Abstract
Xylanases produced by Aspergillus niger are industrially important and many types of xylanases have been reported. Individual xylanases have been well studied for their enzymatic properties, gene cloning, and heterologous expression. However, less attention has been paid to the relationship between xylanase genes carried on the A. niger genome and xylanases produced by A. niger strains. Therefore, we examined xylanase genes encoded on the genome of A. niger E-1 and xylanases produced in culture. Seven putative xylanase genes, xynI–VII (named in ascending order of the molecular masses of the deduced amino acid sequences), were amplified from the strain E-1 genome using primers designed from the genome sequence of A. niger CBS 513.88 by PCR and phylogenetically classified into three clusters. Additionally, culture supernatant analysis by DE52 anion–exchange column chromatography revealed that this strain produced three xylanases, XynII, XynIII, and XynVII, which were identified by N-terminal amino acid sequencing and MALDI-TOF-MS analyses, in culture when gown in 0.5% xylan medium supplemented with 50 mM succinate. Furthermore, XynVII, the only GH family 10 xylanase in A. niger E-1, was purified and characterized. The purified enzyme showed a single band with a molecular mass of 35 kDa by SDS-PAGE. The highest activity of purified XynVII was observed at 55°C and pH 5.5. The enzyme was stable in the broad pH range of 3–10 and up to 60°C and was resistant to most metal ions and modifying regents. XynVII showed high specificity against beechwood xylan with Km and Vmax values of 2.8 mg mL–1 and 127 μmol min–1mg–1, respectively. TLC and MALDI-TOF-MS analyses showed that the final hydrolyzed products of the enzyme from beechwood xylan were xylose, xylobiose, and xylotriose substituted with a 4-o-metylglucuronic acid residue.
Keywords: Aspergillus niger; Endo-β-1,4-xylanase; Glycoside hydrolase family 10; Xylan degradation
Introduction
Xylanolytic enzymes have great biotechnological potential for various industrial processes, including feed, food, pulp, and others (Akpinar et al. 2009; Beg et al. 2000; Camacho and Aguilar 2003; Twomey et al. 2003), and there has been much interest in microorganisms that produce the enzymes, properties of the produced enzymes, and their genes, from applicable standpoints. Many studies on the utilization of the microorganisms, their enzymes, and reaction products make progress in the broad range of industrial applications, and commercial xylanases have been industrially produced in many countries (Beg et al. 2001).
Xylan is the most abundant hemicellulose and a complex polysaccharide composed of a backbone of β-1,4-glycoside–linked xylose residues. Several hydrolytic enzymes are required for complete xylan degradation. Endo-1,4-β-xylanase (EC. 3.2.1.8) plays a major role in the degradation of xylan by cleaving the xylosyl backbone and releasing short xylooligosaccharides, which are further hydrolyzed into xylose units by xylan 1,4-β-xylosidase (EC. 3.2.1.37) (Shallom and Shoham 2003). Many microorganisms, including bacteria, fungi, and yeast, are known to produce these xylanolytic enzymes (Beg et al. 2001). The filamentous fungi Aspergillus and Penicillium are particularly important xylanase producers because they excrete the enzyme into media at higher levels than other microorganisms (Chávez et al. 2006; de Vries and Visser 2001). Since the xylanases from Aspergillus niger, an excellent xylanase producer, were first purified and characterized in 1977 (Gorbacheva and Rodionova 1977), many xylanase purification has been reported, and they were found to differ in molecular masses and pIs (Fournier et al. 1985; Frederick et al. 1981; Frederick et al. 1985; Shei et al. 1985). To date, many xylanase genes have been cloned from newly isolated A. niger, strain and enzymes expressed from the cloned genes were characterized in detail (Deng et al. 2006; Kinoshita et al. 1995). Detergent and organic solvent-stable xylanase has also been studied toward industrial applications (Hmida-Sayari et al. 2012).
In the 21st century, genome sequencing of microorganisms has steadily revealed exhaustive information about the genes found on microbial genomes. The genome sequence of A. niger reported by Pel et al. (2007) includes five candidate xylanase genes: one xylanase belonging to the glycoside hydrolase (GH) family 10 and four other genes homologous to GH family 11 enzymes. Although genome-wide knowledge and enormous individual data on various xylanases and their genes from A. niger have been generated, it is unclear how many xylanase genes are encoded on each genome of isolated A. niger strains and which xylanases are produced in culture. Thus, to our knowledge, there are very few studies that have exhaustively linked the putative xylanase genes encoded on A. niger genome to all xylanases secreted into culture. Furthermore, it appears that the physiological roles of each xylanase in xylan degradation remain poorly characterized. We, therefore, aimed to clarify the relationship between xylanase genes encoded on the genome and xylanases produced in culture.
In a previous study, we isolated A. niger E-1, which produces a high level of xylanase, from gray gentle lemur feces extracts (Takahashi et al. 2012). This report describes the characterization of xylanase genes on the A. niger E-1 genome. In addition, we found three xylanases (XynII, XynIII, and XynVII) in the culture supernatant of A. niger E-1 and purified and characterized XynVII because little was known about this enzyme.
Materials and methods
Microorganisms and culture conditions
Aspergillus niger E-1 was previously isolated from gray gentle lemur feces extracts (Takahashi et al. 2012). For xylanase production, a shaking flask (500 mL) containing 50 mL of 0.5% beechwood xylan (Sigma-Aldrich, St. Louis, MO, USA) medium (pH 5.5) (Takahashi et al. 2012) supplemented with 50 mM sodium succinate was inoculated with spores (5 × 106). Inoculated flasks were incubated at 130 rpm and 37°C. After filtration and centrifugation, the culture supernatant was used as a crude enzyme preparation.
Escherichia coli XL1-Blue was used in molecular biological experiments and cultured in Luria–Bertani medium (Sambrook and Russell 2001) supplemented with ampicillin (100 μg mL–1) and tetracycline (12.5 μg mL–1), and when necessary, isopropyl β-D-thiogalactopyranoside (1 mM) and X-Gal (0.04%) at 37°C under aerobic conditions.
Cloning of xylanase genes from A. niger E-1
To design primers to amplify putative xylanase genes and their homologues encoded on A. niger E-1 genome, xylanase genes on A. niger CBS 513.88 genome that was sequenced completely (Pel et al. 2007), were searched at databases of DNA Data Bank of Japan (DDBJ) using an All-round Retrieval of Sequence and Annotation Program. We found eight candidate xylanase genes, including one gene for GH family 10 xylanase, four genes for GH family 11, and three putative genes showing weak similarity to endoxylanases of other microorganisms. Information on these putative enzymes is summarized in Table 1. We designed primer pairs on the basis of the 5′- and 3′-terminal sequences of the candidate xylanase genes (Table 2). To amplify xynVIII, another primer pair, xynVIII2F and xynVIII2R was also designed on the basis of the internal sequence of putative xynVIII.
