Abstract
The parahippocampal cortex (PHC) has been associated with many cognitive processes, including visuospatial processing and episodic memory. To characterize the role of PHC in cognition a framework is required that unifies these disparate processes. An overarching account was proposed, whereby the PHC is part of a network of brain regions that processes contextual associations. Contextual associations are the principal element underlying many higher-level cognitive processes, and thus are suitable for unifying the PHC literature. Recent findings are reviewed that provide support for the contextual associations account of PHC function. In addition to reconciling a vast breadth of literature, the synthesis presented expands the implications of the proposed account and gives rise to new and general questions about context and cognition.
Keywords: Parahippocampal cortex, contextual processing, associations, episodic memory, spatial processing, scenes
Converging towards a more inclusive view of PHC function
The parahippocampal cortex (PHC) encompasses a large portion of the medial temporal lobe. It is located at the junction between brain regions described as essential to memory formation (e.g., the hippocampus) and high level visual processing (e.g., the fusiform cortex). A significant body of research has provided evidence for a number of different processes related to the signal elicited from the PHC with diverse classes of stimuli, tasks, and environments. Thus, efforts to define a single function for the PHC have been challenging because, like other higher-order cortical regions, it seems to be involved in many different functions and act in concert with many other regions to accomplish those functions. The purpose of this review is to connect the dots across the diverse findings to uncover the overarching process that relies on the PHC and mediates all those functions.
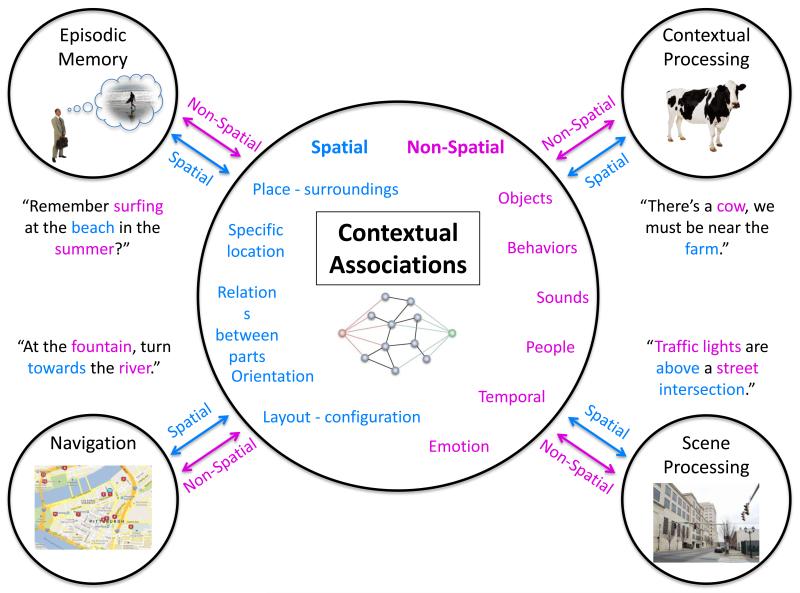
Contextual associations (see Glossary) are proposed to be the building blocks that sit at the foundation of the various functions that have previously been attributed to the PHC (see Figure 1). The analysis and synthesis of the diverse literature presented here is aimed to consolidate our understanding of the PHC and its role in cognition. This proposal advances from previous accounts [1-3] by including many recent studies, and thus represents a broad synthesis of the literature. The contextual processing account integrates differing accounts of the PHC function and provides a basis for generating novel hypotheses and directions for future research.
Figure 1.
Contextual associations can be seen as the buildings blocks for many of the cognitions attributed to the function of the PHC.
PHC anatomy and connectivity
To fully understand the role of a brain region it is necessary to examine not only its functional activity but also its anatomy, because the region’s location and connections can provide valuable cues about its function. The PHC is quite distinct anatomically within the medial temporal lobe (MTL), easily distinguished from other MTL regions such as the perirhinal, entorhinal, and hippocampal cortices. The perirhinal and entorhinal cortices provide the rostral border for the PHC [4,5] with the caudal border in the human brain marked by the first coronal slice in which the calcarine sulcus is visible [6]. Although anatomical comparisons between animals should be made with caution, the PHC has been related to the TH and TF regions in the monkey and postrhinal cortices in the rat [4,7]. Anatomical delineations in the macaque monkey suggest the PHC comprises the medial region TH and the more lateral region TF, both regions thought to be involved in spatial processing and memory [8]. Region TF may be subdivided into a more medial region referred to as either TFm [4] or as an intermediate zone between TF and TH, as TL [9]. And the medial TH region may also be broken down even further in a rostral subregion (THr) and a caudal subregion (THc) [10]. Clarifying the homologues of these anatomical segmentations within the human cortex may provide critical insight into the diversity of results regarding the function of the PHC. Some work in this vein has already begun (e.g., [11]), which we discuss later in this review.
The PHC is part of a large network that connects regions of the temporal, parietal, and frontal cortices. It is connected with unimodal cortex, such as V4 and limited regions of the TE and TEO (of the monkey brain, which are involved in object recognition; [8]), auditory association areas of the superior temporal gyrus (STG). There are also prominent connections with polymodal association areas that include the retrosplenial cortex, lateral regions of the inferior parietal lobule, and the dorsal bank of the superior temporal sulcus (STS) [4]. The PHC has reciprocal connections within the medial temporal lobe. This includes projections with the temporal pole, perirhinal cortex, parahippocampal cortex itself (i.e., TF projects to TH). It also provides a major source of input to the entorhinal cortex, which feeds directly into the hippocampus. There are also direct connections with the hippocampus itself in CA1 and presubiculum, and the amygdala [9]. The PHC is also highly connected with the frontal cortex, including with the medial prefrontal cortex, dorsolateral prefrontal cortex, and orbitofrontal cortex, as well as with the insula [4]. There is some evidence from a human neuroimaging study examining functional connectivity of differences between anterior and posterior regions of the PHC that the anterior regions are more functionally connected with RSC and parietal cortices, whereas the posterior regions are more functionally connected with visual processing regions [12]. This will become more relevant as the proposed functional distinction between posterior and anterior PHC is discussed below.
Given the highly interconnected nature of the PHC, both with unimodal and polymodal cortices, it is not surprising that it seems to be involved in a variety of different cognitive functions, as we review next.
Functional characterizations of the PHC
The PHC has been studied extensively, and has been ascribed many functions. Among those, two prominent groups of research and claims have emerged: episodic memory and visuospatial processing. Specifically, the PHC has been reported to be involved in episodic memory relating to associative memory, source memory, and recollection [13-15], and to visuospatial processing relating to scene perception [16-18], spatial representation [19-21], and navigation [22,23]. But beyond those two dominant accounts, the diversity of findings in the PHC extends to the processing of emotional stimuli [24-27], low-level visual processes such as distinguishing center-periphery of the visual field [28], and even selective processing of high spatial frequencies [29] (Box 1). Although the PHC is commonly thought of as responding to visual stimuli, it has also been found to elicit activity for auditory stimuli [25,26,30,31], and to respond to odor stimuli [32-34].
Box 1: Additional roles attributed to the PHC.
Emotion processing
Emotional processing (e.g., in pictures, music) has been linked to the PHC, both in modulation of response and in impaired behavior when it is damaged [24-27]. Emotion can provide strong contextual cues (e.g., music leading to a scary scene in a movie). However, the converse is also true, emotional inferences often require understanding of context, which include anything from facial, bodily and vocal cues, to visual and aural tone of a scene, to music. For example, the expression of anger and fear are often very similar, and often the affective expression can be deciphered only by context [96]. We propose that the PHC, in conjunction with the RSC and MPFC, and regions implicated in affective responses (e.g., amygdala and hippocampus) mediate this strong connection between contextual processing and emotion, facilitating emotion understanding, and expectations of our environment.
