Abstract
Background
Treatment decisions can be difficult in men with low-risk prostate cancer (PCa).
Objective
To evaluate the ability of a panel of four kallikrein markers in blood—total prostate-specific antigen (PSA), free PSA, intact PSA, and kallikrein-related peptidase 2—to distinguish between pathologically insignificant and aggressive disease on pathologic examination of radical prostatectomy (RP) specimens as well as to calculate the number of avoidable surgeries.
Design, setting, and participants
The cohort comprised 392 screened men participating in rounds 1 and 2 of the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer. Patients were diagnosed with PCa because of an elevated PSA ≥3.0 ng/ml and were treated with RP between 1994 and 2004.
Outcome measurements and statistical analysis
We calculated the accuracy (area under the curve [AUC]) of statistical models to predict pathologically aggressive PCa (pT3–T4, extracapsular extension, tumor volume >0.5 cm3, or any Gleason grade ≥4) based on clinical predictors (age, stage, PSA, biopsy findings) with and without levels of four kallikrein markers in blood.
Results and limitations
A total of 261 patients (67%) had significant disease on pathologic evaluation of the RP specimen. While the clinical model had good accuracy in predicting aggressive disease, reflected in a corrected AUC of 0.81, the four kallikrein markers enhanced the base model, with an AUC of 0.84 (p < 0.0005). The model retained its ability in patients with low-risk and very-low-risk disease and in comparison with the Steyerberg nomogram, a published prediction model. Clinical application of the model incorporating the kallikrein markers would reduce rates of surgery by 135 of 1000 patients overall and 110 of 334 patients with pathologically insignificant disease. A limitation of the present study is that clinicians may be hesitant to make recommendations against active treatment on the basis of a statistical model.
Conclusions
Our study provided proof of principle that predictions based on levels of four kallikrein markers in blood distinguish between pathologically insignificant and aggressive disease after RP with good accuracy. In the future, clinical use of the model could potentially reduce rates of immediate unnecessary active treatment.
Keywords: Prostate-specific antigen/blood, Prostatic neoplasms, Mass screening, Radical prostatectomy, Kallikrein-related peptidases
1. Introduction
A high proportion of patients with screen-detected prostate cancer (PCa) do not have aggressive disease. It can be questioned whether a patient found to have pathologically insignificant disease on pathologic analysis of the radical prostatectomy (RP) specimen should have undergone immediate active treatment or should have been managed with surveillance. A commonly used definition of pathologically insignificant PCa in RP specimens is the one suggested by Epstein et al: organ-confined cancer with a volume ≤0.5 cm3 without poorly differentiated elements (Gleason score ≤6) [1].
Prediction tools exist that take into account several clinical features that help to preoperatively distinguish pathologically insignificant from aggressive disease. These tools include prostate-specific antigen (PSA) and clinical stage, as well as biopsy variables such as transrectal ultrasound prostate volume, Gleason grade, number of positive biopsy cores, percentage of cancer in any core sample, total cancer length, and noncancer tissue in biopsy cores [2–6]. Currently available prediction tools have a discrimination in the area under the curve (AUC) range of 0.70–0.80; hence, there is room for improvement. Current models will classify some men as having pathologically insignificant disease who will eventually present with aggressive disease while on surveillance and will advise men to have invasive treatment who may actually have pathologically insignificant disease. There is a need for more optimal pretreatment decision tools that aim at reducing possible overtreatment of pathologically insignificant disease.
We have previously shown that a model based on levels of four kallikrein markers in blood—total PSA, free PSA, intact PSA, and kallikrein-related peptidase 2 (hK2)—has the ability to predict the result of prostate biopsy and that discrimination is improved for low-grade compared with high-grade disease [7,8]. We therefore hypothesized that this four-kallikrein model would also help distinguish between pathologically insignificant and aggressive disease on pathologic examination of the RP specimens, when used in addition to standard clinical predictors. We also aimed to evaluate whether this model could be used as a treatment decision aid, particularly in low-risk patients.