Table 1.
Putative xylanases encoded onA. nigerCBS 513.88 genome
| Name | Deduced MM (kDa)a | pIa | Accession no. | Protein ID |
|---|---|---|---|---|
| XynI | 19.0 | 3.97 | AM270363-19 | CAK42832.1 |
| XynII | 22.6 | 4.10 | AM270327-78 | CAK46731.1 |
| XynIII | 24.1 | 5.09 | AM269952-5 | CAK43456.1 |
| XynIV | 24.9 | 3.77 | AM269993-14 | CAK44157.1 |
| XynV | 27.9 | 4.56 | AM270343-22 | CAK97322.1 |
| XynVI | 35.4 | 4.45 | AM270229-8 | CAK40644.1 |
| XynVII | 35.5 | 6.19 | AM270045-11 | CAK38067.1 |
| XynVIII | 86.1 | 4.40 | AM270362-17 | CAK48600.1 |
aEach value was calculated on the basis of amino acid sequence containing a putative signal peptide sequence.
Table 2.
Primers designed from putative xylanase gene sequences ofA. nigerCBS 513.88
| Gene | Size (bp)a | Primer name | Primer sequence |
|---|---|---|---|
| xynI | 543 | xynIF | 5′-atgttcttcaagaccatcctt |
| xynIR | 5′-ttaacccagagtaagagtgaa | ||
| xynII | 686 | xynIIF | 5′-atgaaggtcactgcagcttt |
| xynIIR | 5′-taagaagatatcgtgacactg | ||
| xynIII | 746 | xynIIIF | 5′-atgctcaccaagaaccttct |
| xynIIIR | 5′-ttactgaacagtgatggacg | ||
| xynIV | 755 | xynIVF | 5′-tggtcgcctactcgtctc |
| xynIVR | 5′-tagcagctctcctcggtg | ||
| xynV | 826 | xynVF | 5′-atggtgtctttccttggcc |
| xynVR | 5′-ctatgcactaacggtgaagt | ||
| xynVI | 1043 | xynVIF | 5′-atgtcgcacccccaacag |
| xynVIR | 5′-ctacgataaagtcctcccc | ||
| xynVII | 1494 | xynVIIF | 5′-atggttcagatcaaggtagc |
| xynVIIR | 5′-ctagagagcatttgcgatag | ||
| xynVIII | 2962 | xynVIIIF | 5′-tgcgcgtaccgaaccgg |
| xynVIIIR | 5′-tcatatgctccgatgtccc | ||
| xynVIII2Fb | 5′-aacggaggcatacctcaac | ||
| xynVIII2Rb | 5′-tgttgccacccagcagac |
aPutative intron sequences are included in gene sizes.
bxynVIII2F and xynVIII2R were designed from the internal sequence of xynVIII.
Mycelia of A. niger E-1 were harvested from culture grown for 72 h by filtration, and total DNA was extracted by the method of Hamamoto (2001), except for the extraction buffer. We used an extraction buffer of 40 mM ethylene-N,N,N′,N′-diaminetetraacetic acid disodium salt dihydrate (EDTA) and 100 mM Tris–HCl (pH 9.0). Xylanase genes of strain E-1 were amplified by polymerase chain reaction (PCR) using appropriate primer pairs shown in Table 2. PCR was performed using TaKaRa Ex Taq (Takara Bio, Ohtu, Japan). The temperature profile consisted of an initial denaturation step of 2 min at 98°C, followed by 30 cycles of denaturing at 98°C for 30 sec, annealing at 53°C for 30 sec, and extension at 72°C for 4 min. The amplified products were cloned and sequenced by the methods described previously (Takahashi et al. 2012). The sequences obtained were compared with those of the putative A. niger CBS 513.88 xylanase genes using GENETYX-WIN version 3.1.0 (Software Development, Tokyo, Japan).
Multiple sequence alignment and phylogenetic tree construction were performed using ClustalW 2.1 software at the DDBJ. The phylogenetic tree was visualized using TreeView 1.6.6 software available online (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). The DDBJ/EMBL/GenBank accession numbers for the reported sequences in this paper are AB821364–AB821370.
Enzyme assays
Xylanase activity was assayed as described previously (Takahashi et al. 2012). One unit (U) of xylanase activity was defined as the amount of enzyme that releases 1 μmol of reducing sugar as a xylose equivalent per minute. Protein concentrations were estimated by the method of Lowry et al. (1951) using bovine serum albumin (Wako Pure Chemical Industries, Osaka, Japan) as a standard.
Separation and purification of xylanases
The crude enzyme preparation (4,500 U) was concentrated by lyophilization and dissolved in 20 mM acetate buffer (pH 5.5; buffer A). The concentrated sample was dialyzed against buffer A and applied to a DE52 (Whatman, Kent, UK) anion-exchange column (1.4 × 13 cm) equilibrated with buffer A at a flow rate of 50 mL h–1. Unbound proteins were eluted with buffer A and collected as an active xylanase fraction (fraction 1). Purity of the fraction was examined by native- and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and this fraction was used for further characterization experiments. Bound proteins were eluted with a liner gradient of NaCl (0–0.5 M) at a flow rate of 50 mL h–1, and the eluate was separated into tubes (3.2 mL for each). Enzyme activities and protein concentrations were examined in fraction 1 and collected tubes.
Native-PAGE and zymogram analysis
Native-PAGE was performed by the method of Davis (1964) using a 7.5% (w/v) acrylamide gel with Tris–glycine buffer (pH 8.3). After running, the gel was stained with Coomassie brilliant blue R-250 (CBB) or, for zymogram analysis, soaked in 83 mM acetate buffer (pH 5.5) containing 0.4% xylan for 30 min at room temperature with gentle shaking and then incubated for 1 h at 37°C. After incubation in 0.1% (w/v) Congo Red for 15 min at room temperature, the gel was washed with 1 M NaCl and then soaked in 5% acetic acid to visualize clear bands with xylanase activity.
SDS-PAGE and pI determination
SDS-PAGE was performed by the method of Weber and Osborn (1969) using a 12% (w/v) acrylamide gel with Tris–glycine buffer (pH 8.3) containing 0.1% (w/v) SDS. Molecular weight marker, middle range (Wako Pure Chemical Industries) was used as a molecular mass standard. After electrophoresis, the gel was stained with CBB. Isoelectric focusing was performed using an Immobiline DryStrip with a pH range of 6–9 (18 cm; GE Healthcare, Buckinghamshire, UK) equipped with an Immobiline DryStrip kit (GE Healthcare). Electrophoresis was performed using a Multiphor II Electrophoresis System according to the manufacturer’s instructions (GE Healthcare). The isoelectric point was estimated from the position of a protein band on the Immobiline DryStrip after staining with CBB.
