Abstract
Studies in vitro and in vivo continue to identify complex regulated mechanisms leading to overt fibrocalcific aortic valve disease (FCAVD). Assessment of the functional impact of those processes requires careful studies of models of FCAVD in vivo. Although the genetic basis for FCVAD is unknown for most patients with FCAVD, several disease-associated genes have been identified in humans and mice. Some gene products which regulate valve development in utero also protect against fibro-calcific disease during postnatal aging.
Valve calcification can occur via processes that resemble bone formation. But valve calcification can also occur by non-osteogenic mechanisms, such as formation of calcific apoptotic nodules. Anti-calcific interventions might preferentially target either osteogenic or non-osteogenic calcification.
Although FCAVD and atherosclerosis share several risk factors and mechanisms, there are fundamental differences between arteries and the aortic valve, with respect to disease mechanisms and responses to therapeutic interventions. Both innate and acquired immunity are likely to contribute to FCAVD. Angiogenesis is a feature of inflammation, but may also contribute independently to progression of FCAVD, possibly by actions of pericytes that are associated with new blood vessels. Several therapeutic interventions appear to be effective in attenuating development of FCAVD in mice. Therapies which are effective early in the course of FCAVD, however, are not necessarily effective in established disease.
Keywords: Aortic stenosis, aortic valve, calcification
Fibrocalcific aortic valve disease (FCAVD) is a growing health problem in the developed world.1,2 The incidence of overt FCAVD rises exponentially with age, with a steep increase after 65 years of age.3 As developed societies age, therefore, the burden of disease is also expected to increase. Recognition of the emerging importance of FCAVD, together with seminal studies pointing to a complex orchestrated sequence of processes which culminate in a lethal phenotype, have spurred interest in elucidating the nature of the disease and in identifying effective preventive interventions.
In a recent series, mechanisms of cardiovascular calcification were reviewed, including emphasis on events in the aortic valve.4–9 In this Compendium, we will review the rationale for studies in mice, the growing complexity of assessing aortic valve function in vivo, emerging mechanistic themes relating to the pathophysiology of FCAVD, and studies designed to ameliorate the course of FCAVD in mice.
Studies in whole animals
Genetic factors that predispose to FCAVD have been identified.8 Overt FCAVD, however, usually appears after a long latency, with incomplete penetrance. Mechanisms by which a given gene (product) affects the course of FCAVD can not be ascertained fully in vitro. A clinical risk factor for FCAVD, such as hypertension, itself typically has long disease latency. Cellular and molecular influences of a specific mediator of hypertension, e.g. angiotensin II, can be evaluated in vitro. But recapitulation of the complex pro-FCAVD milieu of hypertension can not be replicated in vitro. In addition, it can be difficult to determine whether changes that are observed in vitro are important in the pathophysiology of a disease, or whether they represent a compensatory protective response to disease processes. Studies in whole animals allow integration of biomechanical, humoral, neural, and inflammatory mechanisms into a broader mechanistic understanding of the pathophysiology of FCAVD. Longitudinal studies in experimental animals may serve as a platform for testing of translational hypotheses in humans at risk for development of overt FCAVD.
Functional impact of FCAVD
Foremost among the advantages of studies in whole animals is the ability to assess the impact of risk factors upon aortic valve function and biventricular responses to valve disease. Commonly employed tools for assessment of aortic valve function in humans can be applied in mice to place experimental findings in a clinically relevant context.
Echocardiography is the mainstay of quantitative assessment of aortic valve function in mice. A comprehensive report describes morphologic and functional responses of the aortic valve to aging, from fetal development to senescence.10 Aortic valve cusp motion can be observed using 2D-echocardiography, but quantitation is challenging at physiologic heart rates, even with newer high-resolution equipment. M-mode echocardiography provides greater spatial and temporal resolution than 2D echocardiography, is feasible in almost all mice, and normal values have been defined.11 M-mode assessment of stenosis severity has been validated against hemodynamic measurements in mice, in the absence of aortic regurgitation.12
Technical considerations in mice
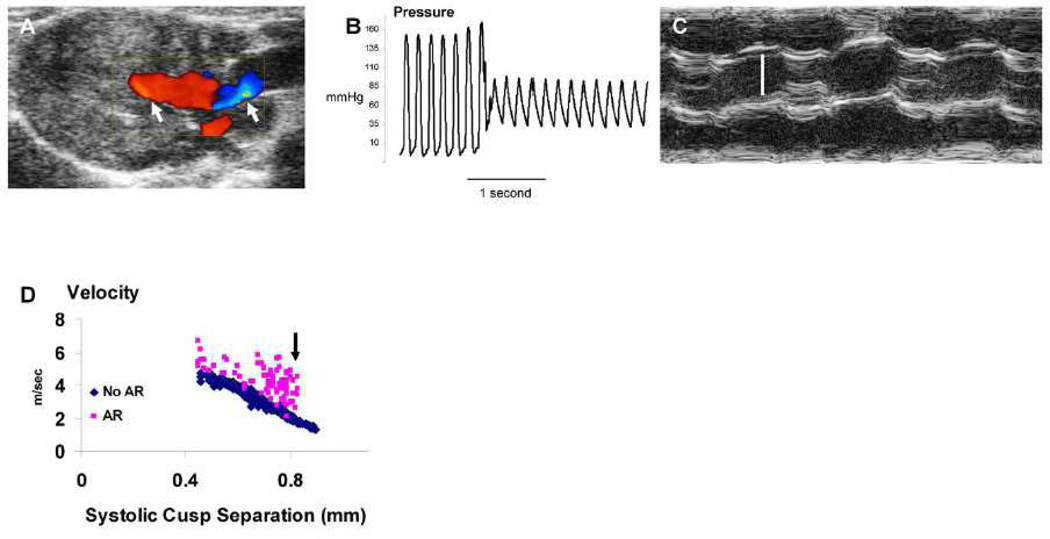
Some technical challenges that apply to studies in mice are summarized in Table 1. Accurate measurement of transvalvular pressure gradient obviously can be useful for assessment of aortic stenosis severity. In humans and mice, intraventricular flow acceleration can produce a significant subvalvular gradient, which is optimally measured in the left ventricular outflow tract, using depth-gated (pulse-wave) Doppler velocimetry. In hyperdynamic states, such as hypertrophic obstructive cardiomyopathy or aortic regurgitation, a very significant intraventricular cavity (subvalvular) pressure gradient can occur. Thus, accurate assessment of the severity of aortic valve stenosis requires subtraction of the intraventricular cavity gradient from the total left ventricle-to-aorta pressure gradient. When aortic regurgitation is known or suspected to be present, reliance solely on assessment of left ventricle-to-aorta pressure gradient will be prone to overestimation of aortic stenosis severity, even when invasive methods are utilized to measure the gradient (Figure 1.)
Table 1.
Technical challenges of echocardiographic assessment of aortic valve function in mice.
| Limitation | Consequence |
|---|---|
| Limited 2-D resolution | M-mode required |
| Limited color Doppler frame rates | Underestimate aortic regurgitation |
| Lack of continuous-wave Doppler* | Pulse wave-derived valve gradients |
| Oversized pulse-wave gates | Overestimate aortic stenosis |
| Non-parallel signal alignment | Overestimate aortic stenosis |
When using ultra-high resolution instruments designed primarily for studies in mice.
Figure 1. Overestimation of aortic stenosis severity in the presence of aortic regurgitation.
A: Color Doppler from an eNOS−/− mouse demonstrates moderate-to-severe aortic regurgitation (arrows). B: LV-to-aorta pressure gradient = 55 mmHg in the same mouse. C: M-mode echocardiogram demonstrates normal aortic cusp separation = 1.2 mm (vertical white bar), i.e. absence of aortic stenosis, in the same mouse. D: Relationship between aortic valve systolic cusp separation and continuous-wave Doppler transvalvular blood velocity in the presence (N = 86) or absence (N = 699) of aortic regurgitation in fat-fed Ldlr−/−Apob100/100/Mttpfl/flMx1Cre+/+ mice, ages 3 – 12 months. Arrow indicates data from mice that have aortic regurgitation, but mild or absent aortic stenosis, with significantly elevated transvalvular velocities. In all cases, the angle of exit between the Doppler line and the systolic blood flow vector was ≤ 60°.
Late in the course of valvular heart disease caused by aortic stenosis, left ventricular systolic function can become significantly impaired. Decreased stroke volume and reduced rate of ejection of blood from the left ventricle can result in a modest transvalvular gradient, even when severe aortic stenosis is present. In humans, that combination of findings, known as “low-flow low-gradient stenosis” is associated with a particularly dire prognosis.13 The phenomenon has been observed anecdotally in mice, but comprehensive studies have not yet been published.
Continuous-wave Doppler is the technique of choice for non-invasive assessment of transvalvular gradients in humans. Continuous-wave Doppler capability, however, is not currently offered on high-resolution echo imagers designed for studies in mice. Use of continuous-wave Doppler in mice thus requires equipment designed for use in humans, which, provides lower spatial and temporal resolution.
