Abstract
Critical limb ischemia (CLI) is a major cause of limb loss and mortality among patients with advanced peripheral artery disease. Our objective was to evaluate the gender-specific differences in patient characteristics and clinical outcomes among patients with CLI. We performed a retrospective analysis of 97 women and 122 men presenting with CLI who underwent angiography from 2006 to 2010. Baseline demographics, procedural details, and lesion characteristics were assessed for each patient. Kaplan–Meier analysis was used to assess long-term patient and lesion-level outcomes. Cox proportional hazard modeling was used to evaluate the relationship between gender and major adverse cardiovascular events (MACE). Compared to men, women were less likely to have a history of coronary artery disease (39% vs 54%, p = 0.02) or diabetes (57% vs 70%, p = 0.05) but had similar baseline medical therapy. At angiography, women were more likely to have significant femoropopliteal (77% vs 67%, p = 0.02) and multi-level infrainguinal disease (63% vs 51%, p = 0.02). Women were also more likely to undergo multi-vessel percutaneous intervention (69% vs 55%, p = 0.05), but had similar rates of limb salvage after percutaneous intervention or surgical bypass (HR 0.94 [95% CI 0.45–1.94], p = 0.9). During follow-up, women had higher rates of subsequent major adverse cardiovascular events (HR 1.63 [95% CI 1.01–2.63], p = 0.04). In conclusion, women with CLI are more likely to present with femoropopliteal and multi-level infrainguinal disease. Despite similar rates of limb salvage, women with CLI have an increased rate of subsequent major adverse cardiovascular events.
Keywords: atherosclerosis, critical limb ischemia, gender variation, interventional cardiology, percutaneous intervention, peripheral artery disease
Introduction
Critical limb ischemia (CLI) is an advanced stage of peripheral artery disease (PAD) characterized by ischemic rest pain, ulcers and gangrene and is associated with an increased risk of limb loss and mortality.1,2 In addition to significant morbidity from limb loss, these patients are also at high risk for subsequent cardiovascular events, including myocardial infarction and stroke.2,3 The overall mortality rate of patients with CLI is approximately 50% at 5 years.2
While gender-related differences in coronary artery disease and stroke have been studied extensively, gender differences in PAD and CLI remain understudied.4–7 Compared to men, women with PAD may present at an older age with atypical symptoms and more advanced atherosclerotic disease.8 Preliminary research has also suggested that women with PAD and CLI have worse long-term outcomes.9–13 The need for additional studies in this area was recently highlighted by a call to action for studies of women and PAD by the American Heart Association.7
The goal of this study was to analyze gender-related differences in baseline characteristics, lesion characteristics and clinical outcomes among patients with CLI who underwent peripheral angiography and subsequent treatment, consisting of endovascular intervention, surgical bypass grafting, or primary amputation. We hypothesized that women with CLI would have more severe angiographic disease and would have higher subsequent rates of major adverse cardiovascular events during long-term follow-up.
Methods
PAD-UCD Registry
The PAD-UCD Registry consists of all patients with a clinical diagnosis of PAD who underwent diagnostic peripheral angiography or therapeutic percutaneous intervention at the University of California at Davis, Medical Center between 1 June 2006 and 1 December 2010. At the time of data extraction 622 patients were included in the overall data registry. For this retrospective chart review the subset of patients in the registry with CLI (219, representing 35% of the total registry) were analyzed. CLI was defined as Rutherford category 4–6 disease (defined as ischemic rest pain, minor tissue loss, or major tissue loss, respectively) based on review of clinic notes, history and physical examination, and hospital discharge summaries. The median length of patient follow-up was 2.2 years.
Data collection and definitions
After approval by the Institutional Review Board at University of California at Davis, Medical Center, baseline data were collected from review of electronic medical record documentation and procedure notes. Pre- and post-procedure clinical notes and the admission history and physical documentation were used to identify clinical presentation as well as post-procedure outcomes and medical management. This information was entered into a pre-specified case report form with standardized data entry. A complete listing of all data definitions can be found in the accompanying supplemental material. Two authors (EA and UJ) reviewed all angiographic images to verify lesion location, presence of chronic occlusion, TransAtlantic Inter-Society Consensus (TASC II) classification, extent of calcification, presence of thrombus, and status of the distal run-off vessels. Quantitative angiography was performed on all target lesions to evaluate the pre- and post-intervention percent diameter stenosis, lesion length, and reference vessel diameter.
Procedural data included whether the lesion was a restenosis, the type of intervention, whether a stent was placed, and whether the intervention involved balloon angioplasty, cutting balloon angioplasty, excisional or rotational atherectomy, thrombolysis, thrombus aspiration, rheolytic throm bectomy, or cryoplasty. Procedural success was defined as < 30% stenosis at the conclusion of the procedure. Lesion post-procedure outcomes were determined by clinical follow-up and duplex ultrasound surveillance performed routinely at 1, 6, and 12 months.
During follow-up, death, myocardial infarction (MI) or stroke were identified through electronic documentation of clinic or inpatient notes as well as the Social Security Death Index. Major amputation was defined as any amputation above the level of the ankle joint. Lesion patency was followed with serial ankle–brachial index (ABI) and toe–brachial index (TBI) measures and peak systolic velocity (PSV) at 0–30 days, 4–6 months, and 9–12 months. ABI and TBI were obtained by measurement of systolic brachial and tibial artery pressures with a standard blood pressure cuff and continuous wave Doppler. The highest pressures in each foot were used to calculate right and left ankle/arm ratios. Photoplethysmography was used to obtain systolic pressure from the great toe. Restenosis (loss of primary patency) following the initial revascularization was defined as the presence of > 50% stenosis at the treatment site or by target vessel revascularization. For each vessel segment the highest peak systolic velocity obtained was used as the sample measure. A peak systolic velocity ratio ≥ 2.0 defined as the peak systolic velocity within the lesion segment divided by the peak systolic velocity in the nearest normal segment was used to define 50% or greater stenosis at each interval duplex ultrasound examination.
Outcomes
The primary endpoint was the occurrence of any major adverse cardiovascular events (MACE), defined as death, MI, or stroke during the follow-up period for all patients with CLI who had undergone peripheral angiography. Patients were included in the primary outcome regardless of whether they subsequently underwent endovascular intervention, surgical bypass, or primary amputation.
Secondary endpoints included primary patency (< 50% stenosis at any point during the follow-up period), primary assisted patency (patency of the target vessel regardless of secondary interventions performed to restore blood flow after restenosis), secondary patency (patency of the target vessel regardless of secondary interventions performed to restore blood flow after reocclusion) and not patent status (100% occlusion without revascularization).
