Abstract
The demand for high-quality islets for transplantation in type I diabetics will increase as the current clinical trials transition into standard of care. The mode of preservation of donor pancreata is critical to this mission since islets are very sensitive to ischemic injury. Hypothermic perfusion preservation (HPP) is being investigated for extended pancreas preservation in light of the beneficial effects reported for other organs. The present pilot study aimed to establish the potency of porcine islets isolated from pancreata after 24 h of HPP at 4–8°C. The study design included a split-lobe pancreas model that permitted paired comparisons of islets isolated from 24-h HPP splenic lobes with nonperfused, fresh control duodenal/connecting lobes stored at 4°C for <3 h. Prior to transplantation, islet viability was assessed in vitro using the ratio of oxygen consumption rate to DNA (OCR/DNA) assay and correlated with subsequent in vivo function by transplantation in diabetic immunodeficient mice. The OCR/DNA (mean ± SD) measured after 7 days of culture and immediately prior to transplantation for islets from the 24-h HPP group was 269 ± 19 nmol/min/mg DNA, which was higher but not statistically different to the mean of 236 ± 43 for the counterpart control group. All four nude mice transplanted with islets from the 24-h HPP group showed diabetes reversal, compared with five of six transplants from the control group. In conclusion, islets isolated from adult porcine pancreata after 24-h HPP exhibited high viability as measured by OCR/DNA and were able to consistently reverse diabetes in a nude mouse bioassay.
Key words: Perfusion, Pancreas, Islet, Oxygen consumption, Viability, Hypothermic perfusion
INTRODUCTION
The field of islet transplantation for the treatment of type I diabetes is currently in the phase of clinical trials at several centers around the world (1,23). The growing recognition that islet transplantation is becoming a viable proposition for clinical therapy has led to an increasing demand for high-quality islets for both research and clinical procedures. Moreover, the potential for xenotransplantation to relieve the demand on an inadequate supply of human pancreases will also depend upon the efficiency of techniques for isolation of islets from alternative sources, of which the pig is highly favored for a number of compelling reasons (16). Islets are highly vulnerable to irreversible damage after prolonged ischemia, in part because they do not possess the enzymatic machinery necessary (lactate dehydrogenase A; LDHa) for ATP generation under anaerobic conditions (2,4–7,17,24) and cold ischemia of the cadaveric pancreas is detrimental to islet yield (3,9,11,13,14,21). In vitro studies have shown a significant reduction in insulin release in response to a glucose challenge even after short periods of conventional static cold storage in University of Wisconsin (UW) solution (9). These observations have been seen in clinical practice as there have been no reports of successful single donor islet transplants with prolonged cold storage beyond 10 h, which is still regarded as the safe limit of cold ischemia (11). Furthermore, Ryan et al. have provided evidence of the detrimental impact of cold ischemia on posttransplant islet function (22).
In light of the recent resurgence of clinical interest in hypothermic perfusion preservation (HPP) of organs, notably for marginal and expanded criteria organs (26), we have recently applied the state-of-the-art technology to pancreas perfusion as a prelude to islet isolation (25,27). It was recently reported that, by comparison with conventional static cold storage, hypothermic machine perfusion preservation allows safe storage of juvenile pig pancreases for 24 h with excellent in vitro structure and function of islets isolated from the perfused pancreata. The present study reports the outcome of a pilot study to validate these in vitro findings using expanded quality assessment including oxygen consumption rate (OCR) analysis (18–20) and in vivo transplantation in immunodeficient “nude” mice.
MATERIALS AND METHODS
Experimental Design
This study was designed to validate, in an adult pig model, the recent findings of Taylor et al. (25,27) concerning the functional integrity of islets prepared from the pancreata of juvenile pigs after 24-h HPP. To this end, the study assessed the outcome of 10 nude mouse transplants using islets derived from three adult pig pancreas perfusions compared with three paired fresh islet isolations. This was achieved using our previously established split-lobe porcine pancreas model as described below (8). Statistical significance was analyzed by Student’s paired t-test using GraphPad Software v5.01 with 95% confidence limits.
