to control participants in both the neutral and punishment conditions. The punishment manipulation significantly improved response inhibition performance for both groups, when compared to the neutral condition, and there was an indication in the data that CD participants may be less able to improve their performance in response to punishment than controls (the interaction effect, p = .09). This pattern of deficits was observed without significant demographic differences (e.g., education, IQ or age).
The response to monetary penalties by the CD group, albeit substantially poorer than controls, was associated with significantly greater tonic and phasic inhibition-related BOLD activity. During the punishment blocks of the inhibition task, CD participants tonically increased activity (in comparison to controls) in both cortical regions associated with successful response inhibition in controls, for example, the dorsal ACC, and those not significantly activated by controls, such as the left inferior frontal gyrus and right superior parietal lobule. These regions and additional ones such as the right IFG, considered critical to response inhibition, also showed significantly greater event-related response inhibition activity for the CD group, when compared to controls. While response inhibition performance remained significantly poorer for the CD group, the pattern of inhibition-related activity during the punishment condition is consistent with them ‘rising to the challenge’ of the increased incentive to perform with greater tonic and phasic levels of control. For example, left IFG activity has been seen in both abstinent drug dependent participants and stroke lesion patients, as a compensatory, but less able substitute, for right IFG dysfunction (Connolly et al., 2011; Nestor et al., 2011; Swick et al., 2008). An important exception to this pattern was the lack of such an improvement in the processing of errors.
In contrast to control participants, CD participants also failed to show post-error slowing of response speed in response to inhibition errors during the punishment condition. Adopting such a cautious pattern of post-error behaviour has been argued to represent an adaptive behavioural response during speeded response tasks such as the Go/No-go task (Hajcak et al., 2003; Kerns et al., 2004). Our results support this contention with increased post-error slowing associated with improved inhibition accuracy. Previous studies have indicated deficits in post-error adaptive behaviour in cocaine users (Franken et al., 2007; Li et al., 2006), though several have found intact post-error slowing during neutral conditions (Hester et al., 2007b; Li et al., 2008). For example, Li and colleagues (Li et al., 2006) showed that the increased stop-signal RTs (SSRT) of cocaine addicted subjects were accompanied by smaller response slowing on trials that followed both errors and successful inhibitions. The group difference in SSRT was eliminated by factoring out the post-trial slowing effect, suggesting that the apparent impulse control deficit of users may be driven by deficits in a proactive rather than reactive control system. In the present data, there also appeared to be a general trend for speed-accuracy tradeoff in the Go trial RT performance, with performance in the punishment condition significantly slower than the neutral condition. While the punishment-related slowing in control participants was greater than CD, consistent with their higher accuracy for this condition, the interaction effect between group and condition for Go Trial RT and inhibition accuracy were both non-significant. Similarly, previous fMRI studies examining speed-accuracy trade-off have found that increased cautiousness results in reduced activity in key parts of the response inhibition network, namely the right IFG and dorsal ACC, during successful inhibition (Forstmann et al., 2008; Jahfari et al., 2012). The present results identified such a pattern when comparing successful stops in the punishment and neutral condition, as well as significant group differences in these regions (CD greater than control), but failed to find significant interaction effects between group and condition that would be consistent with a heightened tendency for control participants to enact proactive cognitive control during the punishment condition and CD participants a reactive control approach.
The isolation of deficient post-error slowing to the punishment condition might also suggest that such adaptive behaviour, or the absence therein, may be particularly sensitive to the contingencies inherent to the task, for example, where post-error slowing offers the greatest advantage to task performance (Bissett and Logan, 2012). The observation that neither group showed post-error slowing during the neutral condition may also be consistent with this theme, as our previous use of the Go/No-go task has also showed post-error speeding (Hester et al., 2007a, 2005). Our version of the task does not present consecutive no-go trials and on average they were 7 trials apart, diminishing the adaptive value, in comparison to other tasks such as the Stroop, Stop-signal and Flanker that often feature consecutive incongruent trials, of immediately slowing responses post-error. The results also appear consistent with the previous demonstrations of reduced sensitivity to monetary loss in cocaine users (Goldstein et al., 2007), but further demonstrate that such insensitivity is associated with an inability to increase impulse control in the face of negative consequences, a core symptom of drug addiction.