Data analysis
Median values with interquartile ranges were used to describe continuous variables, and numerical values (percentages) were used for categorical variables. Univariate analysis was used to identify differences between men and women with CLI. Continuous variables were compared using the Kruskal–Wallis test. Categorical values were compared by the chi-squared or Fisher’s exact tests.
Long-term mortality was analyzed using Kaplan–Meier survival analysis and the log-rank test. A Cox proportional hazard model was developed to explore the relationship between gender and MACE. Several known risk factors for mortality (age, history of coronary artery disease, diabetes,chronic kidney disease) were automatically included in the analysis.In addition, a list of possible confounders was generated using a directed acyclic graph. Confounders from this second group were retained if they were found to be associated with the outcome, using a p-value < 0.1 as a cut-off for inclusion.14 The final variables in the model included age, coronary artery disease, diabetes, chronic kidney disease, and prior coronary artery bypass graft. The proportional hazards assumption was verified using log-log plots. All analyses were performed using STATA Version 11.2 (STATA Corp., College Station, TX, USA). A p-value < 0.05 was considered significant.
Results
Demographics
During the study period, 219 patients (97 women and 122 men) with CLI underwent peripheral artery angiography. Patient characteristics and risk factors are summarized in Table 1. Compared to men, women with CLI were less likely to have a history of diabetes (57% vs 70%, p = 0.05) or coronary artery disease (39% vs 54%, p = 0.02). There were no differences in other demographic characteristics, including tobacco use, heart failure (HF), hypertension, stroke, malignancy, chronic obstructive pulmonary disease (COPD), carotid stenosis, prior amputation, or hemoglobin A1c (HbA1c).
Table 1.
Clinical characteristics of patients with critical limb ischemia
| Variable | Men (n = 122) | Women (n = 97) | p-value |
|---|---|---|---|
| Age, years (IQR) | 66 (58–76) | 66 (58–79) | 0.9 |
| BMI, kg/m2 (IQR) | 27 (23–30) | 26 (22–33) | 0.5 |
| Tobacco, former or current (%) | 83 (68) | 61 (63) | 0.4 |
| CHF (%) | 36 (30) | 25 (26) | 0.6 |
| DM (%) | 86 (70) | 55 (57) | 0.05 |
| GFR, ml/min (IQR) | 56 (35–86) | 48 (24–77) | 0.2 |
| HTN (%) | 106 (87) | 82 (85) | 0.6 |
| CAD (%) | 66 (54) | 38 (39) | 0.02 |
| Stroke/TIA (%) | 31 (25) | 18 (19) | 0.3 |
| Malignancy (%) | 17 (14) | 12 (13) | 0.7 |
| COPD (%) | 12 (10) | 18 (12) | 0.06 |
| AAA (%) | 6 (5) | 3 (3) | 0.4 |
| Carotid stenosis (%) | 11 (9) | 14 (14) | 0.2 |
| Contralateral amputation (%) | 16 (13) | 13 (13) | 0.9 |
| Prior surgical bypass (%) | 10 (8) | 8 (8) | 0.9 |
| Total cholesterol, mg/dl (IQR) | 132 (115–172) | 163 (124–196) | 0.04 |
| LDL, mg/dl (IQR) | 74 (55–102) | 78 (62–102) | 0.4 |
| HDL, mg/dl (IQR) | 32 (25–40) | 41 (31–54) | 0.002 |
| TG, mg/dl (IQR) | 115 (92–161) | 121 (96–173) | 0.6 |
| HbA1c, % (IQR) | 7.6 (6.6–9.4) | 7.5 (6.3–9.1) | 0.9 |
| Statin use (%) | 84 (69) | 60 (62) | 0.3 |
| Hormone use (%) | 1 (1) | 6 (6) | 0.02 |
| ACE inhibitor or angiotensin receptor blocker (%) | 69 (56) | 58 (60) | 0.6 |
| Beta blocker (%) | 69 (56) | 52 (54) | 0.7 |
| Aspirin (%) | 74 (61) | 57 (59) | 0.4 |
| Aspirin use at 30 days (%) | 84 (69) | 73 (75) | 0.7 |
| Clopidogrel (%) | 34 (28) | 21 (22) | 0.3 |
| Clopidogrel use at 30 days (%) | 50 (41) | 46 (47) | 0.3 |
| Rutherford score | 0.2 | ||
| 4% | 12 (10) | 18 (19) | |
| 5% | 97 (80) | 65 (68) | |
| 6% | 12 (10) | 11 (12) | |
| Ankle–brachial index, median (IQR) | 0.51 (0.30–0.74) | 0.40 (0.19–0.60) | 0.07 |
IQR, interquartile range; BMI, body mass index; CHF, congestive heart failure; DM, diabetes mellitus; GFR, glomerular filtration rate; HTN, hypertension; CAD, coronary artery disease; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; AAA, abdominal aortic aneurysm; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; HbA1c, hemoglobin A1c; ACE, angiotensin converting enzyme.
Baseline medical therapy did not significantly differ based on gender, with similar prescription rates of statins, beta-blockers, angiotensin converting enzyme inhibitors, aspirin, and clopidogrel. There also were no significant differences in aspirin or clopidogrel use at 30 days post-procedure. As expected, women were more likely to be taking estrogen hormone therapy (6% vs 1%, p = 0.02). Women with CLI also had a more favorable baseline cholesterol profile, with similar baseline low-density lipoprotein (LDL) (78 vs 74 mg/dl, p = 0.4) and higher baseline high-density lipoprotein (HDL) (41 vs 32 mg/dl, p = 0.002).
Angiographic characteristics and lesion outcomes
Table 2 summarizes the anatomic level of disease as well as lower extremity run-off based on diagnostic angiography. Women were more likely than men to have significant femoropopliteal disease (77% vs 67%, p = 0.02). Women were also more likely to have multi-level infrainguinal disease (63% vs 51%, p = 0.02), defined as significant stenosis in both femoropopliteal and infrapopliteal vessels. There was no significant difference in the presence of aorto-iliac (21% vs 27%, p = 0.6) or infrapopliteal (80% vs 74%, p = 0.6) disease between men and women. Additionally, there was no significant difference in the prevalence of chronic total occlusions between genders.
Table 2.