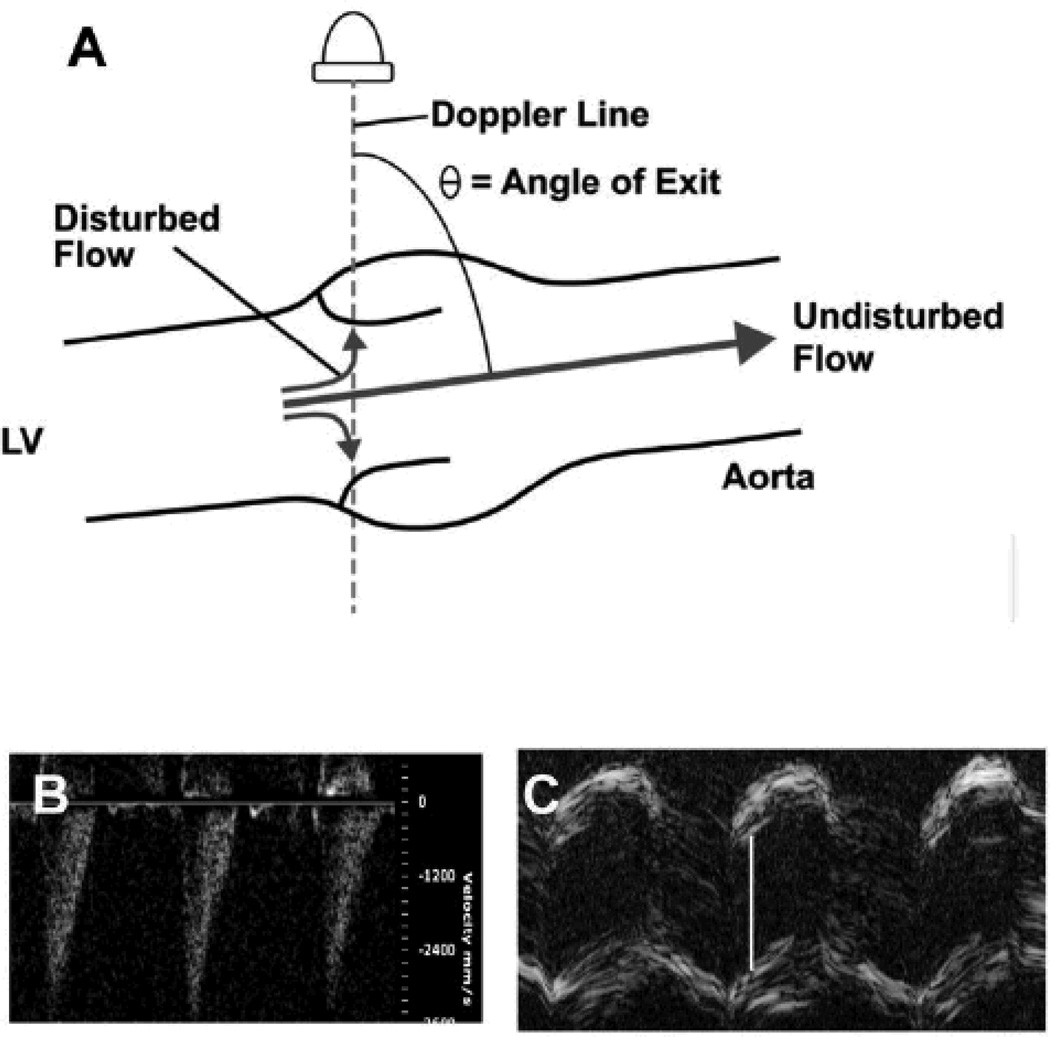
When the direction of blood flow deviates from the Doppler line, angle-correction must be employed in order to report accurate blood velocities. Angle correction is prone to magnification of errors when the angle approaches 90 degrees, especially with disturbed blood flow (Figure 2). These potential problems can be minimized by optimizing the angle of exit between transvalvular blood flow and the Doppler line. If color Doppler screening reveals no significant aortic regurgitation, and 2-D B-mode images confirm the presence of normal left ventricular systolic function, then Doppler velocimetry can produce a reasonable measurement of severity of aortic stenosis (Figure 1D). However, we are not aware of a published, experimentally validated, “maximum acceptable” angle of exit, below which accurate assessments of stenosis severity can be achieved with confidence, using Doppler velocimetry in mice.
Figure 2. Overestimation of stenosis severity.
A. Calculation of transvalvular blood flow velocity requires angle correction when the direction of flow is not colinear with the line of Doppler interrogation. In the illustration, the angle of exit of presumed blood flow is 75° . Thus, measured velocity would be divided by the cosine of 75°, = 0.26, in order to compute actual flow velocity. When transvalvular blood flow is disturbed, a portion of the flow jet will be directed more parallel to the Doppler line, and will be detected as a higher velocity. In this case, correction for a presumed angle of exit of 75° will overestimate true blood velocity by a factor of about 4. B. Doppler velocimetry from a normal mouse, with neither aortic stenosis nor regurgitation. The angle between the Doppler cursor and the presumed direction of transvalvular flow was 75° (image not shown). Peak velocity is reported as = 3.6 m/s, erroneously predicting a peak valve gradient of 52 mmHg. C. M-mode echocardiogram of the aortic valve in the same mouse. White bar indicates aortic cusp separation = 1.3 mm, which is normal. Color Doppler and magnetic resonance imaging both confirmed the absence of aortic regurgitation (images not shown).
Severity of stenosis can also be ascertained by utilizing the excellent spatial resolution provided by high-field magnetic resonance imaging.14 Magnetic resonance imaging carries the added advantage of allowing precise measurements of stroke volumes of both ventricles, which facilitates quantitation of the severity of aortic regurgitation.15 Invasive hemodynamic measurements of left ventricle-to-aorta pressure gradients are of great value, and avoid some of the technical drawbacks of Doppler echocardiography. Nevertheless, invasive measurements are still prone to over- or underestimation of stenosis severity in the presence of aortic regurgitation or left ventricular systolic dysfunction (Figure 1). When severe aortic stenosis is present invasive techniques are technically challenging, require deep general anesthesia, and may result in tissue injury.
A summary of the attributes of methods used for assessment of valvular heart disease in mice in vivo is provided in Table 2.
Table 2.
Assessment of valvular heart disease in mice in vivo.
| M-mode echo |
2D Echo |
Doppler Echo |
MRI | Catheterizatio n |
|
|---|---|---|---|---|---|
| Cost | +++ | +++ | +++ | ++++ | + |
| Depth of Sedation | + | + | + | ++ | ++++ |
| Valve Morphology | ++ | ++ | − | +++ | − |
| Severity of Stenosis | +++ | + | +++A | +++ | ++++A |
| Severity of Regurgitation | − | − | ++ | ++++ | − |
| LV Anatomy | ++ | ++++ | − | ++++ | − |
| LV Function | ++ | ++++ | +++ | ++++ | ++++ |
| RV Function | + | ++ | +++ | ++++ | ++++ |
| Production of Tissue Injury | − | − | − | − | +++ |
when aortic regurgitation is known to be absent
Disadvantages of studies in mice
Although there are anatomical and pathophysiological similarities between murine aortic valves and human aortic valves, there also are important differences. The aortic and ventricular sides of the valve are exposed to different hemodynamic stimuli and, in humans, the thickness of the valve cusps allows a gradient of responses by VICs. The gossamer-like structure of murine aortic valve cusps may not allow a gradient of responses by VICs.
As in humans, there is significant heterogeneity in the severity and rate of progression of FCAVD among individual mice, even in carefully controlled studies of syngeneic mice. In fact there can be significant heterogeneity of histopathological changes within the valve of a single mouse. Postmortem histologic and gene-expression studies offer only a single “snapshot” characterization of disease-associated events. Thus, it can be challenging to identify the cellular and molecular mediators that govern the initiation and propagation of FCAVD, when whole animals are used for study. Newer imaging methods may help clarify the temporal relationship between processes such as inflammation,16 valve calcification, and valve dysfunction.
Mechanistic Themes
Mechanical factors may influence the course of FCAVD
In vascular endothelial cells, longitudinal shear increases the activity and expression of eNOS, and attenuates inflammation.17 Mechanistic similarities between vascular disease and valve disease suggest that longitudinal shear stress could be an important regulator of valve homeostasis.
Congenitally bicuspid aortic valves are more prone than trileaflet valves to develop clinically important FCAVD.1,18 FCAVD occurs at a much younger age in humans with bicuspid aortic valves than in those with trileaflet valves.19 Bicuspid aortic valves often develop predominant aortic stenosis, but predominant aortic regurgitation is also common in patients with bicuspid aortic valves.20 Although not rigorously tested, the prevailing explanation for susceptibility to FCAVD in patients with bicuspid aortic valves relates to disruption of flow across the abnormal valve, resulting in diminished longitudinal shear along the valve surface. The concept is supported by mathematical modeling of regional stresses in bicuspid and trileaflet aortic valves,21 and by ex vivo experimental models that simulate congenital bicuspid aortic valves.22
Asymmetry of aortic valve endothelium and shear stress
In humans, FCAVD usually begins on the aortic side of valve cusps, with relative sparing of the ventricular side, which is exposed to high shear induced by ejection of ventricular blood.19 Recently, several investigators have sought to identify fundamental differences between the endothelium lining the ventricular and aortic sides of the valve. Following careful isolation of endothelium from both sides of the valve,23 targeted approaches and high-throughput molecular screening have provided key insights into molecular mechanisms which regulate paracrine signals that originate from endothelium.
Expression of antioxidant enzymes is relatively high in endothelial cells isolated from the aortic side of the normal valve.24 In contrast, expression of pro-inflammatory molecules does not differ between the aortic and ventricular sides of the valve.24 The findings are surprising because disturbed flow on the aortic-side endothelium would be expected to induce both inflammation and increase oxidative stress, and suggest that increased antioxidant enzyme levels may serve as an adaptive mechanism to protect the valve in this region.
Expression of inhibitors of osteogenic signaling is significantly reduced in endothelium from the aortic side of the valve.24,25 Inhibitors of bone morphogenetic protein (BMP) signaling, such as BMP-binding endothelial regulator, and noggin, are reduced in endothelium from the aortic side of the valve, even in healthy/unstressed aortic valves.25 Molecular mechanisms underlying these changes are not clear. Given the important role of osteogenic morphogens in the initiation and progression of FCAVD, identification of novel interventions to increase expression of BMP antagonists on the aortic side of the valve may prove to be useful in preventing initiation of aortic valve calcification.
Studies to address the importance of endothelial cell asymmetry have usually been performed using valves from large mammals. It is possible that the proximity of the two endothelial cell layers to one another in murine aortic valves exposes diminishes or eliminates some of the endothelial asymmetry observed in valves from larger mammals. We note, however, a study which reported that marrow-derived progenitor cells home preferentially to the ventricular side of the uninjured aortic valve in wild-type mice, indicating the plausibility of asymmetrical responses in murine aortic valves.26
Valve homeostasis is regulated by epigenetic events
Epigenetic modifications are emerging as major regulators of gene expression in health and disease.27–29 Alterations in histone acetylation, histone methylation, and DNA methylation can have a profound impact on transcriptional responses to multiple stimuli, but the interaction between these regulatory mechanisms is not understood well.
Knockdown of SIRT1 (an NAD+-dependent histone deacetylase) accelerates smooth muscle cell calcification in vitro.30 Furthermore, loss-of-function mutations in SIRT1 are associated with coronary artery calcification.31 Depletion of endothelial SIRT1 increases vascular inflammation.32 In thoracic aortic aneurysms associated with bicuspid aortic valves, levels of the injury-response element pSmad2, and its translocation to the nucleus, are increased in smooth muscle cells.33 In addition, hyperacetylation of histone 3K9/14 and hypermethylation of H3K4 (both of which can be permissive for transcription factor binding)appear to contribute to the constitutive expression of Smad2.33 Little is known, however, about the role of histone deacetylases, histone acetyltransferases, and histone methyltransferasesin regulation of aortic valve calcification or fibrosis. Studies which examine structural and functional consequences of altering levels of histone-modifying enzymes in vivo will be critical in determining whether these processes are viable therapeutic targets in FCAVD.