2. Methods
The cohort emerges from the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer (ERSPC). Men were invited for screening every fourth year. PCa was detected by lateralized sextant transrectal ultrasound–guided biopsy because of an elevated PSA ≥3.0 ng/ml. Our cohort comprised 392 screened men participating in rounds 1 and 2 (the first round took place during 1993–2000, and the second round 4 yr later for every participant) who were treated with RP between 1994 and 2004. The outcome, pathologically aggressive (nonindolent) disease on RP specimens, was defined as disease not confined to the prostate, tumor volume >0.5 cm3, or Gleason grade >3.
The TNM 2002 classification was used for PCa staging. The volume of tumor foci was measured by computer-assisted morphometric analysis after carefully encircling tumor areas on the glass slides.
We built a base model predicting pathologically aggressive disease using logistic regression, including the following standard clinical features: age (age at blood draw for 369 men, age at clinical examination for 29 men), total PSA, number of positive biopsy cores, and millimeters of cancerous tissue (all modeled continuously) as well as Gleason score (≤6, 7, and ≥8) and clinical stage (lower than T2b compared with T2b or higher). The length of PCa in single-needle biopsies was calculated based on the tumor percentage of the total microscopic needle biopsy length. Discontinuous cancer foci with a single biopsy were considered as separate foci.
Clinically insignificant tumors preoperatively were defined according to the Epstein criteria [1] as PSA <10 ng/ml, biopsy Gleason score ≤6, clinical stage T2a or lower, two or fewer positive cores, and ≤50% of any one core involved with tumor.
The nomogram developed by Steyerberg et al [2] is frequently used in predicting pathologically insignificant disease on RP, building on the nomograms from Kattan et al [4]. The Steyerberg nomogram, however, requires more extensive pathologic examination (such as the length of noncancerous tissue in individual biopsy cores), which is not common practice at all hospitals. We therefore created our base model on clinical parameters that are available at virtually any hospital in which RPs are performed.
2.1. Laboratory methods
All laboratory analyses were conducted blind to biopsy results [9]. Serum samples were retrieved from the archival serum bank in Rotterdam, The Netherlands, where they were stored frozen at −80°C after their initial processing ≤3 h from venipuncture. The samples were shipped frozen on dry ice to Malmö, Sweden, in 2005–2007. Analyses of free, total, and intact PSA, along with hK2, were done in Hans Lilja’s laboratory at the Wallenberg Research Laboratories (Department of Laboratory Medicine, Lund University, Skåne University Hospital, Malmö, Sweden) during 2005 and 2007. Free and total PSA were measured using the dual-label DELFIA ProStatus Free/Total PSA assay (PerkinElmer, Turku, Finland) [10]. Intact PSA and hK2 were measured using F(ab′)2 fragments of the monoclonal capture antibodies to reduce the frequency of nonspecific assay interference [11].
2.2. Statistics
We first calculated a risk score predicting any PCa, irrespective of grade, for each patient, based on age plus the patient’s four kallikrein levels, using a previously developed model on the Rotterdam cohort (developed on all men undergoing biopsy) [8]. To evaluate the clinical usefulness of this kallikrein risk score in predicting potentially aggressive cancer on RP (ie, in a subcohort of the Rotterdam cohort)—defined as pT3–T4, extracapsular extension, tumor volume >0.5 cm3, or any Gleason grade ≥4—we added the score to, and compared it with, our standard clinical model (base model).
The predictive accuracy was assessed by the AUC. We compared the AUC for the kallikrein model when added to the clinical base model, using the likelihood ratio test. All estimates were corrected for overfit using 10-fold cross-validation.
We conducted a sensitivity analysis applying the Steyerberg nomogram to calculate the probability of pathologically insignificant cancer on RP specimens based on PSA, transrectal ultrasound prostate volume, clinical stage, prostate biopsy Gleason grade, and total length of cancer and noncancer tissue in biopsy cores. The model was originally developed on a subset of our Rotterdam study cohort and included men who underwent RP. The predictive accuracy of this model in our cohort was calculated with and without including the ERSPC-based kallikrein panel score. This model adjusts for Gleason scores <6. In contemporary pathology practices, a Gleason score <6 is no longer reported on prostate biopsies [12]. Therefore, we performed the analysis recategorizing cancers graded <6 as Gleason score 6. All models were additionally tested in two subgroups: low risk (Gleason ≤6) and very low risk (Gleason ≤6, T1c, and PSA <10).