Center-periphery organization
Levy and colleagues [28] discuss PHC function within a framework characterizing the ventral visual stream at large. They suggest the ventral stream is organized along a center-periphery gradient as an extension of the retinal eccentricity of early visual areas. The fusiform cortex processes items that fall within the center of our visual field, and thus are specialized for the perception of single objects and faces. In contrast, more medial regions such as the PHC, process objects that are typically found in the periphery of the visual field, such as buildings and full scenes. As a preliminary hypothesis, one can interpret this effect as important for contextual processing: context comprises regularities that occur in the entire field of view rather than just in the center of our visual field.
High spatial frequencies and expertise
Rajimehr et al. [29] found the PHC more sensitive to high spatial frequencies (HSFs) than to low spatial frequencies (LSFs). Our interpretation of these findings is as follows. The PHC has substantial projections from layers in V1 that receive parvocellular (P) input from the LGN (see Box 2). Because of these P-dominant projections, the PHC can resolve HSFs, such as sharp edges and boundaries that are typically present in images of indoor scenes. More generally, it may be sensitive to detailed information beyond what is carried in low-level visual differences. The PHC has been reported to be activated by details from high-level stimuli, such as emotional scenes, expert chess boards, contextual words, and episodic memory, which is detailed by definition. For example, chess experts recruited the PHC when looking at boards with plausible chess positions, compared with boards where pieces were placed randomly [97]. In corroboration, expert archers recruited more PHC when watching a video of Western-style archery compared with novices [98]. As expertise develops, a network of associations of the given topic is developed, which would automatically engage contextual processing in the PHC [99]. Thus, the PHC function may be to analyze ‘contextual details’ or ‘contextual specifics’, which are then compared to a ‘context frame’ in the other key region in this network, the retrosplenial complex.
How could the PHC be involved in so many different functions? The account that we offer to reconcile these findings is that the PHC can more comprehensively be described as mediating contextual associations. According to this account, the main tasks and stimuli that elicit PHC activation do so because they engage contextual associative processing in one way or another (Figure 1). Before elaborating on why contextual associative processing may be the basic element that bridges the prominent functions attributed to the PHC, we first describe each of those accounts in more detail.
Visuospatial processing in the PHC
The PHC is highly engaged by tasks involving spatial information about the environment. For example, the fMRI BOLD response in the PHC has been correlated with viewing pictures of scenes and landmarks, using spatial maps, and remembering locations of objects [16,22,23,35,36]. Strong activations in the PHC in response to scene stimuli led to naming the posterior region of the PHC the ‘parahippocampal place area’ (PPA) in an early study of neural correlates of place perception [16]. Further evidence was provided by studies of patients with damage to the PHC, which impaired their visuospatial processing. These patients were impaired in landmark identification, spatial orientation, navigation, and spatial memory [37,38]. Finally, there have been a number of studies showing a direct link between the posterior parahippocampal regions and spatial processing in both rodents and in monkeys, where damage to this region selectively impairs visuospatial processing [39,40].
However, what the PHC is actually computing in visuospatial processing is still unclear. Epstein and colleagues [41] put forth the spatial layout hypothesis according to which the PHC processes the surface geometry of a stimulus. In this hypothesis, the function of the PHC is to process the geometrical spatial layout of a scene (such as walls and floors), independent of individual elements in the scene (e.g., furniture) and any influence of experience, memory, or semantics. Alternatively, Mullally and Maguire [19] proposed a spatial defining hypothesis that suggests that the PHC is sensitive to the experience of ‘basic 3D space’. In this view, single objects can evoke a sense of 3D space devoid of other objects, spatial layout, or any contextual elements, and that this ‘sense of space’ is the optimal stimulus for the posterior PHC. Others have suggested that the PHC processes the expanse of space [20,21]: for example, resolving that a mountain range is an open expanse, whereas a cave is a closed expanse. These related theories are intriguing, although they target only what the PHC may be processing, but not how or why it is processing this information. Furthermore, none of these accounts attempts to reconcile spatial processing, episodic memory, and the other functional claims within the same PHC.
Other theories posit that the PHC extracts from scenes useful information for way-finding and spatial orientation. Aguirre and colleagues [22] investigated navigation by using a virtual environment and found that topographical learning engaged the PHC. Similarly, Mellet and colleagues [36] found that mental navigation tasks recruit PHC processing. Location information is not only important for way-finding at large, but can be related to specific sub-processes of navigation. For example, spatial memory, typically defined as remembering the location of an object, can elicit strong fMRI signal in the PHC [42,43]. A causal relation between spatial memory and the PHC has been demonstrated in patients with lesions in the PHC, who have severely impaired spatial memory (e.g., [38]). A similar finding was also found in monkeys who could not learn object-place associations after parahippocampal lesions [40], and rats with postrhinal lesions [39]. Linking spatial memory with navigation, Janzen et al. [23] demonstrated that objects used as salient markers (or association cues) for a navigational decision (e.g., turn right at the vase) were enough to elicit PHC activation.
Taken together, there are many different paradigms that have found a direct relationship between scene and spatial processing and PHC activity. However, the specifics and convergence about what and how it is processed are lacking. Although the theories listed above have used the existing data rigorously and thus provide a promising start, it seems that a theory with more explanatory power is needed. In particular, it is important to develop a theory that can accommodate the seemingly conflicting diversity of findings, and explain, for example, why spatial processing and episodic memory both recruit the PHC.
PHC and episodic memory
A large body of literature has explained PHC activity as dedicated to the encoding and retrieval of episodic memory. It is relevant to keep in mind that an episode is associative in nature, linking objects, relations, places, sounds, and more, in a single compounded construct. Indeed, associative memory, the memory that links different items together (e.g., face-name), rather than memory for a single item, is found to activate the PHC [14,39,44-49]. Thus, the PHC is not involved in just any type of episodic memory, but memory-related processing that involves associations between elements.
In some cases, the PHC is involved in binding a target item with the surrounding context, compared with just remembering the target alone [14,50,51]. This context can be in the form of other items presented with the target item, background scene information, or a particular frame of mind (e.g., a task being done at that time). Such contextual information may provide “source” memory details (i.e., details about the specific episode; [14,15,45,52]). It has been proposed that the PHC provides to the hippocampus the contextual information about the where and when of a target item for memory encoding [53,54].
Associative memory and the PHC function have also been examined in lesion studies of animals and human patients. These studies demonstrate that the PHC is critical for associative memory, and if damaged, can lead to significant impairments [13,38-40,55]. Together, these studies show a clear and widely accepted involvement of the PHC in associations and context from diverse domains.
If the PHC’s function could be explained exclusively as spatial processing, then PHC activations in those episodic memory studies should be observed only when the episodic material included spatial information. Indeed, some studies of episodic memory that have reported selective PHC activations included processes related to spatial and scene memories (e.g., [52,56,57]), but not all episodic memory studies could be tied to spatial information. For example, in a seminal paper by Wagner and colleagues [58], PHC activity was related to the encoding of words, half of which were concrete words and, critically, the other half were abstract words. Henke and colleagues [47] found that the PHC was involved in the memory of associated pairs of abstract nouns (e.g., interest, rule). In addition, Hales et al. [48] demonstrated the PHC was involved in encoding pairs of temporally associated objects, rather than just single objects, and thus using a paradigm that didn’t involve the spatial domain. Moreover, Alvarez et al. [34] demonstrated that rats with lesions in the PHC were impaired in memory for odor-odor associations. Kirwan and Stark [44] showed that memory for an association between a face and name was also mediated by the PHC. In another example of non-spatial memory processed in the PHC, Tendolkar et al. [45] demonstrated that the amount of details (e.g., color) within the reported memories positively correlated with PHC activity. Taken together, studies such as those reviewed here indicate that even episodic memories that are devoid of any clear spatial component activate the PHC, therefore posing a critical challenge to the view that the PHC is exclusively mediating space-related processing.