While there are extensive data implicating changes in DNA methylation in altered transcriptional patterns reported in cancer,34 stroke,35 and atherosclerosis,36, data examining the role of DNA methylation in FCAVD are only beginning to emerge. For example, expression of the pro-inflammatory enzyme 5-lipoxygenase is inhibited by methylation of its promoter,37 and hypomethylation of the 5-lipoxygenase promoter is associated with consequent increases in mRNA levels in inflammatory cells.38 This phenomenon is not likely to be restricted to inflammatory cells, as inhibition of DNA methylation results in increased 5-lipoxygenase expression in cultured VICs.38
There are accumulating data from other tissues suggesting that DNA methylation may play an important role in transcriptional regulation of other genes implicated in FCAVD. First, expression of matrix Gla protein (Mgp) —a potent inhibitor of BMP signaling—can be markedly reduced by promoter hypermethylation.39 Second, expression of alkaline phosphatase is dramatically increased in osteogenic cells following treatment with compounds that induce DNA hypomethylation,40 which suggests that tonic suppression of expression of alkaline phosphatase may protect against initiation of aortic valve calcification.
MicroRNAs contribute to epigenetic control of gene expression. Using high-throughput, non-biased screening methods, 30 shear-sensitive, and 3 side-sensitive (i.e., ventricular- versus aortic-side endothelium) microRNAs have been identified in aortic valve endothelial cells.41 Identifying mechanisms whereby these microRNAs exert their deleterious or protective effects is challenging, because a single microRNA can alter expression of hundreds of target genes.42,43 Prospectively, the combination of experimentally altering microRNA levels with high-throughput molecular screening will be critical to determining whether pharmacological or genetic manipulation of microRNAs can orchestrate a coordinated, protective phenotype and ultimately become an attractive therapeutic target in patients with FCAVD.
Mechanical properties of the aortic valve
Cardiovascular tissue, in general, responds to increased radial stress by increasing its thickness, which acts to buffer or neutralize mechanical stress. However, mechanisms by which mechanical forces invoke cellular and molecular responses within the aortic valve are not yet well understood. The eccentric shape (see Figure 3), heterogeneous deformation of the valve during the cardiac cycle, and oscillating direction of pressure-stress all render mathematical modeling problematic. Clinical translation will therefore require meticulous experimental validation of mathematically modeling of aortic valve mechanics.
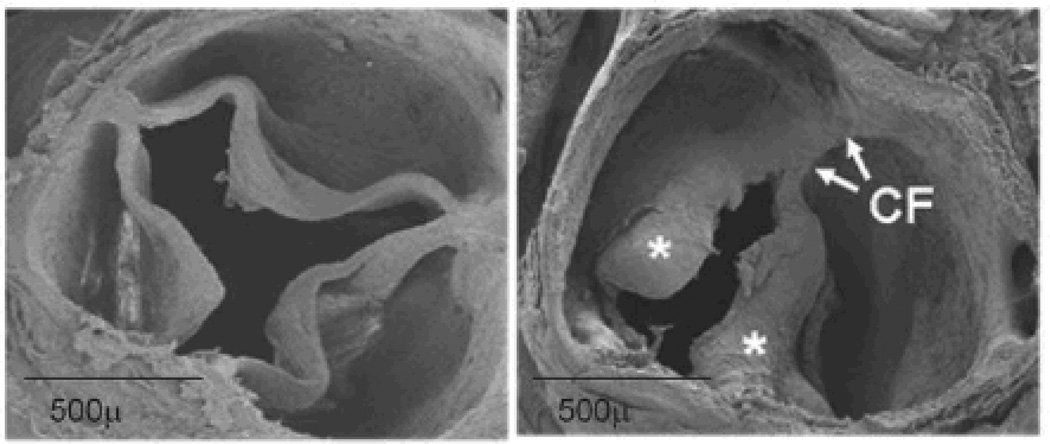
Figure 3. Scanning electron micrographs of trileaflet (left) and bicuspid (right) aortic valves.
The eccentric structure of valve cusps and their attachments to the aortic annulus present challenges for quantitation of mechanical stresses or severity of stenosis. Left: normal trileaflet aortic valve at 12 months of age. Right: congenitally bicuspid aortic valve from an eNOS−/− mouse, at 6 months of age, with cusp thickening (*) and cusp fusion (CF).
Studies in cell culture are clarifying ways in which valve cells respond to changes in their physical environment. VICs that are grown in standard growth media, on a stiff synthetic matrix, rapidly transdifferentiate to myofibroblasts, assemble in nodules, undergo apoptosis, and calcify.44 The processes are attenuated when VICs are cultured on a soft matrix.45 Reduction of matrix stiffness, using photo-responsive material, can actually reverse the processes that lead to formation of calcific nodules.46 Contractile proteins play a critical role in formation of calcific nodules, which are produced by VICs in vitro, after transdifferentiation of fibroblasts to myofibroblasts. Expression47 and polymerization48 of α-smooth muscle actin are critical to formation of calcific nodules.
In a different setting, where VICs are grown on a collagen matrix with variable stiffness, supplementation of culture medium with pro-osteogenic factors (ascorbate, β-glycerophosphate, dexamethasone) induces VICs to differentiate into osteoblast-like cells, which produce calcification of the extracellular matrix.48 Osteogenic calcification, in apparent contrast to apoptosis-related calcification, is decreased by a stiffer matrix composition. The relative importance of the two mechanisms (myofibroblast differentiation with formation of apoptotic calcific nodules vs. osteogenic transdifferentiation leading to bone-like calcification), in vivo, is not known.
Translation of findings regarding the impact of matrix stiffness upon VIC behavior from cell culture to studies in native aortic valves from FCAVD-prone mice could provide significant new insight about mechanisms that are operative in development of FCAVD. In normal valves, matrix elements such as collagen and elastin are organized and stratified. In diseased valves, matrix elements are disorganized, and composition differs from that found in normal valves.49 The impact of matrix remodeling in the aortic valve has been addressed using atomic force microscopy in cryosectioned murine aortic valves, in which increased cusp stiffness correlates with increased abundance of extracellular matrix in several FCAVD-prone mouse strains.50
Studies using micropipette aspiration of excised whole-valves from normal mice demonstrate a gradient of decreasing mechanical stiffness from cusp base to cusp tip.51 Surprisingly, perhaps, mechanical stiffness of the valve annulus and valve cusps appear to decrease with age in normal mice, findings which correlate with replacement of collagen by proteoglycan at the annular site of cusp attachment.51
Individual layers of the valve respond differently to applied mechanical stress. Valve tissue nearest the left ventricle has a lower elastic modulus than tissue nearest the aortic side of the valve.52 The findings provide a mechanical context for the cellular and molecular asymmetries described above. Evolution of methods for assessment of the material properties of the aortic valve will be of great importance for elucidating mechanisms by which mechanical forces are transduced into cellular and molecular events, and vice versa.
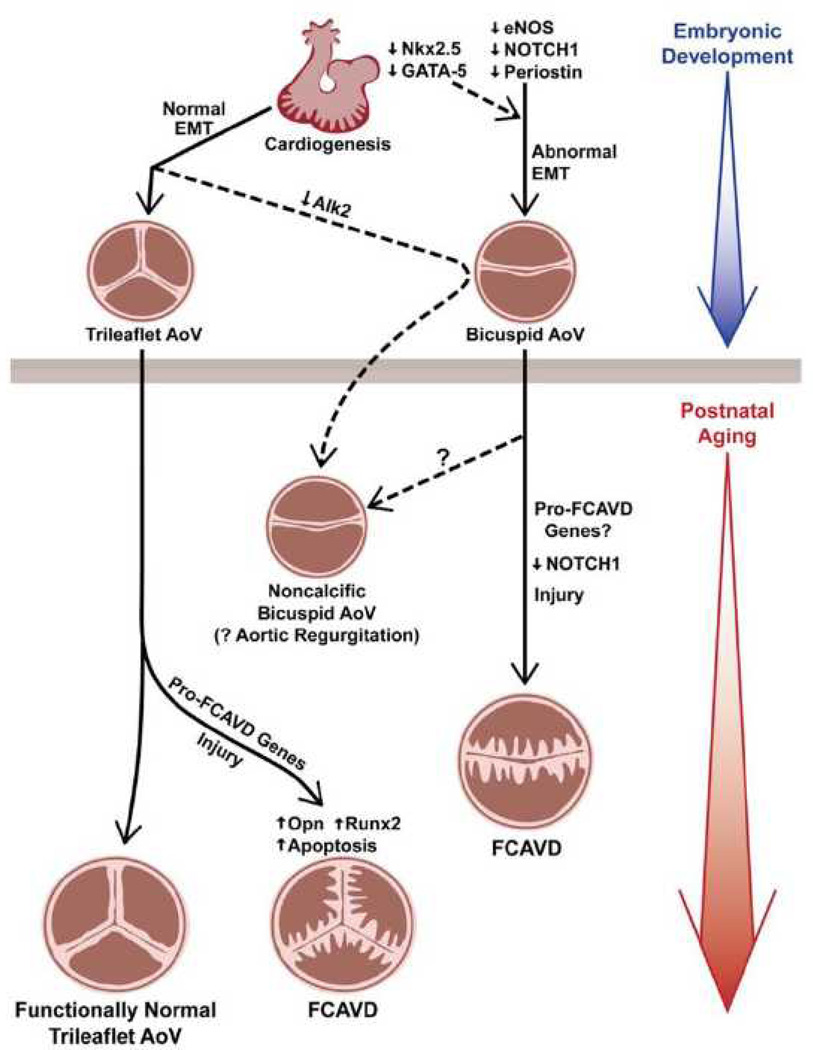
Bicuspid aortic valves (Figure 4)
Figure 4. Interactions between genotype and environmental factors in the development of FCAVD.