Angiographic characteristics of patients with critical limb ischemia
| Variable | Men n = 122 | Women n = 97 | p-value |
|---|---|---|---|
| Level of diseasea | |||
| Aorto-iliac (%) | 26 (21) | 26 (27) | 0.6 |
| Femoropopliteal (%) | 82 (67) | 75 (77) | 0.02 |
| Infrapopliteal (%) | 97 (80) | 72 (74) | 0.6 |
| Multi-level infrainguinal stenosisb (%) |
62 (51) | 61 (63) | 0.02 |
|
Chronic total occlusion |
|||
| Aorto-iliac (%) | 13 (11) | 12 (12) | 0.9 |
| Femoropopliteal (%) | 52 (43) | 47 (48) | 0.2 |
| Infrapopliteal (%) | 97 (80) | 72 (74) | 0.6 |
|
Lower extremity run-off |
0.2 | ||
| 0 (%) | 16 (16) | 14 (17) | |
| 1–2 (%) | 74 (74) | 52 (63) | |
| 3 (%) | 10 (10) | 17 (20) |
Level of disease adds up to > 100%, since some patients had stenoses at multiple levels.
Defined as stenosis > 50% in both femoropopliteal and infrapopliteal vessels.
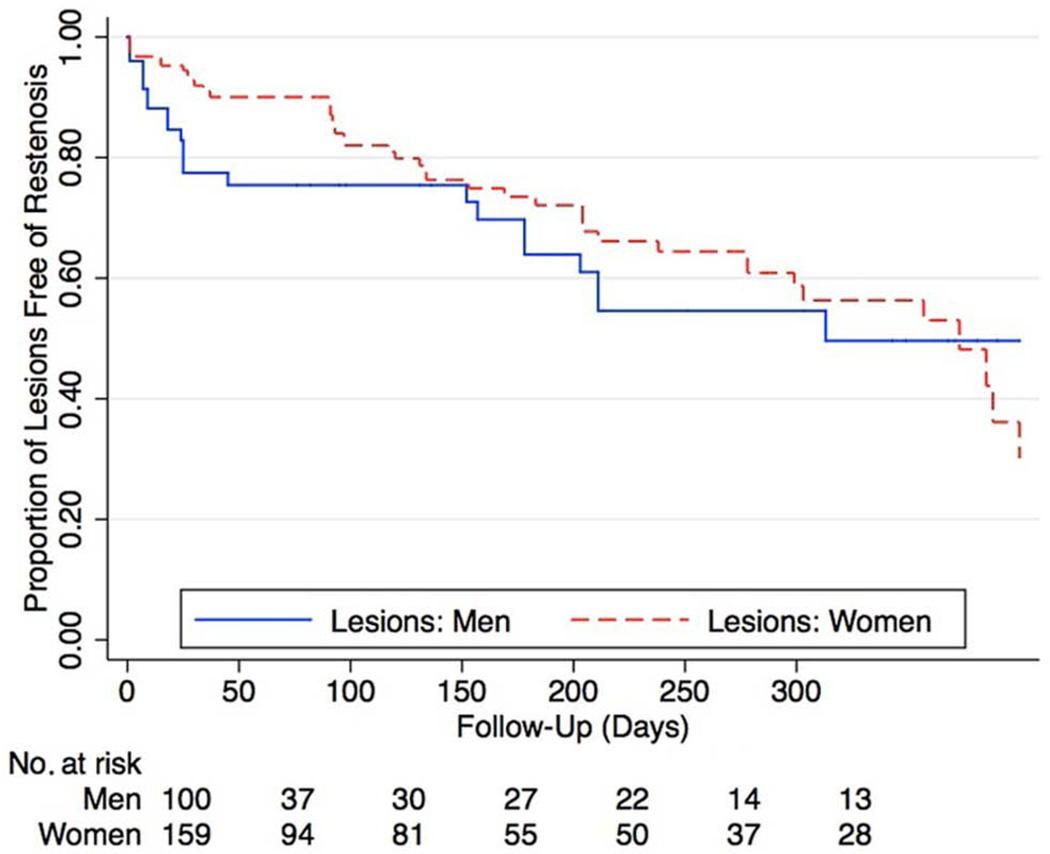
After initial diagnostic angiography, 82 (67%) men and 76 (78%) women underwent percutaneous intervention (p = 0.06). Women were more likely than men to undergo multi-vessel intervention (69% vs 55%, p = 0.05). Table 3 summarizes lesion characteristics among patients who underwent femoropopliteal interventions. When compared to men, women had similar TASC II lesion classification, prevalence of chronic occlusions, and overall lesion length, but were less likely to have severe calcification (9% vs 28%, p = 0.001). Vessel diameter was also smaller in women (4.9 ± 0.9 mm vs 5.5 ± 1.0 mm, p < 0.0001). The initial procedural success rate was higher for women than men (99% vs 91%, p = 0.001). Rates of stent placement were similar between women and men (48% vs 41%, p = 0.3). There were no significant differences in patency on evaluation of post-procedure outcomes at 1 year for primary patency, primary assisted patency or secondary patency (Table 3). This is also demonstrated in Figure 1, showing no gender-related difference in restenosis rates of femoropopliteal lesions over time (hazard ratio (HR) 0.76 [95% CI 0.46–1.26], p = 0.3 for women).
Table 3.
Femoropopliteal lesion characteristics among patients undergoing percutaneous interventions
| Men | Women | p-value | |
|---|---|---|---|
| TASC II Classification | 0.2 | ||
| A (%) | 15 (16) | 16 (12) | |
| B–C (%) | 57 (63) | 82 (59) | |
| D (%) | 19 (21) | 41 (30) | |
| Chronic total occlusions (%) |
41 (42) | 59 (37) | 0.4 |
| Lesion calcification | 0.001 | ||
| None (%) | 9 (9) | 40 (25) | |
| Mild–moderate (%) | 55 (55) | 104 (66) | |
| Severe (%) | 28 (28) | 14 (9) | |
| Thrombus (%) | 2 (2) | 11 (7) | 0.09 |
| Lesion length, mm (IQR) | 80 (40–120) | 80 (40–100) | 0.7 |
| Maximal balloon diameter, mm (IQR) |
6 (5–6) | 5 (4–6) | < 0.001 |
| Stent placed (%) | 41 (41) | 76 (48) | 0.3 |
| Procedural success (%) | 90 (91) | 158 (99) | 0.001 |
| Post-procedure outcomes at 1 year |
|||
| Primary patency | 49% | 53% | 0.3 |
| Primary assisted patency | 55% | 56% | 0.3 |
| Secondary patency | 63% | 65% | 0.3 |
Results are described per lesion. Interventions were performed on 100 lesions in 82 men and 159 lesions in 76 women.
TASC, TransAtlantic Inter-Society Consensus; IQR, interquartile range.
Figure 1.
Kaplan–Meier curves of femoropopliteal lesion patency in patients with CLI. There were no gender-based differences in the primary patency of femoropopliteal lesions (hazard ratio 0.76 [95% CI 0.46–1.26], p = 0.3).