The literature on PHC function is large, but quite contradictory. Both visuospatial processing and episodic memory are strongly tied to this region, as are other processes described in Box 1 (e.g., emotion, low-level visual processing, and expertise). We suggest that these different accounts are encompassed under contextual processing, as discussed in the next session.
Contextual associative processing in the PHC and beyond
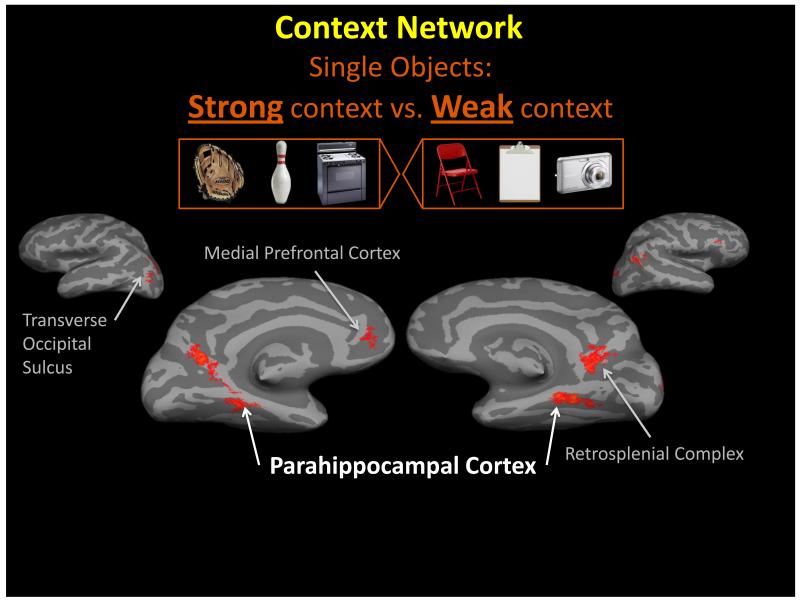
The processing of strong, long-term, contextual associations has also been shown repeatedly to elicit activity within the PHC, as well as the retrosplenial complex (RSC, which includes regions of the retrosplenial cortex, extending into the posterior cingulate cortex, and the precuneus), the medial prefrontal cortex (MPFC), and the transverse occipital sulcus (TOS) regions [2,3,59-67], see Figure 2. Contexts can be formed by repeated exposure to prototypical clustering of objects (both physical or mental), which may or may not include the spatial relations between them and their surroundings, as well as certain expected behaviors in those environments. For example, a ‘kitchen’ context contains key objects such as an oven, a refrigerator and a sink; typical spatial configurations, such as the layout of the cabinets; and expected activities, such as washing dishes, rather than brushing teeth, at the kitchen sink. Usually, not any one item or one spatial configuration defines a context; rather, a critical mass of regularly occurring items and spatial relations among them is needed. Therefore, what is essential to define a context is usually a quorum of associated objects and relations between them that distinguishes this particular context from other contexts. However, once the context has been defined, it may now be represented within a ‘context frame’, which can be triggered by merely a single object [2,68].
Figure 2.
Context cortical network. By comparing fMRI activity elicited for objects with strong contextual associations (left group of objects) with the activity elicited for objects with weak contextual associations (right group of objects) we reveal the network of regions that process contextual associations. This includes the parahippocampal cortex (PHC), retrosplenial complex (RSC), medial prefrontal cortex (MPFC), and the transverse occipital sulcus (TOS). Data from [62].
Contexts can be derived and defined through several domains. A fundamental source of contextual information comes from the place things occur, locations where objects can be found, and the typical configuration found among objects. Contextual information within the spatial domain is a salient cue. However, this is not the only source of contextual information, and critically, contextual processing in non-spatial domains is as fundamental to cognitive processing. Non-spatial contextual associations are associations that do not have a specific location, nor a fixed spatial arrangement. The items in the non-spatial context are associated with each other but not with a specific space or place. A salient cue of non-spatial contextual associations is the co-occurrence of objects or their temporally correlation in the environment. Both spatial and non-spatial contextual information are critical in providing meaning to the environment.
Contexts are important for generating expectations about other objects, the spatial relations between objects, and associated behaviors to be found within a context (e.g., [68,69]). Expectations are not limited to a single modality: a picture of fresh baked cookies, for example, can activate the associated smell and taste, the heat and sound of an oven in which they are baked, and the action of a glass of milk being poured. These expectations, generated through contextual associative processing [70], are critical in guiding us through everyday life and facilitate our interactions with the environment. Contextual associative processing is pervasive and could serve as the basis for spatial processing as well as for episodic memory, because both are necessarily highly associative processes (Figure 1).
Studies across different laboratories have revealed the neural substrates involved in contextual processing. In various studies examining how the brain processes objects with strong contextual associations (e.g., a diving board is strongly related to the context of ‘swimming pool’) compared with processing objects with weak contextual associations (e.g., a garbage can, which can be found in many contexts without being strongly related to any specific context), differential activity was found mainly in the PHC and the RSC, and in subsequent experiments, also in the MPFC and TOS, [2,59-62] see Figure 2. Since these initial studies, other groups have replicated and expanded these results relating contextual processing to these regions of the brain [41,45,63-65,71] using various methods (e.g., multi-voxel pattern analysis; [72,73]), non-human participants [7,40,55,74-76]; and associations within different domains (e.g., temporal associations) [77,78]. Moreover, the PHC has been associated with the processing of co-occurring multisensory information [79,80], which supports the proposal that PHC processes co-occurring items converging within a context. Thus, contextual associative processing has repeatedly elicited activity in the PHC, RSC, and MPFC, using a variety of procedures, analysis methodology, and cognitive perspectives.
Contexts are often linked to a specific place. To distinguish activity evoked by contextual associations from that evoked by scene processing, it was critical to compare contextual processing of objects that are strongly related to a specific place (e.g., an oven is found in a kitchen) with contextual processing of objects that are highly associated with a context but not a specific place (e.g., a birthday cake is found at a birthday party, but the party can take place anywhere - home, restaurant, park). Activity elicited by these two types of contextual objects was compared with the activity elicited for objects that were only weakly associated with many contexts (e.g., a lamp). The resulting differential activity was found in the PHC and the RSC, replicating the previous findings [2]. Activity within the PHC, however, was distributed along an anterior-posterior axis, such that activity related to spatial contexts was focused in the posterior part of the PHC, whereas activity evoked by non-spatial contexts was focused in the more anterior part of the PHC [2,3]. Note that the anterior PHC referred to here is the anterior PHC proper, not necessarily extending into perirhinal regions. These results have been replicated using highly controlled novel stimuli [3], as well as investigations of the differences of object and scene processing within the parahippocampal gyrus at large [12,56]. This result confirmed that the PHC processes contextual associations per se, regardless of whether they are spatial in nature.
Since PHC processing reflects an organization that distinguishes between spatial and non-spatial associations, this suggests that the PHC is sensitive to processing the physical details of context. However, recall that the PHC is only one of three main nodes in the cortical network that mediates contextual associative processing. Results from a collection of studies [2,60,81,82] suggest that another participant in this network, the RSC, may process more schematic, or prototypical, representations of context, previously termed ‘context frames’ (see Glossary) [68]. The RSC has been demonstrated to process an abstracted representation of context through similar activity for single objects and full scenes, both spatial and non-spatial contexts, the activation of non-presented contextual associations, and abstracted representations of scenes [2,60,81]. In these demonstrations, the RSC was not sensitive to what was actually physically perceived but rather reflects an activation of the relevant stored contextual representation (i.e., context frame) that contains the broader network of related contextual associations. The MPFC, on the other hand, may use the information within the context frame to make predictions and generate expectations about what is about to occur in the immediate environment [60-62]. Ongoing studies are examining the specific role of the MPFC, as well as the underexplored TOS, in the network processing contextual associations.