N-terminal amino acid sequencing
After SDS-PAGE, proteins were electroblotted onto a Fluoro Trans membrane (Pall, Port Washington, NY, USA) using a Nihon Eido semidry type electroblotter NA-1512 (Nihon Eido, Tokyo, Japan) according to the manufacturer’s instructions. The membrane was stained with CBB, and protein bands were cut out. The protein bands were sequenced using a Shimadzu protein sequencer PPSQ-31A (Shimadzu, Kyoto, Japan).
Protein identification by MALDI-TOF-MS
To identify purified protein, a gel slice containing a CBB-stained protein band was excised after SDS-PAGE and subjected to digestion with sequencing grade modified trypsin (Promega, Madison, MI, USA) according to the method of Hong et al. (2011). The digested sample and standard peptides for analysis by Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) were prepared using a ProteoMass Peptide & Protein MALDI-MS Calibration Kit (Sigma-Aldrich) according to the manufacturer’s instructions as follows. One microliter of sample containing peptides, which were extracted from the gel slice after trypsin digestion, was mixed with 1 μL of matrix solution [10 mg mL–1 α-cyano-4-hydroxyciannamic acid in 0.1% trifluoroacetic acid solution–acetonitrile (1:1)] on a sample plate and then air-dried under dark condition. MALDI-TOF-MS analysis was performed by a Shimadzu MALDI-TOF-MS AXIMA Performance (Shimadzu) after calibration with bradykinin fragment 1–7 and insulin oxidized B chain (bovine) [(M + H)+: 757.3997 and 3,494.6513, respectively, as monoisotopic molecular masses] as peptide standards. For protein identification, MS spectra data obtained from MALDI-TOF-MS analysis were used to search for protein candidates in the SwissProt database using Mascot software programs (Matrix Science, Boston, MA, USA).
Effects of temperature and pH on xylanase activity and stability
The effects of temperature on xylanase activity were examined under the standard assay conditions, expect that the reaction temperature ranged from 30°C to 70°C. The optimum pH was determined in 20 mM buffers adjusted to different pH, as described in the appropriate figure legend. In order to examine thermostability and pH stability, purified enzyme (1.5 U and 0.25 U, respectively) was incubated at various temperatures for 30 min or in 100 μL of 150 mM buffers adjusted to different pH, for 24 h at 4°C. The residual xylanase activities were assayed under standard conditions.
Effects of metal ions and modifying reagents, and substrate specificity
The effects of chemicals on xylanase activity were examined in the presence of the various chemicals at the final concentration of 2 mM. Relative enzyme activity was calculated as the percentage of activity compared with that without any chemicals.
Cellulolytic and xylanolytic enzyme activities were assayed in a reaction mixture containing oatspelt xylan (Sigma-Aldrich), carboxymethylcellulose sodium salt (CMC, Sigma-Aldrich), crystalline cellulose (Sigmacell Cellulose, Type 20; Sigma-Aldrich), pectin from citrus (Wako Pure Chemical Industries), inulin (Tokyo Chemical Industry, Tokyo, Japan), dextran (Nacalai Tesque, Kyoto, Japan), or dextrin from corn (Sigma-Aldrich) instead of beechwood xylan. Enzyme activity was estimated by measuring the increase in reducing sugar released from each substrate. We also tested p-nitrophenyl α-L-arabinofuranoside (PNPAra, Sigma-Aldrich), p-nitrophenyl β-D-cellobioside (PNPC, Sigma-Aldrich), p-nitrophenyl β-D-glucopyranoside (PNPG, Nacalai Tesque), and p-nitrophenyl β-D-xylopyranoside (PNPX, Nacalai Tesque) using method described previously (Takahashi et al. 2012). Substrate specificity was expressed as relative percentages compared with beechwood xylan as a substrate.
Kinetic parameters
Kinetic parameters were determined using several concentrations (1.25–10 mg mL–1) of beechwood xylan in 83 mM acetate buffer (pH 5.5) under standard assay conditions. The Michaelis–Menten constant (Km) and maximal reaction velocity (Vmax) values were estimated by linear regression from double-reciprocal plots according to the method of Lineweaver and Burk (Lineweaver and Burk 1934).
Analysis of hydrolyzed products
The hydrolyzed products from beechwood xylan were examined by thin-layer chromatography (TLC). Purified xylanase (10 U mL–1) was incubated in 20 mM buffer A containing 1.7% beechwood xylan, xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), or xylohexaose (X6) at 37°C for an appropriate time and the enzyme reactions were stopped by boiling. After centrifugation, the supernatants were concentrated by a vacuum centrifuge and spotted onto a silica gel 60 F254 aluminium plate (Merck, Whitehouse Station, NJ, USA), developed using the solvent system of acetonitrile–ethyl acetate–2-propanol–water (17:5:11:10, v/v/v/v). Xylose and X2–X6 were used as standards. The hydrolysates were detected by spraying with 10% (v/v) sulfuric acid in methanol, followed by heating at 180°C. To reanalyze an unusual product (Xα, see Results section), the hydrolyzed products were also developed according to the method of Kolenová et al. (2006).
Structure of the hydrolyzed products with a molecular mass of more than 550 was analyzed by MALDI-TOF-MS according to the method of Reis et al. (2003). A sample for MALDI-TOF-MS analysis was prepared by mixing 1 μL of the products described above, 1 μL of 2,5-dihydroxybenzoic acid dissolved in a solvent mixture composed of 0.1% trifluoroacetic acid solution–acetonitrile (30:70, v/v), and 1 μL of 5-chloro-2-mercaptobenzothiazole dissolved in tetrahydrofuran–ethanol–water (1:1:1, v/v/v).
Results
Cloning of the xylanase genes of A. niger E-1 and phylogenetic analysis
We found eight putative xylanase genes on the A. niger CBS 513.88 genome and designated them xynI–VIII in ascending order of molecular masses of the deduced amino acid sequences (Table 1). To confirm the presence of these xylanase genes on the A. niger E-1 genome, PCR was performed using primer pairs designed on the basis of the putative A. niger CBS 513.88 xylanase gene sequences (Table 2). The seven putative xylanase genes xynI–VII were amplified and their nucleotide sequences showed 98.4%–100% identities with those of corresponding genes on the A. niger CBS 513.88 genome (data not shown). We found that xynV, showing the lowest identity (98.4%) in comparisons of corresponding nucleotide sequences, was found to possess substitutions of amino acid residues at three positions (A108→T, A110→T, and S161→T in the XynV sequence from A. niger CBS 513.88), and the Asn-38 residue in the XynII sequence of strain CBS 513.88 was also substituted with an Asp residue at the corresponding position in XynII of strain E-1 (99.7% identity in comparison of nucleotide sequence). The other xylanases had no substitutions. We could not amplify strain E-1 xynVIII by PCR using the pair of xynVIII2F and xynVIII2R which were designed from the internal sequence of strain CBS 513.88 xynVIII, in addition to the pair of xynVIII1F and xynVIII1R.
