Abstract
Background
The recommended standard of care calls for treating opioid-dependent pregnant women with methadone and observing neonates exposed in utero for five to seven postnatal days to see if treatment for neonatal abstinence syndrome (NAS) is needed. Data from a large multisite randomized clinical trial comparing buprenorphine vs. methadone for the treatment of opioid dependence during pregnancy suggest buprenorphine-exposed neonates had less severe NAS, but may require pharmacologic treatment for NAS later than methadone-exposed neonates. The present study examined whether time to pharmacologic treatment initiation differed in a relatively large non-blinded clinical sample of buprenorphine- vs. methadone-exposed neonates treated for NAS.
Methods
Medical records for 75 neonates exposed to buprenorphine (n = 47) or methadone (n = 28) in utero who required treatment for NAS were examined. Time elapsed between birth and initiation of pharmacologic treatment was calculated for each neonate and time to treatment initiation compared between groups.
Results
Median time to treatment initiation (hours:minutes, IQR) was significantly later in buprenorphine- vs. methadone-exposed neonates (71:02, 44:21-96:27 vs. 34:12, 21:00-55:41, respectively, p<.001). Estimates of mean time to treatment initiation from parametric analyses that adjusted for maternal and neonatal characteristics were very similar (73:10 (95% CI: 61:00 to 87:18) vs. 42:36 (95% CI: 33:06 to 53:30), respectively, p = .0005). This difference was not dependent on maternal age or neonatal sex, gestational age, or birth weight.
Conclusions
These findings confirm results from randomized clinical trials, adding generality to the observation that buprenorphine-exposed neonates require treatment significantly later than methadone-exposed neonates.
Keywords: Pregnancy, opioids, neonatal abstinence syndrome, methadone, buprenorphine, treatment
1. INTRODUCTION
Methadone is the recommended standard of care for the treatment of opioid dependence during pregnancy (National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998). While maintenance treatment with methadone, a full mu agonist, improves maternal and neonatal outcomes compared to no treatment or medication-assisted withdrawal (Jones et al., 2008; Kaltenbach et al., 1998), in utero exposure can result in a neonatal abstinence syndrome (NAS) characterized by hyperirritability of the central nervous system and dysfunction in the autonomic nervous system, gastrointestinal tract and respiratory system (Finnegan and Kaltenbach, 1992). Left untreated, NAS can result in serious complications (e.g., diarrhea, feeding difficulties, weight loss, seizures) and mortality (Finnegan and Kaltenbach, 1992).
Buprenorphine, a partial mu agonist and kappa antagonist, was approved for the treatment of opioid dependence in non-pregnant adults in 2002 in the U.S. In addition to demonstrating its efficacy as a maintenance medication, studies testing the clinical utility of buprenorphine in non-pregnant adults also reported that abrupt discontinuation of this medication results in a milder withdrawal syndrome that also may have a delayed onset relative to withdrawal from a full opioid agonist (Johnson et al., 2003; Walsh et al., 2003). The promising observations of a milder withdrawal with buprenorphine in adults prompted a number of studies comparing the NAS of neonates exposed in utero to methadone vs. buprenorphine. The largest and most rigorous comparison to date was the MOTHER (Maternal Opioid Treatment: Human Experimental Research) study, a double-blind randomized clinical trial that reported outcomes for 131 neonates, 68 of whom required treatment for NAS (Jones et al., 2010). Primary and secondary analyses of the MOTHER study data indicated that the NAS of buprenorphine-exposed neonates was milder, with less severe central nervous system signs, and that these neonates required significantly less morphine to treat their NAS and had a significantly shorter duration of hospitalization compared to methadone-exposed neonates (Gaalema et al., 2012; Jones et al., 2010). Also consistent with the adult literature, the 27 buprenorphine-exposed neonates who required pharmacologic treatment started that treatment significantly later than the 41 methadone-exposed neonates who required pharmacologic treatment (59 vs. 36 hours, respectively). In summary, while the NAS associated with buprenorphine appears less severe in several respects, these findings do not suggest that buprenorphine-exposed neonates can be monitored for a shorter period of time than methadone-exposed neonates