Procurement
Pancreata were procured from adult (1–2 years old) Landrace pigs using a deceased after cardiac death (DCD) donor model involving total viscerectomy followed by en bloc pancreatectomy. All animal care and handling complied with policies and approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota. Following procurement, the pancreas was divided into two segments for use in paired isolation outcomes. The first segment consisted of the connecting and duodenal lobes (8), which were processed for islet isolation with the least cold ischemia time (CIT) possible (<3 h). The second segment consisted of the splenic lobe procured with the entire vascular network, which was then prepared for HPP according to the technique of Taylor and colleagues (25,27). The splenic lobe was selected for perfusion, as this lobe in contrast to the connecting and duodenal lobes has the vasculature available for perfusion. In our experience with porcine islet isolation, the yields and quality of islets are similar for the connecting, duodenal, and splenic lobes, so that a different outcome based on differences in lobes is not expected.
Perfusion
Both the superior mesenteric artery and the celiac trunk attached to the splenic lobe were cannulated using SealRing™ cannulas (10 × 35 mm; Organ Recovery Systems, Itasca, IL) and connected to a LifePort® Kidney Transporter pulsatile perfusion machine (Organ Recovery Systems) as described previously (25,27). All arterial vessel leaks were identified and ligated. The lobe was then perfused for 24 h with a systolic pressure of 10 mmHg, while the pancreas was immersed in cold perfusion solution [kidney perfusion solution-1 (KPS-1), Organ Recovery Systems] controlled between 4 and 8°C.
Islet Isolation, Purification, and Culture
Islets were isolated from both segments of each pancreas using identical conventional techniques involving collagenase digestion in a modified Ricordi chamber (BioRep Technologies, Miami, FL) as previously described (12,27). In brief, the pancreas segment was chopped into pieces and digested at 35–37°C using Liberase MTF (mammalian tissue free) enzyme (Roche, Indianapolis, IN) at a concentration of 150–167 μg/ml. Islets were purified after isolation using established standard continuous Ficoll gradient separation with a COBE 2991 (Gambro BCT, Lakewood, CO). After purification, islets were cultured between 4 and 12 h and then assessed using OCR and DNA quantitation (18–20). Subsequently, islets were cultured in ME199 media at 37°C, for 7–8 days. After the culture period, islets were again assessed using OCR and DNA quantitation as well as gross morphology using dithizone staining. Aliquots were taken for mouse transplants at this time.
Islet OCR/DNA
Islet viability was assessed by measuring the OCR/DNA of the purified islets using a sealed chamber fiber optic oxygen sensing system (Instech Labs, Newton, MA) as described elsewhere (18–20). For this analysis, 5,000 islet equivalents (IEQ) were allocated immediately after purification and again after 7 days of culture. Islets were suspended in a nonsupplemented, nutrient-rich ME199 and sealed in stainless steel chambers. The oxygen partial pressure inside the chamber was recorded and the consumption rate was calculated based on the rate of oxygen depletion from the chamber. The DNA quantity in each chamber was determined using the Quant-iT Picogreen dsDNA kit (Molecular Probes, Eugene, OR).
Islet Morphology
Islet samples stained with dithizone for counting were also scored for morphology on a 10-point scale. The total score was calculated by summing five categories, each evaluated on a 2-point scale: 1) shape (3D), with more points awarded to more spherical islets; 2) border (2D), with more points awarded to well rounded islets; 3) integrity, with more points awarded to more solid/compact islets; 4) presence of single cells, with more points awarded to preparations with less single cells; 5) diameter, with more points awarded for larger diameter islets.
Nude Mouse Bioassay
Diabetes was induced in nude mice using a 240 mg/kg dose of streptozotocin injected intravenously, following recommendations of the Clinical Islet Transplant (CIT) consortium regarding procedures for the mouse bioassay in testing human Islet cell preparations (150–240mg/kg) (http://www.isletstudy.org/CITDocs/3104,%20A04%20In%20Vivo%20Islets%20Function.pdf). The mice were confirmed diabetic after blood glucose levels were maintained above 500 mg/dl for 2 days. Islets were then transplanted beneath the kidney capsule and distributed along the surface of one side of the kidney. Diabetes is considered to be reversed when blood glucose measurements were less than 200 mg/dl on 2 or more consecutive days. After about 4 weeks the grafts were explanted by nephrectomy to ensure that blood glucose levels in animals that had diabetes reversal returned to diabetic levels. Two mice were transplanted with 2,000 IEQ after 7 days in culture for each pancreas lobe with the exception of the last splenic lobe due to a subcritical mass of islets surviving at 7 days to justify transplantation. A total of 10 mice were transplanted, 4 with islets from 24-h HPP lobes and 6 with islets from controls.