Using the methodology of Vickers and Elkin [13], we calculated the clinical utility (net benefit) of the clinical model alone and of the clinical model plus the kallikrein panel. Net benefit is calculated across a range of threshold probabilities. We chose 20–50% as our range, based on discussions with urologists that few men would opt to undergo treatment if told that their probability of indolent PCa was >80%; similarly, few men would deny treatment if told that they had a >50% chance of potentially aggressive disease. A good model has a high net benefit. The comparison is to regard all patients as true positives and to treat all.
We chose a threshold of 30% risk of potentially aggressive disease on RP to calculate the number of surgeries performed as well as pathologically insignificant cases treated per 1000 men [14]. Statistical analyses were conducted using Stata 12.0 (StataCorp, College Station, TX, USA).
3. Results
Patient characteristics are presented in Table 1. The median age at diagnosis was 64 yr (interquartile range: 61–67). Of the 392 men, more than two-thirds (270 of 392) were diagnosed during the first screening round. Three-quarters of the patients (289) had low-risk disease (biopsy Gleason score ≤6), and 186 of these patients had very-low-risk disease (additionally T1c and PSA <10 ng/ml).
Table 1.
Clinical and pathologic characteristics of 392 men diagnosed with cancer in the Rotterdam branch of the European Randomized Study of Screening for Prostate Cancer and treated with radical prostatectomy
| Characteristics | Results |
|---|---|
| Screening round, no. (%) | |
| 1 | 270 (69) |
| 2 | 122 (31) |
| Age, yr, median (IQR) | 64 (61–67) |
|
| |
| Clinical characteristics | |
|
| |
| Clinically significant disease, no. (%) | 260 (66) |
| Positive DRE, no. (%) | 139 (35) |
| Biopsy cores, no. (%) | |
| 6 | 259 (66) |
| 7 | 133 (34) |
| Positive cores, no. (%) | |
| 0 | 2 (1) |
| 1 | 129 (33) |
| 2 | 104 (27) |
| 3+ | 157 (40) |
| Biopsy Gleason score, no. (%) | |
| ≤6 | 289 (74) |
| 7 | 87 (22) |
| ≥8 | 16 (4) |
| Kallikrein panel, ng/ml, median (IQR) | |
| Total PSA | 4.54 (3.39–6.64) |
| Free PSA | 0.80 (0.54–1.10) |
| Intact PSA | 0.44 (0.32–0.63) |
| hK2 | 0.069 (0.047–0.10) |
| Clinical stage, no. (%) | |
| <T2b | 330 (84) |
| ≥T2b | 61 (16) |
| Prostate volume, ml, median (IQR) | 37 (29–49) |
| Maximum percentage of cancer in any one biopsy core, median (IQR) | 8 (3–19) |
| Cancerous tissue, mm, median (IQR) | 6 (2–13) |
| Benign tissue, mm, median (IQR), n = 387 | 64 (52–71) |
|
| |
| Pathologic characteristics | |
|
| |
| Pathologically significant disease, no. (%) | 261 (67) |
| Tumor volume, cm3, median (IQR) | 0.6 (0.2–1.3) |
| Tumor volume ≥0.5 cm3, no. (%) | 219 (56) |
| Pathologic Gleason score, no. (%), n = 389 | |
| ≤6 | 250 (64) |
| 7 | 123 (32) |
| ≥8 | 16 (4) |
| Pathologic stage ≥T2b, no. (%) | 296 (76) |
IQR = interquartile range; DRE = digital rectal examination; PSA = prostate-specific antigen; hK2 = kallikrein-related peptidase 2.