A few relatively smaller studies have also examined time to treatment initiation in methadone vs. buprenorphine-exposed neonates. Consistent with the MOTHER study results, one small double-blind randomized clinical trial (n = 14) reported earlier time to treatment initiation in methadone- vs. buprenorphine-exposed neonates who required treatment (n = 3 and 5, respectively; Fischer et al., 2006). In contrast, a non-randomized study (n = 36) by this same group reported later time to treatment initiation in methadone- vs. buprenorphine-exposed neonates who required treatment (n = 15 and 3, respectively; Ebner et al., 2007). More data from larger samples are needed to determine whether the observations made in blinded randomized trials generalize to everyday clinical practice. In addition, clarifying the range for time to treatment initiation has important implications for the clinical assessment and timely treatment of opioid-exposed neonates. The American Academy of Pediatrics (AAP) currently recommends that neonates exposed to long-acting opioids like methadone and buprenorphine be monitored for a minimum of 5-7 days (120-168 hours) after delivery, although it is not clear what data this recommendation is based on (Hudak et al., 2012). The present study, a retrospective chart review, examined time to treatment initiation in a non-blinded clinical sample (n = 75) of buprenorphine- vs. methadone-exposed neonates treated for NAS. Based on previous findings from research samples (Gaalema et al., 2012; Fischer et al., 2006) it was hypothesized that time to treatment initiation would occur later in buprenorphine- vs. methadone-exposed neonates.
2. METHODS
All neonates exposed to opioids in utero and delivered at our academic medical center, Fletcher Allen Health Care in Burlington, VT, are hospitalized for a minimum of four days for assessment of NAS. NAS assessments are conducted every 3-4 hours using a 19-item modified Finnegan Scale (Jansson et al., 2009). The clinical protocol calls for initiation of pharmacologic treatment for two consecutive total scores of 9-12 or one score of 13 or higher.
For the present retrospective chart review approval for the examination of electronic medical records was obtained from the University of Vermont Institutional Review Board. Electronic medical records were instituted on the neonatology service in April 2009 and the subsequent three years were searched to find records of neonates exposed prenatally to buprenorphine or methadone who required pharmacologic treatment for NAS. Seventy-five neonates were identified, 47 buprenorphine-exposed and 28 methadone-exposed. Maternal and neonatal characteristics were extracted from the electronic medical records and time of birth and time of treatment initiation were recorded to the nearest minute. Time of pharmacologic treatment initiation was defined as the first time after delivery that the neonate was administered any opioid. It is likely that many, if not all, neonates also received non-pharmacologic treatment (e.g., swaddling, skin-to-skin contact, low light and sound environment) prior to the need for pharmacologic treatment. Pharmacologic treatment was most often initiated with methadone (85%) and in other cases, morphine (8%) or fentanyl (7%), although the vast majority (96%) of the neonates were subsequently stabilized on methadone. Time elapsed between birth and initiation of pharmacologic treatment was calculated for each neonate.
Potential differences in characteristics of mother/neonate pairs maintained on methadone versus buprenorphine were examined using t-tests for continuous variables and Fisher’s exact tests for categorical variables. Median time to treatment initiation between the two groups was compared using a Wilcoxon rank sum test. A generalized linear model based on a gamma distribution utilizing a log-link function was used to evaluate drug differences in mean time to treatment initiation after adjusting for selected neonatal and maternal characteristics. The gamma model, which has been extensively used to model time to event data (Nelson, 1982; Lawless, 2003), had superior fit to other distributions based on goodness of fit criteria (i.e., AIC and BIC). Means presented based on the gamma modeling represent geometric means and associated confidence intervals due to the use of a log link function. Neonatal sex, gestational age, and birth weight were included as covariates as data suggests they may play a role in the development of NAS (Brown et al., 1998; Doberczak et al., 1991; Dysart et al., 2007; Jansson et al., 2007; O’Connor et al., 2013). Because gestational age and birth weight are very highly correlated, size for gestational age z-score (Olsen et al., 2010) was computed for inclusion in the models rather than birth weight to avoid issues relating to multicollinearity. Maternal age was also included as a covariate because it differed between medication conditions. Additional models were run to determine if drug differences were moderated by any of these characteristics.