The diminished behavioural sensitivity to punishment shown by CD participants was associated with hypoactive error-related BOLD responses, when compared to control participants, in the dorsal ACC, right insula and right middle frontal regions. Specifically, CD participant’s error-related response in these regions was not modulated by the presence of punishment, whereas control participants’ response showed a significant BOLD increase during punished errors. While hypoactive error-related activity in CD participants has previously been observed in both the ACC and insula (Franken et al., 2007; Kaufman et al., 2003), along with diminished neural sensitivity to monetary loss (Goldstein et al., 2007), the current data indicates a confluence of these factors that results in a diminished response to punished errors. One potential consequence of this diminished response is the failure to engage adaptive post-error corrective behaviour. The magnitude of error-related dorsal ACC activity has previously been linked with post-error slowing (Danielmeier et al., 2011; Debener et al., 2005; Hester et al., 2007a) and the present data demonstrate this relationship during the punishment condition, within each of the regions showing diminished error-related activity for CD participants.
Previous studies have identified insular cortex activity during punishment (Sanfey et al., 2003; Wachter et al., 2009), with the level of activity linked to the magnitude of (Elliott et al., 2000) and individual sensitivity to punishment (Samanez-Larkin et al., 2008). The region is thought to be generally representative of negative emotional states, including diverse states such as hunger, pain, anger and disgust (Naqvi and Bechara, 2009). Craig (2009) has hypothesised that rather than a reaction to punishment directly, anterior insula activity represents awareness of an outcomes’ salience and our emotional reaction to it. Greater activity in the insula therefore indicates heightened awareness of the emotional significance of an outcome. Recent work has consistently demonstrated insular cortex dysfunction is associated with addiction (Naqvi and Bechara, 2009; Paulus, 2007), particularly poor decision making that may contribute to continued drug taking in the face of significant negative consequences (Paulus et al., 2005). The present result may offer a functional neuroanatomical correlate for the relationship between insular cortex dysfunction and the failure to adapt behaviour following negative outcomes.
Given our small sample size, we were unable to clearly assess the relationship between duration of abstinence and behavioural/imaging findings. Future research to pursue this question is of interest to test the hypothesis that the dysfunction detected in the present study is a pre-existing condition that was not the result of cocaine use, and hence will not likely ‘correct’ over time with the maintenance of abstinence. Recent findings have highlighted that the impulse control problems and cortical dysfunction we identify here in abstinent cocaine users, is present in both stimulant dependent individuals and their non-addicted siblings (Ersche et al., 2012). Longitudinal research has similarly shown that two predictors of developing later drug dependence among children who have yet to consume drugs is their sensitivity to reward and cognitive control dysfunction (Lukasiewicz et al., 2008; Tarter et al., 2003), with mounting evidence that dopamine receptor density (particularly in the striatum) is linked to both these behavioural characteristics and the risk for developing a drug dependence (Volkow et al., 2001). If the present findings are not related to cocaine use per se, nor ameliorated by continued abstinence, there are potential implications for the treatment and prevention of cocaine dependence, particularly the potential for punishment to significantly influence the self-control required to maintain abstinence.
A limitation of the current study was the characterization of the sample as formerly dependent cocaine users. While polydrug use, and co-morbid dependence, is typical in cocaine dependent samples, chronic use of alcohol and heroin have both been shown, like cocaine, to diminish cognitive control broadly, and specifically the error-related response (Forman et al., 2004). While the size of our sample does not adequately cater for examination of these co-morbid effects, it suggests that the diminished response to punishment seen in the error-related response may be influenced by polydrug use, and similarly, that the diminished sensitivity to punishment during cognitive control and error processing may be a general phenomenon seen in drug dependence.