Mutations which interfere with epithelial-to-mesenchymal transformation (EMT) predispose to formation of a congenitally bicuspid valve and, in some cases, predispose to calcific aortic stenosis. Knockdown of Alk2 perturbs valve development following EMT and predisposes to development of a functionally bicuspid aortic valve, but does not produce overt valve calcification. Mild aortic valve sclerosis is a predictable consequence of normal aging. But in the presence of valve injury, or disease-prone genes, congenitally normal valves can develop overt FCAVD. Opn osteopontin. See text for additional abbreviations.
As discussed above, perturbation of blood flow occurs in patients with bicuspid aortic valves,53 and has been proposed as an explanation for the increased incidence and severity of aortic stenosis in patients with bicuspid aortic valves. That longstanding belief, however, has not been rigorously tested in humans or in experimental animals. There is ongoing debate regarding the relative importance of cusp number vs. underlying genotype, in humans with bicuspid aortic valve. In patients with bicuspid aortic valves, predilection for development of aortic root aneurysm follows a strong familial pattern.54 The risk for development of clinically significant aortic valve dysfunction, however, is less predictable, even within single families with high prevalence of bicuspid aortic valve.55 Thus, environmental and epigenetic factors influence the risk for overt valve disease, perhaps more so than the risk for development of aortic aneurysms, in patients with bicuspid aortic valve.
The discovery of mouse strains with increased prevalence of bicuspid aortic valves offers the opportunity to elucidate mechanisms of disease in syngeneic kindreds with either bicuspid or trileaflet aortic valves. About 30% of mice that are deficient in endothelial nitric oxide synthase (eNOS−/− ) are born with bicuspid aortic valves.56,57 When fed a high-fat diet, eNOS−/− mice with bicuspid valves, but not trileaflet valves, develop calcific aortic stenosis by 6 months of age.58 Similarly, preliminary reports suggest that experimentally altering endogenous inhibitors of nitric oxide synthases (e.g., asymmetric dimethylarginine, ADMA) in mice with trileaflet valves does not alter the early initiation phases of CAVD.59 The combination of prolonged hypercholesterolemia and elevated ADMA levels for greater than 6 months, however, does appear to accelerate progression of CAVD in mice with trileaflet valves.59 Those findings suggest that deficiency of endothelium-derived NO alone is not sufficient to cause physiologically important aortic valve calcification in both bicuspid and trileaflet aortic valves, but that NO deficiency augments valve calcification in the presence of hypercholesterolemia.
A preliminary report suggests, however, that eNOS−/− mice with bicuspid valves are prone to develop aortic valve fibrosis and calcification, even in the absence of hypercholesterolemia.60 Interestingly, fibrosis, but not calcification, was increased in both bicuspid and trileaflet aortic valves, which suggests a gene effect, independent of cusp number.
Other genetically engineered mice that are prone to develop bicuspid aortic valves offer the opportunity to understand further the relative role of genotype (or gene dose) vs. aortic cusp number in development of overt FCAVD. Bicuspid valve occurs in up to 11% of mice that are haploinsufficient for the transcription factor Nkx2.5, and the prevalence is influenced by background strain.61 Although loss-of-function mutations in Nkx2.5 are associated with other congenital heart malformations, it is not yet clear whether bicuspid aortic valve is a manifestation of Nkx2.5 deficiency in humans.
Humans that are heterozygous for some decrease-of-function mutations in Notch1, a regulator of cell fate and intercellular communication, are prone to develop bicuspid aortic valves.62 Notch signaling represses osteogenic signaling in the aortic valve postnatally.63,64 Human carriers of some decrease-of-function Notch mutations develop overt FCAVD, even in trileaflet aortic valves.62 In mice, suppression of embryonic Notch1 signaling by knockdown of the gene encoding periostin causes cusp fusion and upregulation of osteogenic signaling, leading to FCAVD in postnatal life.65 Surprisingly, however, fibrotic valve changes caused by a high-fat diet are actually attenuated by knockdown of the gene encoding periostin, a paradox possibly related to compartmentalization of periostin expression in the normal aortic valve.66
The transcription factor GATA-5 regulates both eNOS signaling and Notch1 signaling during embryonic development. Deficiency of GATA-5 in mice results in increased incidence of bicuspid aortic valves, in association with hypoplastic heart syndrome, in mice.67 Bicuspid aortic valves with varying degrees of dysfunction have been reported in humans with rare GATA-5 mutations.68 Thus, factors such as eNOS, Notch-1, and periostin all regulate the critical phase of cardiac morphogenesis known as epithelial-to-mesenchymal transformation (EMT). Deficiency of any one of those factors increases the likelihood of congenital aortic valve anomaly, and also accelerates osteogenic valve calcification during adult life. It is not yet known whether bicuspid aortic valves that develop due to deficiency of Nkx2.5 or GATA-5, which also regulate EMT, are prone to develop overt valve calcification.
Are valve calcification and valve dysfunction mechanistically linked?
One might expect that early stages of aortic valve dysfunction, with alterations in fluid dynamics and mechanical stresses, would promote fibrosis and calcification. At least one study, however, has demonstrated significant aortic valve dysfunction during postnatal life in mice, without evidence of subsequent valve calcification. Knockdown of the gene encoding activin receptor-like kinase-2 (Alk2, also known as activin receptor type I, or Acvr1), which is embryonically activated in the developing heart following epithelial-to-mesenchymal transformation, results in functionally bicuspid aortic valves with a high embryonic penetrance.69 (See Figure 4.) Alk2 knockdown mice that survive to adulthood develop increased systolic pressure gradients across the abnormal aortic valves, and also increased left ventricular stroke volume, which suggests aortic regurgitation and/or mixed valve dysfunction. The knockdown mice also demonstrate some elements of an osteogenic gene expression profile, including increased osteopontin and reduced Sox9. However, expression of the committed osteoblast marker, Runx2, was decreased in Alk2 knockdown mice, and valve calcification was not observed – even during mature adult life.69
Resistance to valve calcification can follow a familial pattern in humans with bicuspid aortic valves, a finding corroborated by absence of NOTCH1 mutations in subjects with bicuspid aortic valve, dilation of the ascending aorta, but absence of detectable aortic valve calcification.70 Those findings in mice and humans suggest that perturbation of flow associated with a bicuspid aortic valve alone is not sufficient to produce valve calcification, and that additional genetic or environmental stimuli may be necessary for development of overt calcific aortic valve disease. It also appears that the specific genetic basis for formation of a bicuspid valve can influence the predilection for valve calcification during postnatal life (Figure 4).
Mice that are haplo-insufficient for elastin (Eln+/−) have normal aortic valve function at birth, and later develop aortic regurgitation.71 The composition and organization of the extracellular matrix are abnormal, and VICs are activated in aortic valves from Eln+/− mice. Neither apoptosis nor valve calcification were reported in that study. Thus, hemodynamically significant valve dysfunction does not inevitably initiate valve calcification, and aortic valve dysfunction is not invariably dependent on valve calcification.
Non-osteogenic calcification
For many years, it was assumed that calcification of the aorta and aortic valve is secondary to cell death, with calcification of “cell debris”.72,73 A simplistic (not necessarily inaccurate) view was that, as cells die in arteries, they may calcify, and “wall off” inflammation.
It seems likely that apoptotic cell death does in fact contribute to calcification of the aorta and aortic valves.74 When smooth muscle cells undergo apoptosis, the cells release matrix vesicles.75 Apoptotic vesicles are present in calcified aortic valves,73 as well as aorta, and may contribute to calcification. TGF-β stimulates, and a caspase inhibitor attenuates, apoptosis and calcification of VICs in vitro.76 Recently, we found that pioglitazone, a peroxisome proliferator associated receptor gamma (PPARγ) agonist, preserves aortic valve function in hypercholesterolemic mice, an effect best explained by attenuation of apoptosis-related calcification in the valve.77
Mgp is expressed in vascular muscle and chondrocytes, and is a potent inhibitor of mineralization.78 Severe arterial calcification develops in Mgp-deficient mice, and the mice usually die within eight weeks of age from aortic rupture. Perhaps paradoxically, apoptotic vesicles that are released by smooth muscle cells contain inhibitors of calcification, including Mgp,75 as well as pro-apoptotic factors. Thus, apoptotic vesicles modulate vascular calcification, but because they contain factors that may both promote and inhibit calcification, their role is not clearly understood.
Based on changes in the signaling pathways that mediate osteogenesis,79 it is clear that “active” calcification is important in the aorta and aortic valve. It is now generally assumed that this mechanism is the predominant mechanism of vascular calcification. We wish to emphasize, however, that the relative importance in development of FCAVD of apoptosis vs. an active osteogenic pathways is not clear.
The aortic valve differs from blood vessels
Aortic stenosis is often associated with dilatation of the ascending aorta. Dilatation of the aorta in patients with aortic stenosis has been attributed to a jet of blood striking the aortic wall, with passive enlargement of the aorta. In our view, an important role for this mechanism is unlikely. A complementary explanation for the association between bicuspid aortic valve and aortic root dilatation is that gene mutations that alter connective tissue in the aortic valve may also alter connective tissue in the aorta.
Several inherited diseases affect both the aortic valve and aorta. Marfan’s syndrome, Ehlers-Danlos syndrome, Loeys-Dietz disease, annuloaortic ectasia, and bicuspid aortic valves are associated with aortic regurgitation and enlargement of the aorta.80 Enlargement of the aorta may precede aortic valve disease, and presumably is the result primarily of aortic disease, rather than valvular disease.81–83
Risk factors for atherosclerosis and aortic valve stenosis are generally similar, but the relative importance of the risk factors differs in relation to association with aortic disease vs. aortic valvular disease. Similarly, the rate and severity of calcification appear to differ in aorta and aortic valve.19
Hypotheses in relation to mechanisms of calcification of the aorta valve often are based on observations in the aorta, with the assumption that mechanisms are similar in the aortic valve and aorta. There are, however, major differences in structure and cellular composition of aortic valve and aorta. Normal aortic valve consists of endothelial cells and valvular interstitial cells. In contrast, the aorta is composed predominantly of smooth muscle cells and connective tissue, with relatively far less endothelium.
Recently, we have reported fundamental differences in responses in mice of the aortic valve and aorta to pioglitazone, a PPARγ ligand.77 Pioglitazone strongly attenuated lipid deposition and calcification of the valve, but had no effect on fibrocalcific events of the aorta.77 There also were striking differences in gene expression between the aortic valve and aorta during hypercholesterolemia and treatment with pioglitazone. The findings imply that one may not be able to predict mechanisms that lead to aortic valve disease based on studies of the aorta.