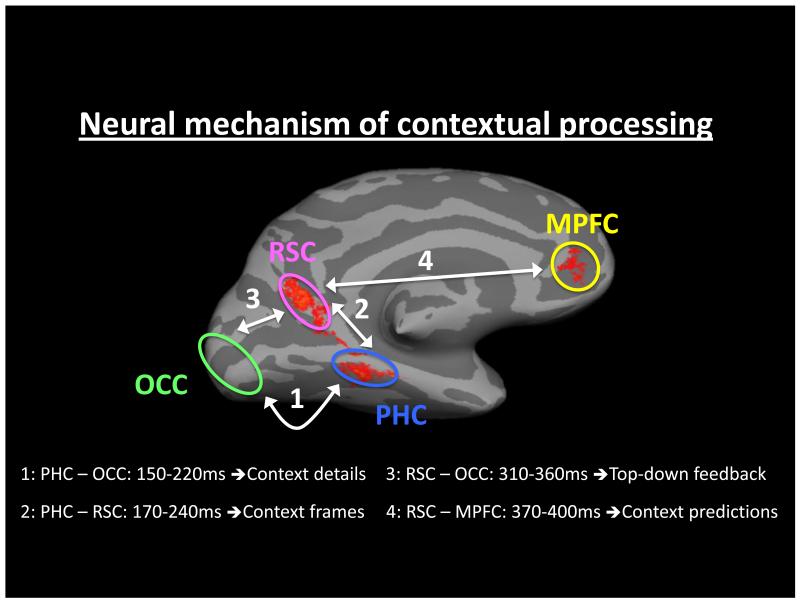
To combine these different regions into a neural mechanism underlying contextual associative processing, the underlying dynamics were investigated using magnetoencephalography (MEG) [62]. Using phase synchrony analysis, the context-sensitive MEG signal was examined between the PHC, RSC, MPFC, and early visual areas, see Figure 3. Results from this study demonstrated that contextual associative processing of visual objects was initiated through activity within the PHC (PHC with early visual areas, 150-220ms), which is suggested to analyze the contextual details of the current stimulus. This context-sensitive activity in the PHC was followed by its phase-locking with the RSC at 170-240ms. Kveraga et al. [62] propose that this reflects the instantiation of the relevant context frame, and the processing of related (offline) contextual associations within the frame. After this stage, the RSC exhibited synchronous activity with the MPFC (370-400ms), which is suggested to use associations within the context frame to generate predictions and expectations of what will be encountered next in the environment. A more in-depth discussion regarding the origins of the division of labor between the PHC and RSC is provided in Box 2, and a potential mechanism mediating contextual processing is elaborated in Box 3.
Figure 3.
Dynamic analysis of the context network. Using MEG and phase synchrony methods the spatiotemporal dynamics within the network of regions processing contextual associations were elucidated. 1. Differential activity relating to contextual processing begins between the parahippocampal cortex (PHC) and early visual regions in the occipital cortex (OCC), 150-220ms. It is suggested that during this period contextual details are extracted from the current environment. 2. Differential activity is next demonstrated between the PHC and the retrosplenial complex (RSC), 170-240ms. It is suggested that this reflects the activation of the relevant context frame. 3. Proceeding this exchange, there is synchronous activity between RSC and OCC (310-360ms), possibly representing feedback processing. 4. The last stage of the neural mechanism lies between communication between the RSC and medial prefrontal cortex (MPFC), 370-400ms, which is suggested to generating contextually related predictions. Data from [62].
Box 2. Different pathways for PHC and RSC?
The PHC seems to be preferentially activated by detailed, high spatial frequencies (HSF) and novel stimuli. In contrast, lesions or inactivation of the RSC do not affect recognition of novel stimuli, including scenes, but severely impair topographical orientation and spatial navigation (see [100] for review). Such processing tendencies suggest that the PHC and RSC may receive projections originating from the parvocellular (P) and magnocellular (M) pathway inputs into V1, respectively. We briefly review these pathways and their processing properties below.
The M and P pathways originate in the retinal ganglion cells of different types: large parasol cells for M, and midget cells for P [101], remaining segregated in different layers of the LGN and V1 (also see [102,103]). The cells that synapse with M neuron inputs in V1 continue to the thick-stripe regions of V2, MT/V5 and MST, and then to the higher-order motion and attention regions in the temporal and parietal cortices, including the posterior STS, the RSC and posterior cingulate cortex [104]. The dorsal M pathway is thought to have at least three branches projecting to premotor regions, prefrontal cortex and medial temporal lobe (see also [104] for a review). While the anatomical route(s) of the extended M pathway to the prefrontal cortex is unknown, M-biased stimuli preferentially activate the orbital prefrontal cortex [102]. P projections form much of the ventral visual “what” stream, though it also has some magnocellular inputs [105,106].
The M-dominant dorsal stream comprises most of the “where/how” pathway that mediates spatial vision and attention, topographical orientation, planning of grasping, reaching and eye movements, and motion perception [105]. Because retinal M cells sample many large diffuse bipolar cells, they have large receptive fields and high sensitivity to luminance (achromatic) contrast [107]. The P retinal ganglion cells receive information from just two (ON/OFF-center) bipolar midget cells connected to a single cone [103] and have higher spatial resolution in the luminance channel and color sensitivity, but need higher luminance contrast than M cells to be activated [107]. The latter property renders the P cells ineffective for achromatic stimuli below ~8% contrast [108]. M cells show transient responses and have higher temporal sensitivity, as well as faster conduction velocities, than P cells [106,107,109], while P cells exhibit tonic response properties and only respond to slow temporal modulation of stimuli [103].
Despite the reported branching M projection to the medial temporal lobe [110], emerging data using direct manipulations show that the PHC may indeed be preferentially activated by P-biased stimuli and HSFs, whereas the RSC may be preferentially activated by M-biased stimuli (Kveraga et al. unpublished). Perhaps, at least in the visual domain, interactions between the PHC and RSC [62] combine allocentric and egocentric reference frames and generate the contextual framework necessary for generating self-relevant predictions in the prefrontal cortex (the putative function of the MPFC). It is important to note however, that such ‘subdivision of labor’ between the PHC and RSC reveals biases to combine information from different channels, not necessarily exclusive processing of a particular type of information.
Box 3. Oscillatory resonance as a potential mechanism for contextual binding.
As the brain learns contextual relations throughout life, the links between related stimuli (co-occurring or proximal in space and/or time) gradually strengthen. When we are exposed to such learned stimuli later, these associative links would become automatically activated and induce oscillations between the linked neuronal assemblies [62,111]. If the activated associations are strong and/or sufficiently numerous, these oscillations synchronize, or resonate, resulting in a temporally coordinated and thus stronger drive on their targets (think of rhythmic clapping vs. white noise-like un-coordinated applause [111]). This may be accomplished by increases in slower rhythms, such as theta, (4-7 Hz), the dominant operating frequency of the hippocampus and the surrounding isocortex) which open temporal processing “windows” at their peak for local processing that may occur in the faster gamma band (>30 Hz) [112]. Interregional binding, e.g. between ventral stream-PHC and dorsal-stream RSC, may be accomplished by synchronization in the beta (13-30 Hz) band [62,113].
This resonance in the parahippocampal/parietal/prefrontal circuit may be what binds contextually congruent cues together and drives the prefrontal circuits to reach a decision that some object is associated with a kitchen and not with a bathroom; that some scene depicts a beach, rather than a desert. The same process could help retrieve a memory or a navigational cue. When many memory cues resonate together, their combined drive is activating the only thing that links them, while wrong or irrelevant cues wither away due to desynchronization.
While this processing-by-resonance is not a unique property of the PHC (or RSC or mPFC), such framing can help to mechanistically explain many of the findings in PHC. Here are some, admittedly speculative, examples:
-
»
why strongly contextual objects invoke the related context better than weakly contextual objects. A stimulus with strong links to a single target (a neuronal assembly representing the concept of a particular context) would result in better synchronization than a stimulus with weaker links to many targets (contexts) that are not congruent. We have shown that the PHC shows increased synchrony with other task-relevant regions [62] for strongly contextual objects, and there is evidence that successful spatial navigation (e.g. [114]) and contextually facilitated episodic memory retrieval [115] also increase parahippocampal theta-band (4-7 Hz) synchronization or theta power.
