Abstract
Purpose of review
The lack of effective treatments for various neurodegenerative disorders has placed huge burdens on society. We review the current status application of pluripotent stem cells (iPSCs) technology for the cellular therapy, drug screening and in vitro modeling of neurodegenerative diseases.
Recent findings
Disease specific iPSCs were derived from patients of several major neurodegenerative diseases, including Parkison' disease, Alzheimer's disease, amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA). Differentiation of the SMA-iPSCs into neurons showed the recapitulation of the in vivo phenotypes, allowing us the future use of drug screening. The murine model of Parkinson's transplanted with human iPSCs showed the functional recovery, showing the potential of iPSC as cell therapy. Direct conversion to neurons was succeeded from skin fibroblasts of Alzheimer's patients.
Summary
We summarize the recent progress in using iPSCs for neurodegenerative diseases, and provide a future perspective in this field.
Keywords: induced pluripotent stem cells, neurodegenerative diseases, reprogramming, ALS, SMA
Introduction
Neurodegenerative diseases are characterized by the chronic and progressive loss of neuronal functions, which in turn results in memory deficit, cognitive impairment and impaired motor coordination. There is a wide range of hereditary and sporadic neurologic disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntingtons’s disease (HD), amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA). Alzheimer’s disease is the most common form of dementia. It was estimated that 24.3 million people worldwide had dementia in 2005 and the number of patients who suffer from that neurologic illness would be increased to 81.1 million by 2040 [1]. In the United States, approximately 7 million patients are affected various neurodegenerative diseases [2]. As life expectancy continues to increase, so does the prevalence of these diseases, along with the socio-economic burden on affected individuals and their families throughout the rest of their lives.
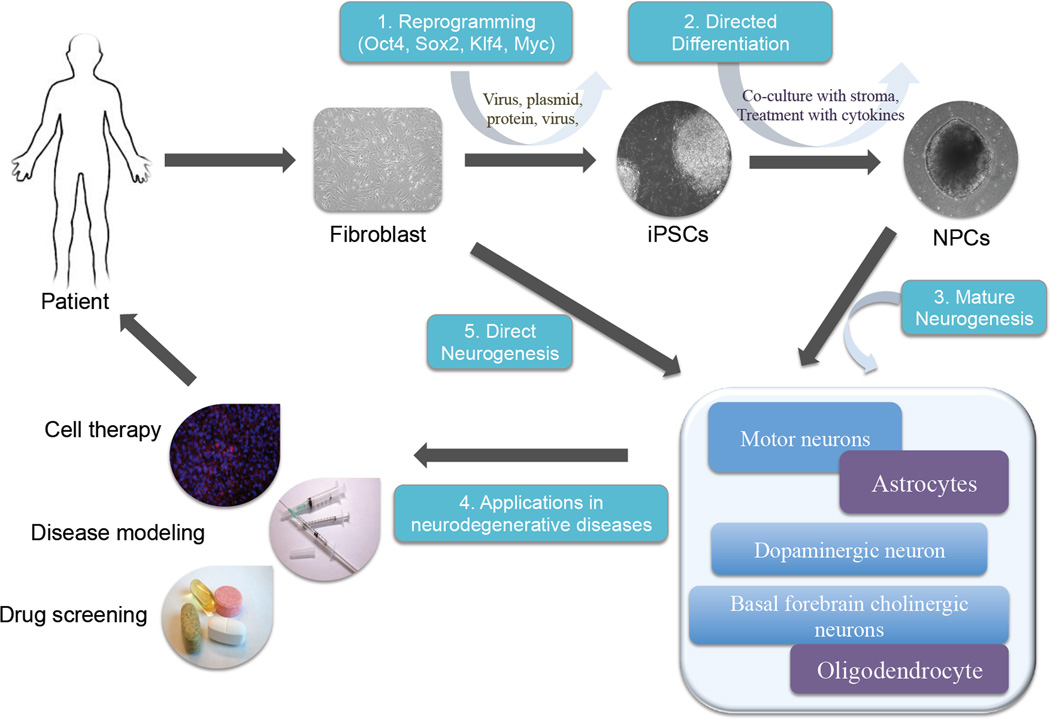
To date, there is no known effective treatment for these neurodegenerative diseases. In addition, there are few cellular models that recapitulate disease pathogenesis and assist in drug screening. Cell replacement therapy using stem cells is considered as a potential approach to treat these diseases, given that the progressive neuronal loss characterizes them. Since Takahashi and Yamanaka reported their achievement in reprogramming mouse and human fibroblasts into pluripotent cells, remarkable progress has been made in the field of pluripotent stem cells [3, 4]. We refer to many recent in-depth reviews on reprogramming and induced pluripotent stem cells (iPSCs) for the interested readers [5–7]. Currently, patient-derived iPSCs are believed to contribute to disease-specific cellular models and drug screening platforms [8, 9]. In addition, iPSCs can be used as autologous source for cell therapy [10](Figure 1). Here, we review the potential of iPSCs in treating neurodegenerative diseases and understanding disease pathogenesis. Furthermore, we will discuss some of the main challenges in application of iPSCs.