Hypertension is a strong risk factor for aortic stenosis in humans.19 It is surprising, perhaps, that the impact of hypertension upon the aortic valve has not been extensively studied in mouse models, and that blood pressure itself has not been consistently reported in studies of mouse models of aortic valve disease.
Redox state regulates aortic valve homeostasis
Oxidative stress is increased in valves from patients with end-stage aortic valve stenosis. Several mechanisms may contribute to increased oxidative stress in the valve,84 with evidence implicating uncoupled nitric oxide synthase and reduced antioxidant enzyme activity, and conflicting evidence regarding the role of increased NAD(P)H oxidase activity. 4,85
Three experimental observations have provided insight into the role of oxidative stress in the pathogenesis of aortic valve calcification. First, increases in reactive oxygen species precede aortic valve dysfunction in a mouse model of FCAVD,14 which suggests that oxidative stress is not merely an epiphenomenon associated with valve calcification and dysfunction. Second, experimentally increasing oxidative stress amplifies osteogenic signaling and accelerates calcification of aortic VICs in vitro.86 Third, reducing blood lipids in mice with advanced FCAVD reduces osteogenic signaling in the aortic valve, but does not reduce oxidative stress in these same regions.87 Collectively, these data suggest that, under some circumstances, oxidative stress can amplify osteogenic signaling in the aortic valve, but that elevated oxidative stress does not always initiate or independently propagate calcification in the aortic valve. Future studies aimed at understanding the role of oxidative stress in different cell types (e.g., endothelium versus valve interstitial cells) and different subcellular compartments (e.g., mitochondria, cytosol, or peroxisomes) will be instrumental in determining the therapeutic usefulness of targeting oxidative stress in CAVD.
Inflammation
Many studies indicate that inflammation is associated with aortic valve disease. There are major gaps, however, in our understanding of mechanisms that produce inflammation in the valve, and the role of inflammation in the pathophysiology of aortic valve disease.
Dendritic cells
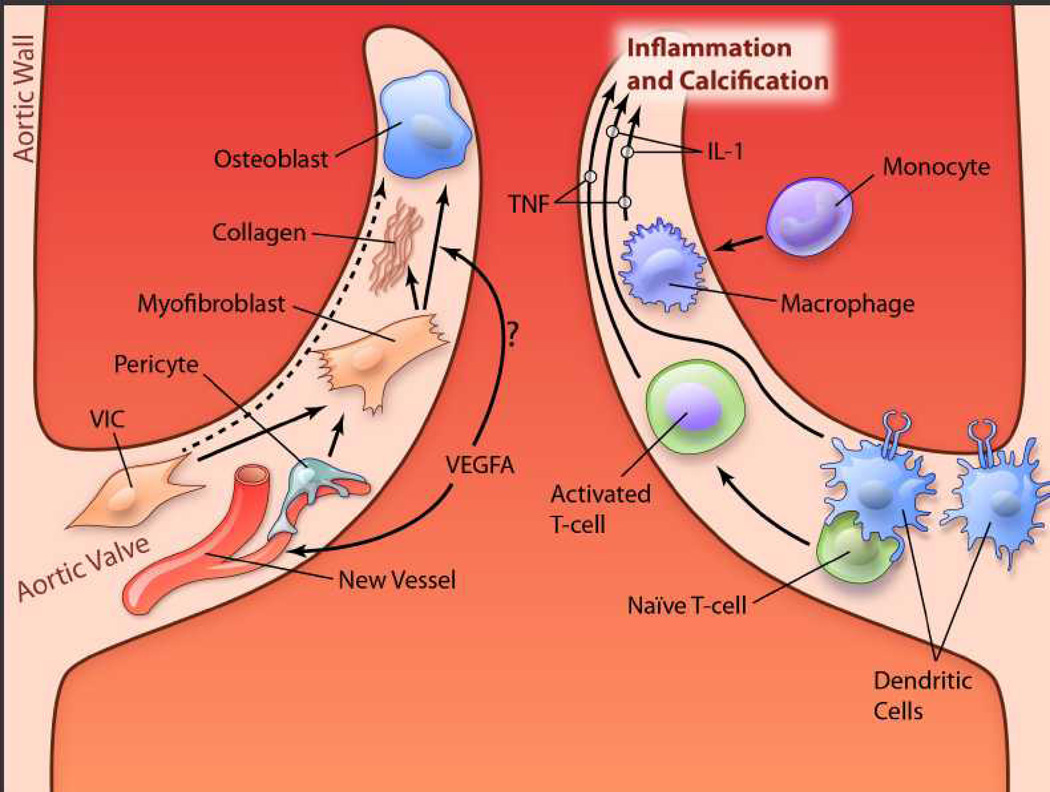
It is intriguing that dendritic cells are not only present in the aortic valve, but are especially dense in the valve base and aortic sinuses.88 (See Figure 5.) Steinman, who received the Nobel Prize for his discovery of dendritic cells, demonstrated that dendritic cells, which are primary subintimal, have processes that extend into the lumen of blood vessels and play a critical role in capture of pathogens and presentation to T-cells.
Figure 5. Speculative and simplified diagram of disease pathways in cusps of the aortic valve.
Left: Valvular interstitial cells (VICs), when activated, transdifferentiate to myofibroblasts or osteoblasts, which produce collagen and calcification. Pericytes, near new blood vessels in the valve, also may transdifferentiate to myofibroblasts. Vascular endothelial growth factor-A (VEGF-A) may stimulate differentiation of myofibroblasts to osteoblasts. Right: When mature dendritic cells, primarily at the base of the valve, bind to naïve T-cells, the T-cells are activated. Interleukin(IL)-1, IL-17, interferon (IFN)-γ and other proinflammatory cytokines produce inflammation and may contribute to calcification. TNF-α, released by monocyte/macrophages and activated T-cells, also plays a key role in inflammation and calcification in the valve. (illustration credit: Ben Smith).
Dendritic cell biology is complex and evolving, and it is now known that there are several types of dendritic cells.89 We will briefly summarize some aspects of dendritic cells, with potential relevance to the aortic valve.
Cardiovascular dendritic cells localize mainly in areas with disturbed flow, including the aortic valve, aortic sinuses, and origin of arterial branches (which are prone to development of atherosclerosis).88 Normal flow patterns may attenuate, and disturbed flow may facilitate, capture of pathogens by dendritic cells. Studies of dendritic cells in valves88 were performed in murine valves, so the authors could not localize dendritic cells to the ventricular or aortic surface of the valves. Considering the predisposition to disturbed blood flow on the aortic side of the valve, we speculate that dendritic cells may predominate on the aortic side, and perhaps contribute to predisposition of aortic valve disease to begin on the aortic side and base of the valve.
The role of dendritic cells, especially in relation to macrophages, in development of atherosclerosis is unsettled.90 Classical dendritic cells (which are strongly immunostimulatory) protect against development of atherosclerosis in the aorta of Ldlr−/− mice.90 The implication is that the immune response may protect against atherosclerosis. It is not clear whether those findings in the aorta, and our speculation, are applicable to the aortic valve.
A second type of dendritic cells, in addition to classical dendritic cells, are macrophage-colony stimulating factor (M-CSF) – dependent, monocyte-derived dendritic cells. During atherogenesis, the number of monocyte-derived dendritic cells, which also strongly stimulate T-cells, increases even more than classical dendritic cells.83 M-CSF is proatherogenic,91 and it is not known whether the proatherogenic effect is mediated through monocyte/macrophages or M-CSF –dependent dendritic cells.
M-CSF, which is one of the links between inflammation and cardiovascular disease, is also critical for formation of osteoclasts. M-CSF, together with receptor activator of NF-κB (RANK) ligand (RANKL), is required for fusion of proosteoclasts into multinucleated osteoclasts, which critically regulate tissue mineralization in bone.92,93 It is not known, however, whether M-CSF regulates osteoclast function and tissue mineralization in the aortic valve.
T cells and Macrophages
Inflammation occurs early in development of aortic stenosis, and is associated with calcification. T-cells and B-cells are present in valves, adjacent to myofibroblasts and preosteoblasts.94–96 Expression of BMP2 and BMP4 occur adjacent to the inflammatory infiltrate,96 which implies that inflammation may contribute to calcification of the valve.
Mice deficient in interleukin-1 receptor antagonist (IL-1 Ra −/−) develop thickening and fibrosis of the aortic valve, with myofibroblast transdifferentiation.97 Transvalvular blood velocity increases, which (to our estimate) predicts a gradient of about 15 mmHg. Thus, unopposed inflammation alone (without other risk factors) produces mild aortic valve dysfunction. Valve disease can be produced by transplantation of T-cells (only) that are deficient in the IL-1R antagonist, which indicates a powerful role for T-cells in FCAVD. TNF-α deficiency prevents FCAVD produced by IL-1R deficiency.
Calcified aortic valves from humans contain many expanded T cell clones, which are also expanded in circulating blood.98 The authors suggest that there is an ongoing systemic adaptive immune response in patients with bicuspid and tricuspid FCAVD, which involves activation of circulating CD8 T cells and clonal expansion, with trafficking of T cells between blood and the aortic valve.98
Atherosclerosis is an inflammatory disease, perhaps triggered by an autoimmune response to LDL.99 To suppress the inflammatory response, dendritic cells treated with apolipoprotein B 100 and interleukin 10 were injected in hypercholesterolemic mice. Injection of tolerogenic dendritic cells reduced the autoimmune response and greatly decreased atherosclerosis.99 Two trials, using methotrexate or an antibody against IL-1B, have been initiated to determine whether inhibition of inflammation prevents atherosclerotic disease.100 The rationale for targeting vascular inflammation as a means to reduce atherosclerosis is reasonable, and it is possible that a similar approach that targets inflammation would protect against development of FCAVD. We speculate, however, that immunomodulatory therapies may (like reduction of lipids) be most effective when initiated prior to development of overt FACAVD.