Clinically indolent disease on biopsy was defined using the Epstein criteria: serum PSA <10, biopsy Gleason score <6, clinical stage T2a or lower, three or fewer positive cores, ≥50% of any one core involved with tumor; pathologically insignificant disease was defined as small tumor volume (<0.5 cm3) confined to the prostate with no focal or established extracapsular extension with Gleason grade ≥3.
A total of 260 patients (66%) had clinically significant cancer on biopsy, as defined by the Epstein criteria, and 261 patients (67%) had significant cancer on pathologic evaluation of the RP specimen. A total of 51 patients (38%) who met Epstein criteria for clinically insignificant tumors on biopsy were reclassified as having aggressive cancer on pathologic evaluation of the corresponding RP.
Table 2 shows the discriminative accuracy of the models after correction for overfit. The clinical model had good discriminative accuracy in predicting aggressive disease, reflected in a corrected AUC of 0.81. AUC increased to 0.84 (p < 0.0005) with the addition of the four kallikrein markers. The improvement in predictive accuracy was somewhat larger in low-risk patients (AUC increased from 0.75 to 0.81) and in patients defined as being at very low risk (AUCs increased from 0.72 to 0.81).
Table 2.
Predictive accuracy (area under the curve) of the clinical model alone and the clinical model together with the kallikrein-based risk score model in predicting potentially aggressive cancer (defined as disease not confined to the prostate, tumor volume >0.5 cm3, or Gleason grade >3) on radical prostatectomy
| No. | Clinical model, AUC (95% CI) | Clinical model plus kallikrein-based risk score model, AUC (95% CI) | p value* | |
|---|---|---|---|---|
| All risk | 392 | 0.81 (0.77–0.85) | 0.84 (0.80–0.89) | <0.0005 |
| Low risk | 289 | 0.75 (0.69–0.80) | 0.81 (0.77–0.86) | <0.0005 |
| Very low risk | 186 | 0.72 (0.65–0.79) | 0.81 (0.75–0.88) | <0.0005 |
AUC = area under the curve; CI = confidence interval; PSA = prostate-specific antigen.
Low risk: Gleason score ≥6; very low risk: Gleason score ≥6, T1c, PSA <10 ng/ml. Clinical model: age, Gleason score, positive cores, millimeters of prostate cancer tissue, stage, PSA. Kallikrein-based risk score model: model based on total PSA, free PSA, intact PSA, kallikrein-related peptidase 2, and age.
The p value obtained using the likelihood ratio test to test the hypothesis that the kallikrein panel improves predictiveness.
Across all risk groups, the Steyerberg model outperformed the clinical base model, particularly in very-low-risk patients (AUC of 0.78 compared with 0.72). The addition of the kallikrein-based risk score to the Steyerberg model improved the AUC, especially in the low- and very low-risk subgroups (AUC of 0.83 and 0.83, respectively). Results were similar when recategorizing Gleason scores ≤6 as 6 or as 4, 5, 6 (Table 3). Results were also similar when the length of benign tissue was added to the clinical model (AUCs improved by 0.03, 0.04, and 0.07 for the whole group, low-risk subgroup, and very-low-risk subgroup, respectively; p < 0.0005 for all). Figure 1 and 2 demonstrate the clinical value (utility) of the results. Although the clinical threshold will vary according to the individual patient’s acceptance of risk, for demonstration purposes we chose a threshold of 30%, suggesting that a patient would opt for surgery if he had a ≥30% risk of aggressive disease. Using the statistical model based on the four-kallikrein panel, for every 1000 men taken to surgery, overtreatment would be avoided in 110 men, and immediate treatment of 26 men with potentially aggressive disease would be delayed. The corresponding numbers for the clinical base model were 48 and 13 per 1000 men, respectively (Table 4).
Table 3.