Supplementary Material
Acknowledgments
Role of Funding Source
This research was supported by USPHS grant from the National Institute on Drug Abuse: R01DA14100, and an Australian Research Council Felloswship (R.H.). Neither funding agency had any further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank The New York State Office of Alcoholism and Substance Abuse Service (OASAS), Tamara Miller-Kammerer, Thomas Miller, Dr. Stephen Kipnis, George Serdinsky, Robert Savino, Dave Samuels, Alan Vassallo and the staff at the Russell E. Blaisdell Addiction Treatment Center and Open Arms Inc. for all of their help in recruitment efforts. The work would simply not have been possible without the dedication of these individuals. We would like to express our sincere gratitude to the participants for giving their time to this effort. Additionally, we would like to thank Raj Sangoi and Emma-Jane Forde for all of their work in data collection.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
RH and HG designed the study and wrote the protocol, RH analysed data and wrote the first draft of the manuscript; RB collected the behavioural and fMRI data, RB, JF and HG assisted with design and interpretation of the data. All authors contributed to and have approved the final manuscript.
Conflicts of Interest
Drs Hester, Bell, Foxe and Garavan reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Post-stop-signal slowing: strategies dominate reflexes and implicit learning. J Exp Psychol Hum Percept Perform. 2012;38:746–757. doi: 10.1037/a0025429. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Smith AR, Hommer DW. Reduced posterior mesofrontal cortex activation by risky rewards in substance-dependent patients. Drug Alcohol Depend. 2008a;95:115–128. doi: 10.1016/j.drugalcdep.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008b;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Buchel C, Smolka MN. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2011;121:45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J Neurosci. 2011;31:1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry. 2010;67:632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty G, Casey BJ, Siegle G, Braver T, Barch D, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci. 2008;28:9790–9796. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hester R, Barre N, Mattingley JB, Foxe JJ, Garavan H. Avoiding another mistake: error and posterror neural activity associated with adaptive posterror behavior change. Cogn Affect Behav Neurosci. 2007a;7:317–326. doi: 10.3758/cabn.7.4.317. [DOI] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Madeley J, Murphy K, Mattingley JB. Learning from errors: error-related neural activity predicts improvements in future inhibitory control performance. J Neurosci. 2009a;29:7158–7165. doi: 10.1523/JNEUROSCI.4337-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009b;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007b;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Bjork JM, Gilman JM. Imaging brain response to reward in addictive disorders. Ann N Y Acad Sci. 2011;1216:50–61. doi: 10.1111/j.1749-6632.2010.05898.x. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Verbruggen F, Frank MJ, Waldorp LJ, Colzato L, Ridderinkhof KR, Forstmann BU. How preparation changes the need for top-down control of the basal ganglia when inhibiting premature actions. J Neurosci. 2012;32:10870–10878. doi: 10.1523/JNEUROSCI.0902-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO/NOGO task as revealed by event-related fMRI. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. Prentice Hall; Englewood Cliffs, New Jersey: 1991. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz M, Neveu X, Blecha L, Falissard B, Reynaud M, Gasquet I. Pathways to substance-related disorder: a structural model approach exploring the influence of temperament, character, and childhood adversity in a national cohort of prisoners. Alcohol Alcohol. 2008;43:287–295. doi: 10.1093/alcalc/agm183. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacol (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. NeuroImage. 2011;56:2258–2275. doi: 10.1016/j.neuroimage.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19:320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Simoes-Franklin C, Hester R, Shpaner M, Foxe JJ, Garavan H. Executive function and error detection: the effect of motivation on cingulate and ventral striatum activity. Hum Brain Mapp. 2010;31:458–469. doi: 10.1002/hbm.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wachter T, Lungu OV, Liu T, Willingham DT, Ashe J. Differential effect of reward and punishment on procedural learning. J Neurosci. 2009;29:436–443. doi: 10.1523/JNEUROSCI.4132-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.