A phylogenetic tree based on the deduced amino acid sequences of A. niger E-1 xylanases was constructed using the Neighbor-Joining method (Figure 1). We found that the A. niger E-1 xylanases constitute independent three clusters. Cluster I consisted of XynI and XynVII although they branched deeply; XynII, XynIII, XynIV, and XynV, which belonged to GH family 11, were classified into another branch, cluster II; and only XynVI was localized in cluster III in the phylogenetic tree.
Figure 1.
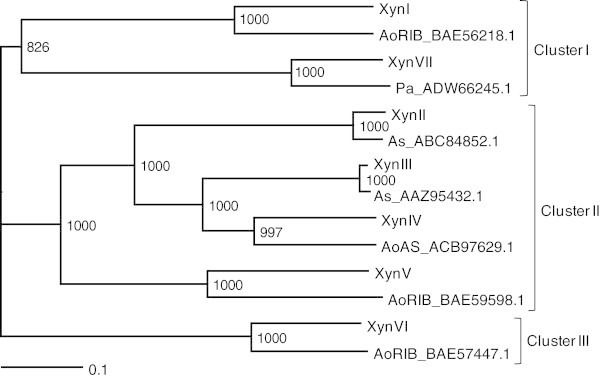
Phylogenetic tree of xylanases fromA. nigerE-1 and the closest related sequences to strain E-1 xylanases. The sequence names consist of the abbreviation of microorganisms and protein ID. Abbreviations: AoRIB, Aspergillus oryzae RIB40; AoAS, Aspergillus oryzae strain AS 3.4382; As, Aspergillus sulphureus; Pa, Paecilomyces aerugineus.
Separation and identification of xylanases from strain E-1
A. niger E-1 produced three xylanases in the culture supernatant when the strain was cultured in 0.5% beechwood xylan medium supplemented with 50 mM sodium succinate (Figure 2a). In order to separate these xylanases, a crude enzyme preparation (4,500 U) was concentrated by lyophilization and applied to a DE52 column after dialysis. An eluate passing through the DE52 column showed xylanase activity and was stored as fraction 1. Two active peaks (fractions 2 and 3), which were eluted with 0.14 or 0.22 M sodium chloride, respectively, were observed when proteins were eluted from the column with a linear gradient of 0–0.5 M sodium chloride (Figure 3). The total activities of fractions 1, 2, and 3 were 670 U, 2,300 U, and 500 U, respectively, and their total amount was calculated as 77% of the crude enzyme preparation (4,500 U).
Figure 2.
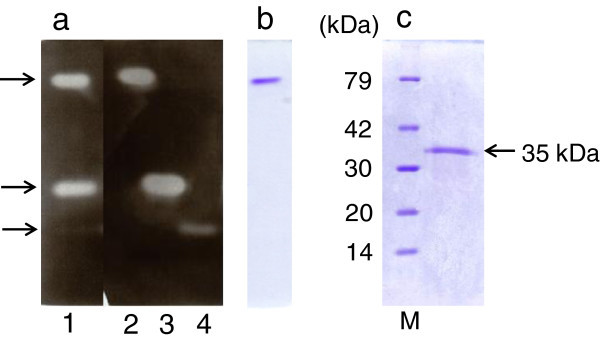
PAGE of xylanase fromA. nigerE-1. (a) Zymogram analysis of crude enzyme preparation (1.6 U, lane 1), fraction 1 (0.3 U, lane 2), fraction 2 (1.9 U, lane 3), and fraction 3 (1.8 U, lane 4). (b) Native-PAGE of fraction 1 (3.2 μg). (c) SDS-PAGE of fraction 1 (2.5 μg). Lane M, molecular mass standard proteins: lysozyme (14 kDa), trypsin inhibitor (20 kDa), carbonic anhydrase II (30 kDa), aldolase (42 kDa), and bovine serum albumin (79 kDa).
Figure 3.
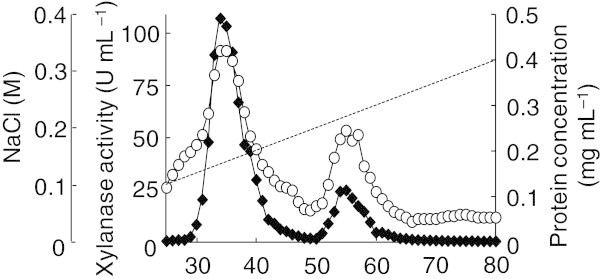
Elution profile of xylanases by DE52 column chromatography. Xylanase activity (filled diamond) and protein concentration (open circle) were assayed for each fraction.
Zymogram analysis revealed that each fraction contained the only one active protein that was represented by a clear zone and corresponded to those in the culture supernatant (Figure 2a). Furthermore, fraction 1 showed only a single band by native- and SDS-PAGE (Figure 2b and 2c, respectively). These results indicate that the three extracellular xylanases produced by A. niger E-1 could be separated from each other, and the xylanase contained in fraction 1 was purified to homogeneity. By contrast, fractions 2 and 3 were found to contain some proteins by native- and SDS-PAGE (data not shown). The purification procedure for fraction 1 is summarized in Table 3. Fraction 1 was purified 1.4-fold with a specific activity of 99 U mg–1 and a yield of 15% of total activity in the culture supernatant.
Table 3.
Summary of XynVII purification
| Purification step | Total act. (U) | Total protein (mg) | Specific act. (U mg–1) | Yield (%) | Purification fold |
|---|---|---|---|---|---|
| Crude enzyme | 4,500 | 65 | 69 | 100 | 1.0 |
| Lyophilization | 4,900 | 34 | 140 | 110 | 2.0 |
| Anion-exchange (DE52) | 670 | 6.8 | 99 | 15 | 1.4 |
Xylanases contained in fractions 1, 2, and 3 were identified by N-terminal amino acid sequencing or MALDI-TOF-MS analysis. N-terminal amino acid sequencing was not available for the identification of xylanase contained in fraction 1 because there were no significant signals of phenylthiohydantoin-amino acid derivatives released from an N-terminal amino acid residue. A trypsinized single band from fraction 1 was analyzed by MALDI-TOF-MS and resulting sequences were queried against the SwissProt database. As a result, 16 peptides were found to exactly correspond to the deduced amino acid sequence of XynVII. The molecular mass of the purified XynVII was 35 kDa by SDS-PAGE (Figure 2c). The isoelectric point of the enzyme was estimated to be 7.0. Furthermore, we characterized XynVII because information on the enzymatic properties of XynVII was limited.