-
»
why contextual scenes, which generally have more associated elements than objects, activate the PHC more strongly than single objects or scenes devoid of content. Contextual scenes have more congruent elements that resonate together than a strong contextual object, or a scene devoid of contextual cues, such as empty rooms, and thus produce a stronger drive on downstream targets.
-
»
why evoking many contextually congruent associations can generate false memories in subsequent recall [60,116]. Congruent associations resonating together should activate (unexposed) congruent items, because they are linked by the same resonance frequency.
This proposed mechanism, and the examples given, should be interpreted with some caution, as they are quite speculative at this point. Future studies will be needed to rigorously test these hypotheses.
Contextual processing, therefore, highlights two important aspects of the PHC function. First, it provides an account for how both spatial and non-spatial information may be processed in the PHC. If the main function of the PHC is to process the binding of associations through repeated exposure to typical contexts, this can involve both spatial and non-spatial associations. However, spatial associations are a frequent, and salient, subcategory of contextual associations in humans, and thus can explain why scene and place processing has been linked to the PHC and may dominate the processing in this region. Moreover, it defines what it is about space that is important – the spatial relation between elements and the association with a location. Second, this explanation provides a way to think about the PHC in the broader framework of a network and how it works in concert with other key regions processing context.
A parsimonious account of PHC activity
How can one reconcile the fact that so many different cognitive processes activate the same general cortical area? We propose that contextual associative processing is a fundamental mechanism that can account for the various tasks and stimuli that have been shown to activate the PHC. Most of the processes that elicit PHC activity can be seen as relying on associative processing at their core. For example, the episodic memory accounts all rely on the association and binding of items belonging to the same episode. Similarly, spatial and scene processing rely on the association between items within scenes or a place (Figure 1).
Of course, it is possible to argue that the same area may be involved in different functions without relying on a common building-block operation as we propose. It could do so via different connectivity and activity patterns. There is no evidence to suggest that this is a likely alternative, especially given the current limits of human neuroimaging, but future research may be able to provide a clearer understanding. As described here, however, the current proposal does acknowledge this alternative, for example, by suggesting that spatial and non-spatial contexts rely on different (posterior and anterior) parts of the PHC.
To clarify why contextual association processing may provide the most parsimonious explanation of PHC activity, especially for spatial and non-spatial contexts, consider the following analogy: the overarching function of the fusiform gyrus, a large cortical area in the ventral visual pathway, is widely believed to process and represent objects. One category of objects, faces, consistently activates a subregion of the fusiform cortex (“fusiform face area”, FFA, [83]). The reason that faces activate this region within the fusiform is because faces share many details across exemplars, and thus consistently activate similar regions of cortex. Any group of objects that share visual features would similarly be expected to activate overlapping cortical regions. So rather than thinking about the FFA as an isolated region for faces, we can think of it as the region that mediates the processing of facial features, including those in face-like objects [84], within a broader framework of object processing. Because faces are such a frequent stimulus in our everyday environment, and because they demand subordinate-level categorization and individuation, the FFA is also a highly trained region [85,86]. In this same vein, it is proposed to think of the general function of the PHC as processing contextual associations. Within it, a subregion of the posterior PHC is particularly optimized to process spatially organized contextual associations, whereas more anterior regions of the PHC are optimized to process contextual associations in other, non-spatial, domains. Spatial contextual associations are a dominant property of places and scenes, and thus scenes consistently activate this posterior subregion of the PHC (e.g., “PPA”, [16]). We of course do not argue that the PHC is not sensitive to spaces, places, and spatial information, but rather that it is sensitive to spatial stimuli because of the associative information (space-related associations) that such stimuli evoke.
Contextual associative processing also accounts for a much larger share of findings, compared with, for example, the episodic memory or spatial processing accounts. Visuospatial theories cannot explain the non-spatial episodic activation of the PHC; and episodic memory theories cannot explain why the PHC would be involved in processing the spatial layout of scenes. Moreover, it will be challenging for visuospatial processing theories to explain differential activity within the PHC that is related to the modulation of information within a scene. Specifically, if the PHC is dedicated to spatial processing it should be indifferent to the specific content of a scene; yet, scene content has been shown to strongly modulate the signal. For example, scene complexity [87], the contextual associations of the main object within a scene (see Figure 4, [59]), the congruency between the object and the background [88], the familiarity of buildings [41], and emotional valence of a scene [27] all modulated activity elicited from the PHC. In addition, although navigation does recruit the PHC region, it has been shown to selectively recruit it based on the context, or strategies engaged [65,89]. Moreover, the visuospatial theories cannot explain activity related to stimuli outside of the spatial domain, e.g., auditory stimuli indicative of material properties [30] or odor stimuli [32].
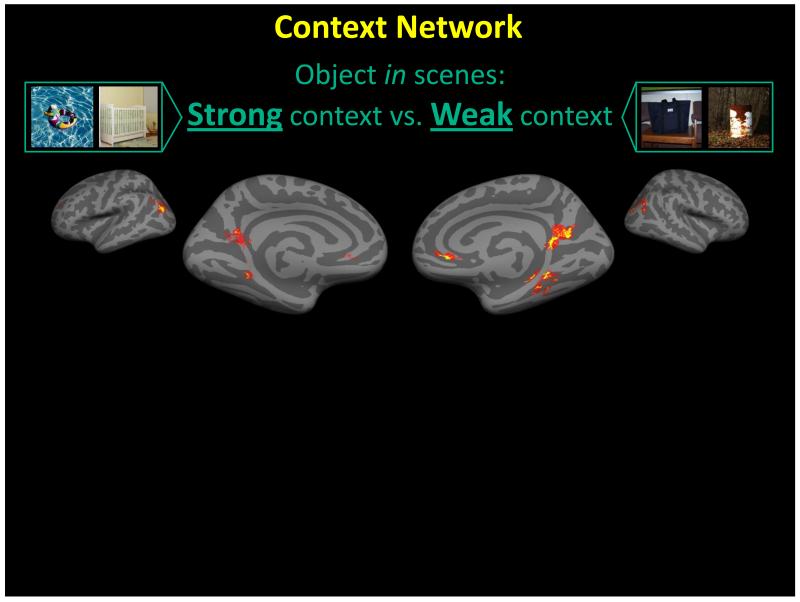
Figure 4.
Scenes compared with scenes. In this experiment, participants passively viewed pictures of scenes which were presented on for 1500ms. The scenes differed in the contextual associations of the foreground object: the foreground object was either strongly associated with a context (e.g., a crib with a nursery [left pictures]), or the foreground object was weakly associated with many contexts (e.g., a bag is not strongly associated with a context [right pictures]). Comparing the activity elicited when viewing the scenes in the different conditions (strong context vs. weak context) revealed differential activity within the context network, and in particular within the PHC, in similar regions as the PPA. The results from this study demonstrate how the contextual associations within a scene can modulate activity within scene selective cortex. This poses a problem for a strictly spatial layout interpretation of the function in these regions. If it was only spatial, there should not be differential activity when comparing scenes to scenes since scenes in both conditions contain spatial layout information. Data from [59].
Likewise, the episodic memory theories also cannot account for many of the results found within the PHC. For example, they cannot explain why stimulus differences, such as scene complexity, which have equivalent familiarity and exposure, would evoke differential activity within the PHC. In contrast, the proposal that contextual associative processing may be the fundamental function of the PHC can explain all these diverse findings.