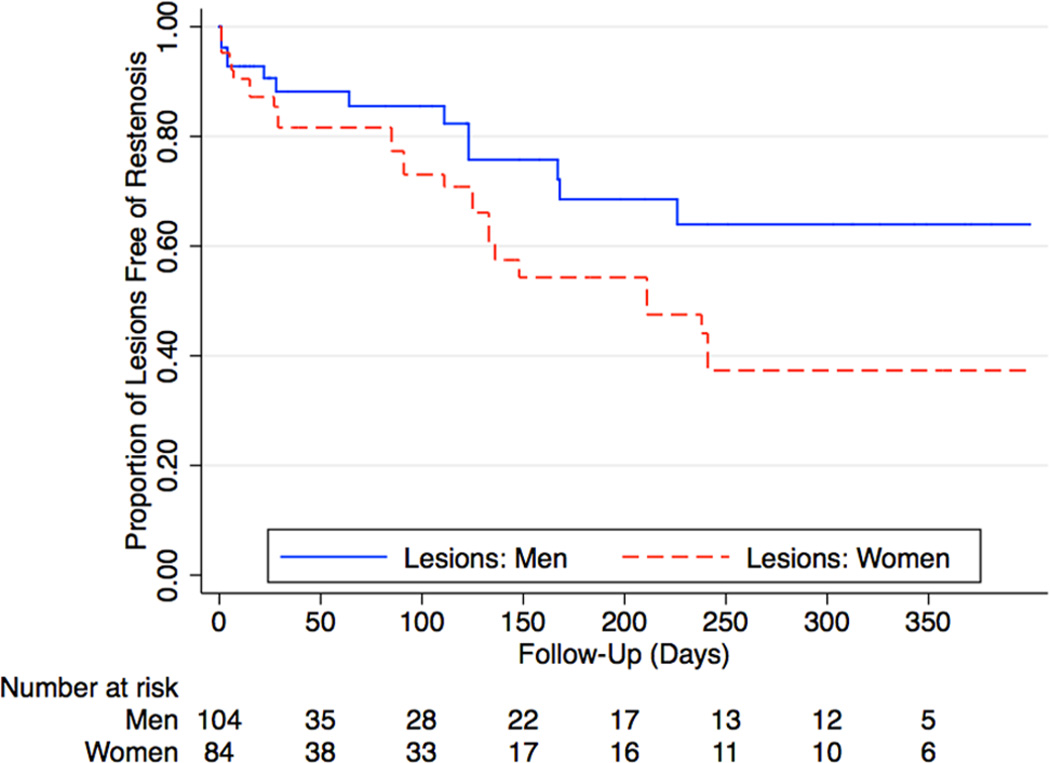
Table 4 summarizes lesion characteristics among patients who underwent infrapopliteal interventions. When compared to men, women had similar lesion lengths, calcification, prevalence of chronic total occlusion, and vessel diameter. Overall procedural success was also similar (89% vs 93%, p = 0.3). Women demonstrated worse post-procedural outcomes than men with significantly lower rates of primary patency and primary assisted patency (Table 4). This is also reflected in Figure 2, with women demonstrating a higher risk for subsequent restenosis of the target vessel. The hazard ratio for restenosis among women undergoing infrapopliteal interventions was 1.9 (95% CI 1.10–3.60, p = 0.04).
Table 4.
Infrapopliteal lesion characteristics among patients undergoing percutaneous interventions
| Variable | Men | Women | p-value |
|---|---|---|---|
| Chronic total occlusions (%) |
45 (43) | 42 (52) | 0.3 |
| Lesion calcification | 0.3 | ||
| None(%) | 63 (61) | 38 (48) | |
| Mild–moderate(%) | 32 (31) | 36 (45) | |
| Severe (%) | 8 (8) | 6 (8) | |
| Thrombus(%) | 5 (5) | 1 (1) | 0.2 |
| Lesion lengths, mm (IQR) | 75 (30–120) | 50 (30–120) | 0.5 |
| Maximal balloon diameter, mm (IQR) | 2.5 (2.5–30) | 2.5 (2.5–30) | 0.7 |
| Stent placed (%) | 4 (4) | 5 (6) | 0.5 |
| Procedural success (%) | 97 (93) | 74 (89) | 0.3 |
| Post-procedure outcomes at 1 year | |||
| Primary patency | 64% | 37% | 0.2 |
| Primary assisted patency |
64% | 37% | 0.2 |
| Secondary patency | 67% | 41% | 0.08 |
Results are described per lesion. Interventions were performed on 104 lesions in 82 men and 84 lesions in 76 women.
Figure 2.
Kaplan–Meier curves of infrapopliteal lesion patency in patients with CLI. There were gender-based differences in the primary patency of infrapopliteal lesions (hazard ratio 1.9 [95% CI 1.1–3.6], p = 0.04).
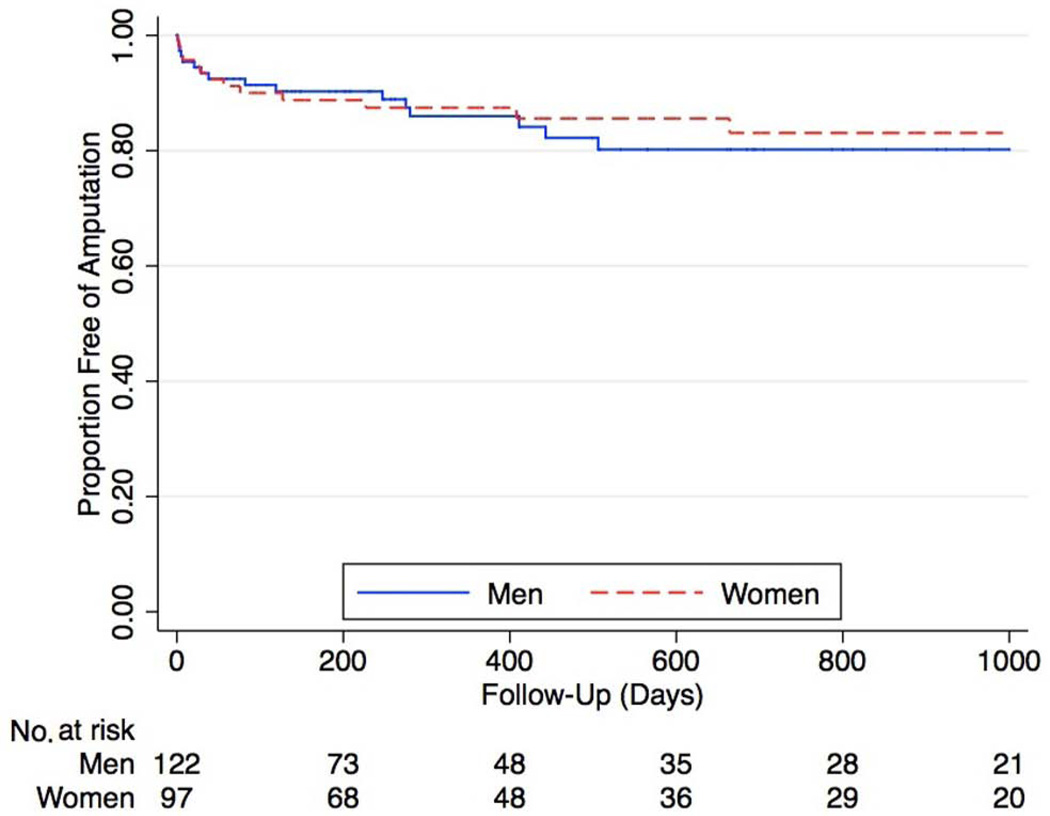
Among the entire cohort of 219 patients with CLI who underwent peripheral angiography, 44 patients (20%) required surgical bypass for limb salvage. Among this group, 20 were women (23% of women) and 24 were men (20% of men, p = 0.9). Despite the fact that women were more likely to have restenosis following infrapopliteal interventions, overall limb salvage was similar in both genders (Figure 3), with no difference in amputation rates over time (HR 0.94 for women [95% CI 0.45–1.94], p = 0.9).