3. RESULTS
There were few differences between mother/neonate pairs in the two medication groups (Table 1). Only age of the mother at delivery differed significantly (27.0 years buprenorphine vs. 29.3 years methadone, t-test, t (73) = 2.27, p = .03). Median time to treatment initiation, differed significantly between groups (Wilcoxon, W = 737, p < .001; Figure 1) with neonates exposed to buprenorphine in utero requiring treatment 37 hours later on average than those exposed to methadone (71:02, IQR: 44:21-96:27 vs. 34:12, IQR:21:00-55:41, respectively). Results of the generalized linear model adjusting for maternal and neonatal characteristics produced similar findings with estimated mean time to treatment of 73:10 (95% CI: 61:00 to 87:18) vs. 42:36 (95% CI: 33:06 to 53:30) hours for buprenorphine- and methadone- exposed neonates respectively (Wald X2 = 12.0, p = .0005). There was no evidence that maternal age, neonatal sex, gestational age, or birth weight was predictive of time to treatment (all p-values > .15). Separate models examining potential interactions between groups and each of the above factors resulted in no evidence that group differences were moderated by any of these factors (all interactions p values > .40).
Table 1.
Characteristics of the mothers and neonates (N = 75).
| Mother | Buprenorphine (N = 47) |
SD | Methadone (N = 28) |
SD | p |
|---|---|---|---|---|---|
| Age (years) | 27.0 | 4.4 | 29.3 | 3.9 | 0.03 |
| Maintenance dose at delivery (mg) | 16.2 | 7.2 | 98.5 | 52.3 | n/a |
| Delivery method (% c-section) | 32 | 43 | 0.46 | ||
| Neonate | |||||
| Birth weight (g) | 3023.3 | 582.0 | 2846.8 | 685.9 | 0.24 |
| EGA (weeks) | 38.9 | 2.6 | 37.6 | 3.1 | 0.06 |
| Size for gestational age (z-score) | 0.31 | 1.48 | 0.13 | 1.49 | 0.60 |
| Gender (% male) | 43 | 50 | 0.63 | ||
| Initial methadone dose (mg) | 0.41 | 0.10 | 0.40 | 0.09 | 0.51 |
Note: Values are means unless otherwise noted. For initial methadone dose n = 46 for buprenorphine and n = 26 for methadone as a total of 3 neonates were initially treated with either fetanyl or morphine rather than methadone. Size for gestational age z-scores based on Olsen et al, 2010.
Figure 1.
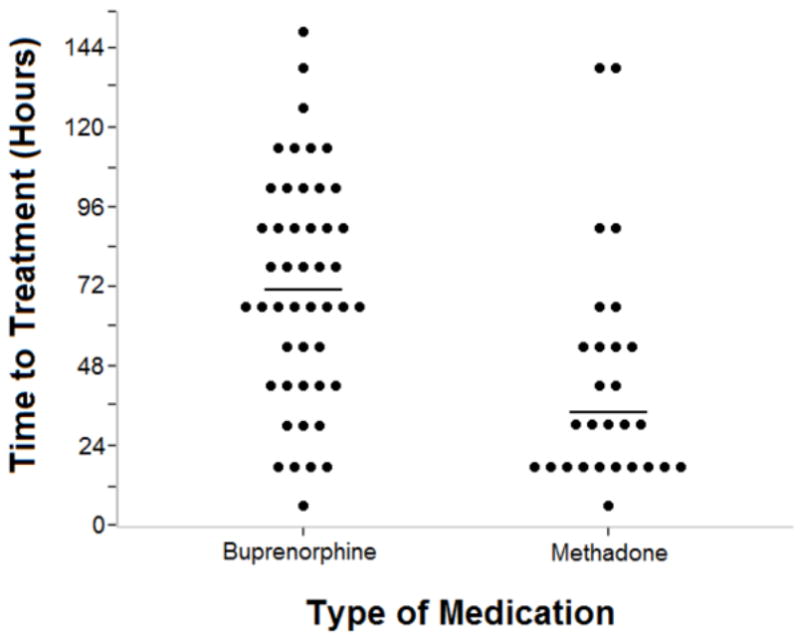
Time to initiation of pharmacological treatment in buprenorphine-and methadoneexposed neonates who required treatment. Each individual neonate’s time of treatment initiation is grouped in the 12-hour range when treatment initiation occurred and is represented by a black dot. The horizontal black line represents the median for each group. Median time to treatment differed significantly between groups (p < .001).