RESULTS AND DISCUSSION
Despite the constraints of islet isolation expense and handling large adult pigs (450–500 lbs), the split lobe model applied in this pilot validation study provided the opportunity to undertake a controlled comparison of islet function from pancreatic lobes exposed to either 24-h HPP or minimal cold ischemia (<3 h). Moreover, the internal control provided by the split-lobe model permitted validation of our prior findings using a small number of adult pigs. In contrast to the previously published study using juvenile pigs, which compared 24-h HPP and 24-h static cold storage with freshly isolated islets (25,27), this validation study was designed to use the merits of the split-lobe model for paired comparison of 24-h HPP with a control minimal cold ischemia group. Based upon the previous study in juvenile pigs, the working hypothesis tested here is that the function of islets prepared from 24-h HPP adult pig pancreata is not compromised compared with control islets obtained from a counterpart pancreatic lobe exposed to minimal cold ischemia.
Islet yields (<1,000 IEQ/g) were typically lower than our experience previously for the adult pig model. This is attributed, in part, to the decision to switch to the new Liberase MTF enzyme preparation, to conform to current clinical practice, and the phasing out of the Liberase PI product used in prior studies. Moreover, this pilot study was undertaken before the optimal concentration of Liberase MTF was determined for this model on the basis that the study design included internal controls for each pancreas by virtue of the split-lobe model. The mean (±SEM) yields from the control and the 24-h HPP were not statistically significantly different (p = 0.4) (Table 1) in the small population of tests in this pilot study. Furthermore, as shown in Table 1, the purity and yields of islets were more than adequate to satisfy the principal objective of this pilot validation study by allowing measurement of viability based on OCR/DNA and in vivo islet function based on transplantation outcome in mice and a paired comparison of these parameters between experimental perfused lobes and non-perfused controls.
Table 1.
Isolation and Quality Control (QC) Data
| Pancreas | Lobe | IEQ/g | Purity % | Day 7 Recovery (%)* | Pretransplant (Day 7) OCR/DNA (ng/min/mg DNA) |
|---|---|---|---|---|---|
| 1 | Conn/Duod (control) | 592 | 90 | 61 | 269 |
| Splenic (24-h HPP) | 128 | 95 | 57 | 279 | |
| 2 | Conn/Duod (control) | 661 | 95 | 27 | 187 |
| Splenic (24-h HPP) | 721 | 90 | 88 | 248 | |
| 3 | Conn/Duod (control) | 858 | 85 | 56 | 253 |
| Splenic (24-h HPP) | 497 | 90 | 63 | 281 |
IEQ, islet equivalent; OCR, oxygen consumption rate; conn, connecting lobes; duod, duodenal lobe; HPP, hypothermic perfusion preservation.
Based on IEQ/DNA.
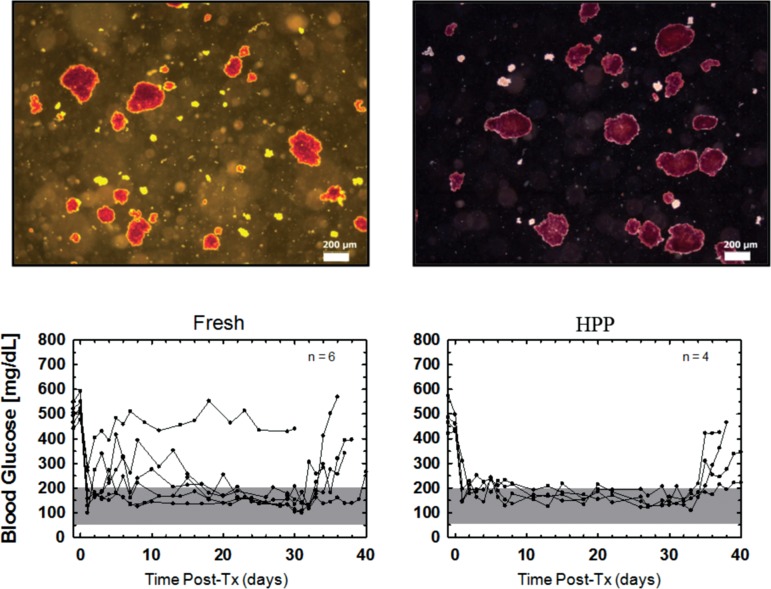
With respect to viability assessed by the OCR/DNA assay, Table 1 shows that the OCR/DNA value of islets at day 7 was comparable between the groups and that the pretransplantation OCR/DNA values all exceeded the acceptable threshold of 175 nm/min/mg DNA currently used as a release criterion for transplantation of porcine islets in nonhuman primates [(10), Papas et al., unpublished observations]. Figure 1 further shows the averaged OCR/DNA data for the two groups immediately after isolation and after 7 days of culture. In both cases, there was no statistically significant difference between the 24-h HPP islets and the controls. Moreover, Figure 