Monocyte/macrophages infiltrate the aortic valve, and almost certainly play a key role in development of FCAVD. In a series of studies, Demer’s group demonstrated that several cytokines released by macrophages stimulate calcification of vascular muscle cells.101 This calcific mechanism presumably can be extrapolated to arteries in vivo, and to smooth muscle cells which are present at the base of the aortic valve. Imaging studies also link macrophages with osteogenesis in development of aortic valve disease.102
Macrophages can internalize basic calcium phosphate crystals in vitro, and trigger release of high concentrations of several inflammatory cytokines, including TNFα.103 TNF-α promotes osteogenic differentiation and vascular calcification.104 The findings imply positive feed-back, with calcification begetting inflammation and then calcification. With advanced calcification of tissues, however, inflammation may decrease.7,102
Vitamin D
Deficiency of vitamin D3 appears to be a risk factor for cardiovascular disease.105 Administration of Vitamin D3 decreases macrophages and CD4+ T-cells in the aortic sinus, decreases dendritic cells and maturation, and attenuates development of atherosclerosis in apoE−/− mice.106 On the other hand, administration of high doses of vitamin D produces aortic valve disease (but with minimal pressure gradient across the valve) in rabbits.107,108 Vitamin D also produces aortic sclerosis in mice with decreased signaling by a downstream effector of Notch 1.109 Thus, effects on the aortic valve of vitamin D deficiency and therapeutic levels of vitamin D are not clear.
Angiogenesis
Normal heart valves are avascular. Inflammation of a heart valve, e.g. from bacterial infection or autoimmune disease, is associated with neovascularization of valves.66 Hypercholesterolemia, which can be viewed as a systemic inflammatory disease, is associated with neovascularization of the aortic valve in apoE−/− mice.110 In humans, neovascularization occurs in stenotic aortic valves, but not in valves without significant calcification.111 In humans, intraleaflet hemorrhage colocalizes with neovascularization, and is associated with rapid decline of aortic valve function.112 In organ culture, abundant endothelial cell migration and tubule formation occur in explanted human stenotic aortic valves, but are minimal in cultured nonstenotic valves.113
The observation that normal articular cartilage is avascular led to isolation of chondromodulin-1, a powerful anti-angiogenic protein that is also present in normal cornea.110,114 Deficiency of chondromodulin-1 produces aortic valve sclerosis and neovascularization in mice.114 Chrondromodulin-1 is expressed in normal human aortic valves, but its expression is decreased in stenotic human valves.
Those findings suggest that neovascularization of the aortic valve is not only a marker for disease, but also may play a role in initiating or propagating the disease, perhaps in part by facilitating entry of cellular and humoral mediators of inflammation into valve interstitium. However, in valve tissue, as in other tissues, angiogenesis is associated with matrix remodeling115 and expression of growth factors, such as vascular endothelial growth factor (VEGF), which may propagate FCAVD in ways that do not depend on formation of new blood vessels.66 In bone, VEGF-A directly mediates osteoblast differentiation via “intracrine” Runx2 and PPARγ signaling.116 It is possible that similar processes occur in diseased aortic valves.
Although neovascularization of the aortic valve is associated with FCAVD, it is not clear whether angiogenesis contributes, or is secondary, to the disease. For example, thickening of the valve may exceed the diffusion distance of oxygen, and thereby stimulate angiogenesis. A similar mechanism has been described in the aorta during development of atherosclerosis.117
Myofibroblasts are present in stenotic aortic valves from humans,118 and play an important role in calcification of the valve. An intriguing observation is that pericytes are present near neovessels in stenotic valves118 (see Figure 5). Pericytes may transform to myofibroblasts.119 We speculate that neovascularization of aortic valves may be accompanied by growth of pericytes, which transform to myofibroblasts, and thereby contribute to calcification and fibrosis of the valve. It has been known for many years that vascular pericytes (from retina) have osteogenic potential. 120,121
Thus, pro-FCAVD features of pericytes may provide a mechanistic link between neovascularization and progression of FCAVD. Confirmation of valve angiogenesis per se as a viable therapeutic target for FCAVD will require studies that isolate the process of neovascularization from the global inflammatory and pro-calcific milieu of FCAVD.
Therapeutic interventions for FCAVD
FCAVD results from diverse complex regulated processes, and it seems likely that specific targets may be identified for therapeutic interventions designed to attenuate FCAVD. Studies in FCAVD-prone mice offer the ability to assess the efficacy of clinically relevant therapeutic interventions within a workable time frame, while controlling for important genetic and environmental co-variables. A few interventions have shown promise in mice that are prone to develop severe aortic stenosis.
Amelioration of the underlying cause of FCAVD
Ldlr−/−Apob100/100 mice are hypercholesterolemic, and are prone to develop severe aortic stenosis in old age.12 A high-fat diet accelerates the disease processes in Ldlr−/−Apob100/100 mice.14 Early in the course of FCAVD, reversal of hypercholesterolemia by means of a “genetic switch” normalizes aortic valve superoxide levels, decreases myofibroblast activation, reduces valvular calcium burden, suppresses pro-osteogenic signaling cascades, and prevents further impairment of aortic valve function, in Ldlr−/− Apob100/100/Mttpfl/flMx1Cre+/+ mice.14 When moderate aortic valve stenosis is present, reversal of hypercholesterolemia significantly reduces pro-osteogenic signaling and valve calcification, in Ldlr−/− Apob100/100/Mttpfl/flMx1Cre+/+ mice.87 Profibrotic signaling in the aortic valve remains elevated, however, after reversal of hypercholesterolemia. The net effect of cholesterol lowering upon aortic valve function is negligible in Reversa mice with advanced FCAVD, despite a 70% reduction in valve calcification. The findings recapitulate findings in studies of lipid lowering in patients with moderate or severe FCAVD.122,123
Two themes emerge from studies of cholesterol lowering in FCAVD-prone mice. First therapeutic efficacy during the early stages of disease does not necessarily predict efficacy for advanced disease. The findings suggest that processes that initiate FCAVD may differ mechanistically from processes that propagate FCAVD. Second, a potential therapeutic intervention may result in differential effects on individual FCAVD processes, e.g. calcification vs. fibrosis, even when the therapy is targeted at the underlying cause of FCAVD.
Exercise
Regular aerobic exercise reduces oxidant stress and improves endothelial function in patients with atherosclerosis,124,125 effects which could also be beneficial for patients with FCAVD. In very young Ldlr−/− mice fed a high-cholesterol diet, regular exercise preserves endothelial integrity, reduces pro-fibrotic and pro-calcific signaling, and protects against development of aortic valve sclerosis.126 Beneficial effects of exercise occur even though plasma cholesterol levels are unaffected by exercise. When the exercise intervention is delayed until 5 months of age, however, after development of aortic valve sclerosis, regular exercise has no demonstrable effect on endothelial integrity, pro-fibrotic signaling, pro-calcific signaling, or valve morphology in cholesterol-fed Ldlr−/− mice.127 The age-dependence of the efficacy of exercise for attenuation of FCAVD reinforces the concept that FCAVD initiation can occur via mechanisms that are different from FCAVD progression, and that efficacy of a therapeutic intervention may depend on the ambient phase of FCAVD.
Targeting a pro-FCAVD gene cluster
PPARɣ is an attractive therapeutic target because it affects a cluster of genes and modulates several mechanisms that may play an important role in the pathophysiology of FCAVD. PPARɣ protects against development of atherosclerosis, inhibits differentiation of progenitor cells to osteoblasts, and is antiinflammatory.77 We77 and others128 have observed that pioglitazone, a PPARɣ ligand, attenuates development of FCAVD. Perhaps a PPARɣ would be useful in patients at high risk for FCAVD, such as patients with a bicuspid aortic valve, in prevention of stenosis.
Prospects for clinical translation
A critical and challenging step will be to apply findings in mice to therapy in patients. One approach will be retrospective analysis of drugs (e.g. PPARɣ ligands or RANKL inhibitors), used for other diseases to determine whether they slow the development or progression of FCAVD. It is unlikely, however, that sensitive methods for evaluation of aortic valve function (echo, MRI) will be available in retrospective studies of treatments prescribed for other indications. Second, although long-term prospective studies will be most valuable, it is not likely that large, long-term studies will be feasible. Studies of a few years duration, as have been performed with statins, may be feasible. Third, imaging methods, including molecular imaging, which target inflammation, calcification, and other mechanisms will be valuable. They are surrogate endpoints, however, and do not provide functional endpoints.
A key question is whether there is positive feedback which, once established, can be interrupted to slow or reverse FCAVD. A concern is that matrix stiffness can influence the propensity for VICs to produce calcification.47,48 It is encouraging that, in at least one experimental model with calcification, VICs in vitro can dedifferentiate away from myofibroblasts when matrix is less stiff.46 It is unknown, however, whether stiffness of the valve can be reduced, and what the net effect on valve calcification would be.
The future holds great promise for further elucidation of mechanisms by which FCAVD develops and progresses, and for development of effective medical therapy for FCAVD. Studies in mice will be critical to assess the functional impact of systemic and local cellular and molecular processes, and effects of therapeutic interventions, upon aortic valve function. Translation of findings from in vitro studies, and from studies in mice, to effective therapies in humans will be challenging, but necessary.
Summary
Studies of mouse models of FCAVD in vivo provide an opportunity to integrate complex mechanical, cellular, and molecular processes that preserve or disrupt valve homeostasis. FCAVD begins and progresses in a setting of complex interactions between mechanical forces and an ever-changing host tissue milieu. Some mouse models of FCAVD recapitulate important features of clinical FCAVD, including overt syndromic calcific aortic stenosis, but there are important differences relating to anatomical scale. Evaluation of aortic valve function in vivo allows examination of cellular and molecular processes and their physiological consequences - hemodynamically significant valve stenosis and/or regurgitation.
Endothelial cells and VICs are the predominant tissue in normal and minimally diseased valves. During evolution of FCAVD, immune cells exert injury-response influences and also regulate the interaction between VICs and ECs. Some genes regulate valvulogenesis, and also regulate postnatal valve homeostasis. Genetic control of processes in the aortic valve is itself regulated by epigenetic, post-transcriptional, and post-translational processes.
The rapidly expanding body of knowledge regarding regulation of valve homeostasis holds great promise for identifying viable therapeutic targets for clinical application. A few therapeutic interventions have shown promise in murine models of FCAVD. Murine models of FCAVD will continue to provide new basic and translational insights in this clinically important disease.
Acknowledgements
We thank Dr Zuhair Ballas for advice about mechanisms of inflammation, Huan Wang for advice about studies of VICs in vitro, and Teresa Ruggle for assistance with preparation of figures. We wish to acknowledge Elise Oehler, Kathy Zimmerman, and Melissa Davis, whose technical expertise was critical for acquisition, analysis, and presentation of echocardiographic data.
Funding Sources: Original studies by the authors were supported by grants from the National Institutes of Health (HL62984, RR026293, HL086160, HL092235, HL111121).