4. DISCUSSION
The results from this retrospective chart review suggest that buprenorphine-exposed neonates who require treatment for NAS have that treatment initiated significantly later than methadone-exposed neonates. Notably, there was no evidence that maternal age, neonatal sex, gestational age, or birth weight were predictive of time to treatment nor were there significant interactions between medication group and any of these factors. This result is consistent with the findings from two double-blind, randomized clinical trials (Fischer et al., 2006; Gaalema et al., 2012) and adds generality to these prior observations, although it should be noted that maternal use of other drugs has typically been low in these studies.
The results of the present study also provide further evidence that the NAS produced by in utero exposure to buprenorphine vs. methadone differs. Not only are such differences of theoretical interest, they also have practical implications for the assessment and treatment of opioid-exposed neonates. While the data to date suggest that the NAS associated with buprenorphine is less severe in several respects (e.g., less severe central nervous system signs, less medication needed to treat NAS), they do not suggest that buprenorphine-exposed neonates can be monitored for a shorter period of time. The results of the present study also help clarify the range for time to treatment initiation among neonates exposed to long-acting opioids like methadone and buprenorphine. The AAP currently recommends that these neonates be monitored for a minimum of 5-7 days (120-168 hours) after delivery, although it is not clear what data this recommendation is based on (Hudak et al., 2012). While the majority of neonates in the present study (70/75, 93%) initiated treatment at less than 120 hours of age, the remaining five neonates (two methadone- and three buprenorphine-exposed) initiated treatment between 120-168 hours, providing support for the AAP’s recommendation.
While consistency is emerging in terms of the direction of the difference in time to pharmacologic treatment initiation among neonates exposed in utero to these two medications (buprenorphine > methadone), there are still large discrepancies in the magnitude of the difference. In the present retrospective chart review, the median time of treatment initiation was 37 hours later in buprenorphine- vs. methadone-exposed neonates. In the large multi-site randomized MOTHER trial, the difference in median time to treatment initiation was 23 hours (Gaalema et al., 2012). Fischer et al. (2006) reported a difference of 12 hours in their small randomized trial, but this is based on mean (vs. median) time to treatment initiation and it was calculated from the time the mother received her last maintenance dose (vs. from delivery). Differences in study design and how this outcome was computed and analyzed undoubtedly contribute to the variability observed across studies. Further complicating comparisons across studies is variability in the terminology used to describe this outcome. For example, Fischer et al. (2006) and Ebner et al. (2007) were both included in the Introduction because both clearly report time to treatment initiation, although they sometimes also referred to this outcome as “onset of NAS” and “onset of clinical NAS symptoms.” Two other studies (Lejeune et al., 2006; Binder and Vavřinková, 2008) report “age of NAS onset,” “onset of NAS symptoms,” or when “NAS was manifested.” It was unclear from the information provided in each paper whether these outcomes were the same as those reported in the other studies cited and attempts to clarify with the corresponding authors were unsuccessful; thus they were not included in the Introduction.
The majority of studies indicate that time to treatment initiation is significantly later in neonates exposed in utero to buprenorphine compared to methadone and provide support for recommendations to observe neonates exposed to long-acting opioids for five to seven postnatal days. Greater standardization of the definition and analysis of time to treatment as an outcome variable are needed to improve the precision of estimates of time to treatment initiation after in utero exposure to these two medications.
Acknowledgments
Funding Source: This study was supported by research grant R01 DA031928 from the National Institutes of Health, National Institute on Drug Abuse.