When a protein with a molecular mass of 23 kDa in fraction 2 was subjected to N-terminal amino acid sequencing, a STPSSTGENNGFYYSFWTDG sequence was identified and found in the deduced amino acid sequence of xynIII, with an estimated molecular mass of 24 kDa. A protein with a molecular mass of 27 kDa in fraction 3 showed the N-terminal sequence of SAGINYVQNYNGNLGDFTYDESTG, which was contained in the deduced amino acid sequence of xynII with an estimated molecular mass of 23 kDa. Further N-terminal amino acid sequencing of other proteins electroblotted from fractions 2 and 3 revealed that they were not putative xylanases, but were other enzymes related to xylan degradation, for example, α-L-arabinofuranosidase and acetyl xylan esterase. These results strongly suggest that XynII and XynIII reflect xylanase activities obtained in fractions 2 and 3, respectively.
The effects of pH and temperature on xylanase activity and stability
XynVII was the most active at 55°C, and its activity rapidly decreased above 60°C. The enzyme maintained more than 85% remaining activity after incubation at 30°C–60°C for 30 min (Figure 4a). The optimal pH for XynVII activity was 5.5, and this enzyme showed more than 70% relative activity in a mildly acidic pH range (pH 4–6) (Figure 4b). Furthermore, the enzyme was stable (approximately 100%) in the broad pH range of 3–10 and retained more than 50% of its activity after incubation at pH 2.5, 10.5, or 11 for 24 h (Figure 4b).
Figure 4.
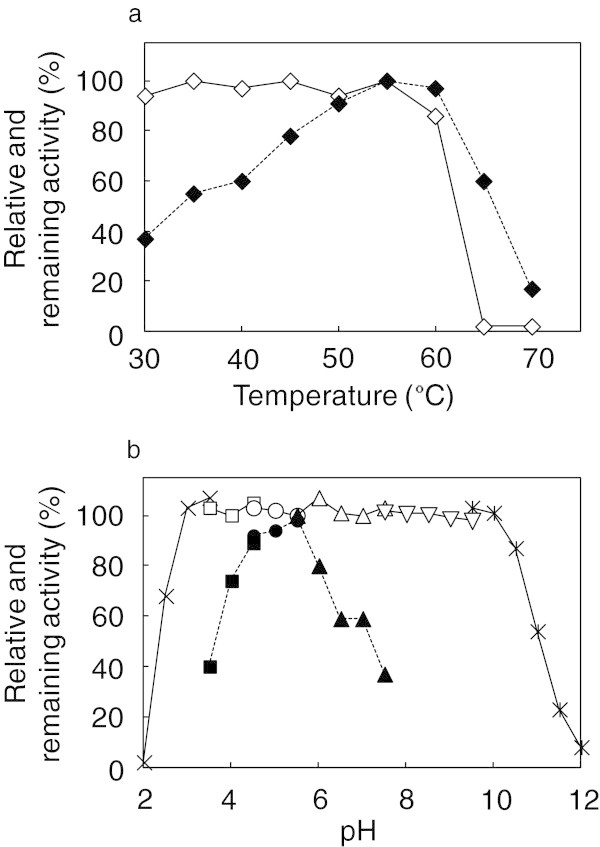
Effects of temperature and pH on XynVII activity. (a) Effects of temperature (filled diamond) and thermostability (open diamond). (b) Effects of pH (filled symbols, dotted line) and pH-stability (open symbols, solid line). The buffers used were 150 mM of glycine–HCl (cross), sodium acetate–HCl (filled square, open square), acetate (filled circle, open circle), sodium–potassium phosphate (filled triangle, open triangle), Tris–HCl (inverted open triangle), and glycine–NaOH (asterisk).
Effects of metal ions and modifying reagents
The effects of various metal ions and modifying reagents on XynVII activity were determined. Hg2+ and N-bromosuccinimide (NBS) strongly inhibited xylanase activity (67% and 5% relative activity, respectively). By contrast, we found that the following metal ions and modifying reagents showed no remarkable effects (relative activity, 86–109%): Ba2+, Ca2+, Cd2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+, Ni2+, Pb2+, Zn2+, 4-chloromercuribenzoic acid, EDTA, and SDS.
Substrate specificity and kinetic parameters
XynVII showed high specificity against beechwood xylan compared with oatspelt xylan (68% relative activity), whereas other hemicellulose-related substrates such as pectin, PNPAra, and PNPX were not suitable substrates for this enzyme (less than 2% relative activity). Moreover, the enzyme showed little activity against dextran, dextrin, or cellulolytic substrates such as CMC, crystalline cellulose, PNPC, or PNPG (less than 2% relative activity). The Km, Vmax, and kcat values of XynVII were 2.8 mg mL–1, 127 μmol min–1mg–1, and 76 s–1, respectively, when beechwood xylan was used as a substrate.
Hydrolysis of beechwood xylan and xylooligosaccharides by purified XynVII
In order to analyze reaction products, beechwood xylan and various xylooligosaccharides (X2–X6) were hydrolyzed with purified XynVII, and the reaction products were analyzed by TLC using a silica gel plate and the solvent system of acetonitrile–ethyl acetate–2-propanol–water (17:5:11:10, v/v/v/v) (Figure 5a). For beechwood xylan, the enzyme initially released xylooligosaccharides with high molecular masses [degree of polymerization (DP) of >5], and the molecular masses became smaller (DP, 1–6) as the enzyme reaction progressed. Finally, the products converged on X1, X2, and Xα, which was localized between X5 and X6. When the final products were developed on a cellulose plate using the solvent system of ethyl acetate–acetic acid–water (3:2:2, v/v/v), Xβ appeared at a position between X2 and X3, instead of Xα, in addition to X1 and X2 (Figure 5b). The position of Xβ was similar to that of X3 substituted with a 4-o-methylglucuronic acid residue (X3MeGlcA) that was reported by Kolenová et al. (2006). In hydrolysis tests of xylooligosaccharides, the enzyme hydrolyzed X3–X6 and produced X1 and X2 as the final hydrolysates after a 6-h incubation, but no reaction products were observed when X2 was used as a substrate (Figure 5c).
Figure 5.