Figure 3.
Kaplan–Meier curves of overall limb salvage in patients with CLI. There was no difference in amputation rates over time (hazard ratio 0.94 [95% CI 0.45–1.94], p = 0.9).
In the overall cohort, 29 patients (16 men and 13 women) eventually required amputation above the level of the ankle joint. Among the 16 men who required amputation, 10 had attempted endovascular intervention, one had surgical bypass first, and five had primary amputation after diagnostic angiography. Among the 13 women who required amputation, eight had attempted endovascular intervention, two had surgical bypass first, and three had primary amputation after diagnostic angiography.
Long-term cardiovascular outcomes
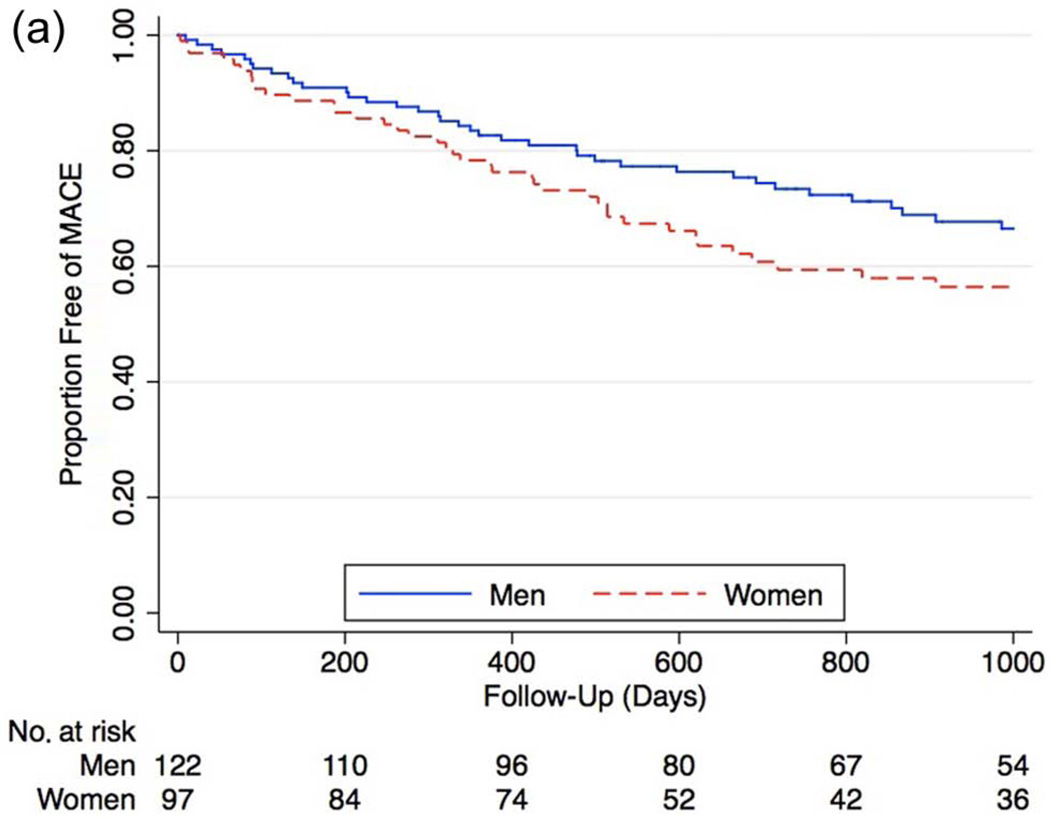