Nonstandard Abbreviations and Acronyms
- ADMA
asymmetric dimethylarginine
- Alk2
activin receptor-like kinase-2, also known as activin receptor type I (ACVR-1)
- BMP
bone morphogenetic protein
- FCAVD
fibrocalcific aortic valve disease
- M-CSF
macrophage-colony stimulating factor
- Mgp
matrix Gla protein
- OPG
osteoprotegerin
- PPARγ
peroxisome proliferator associated receptor gamma
- RANK
receptor activator of nuclear factor-κB
- RANKL
receptor activator of nuclear factor-κB ligand
- VICs
valve interstitial cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: RMW and DDH have received research funding from Amgen Corp.
References
- 1.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner H. Aortic stenosis: medical and surgical management. Heart. 2005;91:1483–1488. doi: 10.1136/hrt.2004.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 4.Demer L, Tintut Y. The roles of lipid oxidation products and receptor activator of nuclear factor-κB signaling in atherosclerotic calcification. Circ Res. 2011;108:1482–1493. doi: 10.1161/CIRCRESAHA.110.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boström KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circ Res. 2011;108:1494–1509. doi: 10.1161/CIRCRESAHA.110.234260. [DOI] [PubMed] [Google Scholar]
- 7.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381–1391. doi: 10.1161/CIRCRESAHA.110.234146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109:578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. doi: 10.1161/CIRCRESAHA.110.234237. [DOI] [PubMed] [Google Scholar]
- 10.Hinton RB, Jr, Alfieri CM, Witt SA, Glascock BJ, Khoury PR, Benson DW, Yutzey KE. Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol. 2008:H2480–H2488. doi: 10.1152/ajpheart.91431.2007. [DOI] [PubMed] [Google Scholar]
- 11.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific Aortic Valve Stenosis in Old Hypercholesterolemic Mice. Circulation. 2006;114:2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 13.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. doi: 10.1016/j.jacc.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 14.Miller JD, Weiss RM, Serrano KM, Brooks RM2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry CJ, Miller JD, McGroary K, Thedens DR, Young SG, Heistad DD, Weiss RM. Biventricular adaptation to volume overload in mice with aortic regurgitation. Cardiovasc Magn Reson. 2009;11:27. doi: 10.1186/1532-429X-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J. 2010;31:1975–1984. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewin MB, Otto CM. The bicuspid aortic valve: adverse outcomes from infancy to old age. Circulation. 2005;111:832–834. doi: 10.1161/01.CIR.0000157137.59691.0B. [DOI] [PubMed] [Google Scholar]
- 19.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 20.Pachulski RT, KL, Chan KL. Progression of aortic valve dysfunction in 51 adult patients with congenital bicuspid aortic valve: assessment and follow up by Doppler echocardiography. Br Heart J. 1993;69:237–240. doi: 10.1136/hrt.69.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conti CA, Della Corte A, Votta E, Del Viscovo L, Bancone C, De Santo LS, Redaelli A. Biomechanical implications of the congenital bicuspid aortic valve: a finite element study of aortic root function from in vivo data. J Thorac Cardiovasc Surg. 2010;140:890–896. doi: 10.1016/j.jtcvs.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Yap CH, Saikrishnan N, Tamilselvan G, Vasilyev N, Yoganathan AP. The congenital bicuspid aortic valve can experience high-frequency unsteady shear stresses on its leaflet surface. Am J Physiol Heart Circ Physiol. 2012;303:H721–H731. doi: 10.1152/ajpheart.00829.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons CA, Zilberberg J, Davies PF. A rapid, reliable method to isolate high quality endothelial RNA from small spatially-defined locations. Ann Biomed Eng. 2004;32:1453–1459. doi: 10.1114/b:abme.0000042360.57960.2b. [DOI] [PubMed] [Google Scholar]
- 24.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ankeny RF, Thourani VH, Weiss D, Vega JD, Taylor WR, Nerem RM, Jo H. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves--association with low BMP antagonists and SMAD6. PLoS One. 2011;6:e20969. doi: 10.1371/journal.pone.0020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–965. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 28.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286:481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 29.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, Eto M, Akishita M, Ouchi Y. Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2054–2062. doi: 10.1161/ATVBAHA.110.216739. [DOI] [PubMed] [Google Scholar]
- 31.Shimoyama Y, Mitsuda Y, Tsuruta Y, Suzuki K, Hamajima N, Niwa T. SIRTUIN 1 gene polymorphisms are associated with cholesterol metabolism and coronary artery calcification in Japanese hemodialysis patients. J Ren Nutr. 2012;22:114–119. doi: 10.1053/j.jrn.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Stein S, Schäfer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Lüscher TF, Matter CM. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging. 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez D, Coyet A, Ollivier V, Jeunemaitre X, Jondeau G, Michel JB, Vranckx R. Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms. Cardiovasc Res. 2011;89:446–456. doi: 10.1093/cvr/cvq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCabe MT, Brandes JC, Vertino PM. Cancer DNA methylation: molecular mechanisms and clinical implications. Clin Cancer Res. 2009;15:3927–3937. doi: 10.1158/1078-0432.CCR-08-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi IA, Mehler MF. Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol. 2010;67:1316–1322. doi: 10.1001/archneurol.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YS, Chou WW, Chen KC, Cheng HY, Lin RT, Juo SH. MicroRNA-152 mediates DNMT1-regulated DNA methylation in the estrogen receptor α gene. PLoS One. 2012;7:e30635. doi: 10.1371/journal.pone.0030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhl J, Klan N, Rose M, Entian KD, Werz O, Steinhilber D. The 5-lipoxygenase promoter is regulated by DNA methylation. J Biol Chem. 2002;277:4374–4379. doi: 10.1074/jbc.M107665200. [DOI] [PubMed] [Google Scholar]
- 38.Nagy E, Andersson DC, Caidahl K, Eriksson MJ, Eriksson P, Franco-Cereceda A, Hansson GK, Bäck M. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123:1316–1325. doi: 10.1161/CIRCULATIONAHA.110.966846. [DOI] [PubMed] [Google Scholar]
- 39.Laenoi W, Uddin MJ, Cinar MU, Phatsara C, Tesfaye D, Scholz AM, Tholen E, Looft C, Mielenz M, Sauerwein H, Schellander K. Molecular characterization and methylation study of matrix gla protein in articular cartilage from pig with osteochondrosis. Gene. 2010;459:24–31. doi: 10.1016/j.gene.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Delgado-Calle J, Sañudo C, Sánchez-Verde L, García-Renedo RJ, Arozamena J, Riancho JA. Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone. 2011;49:830–838. doi: 10.1016/j.bone.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Holliday CJ, Ankeny RF, Jo H, Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301:H856–H867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 43.van Rooij E. The art of microRNA research. Circ Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- 44.Walker GA, Masters KS, Shah DN, Anseth KA, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 45.Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell cultures. J Heart Valve Disease. 2008;17:689–699. [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLOS One. 2012;7:e39969. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benton JA, Kern HB, Mariner PD, Leinwand LA, Anseth KS. Statins inhibit calcific nodule formation to valvular interstitial cells by inhibiting alpha smooth muscle actin expression. Arterioscler Thromb Vasc Biol. 2009;29:1950–1957. doi: 10.1161/ATVBAHA.109.195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 49.Hinton RB, Jr, Lincoln J, Deutsch GH, Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 50.Sewell-Loftin MK, Brown CB, Baldwin HS, Merryman WD. A novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J Heart Valve Dis. 2012;21:513–520. [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnamurthy VK, Guilak F, Narmoneva DA, Hinton RB. Regional structure-function relationships in mouse aortic valve tissue. J Biomech. 2011;44:77–83. doi: 10.1016/j.jbiomech.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao R, Sider KL, Simmons CA. Measurement of layer-specific mechanical properties in multilayered biomaterials by micropipette aspiration. Acta Biomater. 2011 Mar;7(3):1220–1227. doi: 10.1016/j.actbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Meierhofer C, Schneider EP, Lyko C, Hutter A, Martinoff S, Markl M, Hager A, Hess J, Stern H, Fratz S. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur Heart J Cardiovasc Imaging. 2013 doi: 10.1093/ehjci/jes273. in press. [DOI] [PubMed] [Google Scholar]
- 54.Martin LJ, Hinton RB, Zhang X, Cripe LH, Benson DW. Aorta Measurements are Heritable and Influenced by Bicuspid Aortic Valve. Front Genet. 2011;2:61. doi: 10.3389/fgene.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calloway TJ, Martin LJ, Zhang X, Tandon A, Benson DW, Hinton RB. Risk factors for aortic valve disease in bicuspid aortic valve: a family-based study. Am J Med Genet A. 2011;155A:1015–1020. doi: 10.1002/ajmg.a.33974. [DOI] [PubMed] [Google Scholar]
- 56.Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation. 2000;101:2345–2348. doi: 10.1161/01.cir.101.20.2345. [DOI] [PubMed] [Google Scholar]
- 57.Fernández B, Durán AC, Fernández-Gallego T, Fernández MC, Such M, Arqué JM, Sans-Coma V. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol. 2009;54:2312–2318. doi: 10.1016/j.jacc.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 58.Rajamannan NM. Oxidative-mechanical stress signals stem cell niche mediated Lrp5 osteogenesis in eNOS(−/−) null mice. J Cell Biochem. 2012;113:1623–1634. doi: 10.1002/jcb.24031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang B, Oehler EA, Roos CM, Arghami A, Miller JD. Dimethylarginine dimethylaminohydrolase-1 slows progression of fibrocalcific aortic valve disease. Circulation. 2011;124 A17025 (Abstract) [Google Scholar]
- 60.El Accaoui R, Hajj G, Davis M, Chu Y, Lund D, Brooks R, Kraft D, Zimmerman K, Heistad d, Weiss R. Fibrocalcific aortic valve disease in mice is dependent upon both endothelial nitric oxide synthase genotype and valve cusp number. J Am Coll Cardiol. 2012;59(Supplement 1) E2013 (Abstract) [Google Scholar]
- 61.Biben C, Weber R, Kesteven S, Stanley E, McDonald L, Elliott DA, Barnett L, Köentgen F, Robb L, Feneley M, Harvey RP. Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2.5. Circ Res. 2000;87:888–895. doi: 10.1161/01.res.87.10.888. [DOI] [PubMed] [Google Scholar]