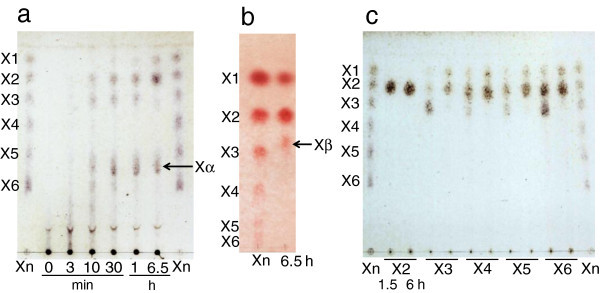
TLC analysis of hydrolysates. After hydrolysis of beechwood xylan (a and b) and xylooligosaccharides (c) with XynVII for various reaction times, hydrolysates were developed on a silica (a and c) and cellulose plate (b). Abbreviations: X1, xylose; X2, xylobiose; X3, xylotriose; X4, xylotetraose; X5, xylopentaose; X6, xylohexaose; Xn, standards mixture of X1–X6. Unknown products are indicated by Xα and Xβ.
To identify the final product (Xβ) appearing at an unusual position in TLC analysis, MALDI-TOF-MS analysis was performed. The MALDI mass spectra obtained from the product Xβ showed more abundant ions at m/z 627 and 649. These ions could be attributed to xylotriose substituted with a 4-o-methylglucuronic acid residue (X3MeGlcA), corresponding to a 23 and 22 Da increase that could be attributed to a single- or double-sodiated ion (m/z). The ions observed at m/z 649 resulted from the substitution of one hydrogen atom by a second sodium atom (Reis et al. 2003). In conclusion, we identified the final reaction products Xα and Xβ as X3MeGlcA based on the results of TLC and MALDI-TOF-MS analyses.
Discussion
Thus far many researchers have reported enzymatic properties, gene cloning, and heterologous expression of individual xylanases from A. niger. However, less attention has been paid to the relationship between xylanase genes carried on A. niger genome and xylanases produced. Therefore, we examined the xylanase genes on A. niger E-1 genome that contribute to xylan degradation though the biosynthesis of functionally active xylanases.
The A. niger E-1 genome encoded seven putative xylanase genes xynI–VII, which are closely similar to those of xylanase genes from A. niger CBS 513.88. On the other hand, strain E-1 produced three xylanases, XynII, XynIII, and XynVII, when this strain was cultured in 0.5% xylan medium supplemented with 50 mM sodium succinate (Figure 2a and Figure 3). There are no reports, to our knowledge, on the expression of xynIV and xynV belonging to cluster II in a phylogenetic tree (Figure 1) or xynI and xynVI in other A. niger strains. These results suggest that these xylanases may play physiologically distinct roles from XynII, XynIII, and XynVII to adapt to quite different environments and assimilate xylan.
XynIII activity represented 51% of total activity in the culture supernatant; XynII and XynVII activities were 15% and 11%, respectively, of the total activity, suggesting that XynIII plays a primary role in the degradation of xylan backbones in this culture condition. This speculation is also supported by the fact that many XynIII and closely related enzymes have been reported from A. niger strains (Krisana et al. 2005; Fu et al. 2012). In addition to XynIII, some enzymes highly homologous to XynII have also been reported (Hmida-Sayari et al. 2012; Yang et al. 2010), but information on XynVII, which belongs to GH family 10 in A. niger, was limited. Therefore, we characterized XynVII in further experiments.
Aspergilli endoxylanases show the maximal activities at a range of 42°C–60°C, and a pH range of 4.0–7.0 (Teixeira et al. 2010). The highest activity of purified XynVII was also observed within these ranges. However, XynVII maintained more than 85% activity after 30 min of incubation at 60°C (Figure 4a), and was more stable than other xylanases from A. niger strains US368 (Hmida-Sayari et al. 2012) and A-25 (Chen et al. 2006), which retained 50% and 10% of activities, respectively. Moreover, the pH stability profile showed that XynVII is highly stable over a wide pH range from 3.0 to 10.0 (Figure 4b), compared with other reported xylanases from A. niger strains (Fu et al. 2012; Hmida-Sayari et al. 2012; Krisana et al. 2005; Yang et al. 2010). In addition, Km and Vmax values of XynVII (2.8 mg mL–1 and 127 μmol min–1mg–1, respectively) were similar to those of GH family 10 xylanases from Penicillium pinophilum strain C1 [4.3 mg mL–1 and 195.4 μmol min–1mg–1, respectively (Cai et al. 2011)] and of GH family 11 xylanase from A. niger strain US368 [1.03 mg mL–1 and 811 μmol min–1mg–1, respectively (Hmida-Sayari et al. 2012)], indicating that XynVII from strain E-1 possesses catalytic properties that are sufficient to contribute to xylan degradation although the total activity of XynVII was smaller than those of other xylanases in the culture supernatant.
As reported for various xylanases from A. niger (Chen et al. 2006; Hmida-Sayari et al. 2012; Yang et al. 2010) and GH family 10 xylanase from Flavobacterium johnsoniae (Chen et al. 2013), XynVII activity was strongly inhibited by Hg2+, which potentially caused inhibition by its interaction with an aromatic ring present in a Trp residue. This finding was consistent with the result that XynVII was strongly inhibited by NBS, which modifies a Trp residue. It is interesting that XynVII was stable in the presence of other metal ions and modifying reagents, particularly Cu2+ and Mn2+, which are well-known inhibitors of xylanases (Chen et al. 2006; Hmida-Sayari et al. 2012).
The N-terminal amino acid sequence determination of purified XynVII was unsuccessful by a gas-phase protein sequencing. Liu et al. (2012) have studied a signal peptide cleavage site in the N-terminal region of A. niger XynB, which is identical to that of XynVII, and proposed that the cleavage of a peptide bond occurs between Arg-25 and Gln-26, releasing the signal peptide. It is likely that the N-terminal Gln residue (Gln-26) of mature E-1 XynVII is changed to pyroglutamate by cyclization after hydrolysis of the peptide bond, yielding a blocked N-terminus (Ito et al. 1992).