Predictive accuracy (area under the curve) of the Steyerberg nomogram alone and together with the kallikrein risk score in predicting potentially aggressive cancer on radical prostatectomy, when recategorizing Gleason scores <6 as 6
| No. | Steyerberg, AUC (95% CI) | Steyerberg plus kallikrein-based risk score, AUC (95% CI) | p value* | |
|---|---|---|---|---|
| All risk | 384 | 0.82 (0.77–0.86) | 0.84 (0.80–0.88) | <0.0005 |
| Low risk | 284 | 0.80 (0.75–0.85) | 0.83 (0.79–0.88) | <0.0005 |
| Very low risk | 183 | 0.78 (0.72–0.85) | 0.83 (0.77–0.89) | <0.0005 |
AUC = area under the curve; CI = confidence interval.
The p value obtained using the likelihood ratio test to test the hypothesis that the kallikrein panel improves predictiveness.
Fig. 1.
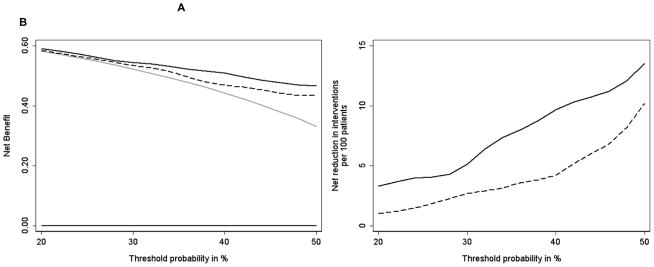
(a) Decision curve comparing the net benefit of models predicting risk of potentially aggressive disease features (non–organ-confined disease, tumor volume >0.5 cm3, or Gleason grade >3) on radical prostatectomy; (b) net reduction in interventions per 100 patients compared with threshold probability of potentially aggressive disease on radical prostatectomy. Thick black line at the bottom = treating no one; thick gray line = treating everyone; dashed line = clinical model; black line = clinical model plus the four-kallikrein panel.
Fig. 2.
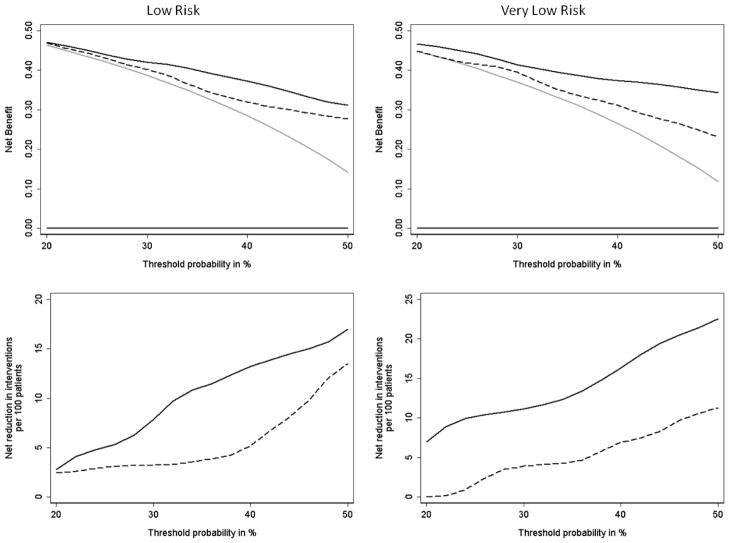
(a) Decision curve comparing the net benefit of models predicting risk of potentially aggressive disease features (non–organ-confined disease, tumor volume >0.5 cm3, or Gleason grade >3) on radical prostatectomy in low-risk subgroups (Gleason grade ≥6) and very-low-risk subgroups (Gleason grade ≥6, T1c, PSA <10 ng/ml); (b) net reduction in interventions per 100 patients compared with threshold probability of potentially aggressive disease on radical prostatectomy. Thick black line at the bottom = treating no one; thick gray line = treating everyone; dashed line = clinical model; black line = clinical model plus the four-kallikrein panel.
Table 4.