Proposing that contextual processing is the fundamental function of the PHC (and RSC) reconciles the discrepancy between the spatial processing literature and the episodic memory literature. This is not the first time that associative processing has been used to explain the functional role of the medial temporal lobe. The hippocampus is another structure that has been strongly linked to both visuospatial processing and to episodic memory, and given its integrative position it may actually play a broader role in context than any other structure. Eichenbaum [90] previously proposed that the main role of the hippocampus more globally is to combine different elements of an episode into a memory that can be linked to a larger memory network. Thus, spatial information is largely processed in the hippocampus, with input from the surrounding cortices, due to the junction of a particular place and a behavior, reward, or other sensory experience (e.g., odor). A place provides the salient stimulus to bind into an association. This interpretation thus posits that the hippocampus is maximally attuned to the conjunction of features, rather than spatial information per se. We extend this theory to the function of the PHC, and describe the main focus of the PHC as processing long-term contextual associations, of which associations linked to a specific place are a large subset.
Eichenbaum, Ranganath and colleagues have proposed a framework connecting the different medial temporal lobe structures, which includes a specific role for the PHC in processing context [53,54]. The model proposed, termed the ‘binding of item and context’ model, suggests that contextual information in the PHC provides the input to the hippocampus in order to bind to new memories and link the memory of that particular episode within a larger network. The hippocampus is known to be an important structure in memory for specific episodes. Thus, it is involved in binding the target of an episode (e.g., remembering a person’s name) with the context in which it is encountered (e.g., work party). This context, however, is processed and analyzed in the PHC, which incorporates the current context with long-term associations of the context built up in memory. Long-term contextual associations are relevant during memory encoding, and likely are used as “fillers” for our episodic memory. The spreading activation of contextual associations during encoding has been found to lead to subsequent false recognition of contextually related items [60], providing support for the role of the PHC within the larger scheme of the medial temporal lobe, and recognition memory.
Future directions in studying the PHC
Establishing the PHC as a node for processing contextual associations offers a new roadmap of future potential insights to explain brain-behavior relations.
The fMRI signal across the brain has a functional profile that does not reflect strict category boundaries. For example, researchers compare the activity elicited by faces and objects with that elicited by scenes to functionally define the “PPA”, and contrast scenes and objects with faces to functionally define the “FFA” [16,83]. However, these regions are responsive to the stimuli used as the contrast as well, just to a lesser a degree (e.g., objects can activate the “PPA” region [2,23] and scenes can activate the “FFA” region [20]). If the PHC were exclusively a scene-processing region, it would be expected to be unresponsive to single object stimuli. Similarly, the “object” processing regions, e.g., the lateral occipital cortex, are typically defined by comparing activity elicited for objects vs. scenes. Outside of the laboratory, we never actually encounter objects in isolation from a scene, and scenes always contain objects, so it is hard to understand what such a contrast represents. With respect to understanding the function of the “PPA” proper, a contextual processing approach can provide a more ecological principle to define this region. Organizing stimuli based on contextual associations provides an axis (i.e., from strongly contextual to weakly contextual) to characterize stimuli along this continuous gradient. This provides a way to define what stimuli are “optimal” to activate this region without using strict categorical boundaries. Moreover, some categories can elude classification. For example, what exactly is a scene? For something to be considered a scene, does it have to depict a navigable place, or just a configuration of parts? Would keys on a keyboard be considered a scene or an object? Characterizing stimuli based on their contextual associations circumvents these definitional problems, and allows them to not necessarily fit specific categorical boundaries.
Scene-selective activity within the PHC reflects the processing of contextual associations. Thus, mechanisms in scene understanding should be investigated using a contextual associative processing approach. When viewing a scene, we are not just viewing the stimulus as is, but also processing it within a larger context in which we have experienced it. It is the contextual associations of a scene that can affect cognition. For example, contextual associations facilitate the recognition of objects, and can facilitate the ability to segment a scene, which may be extremely difficult without processing the context. Cutting-edge computer vision algorithms in scene understanding are not able to achieve anywhere close to the human processing, largely due to the lack of ability to process context. Context has been shown to significantly help computer vision models of scene understanding [91]. Scene processing should not be thought of as exclusively a visual process, but rather a highly associative process that invokes prior experiences and context frames.
The PHC is also identified as a region within the ‘default network’, along with the medial parietal and prefrontal regions, usually defined as a set of regions that becomes more activity when the participant is “resting” in the scanner, compared with the activity while the participant is performing some experimental task. However, tasks involving contextual associative processing increase the activity of these regions (PHC & RSC) above their already high resting baseline, rather than deactivating them, which suggests that these regions are engaged most when processing contextual associations [61]. At rest, or at baseline, humans’ thoughts do not cease, but rather engage in typical, active “mind-wandering” [92], which requires concentrated efforts (such as meditation) to quell. Mind-wandering engages associative processing, such as a free-association train of thought. Thus, the default network doesn’t overlap with the regions that process contextual associations by coincidence, but rather necessarily so, because contextual associations are a critical component of the thought processes occurring at “rest”.
Recently a link has been made between the breadth of associative activation and mood [93,94]. That PHC is part of a network, and that a limit on the extent of associations, as in rumination that happens in depression, may be a result of inhibition, gives rise to interesting new examinations of the interplay between excitation and inhibition in the activation of associations for predictions.
While the contextual processing account cannot account for all the findings (see [19,41]), it seems to be the framework that can explain the largest number of findings so far, and thus is the most parsimonious account of PHC activity to date. Some of the discrepancies within the PHC findings may lie within its anatomical subdivisions (see point 6), whereas others may be task-specific.
The PHC is a sizable region of cortex, and it is possible that an alternative account to the one endorsed here is that the PHC mediates all those difference processes and there is no one overarching process that explains all of PHC functioning. While the resolution of fMRI has significantly improved over the last twenty years, even a single voxel nonetheless contains a very large number of neurons and supporting cells. Fine-grained processing biases in populations of neurons within the PHC, which fMRI cannot yet detect, may also be the root of these different findings. However, more acute localizations of the foci of activation for these different processes may help to better delineate subregions of the PHC. How well do locations of the diverse findings across the literature correspond to each other? Can a more precise description of where within the PHC these foci are provide better understanding to the overall organization for this region of cortex? The PHC clearly can be subdivided into functional subregions - for example anterior and posterior regions [3,12], lateral and medial regions [4], and by multiple visual fields (PHC1 and PHC2) [95]. Do the different foci of activity from the diverse findings correspond to these different subregions?
Strong contextual associations typically activate medial regions of the PHC, whereas scene-selective activity was found more closely focused within the lateral portions/medial fusiform regions of the PHC [11]. Using the term “parahippocampal place area” biases one to assume that the activity is confined within the parahippocampal gyrus. But, as Nasr and colleagues [11] have demonstrated, the activity within this region is concentrated more within the collateral sulcus, and even extending well into the medial fusiform gyrus. Thus, the activity related to scene processing within the region is more widespread than its anatomic toponym implies. Similar arguments may be made for the TOS. Using rigorous anatomical landmarks to describe the location of activity will help to reveal an organization within the cortex to certain stimuli and tasks, or in contrast, demonstrate the activity elicited is widespread across many regions.
Conclusions
A contextual processing framework can account for many of the findings related to the PHC. Moreover, a contextual processing framework provides a parsimonious explanation for linking the posterior parahippocampal gyrus (PHG) with anterior regions of the PHG, linking the PHC with other medial temporal lobe structures, linking the PHC with the greater network it is connected with (e.g., RSC and MPFC), and for reconciling why both episodic memory and spatial processing engage the same region of the cortex.
This account may be simple and powerful enough to encompass the main proposals in the literature, demonstrating that we have all been looking at the same circuitry and functionality, and that the best way to understand the function of the PHC might be converging our perspectives.
Box 4: Questions for future research.
- How can a contextual processing account of the PHC provide a means for enhanced understanding of:
- The mechanisms underlying scene understanding?
- The organization of, and inherent links between, the representation of objects and scenes?
- The role of long-term associative memory in top-down processes involved in visual perception?
- Why the PHC is found within the “default network”?
- A potential mechanism of abnormal associative processing that may underlie some mood disorders?