1 shows that the freshly isolated islets from both groups demonstrated mean OCR/DNA ratios >100 nm/min/mg DNA but failed to meet the predetermined “release criteria” derived in earlier studies for correlating the OCR/DNA index with transplantation outcome. In sharp contrast, islets from both groups exceeded this threshold after 7 days of culture. This is actually reflecting the clearance of dead tissue (DNA) originally present postisolation and reflected in less than 100% IEQ recovery. Basically, culture clears dead islet tissue (that did not survive the isolation process) while viable tissue is maintained therefore viability (OCR/DNA) increases. Most importantly, the recovery and viability of islets from the 24-h HPP pancreata, which was not significantly different to controls, was further corroborated by the transplantation outcome, as shown in Figure 2. Here the individual and mean data for the nude mice transplants shows that all four mice receiving islets of the HPP group reversed diabetes: the efficacy of the islets was proven by the return to the diabetic state after graftectomy. Mice receiving islets from the control group showed diabetes reversal in five out of six cases.
Figure 1.
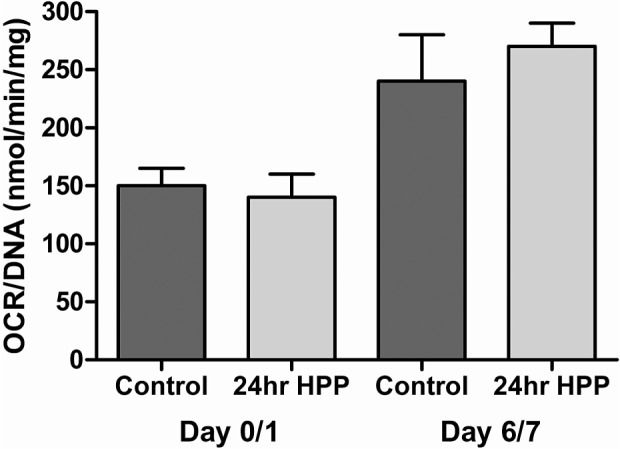
Average oxygen consumption rate (OCR)/DNA for all experiments by condition for day 0/1 and day 7. Error bars represent the SEM.
Figure 2.
Morphology and function of transplanted islets. Upper panels show brightfield micrographs of dithizone-stained islet aliquots immediately prior to transplantation. (A) Representative islets from the control group, which was assigned a mean morphology score of 7.2 ± 0.6 (n = 3); (B) islets from the hypothermic perfusion preservation (HPP) group with a mean morphology score of 7.5 ± 0.5 (n = 3). Lower panels show individual blood glucose levels for mice transplanted on day 7 for each condition. The number of animals in which diabetes was reversed is indicated. In animals that reversed diabetes after islet transplantation, the effect of the transplant was proven by graftectomy 4 weeks after transplantation followed by an increase in blood glucose confirming the diabetic state.
SUMMARY AND CONCLUSION
Islets are highly sensitive to ischemia such that various modes of pancreas preservation are currently under investigation. It is generally assumed that using conventional preservation media, pancreata do not survive periods of 24-h cold ischemia with insufficient yields in islet processing and preparation. The present results give a strong indication that 24-h HPP provides sufficient protection for adult porcine pancreata so that islets in sufficient quantity and of high quality can be prepared. Twenty-four-hour HPP of adult porcine pancreas yielded high-quality islets with a robust in vitro viability (based on OCR/DNA) that was equal to nonperfused fresh controls and exceeded the established threshold set as a release criterion for transplantation in nonhuman primates. The in vivo functional quality of the islets derived from 24-h HPP pancreas was sufficient to consistently reverse diabetes in a nude mouse bioassay. Hypothermic perfusion preservation (HPP) is now favored as the method of choice for clinical kidney preservation when applied to marginal or expanded criteria organs that have suffered a warm ischemic insult (15,26). HPP is also under investigation for pancreas preservation and this study set out to validate recent findings that 24-h HPP is tolerated in a porcine model (25,27).