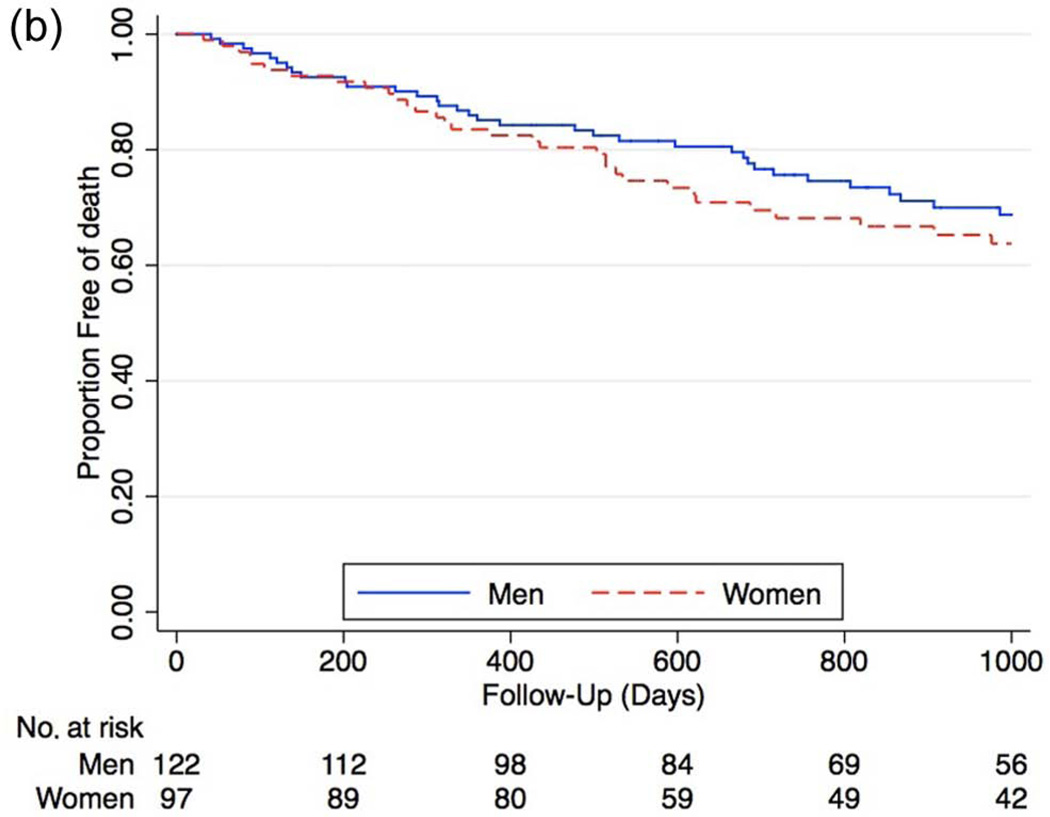
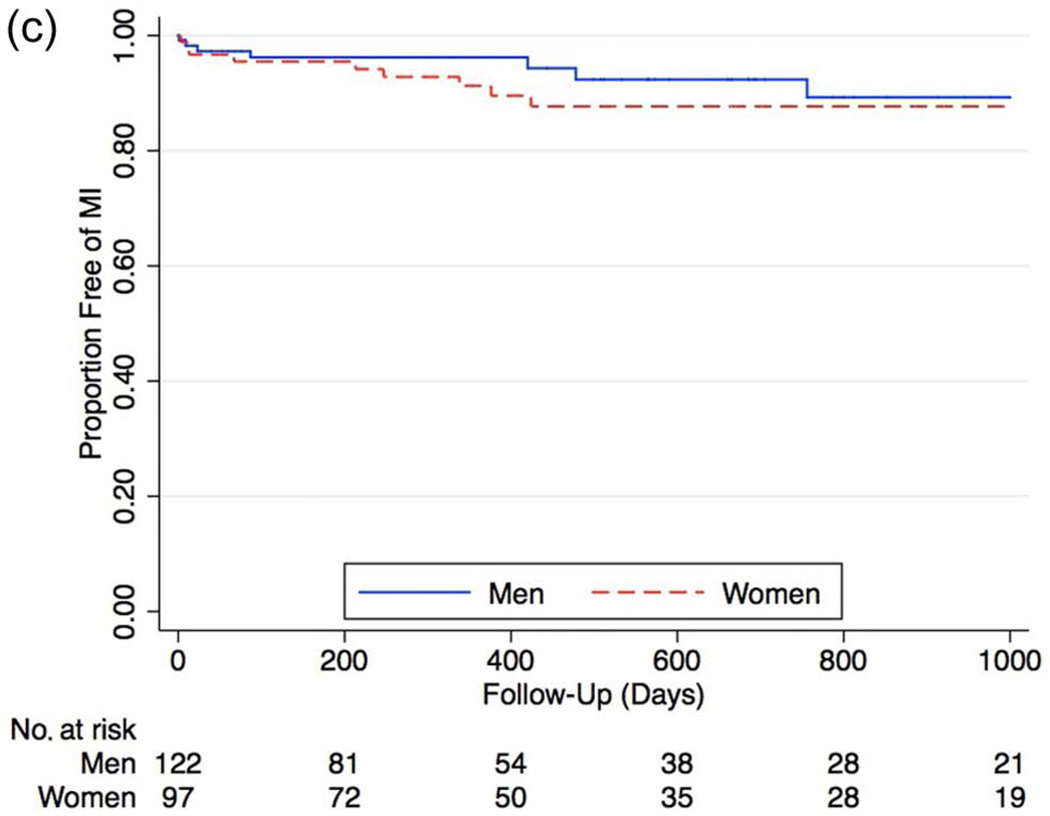
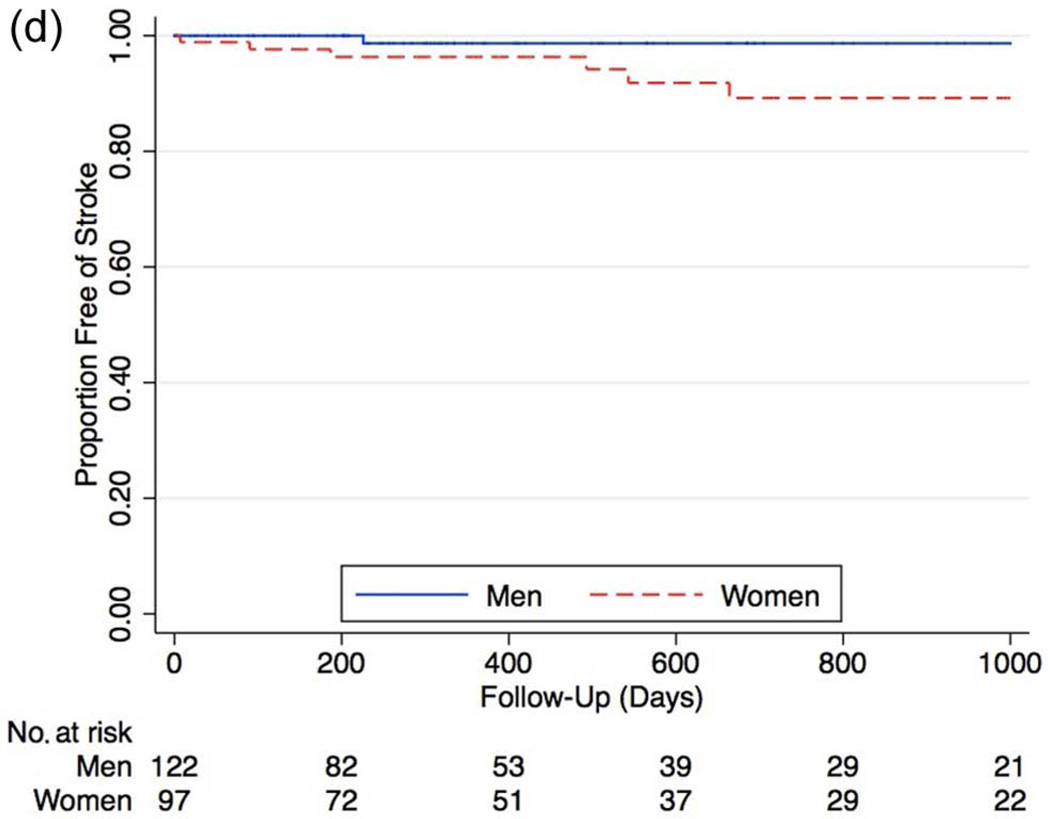
During long-term follow-up, the rate of MACE was higher in females (Figure 4a), with a hazard ratio of 1.63 compared to men (95% CI 1.01–2.63, p = 0.04). After Cox proportional hazard modeling, female gender remained associated with significantly higher rates of subsequent MACE (HR 1.81 [95% CI 1.08–3.05], p = 0.02). Analysis of the individual components of MACE showed increased, though not statistically significant overall rates of all major outcomes, including increased mortality (HR 1.24 [95% CI 0.77–2.01], p = 0.3), increased MI (HR 1.66 [95% CI 0.60– 4.69], p = 0.3), and stroke (HR 6.66 [95% CI 0.88–55], p = 0.07) among women (Figures 4b–d).
