Abstract
This study aimed to analyze the role of endothelial progenitor cell (EPC)-derived angiogenic factors and chemokines in the multistep process driving angiogenesis with a focus on the recently discovered macrophage migration inhibitory factor (MIF)/chemokine receptor axis. Primary murine and murine embryonic EPCs (eEPCs) were analyzed for the expression of angiogenic/chemokines and components of the MIF/CXC chemokine receptor axis, focusing on the influence of hypoxic versus normoxic stimulation. Hypoxia induced an upregulation of CXCR2 and CXCR4 but not CD74 on EPCs and triggered the secretion of CXCL12, CXCL1, MIF, and vascular endothelial growth factor (VEGF). These factors stimulated the transmigration activity and adhesive capacity of EPCs, with MIF and VEGF exhibiting the strongest effects under hypoxia. MIF-, VEGF-, CXCL12-, and CXCL1-stimulated EPCs enhanced tube formation, with MIF and VEGF exhibiting again the strongest effect following hypoxia. Tube formation following in vivo implantation utilizing angiogenic factor-loaded Matrigel plugs was only promoted by VEGF. Coloading of plugs with eEPCs led to enhanced tube formation only by CXCL12, whereas MIF was the only factor which induced differentiation towards an endothelial and smooth muscle cell (SMC) phenotype, indicating an angiogenic and differentiation capacity in vivo. Surprisingly, CXCL12, a chemoattractant for smooth muscle progenitor cells, inhibited SMC differentiation. We have identified a role for EPC-derived proangiogenic MIF, VEGF and MIF receptors in EPC recruitment following hypoxia, EPC differentiation and subsequent tube and vessel formation, whereas CXCL12, a mediator of early EPC recruitment, does not contribute to the remodeling process. By discerning the contributions of key angiogenic chemokines and EPCs, these findings offer valuable mechanistic insight into mouse models of angiogenesis and help to define the intricate interplay between EPC-derived angiogenic cargo factors, EPCs, and the angiogenic target tissue.
Keywords: Macrophage migration inhibitory factor (MIF), angiogenesis, hypoxia, embryonic endothelial progenitor cell (eEPC), chemokine, CXC
Introduction
Despite considerable progress, cardiovascular disease (CVD) represents the number one cause of death worldwide. Stem cell or progenitor cell therapy, using embryonic stem cells or endothelial progenitor cells (EPCs) has proven to have a substantial potential to regenerate injured cardiac and vascular tissues [25, 41]. Nevertheless, the underlying mechanisms are not clearly understood and are subject of intensive investigation. Vascular endothelial growth factor (VEGF) is the most prominent pro-angiogenic factor [14], which promotes angiogenesis in vitro [15, 36, 45] and in various in vivo models [8, 37]. The stromal cell-derived factor-1α (SDF-1α)/CXCL12-CXCR4 chemokine ligand–receptor axis, besides governing hematopoietic cell trafficking and critical developmental effects, has been shown to promote tissue regeneration by mediating the recruitment of progenitor cells into ischemic areas [10, 26, 33]. While a role for HIF-1α-induced endothelium-derived CXCL12 in EPC recruitment along hypoxic gradients has been well established [10], the actual role of CXCL12 in vessel formation has been unclear. Also, CXCL12 is known to play a critical role in the trafficking of hematopoietic and lymphopoietic stem cells and stem cell progenitors, increasing cell survival, development and differentiation [27]. It not only increases angiogenesis and vasculogenesis [31] but also is decisive for vascular wall repair after injury [40].
The ELR motif-containing CXC chemokine keratinocyte-derived growth factor (KC) or CXCL1 [an ortholog of human CXCL8/interleukin-8 (IL-8)], best known for its chemotactic activity towards neutrophils and monocytes/macrophages, is also a potent angiogenic factor [5]. In a cardiovascular context, KC/CXCL8 has been found to be crucial for endothelial recovery after vascular injury by a CXCR2-dependent mechanism [32]. Macrophage migration inhibitory factor (MIF) is a pleiotropic inflammatory cytokine with chemokine-like functions [34, 47], which was recently shown to act as a non-cognate, alternative, ligand of CXCR2 and CXCR4 [6]. MIF/CXCR2 is critically involved in atherogenic monocyte and inflammatory neutrophil recruitment, while the MIF/CXCR4 axis contributes to atherogenic T cell recruitment and fibroblast migration [6, 13]. Of note, endothelial cell-derived MIF secreted in a bimodal manner upon hypoxic stimulation, promotes EPC chemotaxis in a CXCR4-dependent manner [42]. MIF mediates atherogenesis through its interaction with CXCR2/4 [47], and has been more broadly implicated in tumor angiogenesis as well [1, 11].