GH family 10 and GH family 11 xylanases differ in produced xylooligosaccharides. When a glucuronoxylan, such as beechwood xylan, is digested with GH family 11 xylanases, xylotetraose substituted with a 4-o-methylglucuronic acid residue (X4MeGlcA) accumulates as the final reaction product (Biely et al. 1997; Kolenová et al. 2006). X4MeGlcA generally show resistance against hydrolysis with xylosidase, when substitution by glucuronic acid occurs at X1 of the non-reducing end or the second X1 next to the non-reducing end (Tenkanen et al. 1996; Rasmussen et al. 2012). Although glucuronic acid is released from short xylooligosaccharides by the actions of α-glucuronidase, X4MeGlcA is too large to be hydrolyzed with the enzyme (Kolenová et al. 2006). As a result, it is assumed that X4MeGlcA remains and complete glucuronoxylan degradation is unsuccessful when glucuronoxylan is digested with GH family 11 xylanases. On the other hand, X3MeGlcA, which is produced by GH family 10 xylanase reaction, is hydrolyzed with α-glucuronidase, and the resulting X3 is acceptable for hydrolysis of xylosidase. Thus, the GH family 10 xylanase XynVII appears to contribute to efficient glucuronoxylan assimilation by strain E-1. Zheng et al. (2013) recently cloned a cDNA encoding GH family 10 xylanase from A. niger and characterized the purified recombinant enzyme. Characteristics of the recombinant enzyme were similar to those of E-1 XynVII. In addition to these characteristics, we identified the reaction products of E-1 enzyme and suggested the physiological significance of XynVII in glucuronoxylan assimilation based on the reaction products as described above.
In this study, we found only one gene encoding GH family 10 xylanase and four genes encoding GH family 11 xylanases from A. niger E-1. Wakiyama et al. (2008) showed that GH family 10 xylanases from Penicillium spp. and Aspergillus spp. could be classified into two groups by phylogenetic analysis: one is a xylanase group from Penicillium spp. and black aspergilli such as A. kawachii and A. niger, and another is from other aspergilli, such as A. fumigatus, A. nidulans, and A. oryzae. In the aspergilli described above, it has been reported that A. fumigatus, A. nidulans, and A. oryzae, of which the genomes have been sequenced, possess more than two GH family 10 genes on their genome. By contrast, the genome of the black aspergillus A. niger carried only one GH family 10 xylanase gene in both strains E-1 and CBS 513.88. Genome information about other black aspergilli is not available for database search, but numbers of GH family 10 xylanase genes on each aspergillus genome may be related to the diversity of GH family 10 xylanases in the evolution processes of aspergilli genomes.
A. niger E-1 possesses seven putative xylanase genes (xynI–VII), but only three xylanases (XynII, XynIII, and XynVII) were produced, strongly suggesting that potential regulatory mechanisms control the expression of the other four xylanase genes, xynI, xynIV, xynV, and xynVI, for which no translated products were detected. Further research is needed to comprehensively understand xylan degradation by A. niger E-1.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YT performed the experiments, analyzed the data, and drafted the manuscript as part of her Dr. course research program at Meiji University under the supervision of SM. HK contributed to MALDI-TOF-MS analysis. All authors read and approved the final manuscript.
Contributor Information
Yui Takahashi, Email: brownswiss_510@yahoo.co.jp.
Hiroaki Kawabata, Email: kawabata@isc.meiji.ac.jp.
Shuichiro Murakami, Email: smura@isc.meiji.ac.jp.
References
- Akpinar O, Erdogan K, Bostanci S. Enzymatic production of Xylooligosaccharide from selected agricultural wastes. Food Bioprod Process. 2009;87:145–151. doi: 10.1016/j.fbp.2008.09.002. [DOI] [Google Scholar]
- Beg QK, Bhushan B, Kapoor M, Hoondal GS. Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in biobleaching of eucalyptus kraft pulp. Enzyme Microb Technol. 2000;27:459–466. doi: 10.1016/S0141-0229(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Beg QK, Kapoor M, Mahajan L, Hoondal GS. Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- Biely P, Vršanská M, Tenkanen M, Kluepfel D. Endo-beta-1,4-xylanase families: differences in catalytic properties. J Biotechnol. 1997;57:151–166. doi: 10.1016/S0168-1656(97)00096-5. [DOI] [PubMed] [Google Scholar]
- Cai HY, Shi PJ, Bai YG, Huang HQ, Yuan TZ, Yang PL, Luo HY, Meng K, Yao B. A novel thermoacidophilic family 10 xylanase from Penicillium pinophilum C1. Process Biochem. 2011;46:2341–2346. doi: 10.1016/j.procbio.2011.09.018. [DOI] [Google Scholar]
- Camacho NA, Aguilar OG. Production, purification, and characterization of a low-molecular-mass xylanase from Aspergillus sp. and its application in baking. Appl Biochem Biotechnol. 2003;104:159–171. doi: 10.1385/ABAB:104:3:159. [DOI] [PubMed] [Google Scholar]
- Chávez R, Bull P, Eyzaguirre J. The xylanolytic enzyme system from the genus Penicillium. J Biotechnol. 2006;123:413–433. doi: 10.1016/j.jbiotec.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Chen HG, Yan X, Liu XY, Wang MD, Huang HM, Jia XC, Wang JA. Purification and characterization of novel bifunctional xylanase, XynIII, isolated from Aspergillus niger A-25. J Microbiol Biotechnol. 2006;16:1132–1138. [Google Scholar]
- Chen SC, Kaufman MG, Miazgowicz KL, Bagdasarian M, Walker ED. Molecular characterization of a cold-active recombinant xylanase from Flavobacterium johnsoniae and its applicability in xylan hydrolysis. Bioresour Technol. 2013;128:145–155. doi: 10.1016/j.biortech.2012.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ. Disc electrophoresis-II method and application to human serum proteins. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- de Vries RP, Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev. 2001;65:497–522. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Li DF, Cao YH, Lu WQ, Wang CL. Cloning of a gene encoding an acidophilic endo-beta-1,4-xylanase obtained from Aspergillus niger CGMCC1067 and constitutive expression in Pichia pastoris. Enzyme Microb Technol. 2006;39:1096–1102. doi: 10.1016/j.enzmictec.2006.02.014. [DOI] [Google Scholar]
- Fournier R, Frederick MM, Frederick JR, Reilly PJ. Purification and characterization of endo-xylanases from Aspergillus niger. III. an enzyme of pI 3.65. Biotechnol Bioeng. 1985;27:539–546. doi: 10.1002/bit.260270422. [DOI] [PubMed] [Google Scholar]