Figure 4.
a. Kaplan–Meier curve for proportion of men and women free of major adverse cardiovascular events (MACE) after percutaneous intervention (myocardial infarction, stroke, death) (hazard ratio 1.63 [95% CI 1.01–2.63], p = 0.04). After Cox proportional hazard modeling, female gender remained associated with significantly higher rates of subsequent major adverse cardiovascular events (hazard ratio 1.81 [95% CI 1.08–3.05], p = 0.02).
b. Kaplan–Meier curves of overall mortality between genders in all patients who received angiography (HR 1.24 [95% CI 0.77–2.01], p = 0.3).
c. Kaplan–Meier curves of myocardial infarction between genders in all patients who received angiography (HR 1.66 [95% CI 0.60–4.69], p = 0.3).
d. Kaplan–Meier curves of stroke rates between genders in all patients who received angiography (HR 6.66 [95% CI 0.88–55], p = 0.07).
Discussion
Women with PAD represent a growing population of patients.7 Prior studies, primarily based on surgical literature, have demonstrated that women often present later in the disease process with more advanced disease and have lower rates of bypass graft patency.9–13 Because patients are increasingly treated with less invasive endovascular procedures, these data may not represent the current outcomes of all patients with CLI. This contemporary cohort of patients with CLI has three major findings. First, we found that, compared to men, women with CLI had a higher composite rate of major adverse cardiovascular events. Second, women were more likely to have femoropopliteal disease as well as multi-level infrainguinal disease. Third, women were more likely to undergo multi-level infrainguinal interventions and develop infrapopliteal restenosis after endovascular intervention, yet major amputation rates were similar between women and men.
In our cohort, female gender was associated with significantly increased rates of subsequent MACE. To our knowledge, this is the first study to evaluate the influence of gender in CLI on MACE as a whole and utilizing individual components of adverse outcomes.7 Prior work has demonstrated that patients with PAD have a significantly increased risk of cardiovascular events when compared to the overall population.15 Evaluations of gender differences in patients with PAD have suggested that women have a higher risk of mortality, particularly when they have a history of cerebrovascular or coronary disease.9 Historically, in studies of surgical graft bypass outcomes, women have been found to have either equivalent or higher mortality rates when compared to men.2,9,13 Our findings are particularly compelling because at the time of angiography women had a lower prevalence of known CAD and a higher baseline HDL than men. There are several potential explanations for our finding of increased MACE in women. First, this could be due to the continued lack of recognition of CAD in women, whether due to bias in diagnosis or differences in presentation compared with men. Other studies of PAD and CLI have also reported a significantly lower prevalence of CAD in women.16,17 Second, women with PAD and CLI may have more extensive overall atherosclerotic burden, thereby raising their rates of subsequent stroke and mortality. Third, the differences in observed outcomes may be attributable primarily to female gender. Possible etiologies could include hormonal differences between men and women and differences in underlying disease etiology, as has been hypothesized in CAD.18–21 Regardless, these findings are clinically important and emphasize the need for increased secondary risk factor modification and identification of gender-based variation in disease among women with CLI.
Compared to prior studies of patients with both PAD and CLI, this cohort of patients with CLI demonstrated some notable differences. Prior studies have included an older overall population, with women on average being older than men with CLI.8,13,16,21,22 Similar to prior studies, women in our cohort were less likely to have a history of diabetes or coronary artery disease than men.12,16,17 While this finding could represent under-diagnosis or under-treatment of comorbidities, women had similar rates of statin, antiplatelet, and angiotensin converting enzyme (ACE) inhibitor treatment, suggesting that differential prescription of these medications did not affect outcomes.23 We were also able to quantify the number of patients who were actively receiving hormone replacement therapy at the time of intervention. Though there is much speculation about the impact of hormone replacement therapy on the development of PAD and CLI, the clinical impact of hormone replacement therapy in this study was likely small, given the low overall prescription rates (6% among women).24–26
Recent studies have provided conflicting data on the impact of gender on lesion characteristics among patients with PAD. Similar to our findings, a recent report from Europe found that women with CLI are more likely to have both diffuse disease and femoropopliteal lesions.21 Two other international studies found that men with CLI were more likely to have iliac lesions, and that women with CLI had a higher prevalence of infrapopliteal disease.22,27 Other studies have not found gender-related differences in lesion characteristics, but these other studies included a heterogeneous group of patients with both stable claudication and CLI. By focusing on patients only with CLI, we were better able to distinguish differences in presentation as a function of gender.
When we focused on femoropopliteal lesions and their characteristics, we found that though there were no differences in the TASC II classification, women demonstrated less severe lesion calcification. For infrapopliteal disease we did not find any gender-based differences in baseline lesion characteristics, but women had increased rates of restenosis following intervention. Of interest, increased rates of restenosis following infrapopliteal interventions in women were not associated with an increased risk of limb loss. This finding is consistent with previous studies of endovascular interventions for CLI which demonstrated that restenosis is not directly linked to an increased risk of amputation.28
The patient population in this study had lower than expected long-term follow-up due to a lack of repeat duplex ultrasound monitoring. However, there was no differential in the follow-up pattern and therefore we do not feel that this created a systematic bias in the results. Further prospective studies should be performed to confirm our findings.
This study has several potential limitations. First, this was a retrospective analysis. This study also represents the experience of a single academic institution and therefore may not be generalizable to all clinical settings. Patients in the study were identified as having CLI based on physical exam and patient-reported medical history, which may be subject to recall bias. ABI was not used to determine the classification of patients given that not all patients were able to receive ABI prior to angiography and the technical limitations of interpreting ABI values in a patient population with a high percentage of medial calcinosis and non-compressible arteries. However, > 80% of the patients described as having CLI in this study had evidence of non-healing lower-extremity ulceration or gangrene on exam, which categorizes them as Rutherford class 5 or greater. Additionally, it is possible that due to differences in presentations women may present later in the disease process than men and thus have more advanced disease. While we did not identify any gender-specific patterns of medication prescriptions, the overall use of medications proven to reduce MACE among patients with PAD, including aspirin, clopidogrel, ACE inhibitors and statins, remained low. These findings emphasize the need to improve physician treatment of cardiovascular risk factors among patients with advanced PAD. Finally, the patient population included in this study was CLI patients who underwent angiography prior to planned revascularization. While this included the great majority of CLI patients evaluated at our institution, the results of this study may not be generalizable to all patients with CLI.