Figure 1.
Overview of the use of induced pluripotent stem cell (iPSC) research for neurodegenerative diseases.
The somatic cells from patients can be reprogrammed to iPSCs (1) using retroviral vectors, mRNA or proteins to express a set of defined factors (Oct4, Sox2, Klf4 and Myc). Co-culture with stromal cells or treatment with growth factors direct the differentiation of iPSCs into neuronal lineages (2). Further maturation of neurons with cytokines produces the neurons in need, such as motor neurons in ALS, and dopaminergic neurons in PD (3). The in vitro differentiated neurons are used for in vitro disease modeling, drug screening and cell therapy (4). Recent direct neurogenesis from fibroblasts showed the another revenue to generate patient's specific neurons (5).
Disease modeling
Studies aiming to elucidate the pathogenesis of various human neurological diseases have utilized post-mortem tissues and transgenic animal models [11]. However, post-mortem tissues are not always available and often represent the end stage of the disease. The murine models have vastly contributed to understanding human neurodegeneration [12], but they do not fully recapitulate the human neural phenotype. The first and most fundamental step to construct cellular models is to generate iPS cell lines from patients. In 2008, Park et al. derived disease-specific iPSCs from patients with a variety of genetic diseases including PD and HD [9]. iPSCs from HD showed the expanded (CAG)n polyglutamine triplet repeat sequences, maintaining the genotype of patients with HD. Thus far, various iPS cell lines from patients with various neurodegenerative disorders including AD, PD, HD, ALS, and SMA have been generated [9, 13–15]. Some showed the in vitro phenotypes, while for others the further validation of the phenotypes is needed.
SMA is one of the first human neurodegenerative diseases that are well characterized in iPSC-based in vitro disease model. SMA is an autosomal recessive neurological disease characterized by degeneration of spinal motor neurons, muscular atrophy and generalized weakness [16]. The majority of patients with SMA have mutations in SMN1 (survival motor neuron 1), resulting in the selective degeneration of lower α-motor neurons. SMN2, an SMN1 homologue, compensates for the abnormal production of SMN1, the degree of which correlates with the disease severity. Ebert et al. generated iPSCs from fibroblasts of SMA patient and the patient’s unaffected mother as normal control with lentiviral transduction systems expressing OCT4, SOX2, NANOG and LIN28 [14]. To examine the effect of the reduced SMN1 expression on neuronal differentiation and survival, iPSCs from SMA patient and control were differentiated into motor neurons. The motor neurons derived from iPSCs were confirmed through immunostaining for the nonphosphorylated neurofilament SMI-32 and choline acetyltransferase, the established markers for mature motor neurons. Until four weeks of differentiation, there was no significant difference in the number and size of the neurons between the iPSCs of patient and control. With an additional 2 weeks of maturation, the motor neurons from SMA iPSCs showed the less number and smaller size. There was no difference in total number of Tuj1-positive neurons between SMA and normal iPSCs, confirming the selective negative impact of SMN on motor neurons. This study showed the proof of principle of using iPSC derived motor neurons to study the pathogenesis of SMA, which will be a novel platform for screening chemicals as discussed below.