- 62.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 63.Acharya A, Hans CP, Koenig SN, Nichols HA, Galindo CL, Garner HR, Merrill WH, Hinton RB, Garg V. Inhibitory role of Notch1 in calcific aortic valve disease. PLoS One. 2011;6:e27743. doi: 10.1371/journal.pone.0027743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nigam V, Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol. 2009;47:828–834. doi: 10.1016/j.yjmcc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tkatchenko TV, Moreno-Rodriguez RA, Conway SJ, Molkentin JD, Markwald RR, Tkatchenko AV. Lack of periostin leads to suppression of Notch1 signaling and calcific aortic valve disease. Physiol Genomics. 2009;39:160–168. doi: 10.1152/physiolgenomics.00078.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2010;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laforest B, Andelfinger G, Nemer M. Loss of Gata5 in mice leads to bicuspid aortic valve. J Clin Invest. 2009;121:2876–2887. doi: 10.1172/JCI44555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Padang R, Bagnall RD, Richmond DR, Bannon PG, Semsarian C. Rare non-synonymous variations in the transcriptional activation domains of GATA5 in bicuspid aortic valve disease. J Mol Cell Cardiol. 2012;53:277–281. doi: 10.1016/j.yjmcc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Thomas PS, Sridurongrit S, Ruiz-Lozano P, Kaartinen V. Deficient signaling via Alk2 (Acvr1) leads to bicuspid aortic valve development. PLoS One. 2012;7:e35539. doi: 10.1371/journal.pone.0035539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kent KC, Crenshaw ML, Goh DL, Dietz HC. Genotype-phenotype correlation in patients with bicuspid aortic valve and aneurysm. J Thorac Cardiovasc Surg. 2013 doi: 10.1016/j.jtcvs.2012.09.060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hinton RB, Adelman-Brown J, Witt S, Krishnamurthy VK, Osinska H, Sakthivel B, James JF, Li DY, Narmoneva DA, Mecham RP, Benson DW. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ Res. 2010;107:549–557. doi: 10.1161/CIRCRESAHA.110.221358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgan AJ. Mineralized deposits in the thoracic aorta of aged rats: ultrastructural and electron probe X-ray microanalysis study. Exp Gerontol. 1980;15:563–5733. doi: 10.1016/0531-5565(80)90009-1. [DOI] [PubMed] [Google Scholar]
- 73.Kim KM, Huang S. Ultrastructural study of calcification of human aortic valve. Lab Invest. 1971;25:357–366. [PubMed] [Google Scholar]
- 74.Schinke T, Karsenty G. Vascular calcification - a passive process in need of inhibitors. Nephrol Dial Transplant. 2000;15:1272–1274. doi: 10.1093/ndt/15.9.1272. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 76.Jian B, Narula N, Li Q, Mohler E, Levy R. Progression of Aortic Valve Stenosis: TGF-β1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 77.Chu Y, Lund D, Weiss R, Brooks R, Doshi H, Hajj G, Sigmund C, Heistad D. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:523–532. doi: 10.1161/ATVBAHA.112.300794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo G, Ducy P, McKee MD. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 79.Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Habashi JP, Judge D, Holm T, Cohn R, Loeys B, Cooper T, Myers L, Klein E, Liu G, Calvi C, Podowski M, Neptune E, Halushka M, Bedja D, Gabrielson K, Rifkin D, Carta L, Ramirez F, Huso D, Dietz H. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart. 1999;82:19–22. doi: 10.1136/hrt.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Della Corte A, Bancone C, Quarto C, Dialetto G, Covino F, Scardone M, Caianiello G, Cotrufo M. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. European Journal of Cardiothoracic Surgery. 2007;31:397–405. doi: 10.1016/j.ejcts.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Linhartová K, Beránek V, Šefrna F, Hanišová I, Štěrbáková G, Pešková M. Aortic Stenosis Severity is not a Risk Factor for Poststenotic Dilatation of the Ascending Aorta. Circ Journal. 2007;71:84–88. doi: 10.1253/circj.71.84. [DOI] [PubMed] [Google Scholar]
- 84.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Jr, Pomerantzeff PM, Laurindo FR. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 86.Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33:e66–e74. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller JD, Weiss RM, Serrano KM, Castaneda LE, Brooks RM, Zimmerman K, Heistad DD. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol. 2010;30:2482–2486. doi: 10.1161/ATVBAHA.110.211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 91.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 93.Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. C-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest. 2003;111:749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion in “degenerative” valvular aortic stenosis: histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 95.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 96.Mohler ER, III, Gannon F, Reynolds C, Zimmerman R, Keane M, Kaplan F. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 97.Isoda K, Matsuki T, Kondo H, Iwakura Y, Ohsuzu F. Deficiency in Interleukin-1 Receptor Antagonist Induces Aortic Valve Disease in BALB/c Mice. Arterioscler Thromb Vasc Biol. 2010;30:708–715. doi: 10.1161/ATVBAHA.109.201749. [DOI] [PubMed] [Google Scholar]
- 98.Winchester R, Wiesendanger M, O’Brien W, Zhang H, Maurer M, Gillam L, Schwartz A, Marboe C, Stewart A. Circulating Activated and Effector Memory T Cells are Associated with Calcification and Clonal Expansions in Bicuspid and Tricuspid Valves of Calcific Aortic Stenosis. J Immunol. 2011;187:1006–1014. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hermansson A, Johansson D, Ketelhuth D, Andersson J, Zhou X, Hansson G. Immunotherapy With Tolergenic Apolipoprotein B-100-Loaded Dendritic Cells Attenuates Atherosclerosis in Hypercholesterolemic Mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 100.Tabas I, Glass C. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 103.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CDW, McCarthy GM, Landis RC, Haskard DO. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase c and MAP Kinase Pathways. Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 104.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 105.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takeda M, Yamashita T, Sasaki N, Nakajima K, Kita T, Shinohara M, Tatsuro I, Hirata K. Oral Administration of an Active Form of Vitamin Dȝ (Calcitriol) Decreases Atherosclerosis in Mice by Inducing Regulatory T Cells and Immature Dendritic Cells with Tolerogenic Functions. Arterioscler Thromb Vasc Biol. 2010;30:2495–2503. doi: 10.1161/ATVBAHA.110.215459. [DOI] [PubMed] [Google Scholar]
- 107.Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol. 2003;41:1211–1217. doi: 10.1016/s0735-1097(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 108.Ngo DT, Stafford I, Kelly DJ, Sverdlov AL, Wuttke RD, Weedon H, Nightingale AK, Rosenkranz AC, Smith MD, Chirkov YY, Kennedy JA, Horowitz JD. Vitamin D(2) supplementation induces the development of aortic stenosis in rabbits: interactions with endothelial function and thioredoxin-interacting protein. Eur J Pharmacol. 2008;590:290–296. doi: 10.1016/j.ejphar.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 109.Nus M, MacGrogan D, Martínez-Poveda B, Benito Y, Casanova JC, Fernández-Avilés F, Bermejo J, de Pompala JL. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arterioscler Thromb Vasc Biol. 2011;31:1580–1588. doi: 10.1161/ATVBAHA.111.227561. [DOI] [PubMed] [Google Scholar]
- 110.Hakuno D, Fukuda K. Role of anti-angiogenic factor chondromodulin-I for maintaining cardiac valvular function. Clin Calcium. 2007;17:361–372. [PubMed] [Google Scholar]
- 111.Soini Y, Salo T, Satta J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum Pathol. 2003;34:756–763. doi: 10.1016/s0046-8177(03)00245-4. [DOI] [PubMed] [Google Scholar]
- 112.Akahori H, Tsujino T, Naito Y, Matsumoto M, Lee-Kawabata M, Ohyanagi M, Mitsuno M, Miyamoto Y, Daimon T, Hao H, Hirota S, Masuyama T. Intraleaflet haemorrhage is associated with rapid progression of degenerative aortic valve stenosis. Eur Heart J. 2011;32:888–896. doi: 10.1093/eurheartj/ehq479. [DOI] [PubMed] [Google Scholar]
- 113.Chalajour F, Treede H, Ebrahimnejad A, Lauke H, Reichenspurner H, Ergun S. Angiogenic activation of valvular endothelial cells in aortic valve stenosis. Exp Cell Res. 2004;298:455–464. doi: 10.1016/j.yexcr.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 114.Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, Shukunami C, Okada Y, Mukai M, Shin H, Yozu R, Sata M, Ogawa S, Hiraki Y, Fukuda K. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12:1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 115.Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, Vahanian A, Jacob MP. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–1341. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heistad DD, Armstrong ML, Marcus ML. Hyperemia of the aortic wall in atherosclerotic monkeys. Circ Res. 1981;48:669–675. doi: 10.1161/01.res.48.5.669. [DOI] [PubMed] [Google Scholar]
- 118.Syvaranta S, Helske S, Laine M, Lappalainen J, Kupari M, Mayranpaa M, Lindstedt K, Kovanen P. Vascular endothelial growth factor-secreting mast cells and myofibroblasts. Arterioscler Thromb Vasc Biol. 2010;30:1220–1227. doi: 10.1161/ATVBAHA.109.198267. [DOI] [PubMed] [Google Scholar]
- 119.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nature Medicine. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 120.Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990;97:449–461. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- 121.Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 122.Rossebø AB, Pedersen TR, Boman K, et al. SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 123.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. ASTRONOMER Investigators. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 124.Adams V, Linke A, Kränkel N, Erbs S, Gielen S, Möbius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 125.Lewis TV, Dart AM, Chin-Dusting JP, Kingwell BA. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 1999;19:2782–2787. doi: 10.1161/01.atv.19.11.2782. [DOI] [PubMed] [Google Scholar]
- 126.Matsumoto Y, Adams V, Jacob S, Mangner N, Schuler G, Linke A. Regular exercise training prevents aortic valve disease in low-density lipoprotein-receptor-deficient mice. Circulation. 2010;121:759–767. doi: 10.1161/CIRCULATIONAHA.109.892224. [DOI] [PubMed] [Google Scholar]
- 127.Schlotter F, Matsumoto Y, Mangner N, Schuler G, Linke A, Adams V. Regular exercise or changing diet does not influence aortic valve disease progression in LDLR deficient mice. PLoS One. 2012;7:e37298. doi: 10.1371/journal.pone.0037298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li F, Cai Z, Chen F, Shi X, Zhang Q, Chen S, Shi J, Wen Wang D, Dong N. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res Cardiol. 2012;107:306. doi: 10.1007/s00395-012-0306-0. [DOI] [PubMed] [Google Scholar]