Number of surgeries performed and pathologically insignificant cases treated per 1000 men, with a threshold of 30% risk of potentially aggressive disease on radical prostatectomy
| Treatment | Pathologically insignificant cancers | Potentially aggressive cancers | ||||
|---|---|---|---|---|---|---|
| Treated, no. | Not treated, no. | Treated, no. | Not treated, no. | Treated, no. | Not treated, no. | |
| Treat everyone | 1000 | 0 | 334 | 0 | 666 | 0 |
| Clinical model | 939 | 61 | 286 | 48 | 653 | 13 |
| Clinical model plus kallikrein-based risk score model | 865 | 135 | 224 | 110 | 640 | 26 |
The table displays rounded numbers. Clinical model: age, Gleason score, positive cores, millimeters of prostate cancer tissue, stage, PSA. Kallikrein-based risk score model: model based on total PSA, free PSA, intact PSA, kallikrein-related peptidase 2, and age. Potentially aggressive cancers: non–organ-confined disease, tumor volume >0.5 cm3, or Gleason grade >3.
The decision curves show that the model with the kallikrein risk score added has a higher net benefit (Fig. 1) compared with the clinical model across all evaluated thresholds in all risk groups. Figure 2 expresses these results in terms of the net reduction in avoidable RPs. For example, at a 40% threshold probability, use of the kallikrein model is equivalent to use of a model that reduced the number of RPs by 10% while advising no men with aggressive cancer to delay treatment.
4. Discussion
We sought to determine whether a statistical model based on levels of four kallikrein markers in blood added to information available at biopsy could distinguish between pathologically insignificant and aggressive disease at the time of RP. We demonstrated that the kallikrein-based risk score was able to accurately provide information about this risk when used in addition to either a clinical base model or the Steyerberg nomogram.
Using decision analytic methods, we showed that using the panel of four kallikrein markers in blood to make a decision about surgery would lead to clinically superior outcomes compared with current clinical practice. This panel could be used as a decision aid in men with low-risk disease, in whom treatment decisions are most difficult. Adding the kallikrein panel to the decision-making process may reduce the number of immediate radical treatments performed for low-risk disease, bearing in mind that delayed treatment is still an option under surveillance [15]. This approach could save many men years of suffering from adverse effects of treatment, including incontinence and erectile dysfunction, and thus improve quality of life while advising few men with potentially aggressive cancers against immediate surgery.
The results we report for our reference model are consistent with previous studies evaluating the use of standard pretreatment characteristics in distinguishing pathologically insignificant from potentially aggressive disease on RP [4–6,16]. We have no reason to believe that the markers were found to be of value only because of poor performance of our comparison model. The incremental value of the kallikrein panel was consistent in all variants of the reference models. A strength of the kallikrein panel is that it does not require collection of additional or nonstandard blood tubes, additional pathologic ascertainment, or extra clinical procedures such as magnetic resonance imaging; aliquots required to measure the three additional biomarkers (free PSA, intact PSA, and hK2) can be accessed from the same blood draw used for measuring total PSA. Nor does the kallikrein panel require additional analysis of biopsy specimens (as genetic approaches do) or novel procedures (such as prostatic massage for the prostate cancer antigen 3 gene). As such, the panel is easy, is acceptable, and comes at low cost. However, the panel is not yet commercially available.
Although the fact that the model investigated was developed [8] and tested on the same cohort (Rotterdam) could be viewed as a limitation, we do not believe this fact influences our results. The model was developed in all men undergoing biopsy within the Rotterdam cohort, and we tested it in a subcohort of men who underwent RP. The outcome is not the same. The model was developed to predict the probability of PCa on biopsy, regardless of grade, while we tested its ability to predict pathologically insignificant cancer after RP. Another possible limitation is that while current guidelines recommend 10–12 cores, this analysis was based on sextant biopsies, which may have made the grading less ideal and affected the performance of the base model. In addition, a model based on clinical parameters (digital rectal examination, nonstandardized biopsies, and pathology-dependent evaluation) is limited by subjective variability.
Few studies have evaluated the potential of biomarkers in predicting pathologically insignificant disease at RP. Haese et al evaluated the ability of total PSA, free PSA, and hK2 to predict organ-confined disease and pathologic stage among men with total PSA <10 ng/ml undergoing RP for localized PCa. The AUC was 0.64 for hK2 and 0.68 for a statistical model including hK2 and the ratio of free to total PSA, both of which outperformed total PSA alone (AUC: 0.55) [17]. Our present study confirms the improved accuracy of combining biomarkers into a single statistical model. Together, these results suggest that the prediction of pathologically insignificant cancer relies on the combination of these biomarkers.