How anatomically similar are the activation patterns that arise for processing contextual associations, episodic memory, and visuospatial material? Do these processes cluster in different subregions of the PHC? Does this potential anatomical organization reveal inherent similarities and differences between the cognitions?
Highlights.
A synthesis of current research characterizing the functional roles of the PHC.
Contextual associative processing is proposed as the core function of this region.
This proposal unifies findings of the PHC, creating convergence for future research.
Acknowledgments
Supported by NSF grant BCS-0842947 and the Israeli Center of Research Excellence in Cognition (ICORE 51/11) to MB, NIH K01MH084011 to KK, and Office of Navy Research MURI N000141010934 with CNBC and Department of Robotics at Carnegie Mellon University to EA.
Glossary
- Context
A conglomerate of conditions that help to define, represent and bring meaning to the environment. The conditions carry long-term associations built up over repeated exposure. Although this paper focuses on conditions defined by objects and configurations, conditions can also be defined in other domains such as temporal (e.g., order of events), behavioral (e.g., mindset, goal orientation), or emotional.
- Contextual association
The link between contextual items. For example, the link between objects strongly associated with the same context (e.g., bathtub and sink), or the spatial relation between items (e.g., a keyboard is found below, and in front, of the monitor), or the configuration associated with a context (e.g., a conference room contains a long narrow table with chairs on either side).
- Contextual frame
The prototypical memory representation that contains the network of associations that define a context, such as the key objects and the spatial relations between them. This prototypical representation is generic enough to be applied to different exemplars of the same context. A single key association can initiate the processing of a context frame, which may manifest by a spreading activation through the network of associations. For example, seeing a surfboard can trigger activation of a beach context. Context frames can also be activated by a gist (e.g., LSF) [1]. These memory structures serve for both efficient storage and the generations of predictions.
- Non-Spatial context
A context defined in domains independent of spatial relations, configurations, or location.
- Spatial context
The context provided by the spatial configuration or location of items.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bar M. Visual objects in context. Nat Rev Neurosci. 2004;5:617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- 2.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 3.Aminoff E, et al. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki WA. Comparative analysis of the cortical afferents, intrinsic projections and interconnections of the parahippocampal region in monkeys and rats. In: Gazzaniga MS, editor. The cognitive neurosciences IV. MIT Press; 2009. pp. 659–674. [Google Scholar]
- 5.Insausti R, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 6.Reber P, et al. Encoding activity in the medial temporal lobe examined with anatomically constrained fMRI analysis. Hippocampus. 2002;12:363–376. doi: 10.1002/hipo.10018. [DOI] [PubMed] [Google Scholar]
- 7.Furtak SC, et al. Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron. 2012;76:976–988. doi: 10.1016/j.neuron.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki WA. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: Organization of cortical inputs and interconnections with amygdala and striatum. Sem Neurosci. 1996;8:3–12. [Google Scholar]
- 9.Blatt GJ, et al. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: an anatomical and neurophysiological study. J Comp Neurol. 2003;466:161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur J Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- 11.Nasr S, et al. Scene-selective cortical regions in human and nonhuman primates. J Neurosci. 2011;31:13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldassano C, et al. Differential connectivity within the Parahippocampal Place Area. NeuroImage. 2013;75:236–245. doi: 10.1016/j.neuroimage.2013.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zola-Morgan S, et al. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davachi L, et al. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diana RA, et al. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J Cogn Neurosci. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 17.Ekstrom AD, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 18.Stevens WD, et al. Hemispheric asymmetry of visual scene processing in the human brain: evidence from repetition priming and intrinsic activity. Cereb Cortex. 2012;22:1935–1949. doi: 10.1093/cercor/bhr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. J Neurosci. 2011;31:7441–7449. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, et al. Disentangling scene content from spatial boundary: complementary roles for the parahippocampal place area and lateral occipital complex in representing real-world scenes. J Neurosci. 2011;31:1333–1340. doi: 10.1523/JNEUROSCI.3885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kravitz DJ, et al. Real-world scene representations in high-level visual cortex: it’s the spaces more than the places. J Neurosci. 2011;31:7322–7333. doi: 10.1523/JNEUROSCI.4588-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguirre GK, et al. The parahippocampus subserves topographical learning in man. Cereb Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- 23.Janzen G, et al. Neural representation of navigational relevance is rapidly induced and long lasting. Cereb Cortex. 2007;17:975–981. doi: 10.1093/cercor/bhl008. [DOI] [PubMed] [Google Scholar]
- 24.Smith APR, et al. fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 25.Gosselin N, et al. Emotional responses to unpleasant music correlates with damage to the parahippocampal cortex. Brain. 2006;129:2585–2592. doi: 10.1093/brain/awl240. [DOI] [PubMed] [Google Scholar]
- 26.Mitterschiffthaler MT, et al. A functional MRI study of happy and sad affective states induced by classical music. Hum Brain Mapp. 2007;28:1150–1162. doi: 10.1002/hbm.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stock den JV, et al. Affective scenes influence fear perception of individual body expressions. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22195. DOI: 10.1002/hbm.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy I, et al. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- 29.Rajimehr R, et al. The “parahippocampal place area” responds preferentially to high spatial frequencies in humans and monkeys. Plos Biol. 2011;9:e1000608. doi: 10.1371/journal.pbio.1000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnott SR, et al. Crinkling and crumpling: An auditory fMRI study of material properties. NeuroImage. 2008;43:368–378. doi: 10.1016/j.neuroimage.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Engelien A, et al. Functional neuroanatomy of non-verbal semantic sound processing in humans. J Neural Transm. 2006;113:599–608. doi: 10.1007/s00702-005-0342-0. [DOI] [PubMed] [Google Scholar]
- 32.Kjelvik G, et al. The human brain representation of odor identification. J Neurophysiol. 2012;108:645–657. doi: 10.1152/jn.01036.2010. [DOI] [PubMed] [Google Scholar]
- 33.Cerf-Ducastel B, Murphy C. Age-related differences in the neural substrates of cross-modal olfactory recognition memory: an fMRI investigation. Brain Res. 2009;1285:88–98. doi: 10.1016/j.brainres.2009.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez P, et al. Differential effects of damage within the hippocampal region on memory for a natural, nonspatial odor-odor association. Learn Mem. 2001;8:79–86. doi: 10.1101/lm.38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maguire EA, et al. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 36.Mellet E, et al. Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. NeuroImage. 2000;12:588–600. doi: 10.1006/nimg.2000.0648. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122:1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 38.Ploner CJ, et al. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex. 2000;10:1211–1216. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- 39.Eacott MJ, Gaffan EA. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Q J Exp Psychol B. 2005;58:202–217. doi: 10.1080/02724990444000203. [DOI] [PubMed] [Google Scholar]
- 40.Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein RA, Ward EJ. How reliable are visual context effects in the parahippocampal place area? Cereb Cortex. 2010;20:294–303. doi: 10.1093/cercor/bhp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maguire EA, et al. Knowing where things are: Parahippocampal involvement in encoding object locations in virtual large-scale space. J Cogn Neurosci. 1998;10:61–76. doi: 10.1162/089892998563789. [DOI] [PubMed] [Google Scholar]
- 43.Sommer T, et al. Dissociable contributions within the medial temporal lobe to encoding of object-location associations. Learn Mem. 2005;12:343–351. doi: 10.1101/lm.90405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tendolkar I, et al. Contributions of the medial temporal lobe to declarative memory retrieval: manipulating the amount of contextual retrieval. Learn Mem. 2008;15:611–617. doi: 10.1101/lm.916708. [DOI] [PubMed] [Google Scholar]