Despite potential in disease modeling, there are some issues involved in using iPSCs. One is the clonal variation among pluripotent stem cells. The seemingly normal hESCs derived from human blastocysts showed the marked difference in their differentiation potential [17], and the iPSCs derived from the same donor fibroblasts showed the variable neuronal differentiation potential [18]. However, use of the chemical inhibitors of TGFβ and BMP signaling minimizes the neuronal differentiation variation regardless of cellular sources [19, 20]. Likewise, recent large scale characterization of six hESC lines and 16 iPSC lines from ALS patients showed that the standard neuronal differentiation condition using retinoic acid (RA), BDNF, GDNF, and CNTF revealed the significant quantitative difference in motor neuron differentiation among different cell lines, while the use of TGFβ and BMP inhibitors markedly reduced the difference [8]. Thus, it is critical to improve the in vitro differentiation condition to recapitulate the phenotypes of the given diseases, while minimizing the cellular bias.
The unique features of the late onset and the polygenic traits are other important considerations, when modeling neurodegenerative diseases in vitro. It is estimated that the diseases with early onset and the familial cases account for the less than 10 % of each neurodegenerative disease [21]. The late onset phenotype is difficult to recapitulate in vitro, as reported in ALS [8]. Mimicking the physiological aging in vitro to expedite the manifestation of the phenotypes will be needed to succeed in modeling. Alternatively, using the iPSCs derived from patients with the well-defined familial genetic mutation and having the relatively early onset will elucidate the common pathogenesis of the diseases shared with the late onset ones, like the early onset presenilin 1 and presenilin 2 mutant Alzheimer's patients [22].
Drug screening
The cost of drug development was estimated to be $900 million [23]. The vast majority of failure occurred in later stages in drug development during phase IIb and III clinical trials. Approximately 90% of drugs in human clinical trials are not approved for marketing. The underlying causes of this high rate of failure include the lack of efficacy and clinical safety in patients due to the current limits in disease models in recapitulating the human disease and in testing drug safety. In this respect, disease specific iPSCs provide a unique opportunity for drug discovery. Firstly, as a material of screening iPSCs are the exact human cells that are affected in the diseases, but not the unrelated immortalized cell lines artificially modified to mimic the disease. Secondly, as target of screening, iPSCs can be differentiated into the specific neuronal subtypes that are most relevant to disease phenotypes, such as dopaminergic neurons in PD, or motor neurons in ALS. Thus, the small molecules screened as effective for the given target cells are expected to give the similar efficacy when treated to the patients.
Toxicity of the drug can be tested directly using iPSC derivatives. During screening of the chemicals for their efficacy, the neurotoxicity is simultaneously tested. Moreover, cell types, such as cardiomyocytes and hepatocytes, which are critical in determining the cytotoxicity of the chemicals are readily differentiated from iPSCs [24, 25]. The proper screening using iPSCs for highly efficient chemicals with low toxicity would reduce the large amount of drug development cost that will be used in human clinical trials.
Disease treatment
Cell therapy in neurodegenerative disease involves introducing functional cells to restore damaged neural tissues. Thus far, the extensive efforts by researchers to develop cell transplantation therapies have led to using neuronal stem cells (NSCs), mesenchymal stem cells (MSCs), and hESC-derived neuronal cells [26–29]. NSCs and MSCs can be differentiated into neural lineages. However, both cell types show restricted potential of self-renewal and lineage differentiation. The hESCs are a source of neural progenitor cells (NPCs) that can be further differentiated into a wide variety of functional neuron and glia [30]. With the recent advances in the differentiation process of neuronal development, specific neurons have been successfully generated from hESCs [31, 32]. Human iPSCs are close to hESCs, and the experimental approaches developed for use of hESCs can be applied to iPSCs without major modification. In addition, human iPSCs do not have issues in the immunologic incompatibility between donors and recipients reported in hESCs [33]. Thus, they have emerged as an autologous cell source of replacing affected neurons and glias in neuronal diseases.