Glossary
- NAS
neonatal abstinence syndrome
Footnotes
Contributor’s Statements:
Authors Gaalema, Heil and Johnston conceptualized and designed the study. Authors Johnston and Metayer collected the data. Authors Badger, Gaalema and Metayer performed the statistical analyses. Authors Gaalema and Heil drafted the initial manuscript. Authors Badger, Gaalema, Heil and Johnston reviewed and revised the manuscript. All authors contributed to and have approved the final manuscript.
Financial Disclosure: All authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: All authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binder T, Vavrinkova B. Prospective randomized comparative study of the effect of buprenorphine, methadone and heroin on the course of pregnancy, birthweight of newborns, early postpartum adaptation and course of the neonatal abstinence syndrome (NAS) in women followed up in the outpatient department. Neuro Endocrinol Lett. 2008;29:80–86. [PubMed] [Google Scholar]
- Brown HL, Britton KA, Mahaffey D, Brizendine E, Hiett AK, Turnquest MA. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179:459–463. doi: 10.1016/s0002-9378(98)70379-5. [DOI] [PubMed] [Google Scholar]
- Doberczak TM, Kandall SR, Wilets I. Neonatal opiate abstinence syndrome in term and preterm infants. J Pediatr. 1991;118:933–937. doi: 10.1016/s0022-3476(05)82214-0. [DOI] [PubMed] [Google Scholar]
- Dysart K, Hsieh HC, Kaltenbach K, Greenspan JS. Sequela of preterm versus term infants born to mothers on a methadone maintenance program: differential course of neonatal abstinence syndrome. J Perinat Med. 2007;35:344–346. doi: 10.1515/JPM.2007.063. [DOI] [PubMed] [Google Scholar]
- Ebner N, Rohrmeister K, Winklbaur B, Baewert A, Jagsch R, Peternell A, Thau K, Fischer G. Management of neonatal abstinence syndrome in neonates born to opioid maintained women. Drug Alcohol Depend. 2007;87:131–138. doi: 10.1016/j.drugalcdep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson NM, Weitzman ML, Wilson MH, editors. Primary Pediatric Care. 2. Mosby; St Louis: 1992. pp. 1367–78. [Google Scholar]
- Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, Aschauer H. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- Gaalema DE, Scott TL, Heil SH, Coyle MG, Kaltenbach K, Badger GJ, Arria A, Stine SM, Martin PR, Jones HE. Differences in the profile of neonatal abstinence syndrome signs in methadone- vs. buprenorphine-exposed infants. Addiction. 2012;107:S53–62. doi: 10.1111/j.1360-0443.2012.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak ML, Tan RC. The Committee on Drugs, The Committee on Fetus and Newborn. Neonatal drug withdrawal Pediatrics. 2012;129:e540–e560. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Dipietro JA, Elko A, Velez M. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. J Matern Fetal Neonat Med. 2007;20:677–685. doi: 10.1080/14767050701490327. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Velez M, Harrow C. The opioid exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5:47–55. [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Jones HE, O’Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict. 2008;17:372–386. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: effects and management. Obstet Gynecol Clin North Am. 1998;25:139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Lawless JF. Statistical Model and Methods for Lifetime Data. Second Edition. John Wiley & Sons; New York: 2003. [Google Scholar]
- Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S Groupe d’Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenorphine substitution. Drug Alcohol Depend. 2006;82:250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. JAMA. 1998;280:1936–1943. [PubMed] [Google Scholar]
- Nelson W. Applied Life Data Analysis. John Wiley & Sons; New York: 1982. [Google Scholar]
- O’Connor AB, O’Brien L, Alto WA. Are there gender related differences in neonatal abstinence syndrome following exposure to buprenorphine during pregnancy? J Perinat Med. 2013;11:1–3. doi: 10.1515/jpm-2012-0288. [DOI] [PubMed] [Google Scholar]
- Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration/Center for Substance Abuse Treatment (SAMHSA/CSAT) Treatment Improvement Protocol 5: Improving Treatment for Drug-Exposed Infants. Center for Substance Abuse Treatment. Substance Abuse and Mental Health Services Administration; Rockville, MD: 1993. [PubMed] [Google Scholar]
- Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70:S13–S27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]