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Henk Schuurman for his critical reading of the manuscript. This work was funded in part by a grant from the NIH (R44DK076326). Some authors (M.J.T. and S.C.B.) are presently or were previously employed by Cell and Tissue Systems, Inc. as indicated.
REFERENCES
- 1. Alejandro R.; Barton F. B.; Hering B. J.; Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation 86:1783–1788; 2008. [DOI] [PubMed] [Google Scholar]
- 2. Belzer F. O.; Ploeg R. J.; Knechtle S. J.; D’Alessandro A. M.; Pirsch J. D.; Kalayoglu M. M.; Sollinger H. W. Clinical pancreas preservation and transplantation. Transplant. Proc. 26:550–551; 1994. [PubMed] [Google Scholar]
- 3. Benhamou P. Y.; Watt P. C.; Mullen Y.; Ingles S.; Watanabe Y.; Nomura Y.; Hober C.; Miyamoto M.; Kenmochi T.; Passaro E. P.; Zinner M. J.; Brunicardi F. C. Human islet isolation in 104 consecutive cases. Factors affecting isolation success. Transplantation 57:1804–1810; 1994. [PubMed] [Google Scholar]
- 4. Carlsson P. O.; Palm F.; Andersson A.; Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation 69:761–766; 2000. [DOI] [PubMed] [Google Scholar]
- 5. Carlsson P. O.; Palm F.; Andersson A.; Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 50:489–495; 2001. [DOI] [PubMed] [Google Scholar]
- 6. Delmonico F. L.; Jenkins R. L.; Auchincloss H. Jr.; Etienne T. J.; Russell P. S.; Monaco A. B.; Cosimi A. B. Procurement of a whole pancreas and liver from the same cadaveric donor. Surgery 105:718–723; 1989. [PubMed] [Google Scholar]
- 7. Dionne K. E.; Colton C. K.; Yarmush M. L. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42:12–21; 1993. [DOI] [PubMed] [Google Scholar]
- 8. Ferrer J.; Scott W. E. 3rd.; Weegman B. P.; Suszynski T. M.; Sutherland D. E.; Hering B. J.; Papas K. K. Pig pancreas anatomy: implications for pancreas procurement, preservation, and islet isolation. Transplantation 86:1503–1510; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gotoh M.; Maki T.; Satomi S.; Porter J.; Monaco A. P. Immunological characteristics of purified pancreatic islet grafts. Transplantation 42:387–390; 1986. [DOI] [PubMed] [Google Scholar]
- 10. Graham M. L.; Janecek J. L.; Kitteridge J. A.; Hering B. J.; Schuurman H. J. The streptozotocin-induced diabetic nude mouse model: Differences between source animals. Comp. Med. 61:356–360; 2011. [PMC free article] [PubMed] [Google Scholar]
- 11. Hering B. J.; Ricordi C. Islet transplantation for patients with type 1 diabetes: Results, research priorities and reasons for optimism. Graft 2:12–27; 1999. [Google Scholar]
- 12. Hering B. J.; Wijkstrom M.; Graham M. L.; Hardstedt M.; Aasheim T. C.; Jie T.; Ansite J. D.; Nakano M.; Cheng J.; Li W.; Moran K.; Christians U.; Finnegan C.; Mills C. D.; Sutherland D. E.; Bansal-Pakala P.; Murtaugh M. P.; Kirchhof N.; Schuurman H. J. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat. Med. 12:301–303; 2006. [DOI] [PubMed] [Google Scholar]
- 13. Ketchum R. J.; Nicolae M.; Jahr H.; Friedman A.; Naji A.; Barker C. F.; Brayman K. L. Analysis of donor age and cold ischemia time as factors in cadaveric human islet isolation. Transplant. Proc. 26:596–597; 1994. [PubMed] [Google Scholar]
- 14. Lakey J. R.; Rajotte R. V.; Warnock G. L.; Kneteman N. M. Human pancreas preservation prior to islet isolation. Cold ischemic tolerance. Transplantation 59:689–694; 1995. [DOI] [PubMed] [Google Scholar]