PD is one of the diseases that iPSC-based cell therapy is most effectively applied. PD results from selective loss of dopaminergic neurons in the substantia nigra. The replacement of the lost dopaminergic neurons is expected to alleviate the PD symptoms. Patients experience progressive motor dysfunction, such as tremors, gait disturbance, and rigidity. Cognitive dysfunction and dementia may arise in the advanced stages. Using the murine model of PD, Wernig et al. showed that neurons from normal iPSCs reduced the PD symptoms, confirming the effectiveness of iPSC-derived neurons for PD [34]. In another study, Hargus et al. differentiated iPSCs from PD patient into neural precursor cells and transplanted into striatum of the normal and PD rat models. The donor PD cells differentiated into dopaminergic neurons and survived in the rodent brain over several months. In their study, PD iPSC-derived dopaminergic or non-dopaminergic neurons did not show alpha-synuclein positive inclusion bodies in normal mouse, but reduced the PD symptoms in PD rat model [35]. This result further showed the proof of principle of using iPSC-derived neurons for neurodegenerative diseases.
In order to use iPSCs in treating human disease, it is important to first assess the safety of the cells for clinical applications. In general, retro- or lentiviral transduction systems are used to generate iPSCs. However, those viral systems can cause random chromosomal integration, which may result in unpredictable genetic dysfunction [10]. To avoid insertional mutagenesis by retroviral vector, several approaches have been developed to generate iPSCs, such as plasmid transfection, non-integrating episomal vector transfection, and piggybag transposon [36–38]. Despite of these efforts, there were still safety concerns related to the use of virus and potentially harmful chemicals. Kim et al. successfully generated hiPSCs by directly delivering defined reprogramming proteins into fibroblasts [39]. The authors used cell-penetrating peptides that contain high proportion of basic amino acids to deliver the defined proteins into the cells through the cell membranes. The hiPSCs were then differentiated into functional dopaminergic neurons [40]. NPCs derived from protein-based iPSCs showed similar expandability compared to those derived from hESCs. Dopaminergic neurons derived from these iPSCs showed gene expression and electrophysiologic property similar to midbrain dopaminergic neurons. Furthermore, the neuronal cells showed the rescue of motor deficit when transplanted into PD rat models, showing the functional significance of the in vitro differentiated cells. However, this protein-based reprogramming approach must overcome the extremely low reprogramming efficiency of 0.001%, which is 10 – 100 times lower than that of virus based protocols [39]. Secondly, selection of desired and terminally differentiated cells is essential for clinical safety. Research studies reporting the tumorigenicity of mouse iPSCs have been published [41]. The iPSCs were differentiated into secondary neurospheres to examine the propensity to form teratoma based on the reprogramming method and differentiation potential. The result showed that the aggressiveness of tumor formation correlated with the number of residual iPSCs in neurospheres. In addition, in vivo study where dopaminergic neurons derived from protein-based iPSCs were used, three out of 12 transplanted rats died of tumor growth before 8 weeks of graft. This suggests that additional selection is needed to eliminate immature neuronal cells even when protein-based reprogramming approaches are used to make iPSCs [40].
Conclusion
In recent years, direct conversion from differentiated cells into specific neurons was reported [42, 43]. Pfisterer et al. directly reprogrammed human fibroblasts into the dopaminergic neurons with neuronal transcription factors of Ascl1, Brm2 and Myt1l, and two additional genes Lmx1a and FoxA2 [42]. AD patient fibroblasts were converted into functional neurons and the authors suggested the possibility of direct reprogramming as an alternative disease model [44]. Son et al. generated motor neurons also using the direct reprogramming approach [45]. The authors demonstrated the functionality of induced motor neurons (iMNs) and migration of the transplanted iMNs into the ventral horn of the spinal cord of the chick embryo. The similar response to a degenerative stimulus resembling ALS was achieved in iMNs like the embryo-derived motor neurons, demonstrating the significance of direct reprogramming for in vitro modeling. Thus, the direct neurogenesis is an appealing alternative to iPSCs.
The potential of iPSCs to treat the patients with neurodegenerative disease is enormous. Induced stem cells can be introduced into clinical applications in several different ways, such as disease modeling, drug screening, and cell replacement therapy. However, challenges such as those ensuring clinical safety, should be overcome. The protocols of reprogramming and differentiation into desired cells should be optimized to increase efficiency and to eliminate tumor formation. Even though it may take a long time to address the drawbacks of this technology, iPSC-based applications may hold the key to curing neurodegenerative disorders.