- 46.Düzel E, et al. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci. 2003;23:9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henke K, et al. Human hippocampus associates information in memory. Proc Natl Acad Sci USA. 1999;96:5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hales JB, et al. Dissociation of frontal and medial temporal lobe activity in maintenance and binding of sequentially presented paired associates. J Cogn Neurosci. 2009;21:1244–1254. doi: 10.1162/jocn.2009.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, et al. Decreased parahippocampal activity in associative priming: evidence from an event-related fMRI study. Learn Mem. 2008;15:703–710. doi: 10.1101/lm.900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes SM, et al. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17:873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W-C, et al. Dissociable neural correlates of item and context retrieval in the medial temporal lobes. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.05.029. DOI: 10.1016/j.bbr.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staresina BP, et al. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 2011;31:8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eichenbaum H, et al. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diana RA, et al. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Burwell R, et al. Perirhinal and postrhinal contributions to remote memory for context. J Neurosci. 2004;24:11023. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litman L, et al. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19:308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brewer JB, et al. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 58.Wagner AD, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 59.Bar M, et al. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci. 2008;28:8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aminoff E, et al. The cortical underpinnings of context-based memory distortion. J Cogn Neurosci. 2008;20:2226–2237. doi: 10.1162/jocn.2008.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bar M, et al. The units of thought. Hippocampus. 2007;17:420–428. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- 62.Kveraga K, et al. Early onset of neural synchronization in the contextual associations network. Proc Natl Acad Sci USA. 2011;108:3389–3394. doi: 10.1073/pnas.1013760108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters J, et al. Associations evoked during memory encoding recruit the context-network. Hippocampus. 2009;19:141–151. doi: 10.1002/hipo.20490. [DOI] [PubMed] [Google Scholar]
- 64.Norman-Haignere SV, et al. Category-selective background connectivity in ventral visual cortex. Cereb Cortex. 2012;22:391–402. doi: 10.1093/cercor/bhr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown TI, Stern CE. Contributions of Medial Temporal Lobe and Striatal Memory Systems to Learning and Retrieving Overlapping Spatial Memories. Cereb Cortex. 2013 doi: 10.1093/cercor/bht041. DOI: 10.1093/cercor/bht041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diana RA, et al. Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia. 2012;50:3062–3069. doi: 10.1016/j.neuropsychologia.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M, et al. Associative Information Processing in Parahippocampal Place Area (PPA): An fMRI Study. Brain Informatics. 2012;7670:1–9. [Google Scholar]
- 68.Bar M, Ullman S. Spatial context in recognition. Perception. 1996;25:343–352. doi: 10.1068/p250343. [DOI] [PubMed] [Google Scholar]
- 69.Biederman I, et al. Scene perception: detecting and judging objects undergoing relational violations. Cognitive Psychol. 1982;14:143–177. doi: 10.1016/0010-0285(82)90007-x. [DOI] [PubMed] [Google Scholar]
- 70.Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Szpunar KK, et al. Contextual processing in episodic future thought. Cereb Cortex. 2009;19:1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- 72.Diana RA, et al. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus. 2008;18:536–541. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hannula DE, et al. Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.02.011. DOI: 10.1016/j.neuropsychologia.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson S, et al. Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behav Neurosci. 2011;125:578–587. doi: 10.1037/a0024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keene CS, Bucci DJ. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behav Neurosci. 2008;122:651–658. doi: 10.1037/0735-7044.122.3.651. [DOI] [PubMed] [Google Scholar]
- 76.Smith DM, et al. Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus. 2012;22:1121–1133. doi: 10.1002/hipo.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turk-Browne NB, et al. Scene representations in parahippocampal cortex depend on temporal context. J Neurosci. 2012;32:7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diaconescu AO, et al. The co-occurrence of multisensory facilitation and cross-modal conflict in the human brain. Journal of Neurophysiology. 2011;106:2896–2909. doi: 10.1152/jn.00303.2011. [DOI] [PubMed] [Google Scholar]
- 80.Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 81.Park S, et al. Beyond the edges of a view: boundary extension in human scene-selective visual cortex. Neuron. 2007;54:335–342. doi: 10.1016/j.neuron.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Epstein RA, Higgins JS. Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cereb Cortex. 2007;17:1680–1693. doi: 10.1093/cercor/bhl079. [DOI] [PubMed] [Google Scholar]
- 83.Kanwisher N, et al. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadjikhani N, et al. Early (M170) activation of face-specific cortex by face-like objects. Neuroreport. 2009;20:403–407. doi: 10.1097/WNR.0b013e328325a8e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gauthier I, et al. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- 86.Nestor A, et al. Unraveling the distributed neural code of facial identity through spatiotemporal pattern analysis. Proc Natl Acad Sci USA. 2011;108:9998–10003. doi: 10.1073/pnas.1102433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chai XJ, et al. Scene complexity: influence on perception, memory, and development in the medial temporal lobe. Front Hum Neurosci. 2010;4:21. doi: 10.3389/fnhum.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jenkins LJ, et al. Cultural differences in the lateral occipital complex while viewing incongruent scenes. Soc Cogn Affect Neurosci. 2010;5:236–241. doi: 10.1093/scan/nsp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rauchs G, et al. Partially segregated neural networks for spatial and contextual memory in virtual navigation. Hippocampus. 2008;18:503–518. doi: 10.1002/hipo.20411. [DOI] [PubMed] [Google Scholar]
- 90.Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 91.Divvala SK, et al. An empirical study of context in object detection. IEEE Conference of Computer Vision and Pattern Recognition 2009. 2009:1271–1278. [Google Scholar]
- 92.Mason M, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bar M. A cognitive neuroscience hypothesis of mood and depression. Trends Cogn Sci. 2009;13:456–463. doi: 10.1016/j.tics.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shenhav A, et al. Affective value and associative processing share a cortical substrate. Cogn Affect Behav Neurosci. 2012 doi: 10.3758/s13415-012-0128-4. DOI: 10.3758/s13415-012-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arcaro MJ, et al. Retinotopic organization of human ventral visual cortex. J Neurosci. 2009;29:10638–10652. doi: 10.1523/JNEUROSCI.2807-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barrett LF, et al. Context in Emotion Perception. Curr Dir Psychol Sci. 2011;20:286–290. [Google Scholar]
- 97.Bilalić M, et al. Mechanisms and neural basis of object and pattern recognition: a study with chess experts. J Exp Psychol Gen. 2010;139:728–742. doi: 10.1037/a0020756. [DOI] [PubMed] [Google Scholar]
- 98.Kim Y-T, et al. Neural correlates related to action observation in expert archers. Behav Brain Res. 2011;223:342–347. doi: 10.1016/j.bbr.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 99.Cheung OS, Bar M. Visual prediction and perceptual expertise. Int J Psychophysiol. 2012;83:156–163. doi: 10.1016/j.ijpsycho.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vann SD, et al. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 101.Boycott B, Wassle H. Morphological Classification of Bipolar Cells of the Primate Retina. Eur J Neurosci. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 102.Kveraga K, et al. Magnocellular projections as the trigger of top-down facilitation in recognition. J Neurosci. 2007;27:13232–13240. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaplan E. The M, P, and K pathways of the primate visual system. In: Chalupa L, Werner J, editors. The visual Neuroscience. MIT Press; 2004. pp. 481–494. [Google Scholar]
- 104.Kravitz DJ, et al. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 106.Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- 107.Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci USA. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tootell RB, et al. Functional anatomy of macaque striate cortex. IV. Contrast and magno-parvo streams. J Neurosci. 1988;8:1594–1609. doi: 10.1523/JNEUROSCI.08-05-01594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- 110.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 111.Buzsaki G. Rhythms of the Brain. 2006 Rhythms of the Brain. [Google Scholar]
- 112.Canolty RT, et al. High Gamma Power Is Phase-Locked to Theta Oscillations in Human Neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engel AK, et al. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 114.Cornwell BR, et al. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Atienza M, et al. Semantic congruence enhances memory of episodic associations: role of theta oscillations. J Cogn Neurosci. 2011;23:75–90. doi: 10.1162/jocn.2009.21358. [DOI] [PubMed] [Google Scholar]
- 116.Deese J. On the structure of associative meaning. Psychol Rev. 1962;69:161–175. doi: 10.1037/h0045842. [DOI] [PubMed] [Google Scholar]