Conclusion
Women with critical limb ischemia are at increased risk of cardiovascular morbidity and mortality and present more frequently with femoropopliteal and multi-level infrainguinal disease compared with men. As the population of women with advanced PAD continues to increase, it will be critical to address these gender-specific issues with aggressive medical and interventional therapies.
Supplementary Material
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material to this paper can be found at vmj.sagepub.com
Author contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
John Laird is a consultant/board member for Boston Scientific, Medtronic, Covidien, Abbott Vascular, and Bard Peripheral Vascular. All other authors report no conflicts of interest related to this study.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 3.Diehm N, Silvestro A, Baumgartner I, et al. Chronic critical limb ischemia: European experiences. J Cardiovasc Surg (Torino) 2009;50:647–653. [PubMed] [Google Scholar]
- 4.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3 suppl):S62–S72. doi: 10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Forster A, Gass A, Kern R, et al. Gender differences in acute ischemic stroke: Etiology, stroke patterns and response to thrombolysis. Stroke. 2009;40:2428–2432. doi: 10.1161/STROKEAHA.109.548750. [DOI] [PubMed] [Google Scholar]
- 6.Lawton JS. Sex and gender differences in coronary artery disease. Semin Thorac Cardiovasc Surg. 2011;23:126–130. doi: 10.1053/j.semtcvs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch AT, Allison MA, Gomes AS, et al. A Call to Action: Women and peripheral artery disease: A scientific statement from the American Heart Association. Circulation. 2012;125:1449–1472. doi: 10.1161/CIR.0b013e31824c39ba. [DOI] [PubMed] [Google Scholar]
- 8.Ortmann J, Nuesch E, Cajori G, et al. Benefit of immediate revascularization in women with critical limb ischemia in an intention-to-treat analysis. J Vasc Surg. 2011;54:1668–1678. doi: 10.1016/j.jvs.2011.06.110. [DOI] [PubMed] [Google Scholar]
- 9.Egorova N, Vouyouka AG, Quin J, et al. Analysis of gender-related differences in lower extremity peripheral arterial disease. J Vasc Surg. 2010;51:372–378. doi: 10.1016/j.jvs.2009.09.006. e1; discussion 378–379. [DOI] [PubMed] [Google Scholar]
- 10.Pulli R, Dorigo W, Pratesi G, et al. Gender-related outcomes in the endovascular treatment of infrainguinal arterial obstructive disease. J Vasc Surg. 2012;55:105–112. doi: 10.1016/j.jvs.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Eugster T, Gurke L, Obeid T, Stierli P. Infrainguinal arterial reconstruction: Female gender as risk factor for outcome. Eur J Vasc Endovasc Surg. 2002;24:245–248. doi: 10.1053/ejvs.2002.1712. [DOI] [PubMed] [Google Scholar]
- 12.Vouyouka AG, Egorova NN, Salloum A, et al. Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. J Vasc Surg. 2010;52:1196–1202. doi: 10.1016/j.jvs.2010.05.106. [DOI] [PubMed] [Google Scholar]
- 13.Magnant JG, Cronenwett JL, Walsh DB, et al. Surgical treatment of infrainguinal arterial occlusive disease in women. J Vasc Surg. 1993;17:67–76. discussion 76–78. [PubMed] [Google Scholar]
- 14.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: Directed acyclic graphs and the changein-estimate procedure. Am J Epidemiol. 2009;169:1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 15.Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: Long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi: 10.1186/1471-2261-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher KA, Meltzer AJ, Ravin RA, et al. Gender differences in outcomes of endovascular treatment of infrainguinal peripheral artery disease. Vasc Endovascular Surg. 2011;45:703–711. doi: 10.1177/1538574411418008. [DOI] [PubMed] [Google Scholar]
- 17.DeRubertis BG, Vouyouka A, Rhee SJ, et al. Percutaneous intervention for infrainguinal occlusive disease in women: Equivalent outcomes despite increased severity of disease compared with men. J Vasc Surg. 2008;48:150–157. doi: 10.1016/j.jvs.2008.03.007. discussion 157–158. [DOI] [PubMed] [Google Scholar]
- 18.Gardner AW, Montgomery PS, Blevins SM, Parker DE. Gender and ethnic differences in arterial compliance in patients with intermittent claudication. J Vasc Surg. 2010;51:610–615. doi: 10.1016/j.jvs.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nugent L, Mehta PK, Bairey Merz CN. Gender and microvascular angina. J Thromb Thrombolysis. 2011;31:37–46. doi: 10.1007/s11239-010-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassalle C, Sciarrino R, Bianchi S, et al. Sex-related differences in association of oxidative stress status with coronary artery disease. Fertil Steril. 2012;97:414–419. doi: 10.1016/j.fertnstert.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 21.Ortmann J, Nuesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012;55:98–104. doi: 10.1016/j.jvs.2011.07.074. [DOI] [PubMed] [Google Scholar]
- 22.Kumakura H, Kanai H, Araki Y, et al. Sex-related differences in Japanese patients with peripheral arterial disease. Atherosclerosis. 2011;219:846–850. doi: 10.1016/j.atherosclerosis.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Aiello FA, Khan AA, Meltzer AJ, et al. Statin therapy is associated with superior clinical outcomes after endovascular treatment of critical limb ischemia. J Vasc Surg. 2012;55:371–379. doi: 10.1016/j.jvs.2011.08.044. discussion 380. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Higgins JA. Epidemiology of peripheral arterial disease in women. J Epidemiol. 2003;13:1–14. doi: 10.2188/jea.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockman CB, Maldonado TS, Jacobowitz GR, Adelman MA, Riles TS. Hormone replacement therapy is associated with a decreased prevalence of peripheral arterial disease in postmenopausal women. Ann Vasc Surg. 2012;26:411–418. doi: 10.1016/j.avsg.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Davies RS, Vohra RK, Bradbury AW, Adam DJ. The impact of hormone replacement therapy on the pathophysiology of peripheral arterial disease. Eur J Vasc Endovasc Surg. 2007;34:569–575. doi: 10.1016/j.ejvs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Diehm N, Shang A, Silvestro A, et al. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg. 2006;31:59–63. doi: 10.1016/j.ejvs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Romiti M, Albers M, Brochado-Neto FC, et al. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–981. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.