Key points.
The technology of reprogramming into the induced pluripotent stem cells provides a novel opportunity for modeling the neurodegenerative diseases.
Human iPSCs from neurodegenerative diseases will be used for screening novel compound for neurologic illness.
Specific cells derived from iPSCs might be used as the cellular resources for the treatment of neurodegenerative diseases.
Reprogramming methods need improvement to isolate clinically safe iPSCs.
Direct reprogramming to neuronal cells is the alternative to compensating the shortcomings of iPSCs technologies.
ACKNOWLEDGEMENT
IHP is supported by Yale School of Medicine, Child Health Research Award from Charles Hood Foundation and NIGMS GM099130-01.
Footnotes
Conflict of Interest:
There are no conflicts of interest.
References
* of special interest
** of outstanding interest
- 1.Ferri CP, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunn JS, et al. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70(3):353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13(5):497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143(4):508–525. doi: 10.1016/j.cell.2010.10.008. This is an excellent review on reprogramming and pluripotency. Readers who do not have much background in stem cell field are recommended to read this review article.
- 7.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136(4):509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulting GL, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Young AB. Four decades of neurodegenerative disease research: how far we have come! J Neurosci. 2009;29(41):12722–12728. doi: 10.1523/JNEUROSCI.3767-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips W, et al. Animal models of neurodegenerative diseases. Methods Mol Biol. 2009;549:137–155. doi: 10.1007/978-1-60327-931-4_10. [DOI] [PubMed] [Google Scholar]
- 13. Dimos JT, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. This is the first article to generate iPSCs from fibroblasts of ALS patients.
- 14.Ebert AD, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soldner F, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorson CL, Rindt H, Shababi M. Spinal muscular atrophy: mechanisms and therapeutic strategies. Human molecular genetics. 2010;19(R1):R111–R118. doi: 10.1093/hmg/ddq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osafune K, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26(3):313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 18.Hu BY, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DS, et al. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 2010;6(2):270–281. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- 20.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. The Journal of clinical investigation. 2005;115(6):1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yagi T, et al. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20(23):4530–4539. doi: 10.1093/hmg/ddr394. This paper examplfies the strategy using the iPSCs from a defined and familial patient to study the late on-set Alzheimer's disease.
- 23.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 24.Laustriat D, Gide J, Peschanski M. Human pluripotent stem cells in drug discovery and predictive toxicology. Biochem Soc Trans. 2010;38(4):1051–1057. doi: 10.1042/BST0381051. [DOI] [PubMed] [Google Scholar]
- 25.Braam SR, Passier R, Mummery CL. Cardiomyocytes from human pluripotent stem cells in regenerative medicine and drug discovery. Trends Pharmacol Sci. 2009;30(10):536–545. doi: 10.1016/j.tips.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Karussis D, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J, et al. Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neuroscience letters. 2007;423(1):58–61. doi: 10.1016/j.neulet.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 28.Kelly S, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corti S, et al. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. The Journal of clinical investigation. 2008;118(10):3316–3330. doi: 10.1172/JCI35432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SC, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 31.Perrier AL, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(34):12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XJ, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23(2):215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 33.Swijnenburg RJ, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wernig M, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hargus G, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaji K, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458(7239):771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rhee YH, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. The Journal of clinical investigation. 2011;121(6):2326–2335. doi: 10.1172/JCI45794. The reprogramming using proteins is considered as a clincally safe approach to produce patient's iPSCs. However, in this report when the protein-derived iPSCs were differentiated into neurons for use as cell therapy in murine Parkinsonian model, tumor formation was still observed.
- 41.Miura K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 42.Pfisterer U, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambasudhan R, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9(2):113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qiang L, et al. Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell. 2011;146(3):359–371. doi: 10.1016/j.cell.2011.07.007. Direct reprogramming is an emerging technique to skip the intermediate pluripotent stem cell stages to convert the differentiated cells into another cell types of interest. In this paper, authors showed the direct neurogenesis of Alzheimers' patients skin cells.
- 45.Son EY, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9(3):205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]