- 15. Moers C.; Smits J. M.; Maathuis M. H.; Treckmann J.; van Gelder F.; Napieralski B. P.; Kasterop-Kutz M.; van der Heide J. J.; Squifflet J. P.; van Heurn E.; Kirste G. R.; Rahmel A.; Leuvenink H. G.; Paul A.; Pirenne J.; Ploeg R. J. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 360:7–19; 2009. [DOI] [PubMed] [Google Scholar]
- 16. O’Neil J. J.; Stegemann J. P.; Nicholson D. T.; Gagnon K. A.; Solomon B. A.; Mullon C. J. The isolation and function of porcine islets from market weight pigs. Cell Transplant. 10:235–246; 2001. [DOI] [PubMed] [Google Scholar]
- 17. Papas K. K.; Colton C. K.; Gounarides J. S.; Roos E. S.; Jarema M. A.; Shapiro M. J.; Cheng L. L.; Cline G. W.; Shulman G. I.; Wu H.; Bonner-Weir S.; Weir G. C. NMR spectroscopy in beta cell engineering and islet transplantation. Ann. NY Acad. Sci. 944:96–119; 2001. [DOI] [PubMed] [Google Scholar]
- 18. Papas K. K.; Colton C. K.; Nelson R. A.; Rozak P. R.; Avgoustiniatos E. S.; Scott W. E. III; Wildey G. M.; Pisania A.; Weir G. C.; Hering B. J. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am. J. Transplant. 7:707–713; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papas K. K.; Pisania A.; Wu H.; Weir G. C.; Colton C. K. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol. Bioeng. 98:1071–1082; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papas K. K.; Suszynski T. M.; Colton C. K. Islet assessment for transplantation. Curr. Opin. Organ Transplant. 14:674–682; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robertson G. S.; Chadwick D.; Thirdborough S.; Swift S.; Davies J.; James R.; Bell P. R.; London N. J. Human islet isolation—a prospective randomized comparison of pancreatic vascular perfusion with hyperosmolar citrate or University of Wisconsin solution. Transplantation 56:550–553; 1993. [DOI] [PubMed] [Google Scholar]
- 22. Ryan E. A.; Lakey J. R.; Rajotte R. V.; Korbutt G. S.; Kin T.; Imes S.; Rabinovitch A.; Elliott J. F.; Bigam D.; Kneteman N. M.; Warnock G. L.; Larsen I.; Shapiro A. M. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 50:710–719; 2001. [DOI] [PubMed] [Google Scholar]
- 23. Shapiro A. M.; Ricordi C.; Hering B. J.; Auchincloss H.; Lindblad R.; Robertson R. P.; Secchi A.; Brendel M. D.; Berney T.; Brennan D. C.; Cagliero E.; Alejandro R.; Ryan E. A.; DiMercurio B.; Morel P.; Polonsky K. S.; Reems J. A.; Bretzel R. G.; Bertuzzi F.; Froud T.; Kandaswamy R.; Sutherland D. E.; Eisenbarth G.; Segal M.; Preiksaitis J.; Korbutt G. S.; Barton F. B.; Viviano L.; Seyfert-Margolis V.; Bluestone J.; Lakey J. R. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355:1318–1330; 2006. [DOI] [PubMed] [Google Scholar]
- 24. Tanioka Y.; Sutherland D. E.; Kuroda Y.; Suzuki Y.; Matsumoto I.; Deai T. Preservation of dog pancreas before islet isolation with the two-layer method. Transplant. Proc. 30:3419–3420; 1998. [DOI] [PubMed] [Google Scholar]
- 25. Taylor M. J.; Baicu S. Hypothermic perfusion of pancreas: Emphasis on preservation prior to islet isolation. In: Uygun K.; Lee C. Y., eds. Organ preservation and reengineering. Boston, MA: Artech House Publisher; 2011:85–104. [Google Scholar]
- 26. Taylor M. J.; Baicu S. C. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective. Cryobiology 60:S20–S35; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor M. J.; Baicu S. C.; Greene E.; Vazquez A.; Brassil J. Islet isolation from juvenile porcine pancreas after 24-hour hypothermic machine perfusion preservation. Cell Transplant. 19:613–628; 2010. [DOI] [PubMed] [Google Scholar]