Our study used decision analytic techniques that allow for the consideration of a patient’s preferences. If a patient is expressing a strong aversion to the adverse effects of active treatment, he and his surgeon can engage in a conversation about how high a risk of potentially aggressive cancer the patient is willing to accept in order to delay or undergo treatment. We chose to picture the results of our model using a threshold probability of potentially aggressive cancer of 30%. While this may seem higher than would be acceptable to most men, the decision to forgo active treatment is not irrevocable. Men who would be advised, based on the kallikrein model, to enroll in an active surveillance program and who subsequently progressed could still opt for treatment within a window of curable disease. It should be noted that our definition of potentially aggressive cancer includes tumors ≥0.5 cm3 or a Gleason grade >3, disease features that do not necessarily confer aggressive biology. In fact, clinically insignificant PCa may include tumors with volumes as high as 1.3 cm3 [18].
Our decision analysis showed that use of the kallikrein model could reduce the number of unnecessary surgeries. A historical limitation of the present study is that clinicians may be hesitant to make recommendations against active treatment on the basis of a statistical model. Another caveat is that the long-term natural history of PSA-detected low-volume, low-grade disease is still poorly understood, and local progression and distant metastasis can develop over the long term among low-risk patients [19]. Once surveillance cohorts mature, determination of outcomes such as metastatic disease, disease recurrence, and disease-specific death may be feasible.
Our study is based on a rather small cohort of men who were diagnosed by means of sextant biopsy and treated a decade ago, which raises concerns about generalizability. Nonetheless, the current study provides proof of principle for the use of serum markers to aid in surgery decisions. The next step will be to perform an external validation study on an independent cohort.
5. Conclusions
Predictions based on levels of four kallikrein markers in blood accurately distinguish between pathologically insignificant and aggressive disease after RP when used in addition to either a clinical base model or the Steyerberg nomogram. The next step will be to validate our findings. In the future, use of this kallikrein model in clinical practice could potentially guide treatment decisions.
Take-home message.
Predictions based on levels of four kallikrein markers in blood—total prostate-specific antigen (PSA), free PSA, intact PSA, and kallikrein-related peptidase 2—can accurately distinguish between pathologically insignificant and aggressive disease after radical prostatectomy. Clinical use of the model could reduce rates of unnecessary surgery.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Hans Lilja and Monique J. Roobol had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vickers, Lilja, Roobol.
Acquisition of data: Roobol, Schröder, van der Kwast, van Leenders, Lilja.
Analysis and interpretation of data: Carlsson, Maschino, Vickers, Steyerberg, Roobol, Lilja.
Drafting of the manuscript: Carlsson, Maschino, Vickers.
Critical revision of the manuscript for important intellectual content: Carlsson, Maschino, Schröder, Bangma, Steyerberg, van der Kwast, van Leenders, Vickers, Lilja, Roobol.
Statistical analysis: Carlsson, Maschino, Vickers.
Obtaining funding: Roobol, Schröder, Vickers, Lilja.
Administrative, technical, or material support: Roobol, Schröder, van der Kwast, van Leenders, Lilja.
Supervision: Schröder, Steyerberg, Vickers, Lilja, Roobol.
Other (specify): None.