- Frederick MM, Frederick JR, Fratzke AR, Reilly PJ. Purification and characterization of a xylobiose-producing and xylose-producing endo-xylanase from Aspergillus niger. Carbohydr Res. 1981;97:87–103. doi: 10.1016/S0008-6215(00)80527-3. [DOI] [Google Scholar]
- Frederick MM, Kiang CH, Frederick JR, Reilly PJ. Purification and characterization of endo-xylanases from Aspergillus niger. I. Two isozymes active on xylan backbones near branch-points. Biotechnol Bioeng. 1985;27:524–532. doi: 10.1002/bit.260270420. [DOI] [PubMed] [Google Scholar]
- Fu GH, Wang YT, Wang DD, Zhou CY. Cloning, Expression, and Characterization of an GHF 11 Xylanase from Aspergillus niger XZ-3S. Indian J Microbiol. 2012;52:682–688. doi: 10.1007/s12088-012-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbacheva IV, Rodionova NA. Studies on xylan degrading enzymes. I. purification and characterization of endo-1,4-beta-xylanase from Aspergillus niger STR-14. Biochim Biophys Acta. 1977;484:79–93. doi: 10.1016/0005-2744(77)90114-0. [DOI] [PubMed] [Google Scholar]
- Hamamoto M. DNA preparation, yeast and filamentous fungi. In: Suzuki K, Hiraishi A, Yokota A, editors. The methods of classification and identification for microorganisms. 1. Tokyo: Springer Japan; 2001. pp. 22–27. [Google Scholar]
- Hmida-Sayari A, Taktek S, Elgharbi F, Bejar S. Biochemical characterization, cloning and molecular modeling of a detergent and organic solvent-stable family 11 xylanase from the newly isolated Aspergillus niger US368 strain. Process Biochem. 2012;47:1839–1847. doi: 10.1016/j.procbio.2012.06.010. [DOI] [Google Scholar]
- Hong D, et al. Quantitative proteomic analysis of dexamethasone-induced effects on osteoblast differentiation, proliferation, and apoptosis in MC3T3-E1 cells using SILAC. Osteoporos Int. 2011;22:2175–2186. doi: 10.1007/s00198-010-1434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ikemasu T, Ishikawa T. Cloning and sequencing of the xynA gene encoding xylanase A of Aspergillus kawachii. Biosci Biotechnol Biochem. 1992;56:906–912. doi: 10.1271/bbb.56.906. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Takano M, Koseki T, Ito K, Iwano K. Cloning of the xynNB gene encoding xylanase B from Aspergillus niger and its expression in Aspergillus kawachii. J Ferment Bioeng. 1995;79:422–428. doi: 10.1016/0922-338X(95)91255-4. [DOI] [Google Scholar]
- Kolenová K, Vršanská M, Biely P. Mode of action of endo-beta-1,4-xylanases of families 10 and 11 on acidic xylooligosaccharides. J Biotechnol. 2006;121:338–345. doi: 10.1016/j.jbiotec.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Krisana A, Rutchadaporn S, Jarupan G, Lily E, Sutipa T, Kanyawim K. Endo-1,4-beta-xylanase B from Aspergillus cf. niger BCC14405 isolated in Thailand: Purification, characterization and gene isolation. J Biochem Mol Biol. 2005;38:17–23. doi: 10.5483/BMBRep.2005.38.1.017. [DOI] [PubMed] [Google Scholar]
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. doi: 10.1021/ja01318a036. [DOI] [Google Scholar]
- Liu LW, Chen L, Tian H, Yang HY, Zhao L. Using signal peptide prediction with caution, a case study in Aspergillus niger xylanase. Process Biochem. 2012;47:2527–2530. doi: 10.1016/j.procbio.2012.07.004. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Pel HJ, et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol. 2007;25:221–231. doi: 10.1038/nbt1282. [DOI] [PubMed] [Google Scholar]
- Rasmussen LE, Xu C, Sørensen JF, Nielsen MK, Meyer AS. Enzyme kinetics and identification of the rate-limiting step of enzymatic arabinoxylan degradation. Biochem Eng J. 2012;69:8–16. doi: 10.1016/j.bej.2012.08.004. [DOI] [Google Scholar]
- Reis A, Domingues MRM, Ferrer-Correia AJ, Coimbra MA. Structural characterisation by MALDI-MS of olive xylo-oligosaccharides obtained by partial acid hydrolysis. Carbohydr Polym. 2003;53:101–107. doi: 10.1016/S0144-8617(03)00007-9. [DOI] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning a laboratory manual. 3. NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Shallom D, Shoham Y. Microbial hemicellulases. Curr Opin Microbiol. 2003;6:219–228. doi: 10.1016/S1369-5274(03)00056-0. [DOI] [PubMed] [Google Scholar]
- Shei JC, Fratzke AR, Frederick MM, Frederick JR, Reilly PJ. Purification and characterization of endo-xylanases from Aspergillus niger. II. An enzyme of pI 4.5. Biotechnol Bioeng. 1985;27:533–538. doi: 10.1002/bit.260270421. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nomura M, Komiya T, Goto M, Murakami S. Screening of lignocelluloce-degrading fungi from gray gentle lemur (Hapalemur griseus) feces. Bull School Agric, Meiji Univ. 2012;61:77–86. [Google Scholar]
- Teixeira RSS, Siqueira FG, de Souza MV, Ferreira EX, Bon EPD. Purification and characterization studies of a thermostable β-xylanase from Aspergillus awamori. J Ind Microbiol Biotechnol. 2010;37:1041–1051. doi: 10.1007/s10295-010-0751-4. [DOI] [PubMed] [Google Scholar]
- Tenkanen M, Luonteri E, Teleman A. Effect of side groups on the action of β-xylosidase from Trichoderma reesei against substituted xylo-oligosaccharides. FEBS Lett. 1996;399:303–306. doi: 10.1016/S0014-5793(96)01313-0. [DOI] [PubMed] [Google Scholar]
- Twomey LN, Pluske JR, Rowe JB, Choct M, Brown W, McConnell MF, Pethick DW. The effects of increasing levels of soluble non-starch polysaccharides and inclusion of feed enzymes in dog diets on faecal quality and digestibility. Anim Feed Sci Technol. 2003;108:71–82. doi: 10.1016/S0377-8401(03)00161-5. [DOI] [Google Scholar]
- Wakiyama M, Tanaka H, Yosbihara K, Hayashi S, Ohta K. Purification and properties of family-10 endo-1,4-β-xylanase from Penicillium citrinum and structural organization of encoding gene. J Biosci Bioeng. 2008;105:367–374. doi: 10.1263/jbb.105.367. [DOI] [PubMed] [Google Scholar]
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- Yang YL, Zhang W, Huang JD, Lin L, Lian HX, Lu YP, Wu JD, Wang SH. Purification and characterization of an extracellular xylanase from Aspergillus niger C3486. Afr J Microbiol Res. 2010;4:2249–2256. [Google Scholar]
- Zheng J, Guo N, Wu L, Zhou H. Biotechnol Lett. 2013. Characterization and constitutive expression of a novel endo-1,4-β-D-xylanohydrolase from Aspergillus niger in Pichia pastries. [DOI] [PubMed] [Google Scholar]