Financial disclosures: Hans Lilja and Monique J. Roobol certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Hans Lilja holds patents for free prostate-specific antigen (PSA), kallikrein-related peptidase 2, and intact PSA assays. Sigrid Carlsson is supported by grants from the Swedish Cancer Society, the Sweden America Foundation, the Swedish Council for Working Life and Social Research, and the Swedish Society for Medical Research. Other grant support was received from the National Cancer Institute (grants R33 CA 127768-02, P50-CA92629, and R01 CA160816); Swedish Cancer Society (grant 11-0624); Sidney Kimmel Center for Prostate and Urologic Cancers; David H. Koch through the Prostate Cancer Foundation, the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford, and Fundación Federico SA; Rotterdam: The Dutch Cancer Society (grants KWF 94-869, 98-1657, 2002-277, 2006-3518, and 2010-4800); and The Netherlands Organization for Health Research and Development (grants ZonMW-002822820, 22000106, 50-50110-98-311, and 62300035). Ewout W. Steyerberg was supported by the Center for Translational Molecular Medicine (PCCM project, grant 030-203).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein JI, Chan DW, Sokoll LJ, et al. Nonpalpable stage T1c prostate cancer: prediction of insignificant disease using free/total prostate specific antigen levels and needle biopsy findings. J Urol. 1998;160:2407–11. [PubMed] [Google Scholar]
- 2.Steyerberg EW, Roobol MJ, Kattan MW, van der Kwast TH, de Koning HJ, Schroder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177:107–12. doi: 10.1016/j.juro.2006.08.068. discussion 112. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–74. [PubMed] [Google Scholar]
- 4.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792–7. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi H, Wang X, Ochiai A, et al. A nomogram for predicting low-volume/low-grade prostate cancer: a tool in selecting patients for active surveillance. Cancer. 2007;110:2441–7. doi: 10.1002/cncr.23055. [DOI] [PubMed] [Google Scholar]
- 6.Chun FK, Haese A, Ahyai SA, et al. Critical assessment of tools to predict clinically insignificant prostate cancer at radical prostatectomy in contemporary men. Cancer. 2008;113:701–9. doi: 10.1002/cncr.23610. [DOI] [PubMed] [Google Scholar]
- 7.Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008;6:19. doi: 10.1186/1741-7015-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickers A, Cronin A, Roobol M, et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol. 2010;28:2493–8. doi: 10.1200/JCO.2009.24.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickers AJ, Cronin AM, Roobol MJ, et al. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin Cancer Res. 2010;16:3232–9. doi: 10.1158/1078-0432.CCR-10-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 11.Vaisanen V, Peltola MT, Lilja H, Nurmi M, Pettersson K. Intact free prostate-specific antigen and free and total human glandular kallikrein 2: elimination of assay interference by enzymatic digestion of antibodies to F(ab′)2 fragments. Anal Chem. 2006;78:7809–15. doi: 10.1021/ac061201+. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL, Committee IG. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 13.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Vugt HA, Roobol MJ, van der Poel HG, et al. Selecting men diagnosed with prostate cancer for active surveillance using a risk calculator: a prospective impact study. BJU Int. 2012;110:180–7. doi: 10.1111/j.1464-410X.2011.10679.x. [DOI] [PubMed] [Google Scholar]
- 15.van den Bergh RC, Steyerberg EW, Khatami A, et al. Is delayed radical prostatectomy in men with low-risk screen-detected prostate cancer associated with a higher risk of unfavorable outcomes? Cancer. 2010;116:1281–90. doi: 10.1002/cncr.24882. [DOI] [PubMed] [Google Scholar]
- 16.Rubin MA, Mucci NR, Manley S, et al. Predictors of Gleason pattern 4/5 prostate cancer on prostatectomy specimens: can high grade tumor be predicted preoperatively? J Urol. 2001;165:114–8. doi: 10.1097/00005392-200101000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Haese A, Graefen M, Steuber T, et al. Human glandular kallikrein 2 levels in serum for discrimination of pathologically organ-confined from locally-advanced prostate cancer in total PSA-levels below 10 ng/ml. Prostate. 2001;49:101–9. doi: 10.1002/pros.1123. [DOI] [PubMed] [Google Scholar]
- 18.Wolters T, Roobol MJ, van Leeuwen PJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011;185:121–5. doi: 10.1016/j.juro.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 19.Popiolek M, Rider JR, Andren O, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol. 2013;63:428–35. doi: 10.1016/j.eururo.2012.10.002. [DOI] [PubMed] [Google Scholar]