Endothelial progenitor cells were first isolated by Asahara et al. [4] from human peripheral blood using anti-CD34-magnetic microbeads. Later on, Hur et al. [22, 46] cultured two different types of EPCs from adult peripheral blood: early EPCs, which are highly adhesive but show low proliferative capacity, and late EPCs, which are weakly adhesive, and in turn exhibit high proliferative properties. Recently, embryonic EPCs (eEPCs) have been found to provide cardioprotection against acute ischemia-reperfusion injury in preclinical models.
These studies as well as an earlier mechanistic study also demonstrated that EPCs not only contribute to angiogenic remodeling induced by exogenous angiogenic stimuli but also are able to express angiogenic factors on their own [29, 30]. Thymosin-β4 is one of these EPC-derived angiogenic paracrine ‘cargo’ factors, but eEPCs were also found to express MIF mRNA, while it has remained unknown whether (e)EPCs can produce and secrete MIF as well as perhaps other angiogenic proteins [21, 29].
Generally, stem cell therapy requires cell transplantation into injured tissue with low oxygen reserve. However, the behavior of EPCs under hypoxic conditions is far from being understood. In particular, the complex mechanistic interplay between the angiogenic stimuli produced by the affected ischemic tissue and the angiogenic cargo of the EPCs has not been understood. Thus, we have analyzed the role of known protagonistic angiogenic factors in this context in various stages of angiogenesis under both normoxic and hypoxic conditions applying in vitro and in vivo models and have compared them with MIF, a recently defined angiogenic EPC-derived factor. As eEPCs have recently been suggested to represent a promising cellular tool, we compared primary murine EPCs with a murine eEPC line. Embryonic EPCs are characterized by a favorable growth behavior and genetic manipulability [20, 44]. Importantly, they express early endothelial markers, can specifically differentiate into endothelial cells and can be recruited into hypoxic areas, improving tissue remodeling [29].
Methods
Isolation and culture conditions of eEPCs and EPCs
Embryonic EPCs were kindly provided by PD Dr. C. Kupatt, Ludwig-Maximilians-University (LMU) Munich, Germany, and were cultured as described previously [29]. Murine EPCs were derived from spleens of 6- to 8-week-old male C57BL6/6J mice, which were minced and filtered through a mesh ring (30 μm). The mononuclear fraction was isolated by Lympholyte reagent (Cedarlaine Lab., Burlington, USA) and cultured on fibronectin-coated plates in microvascular endothelial growth medium MV2 (PromoCell, Heidelberg, Germany). Non-adherent cells were removed after 4 days and the adherent cells were cultured until day 14. The EPC phenotype was verified by flow cytometric analysis using established markers (see below and Fig. 1a). From one spleen, 50,000–100,000 EPCs could be isolated. Animal studies were approved by the local authorities and complied with German animal protection law.
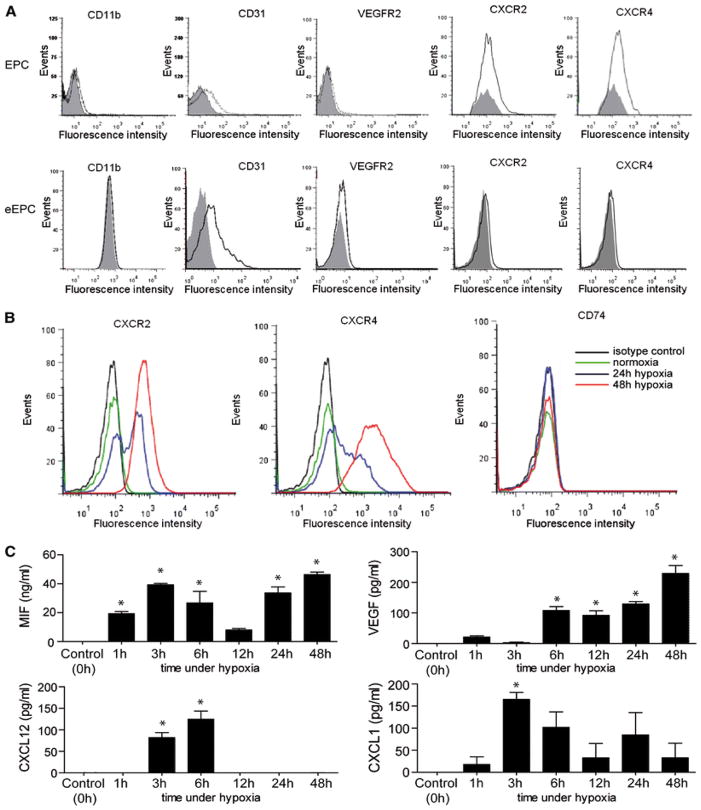
Figure 1.
Characterization of EPCs and upregulation of chemokine receptor expression and angiogenic chemokine/mediator secretion in EPCs. a Characterization of the EPCs used in this study. Flow cytometric analysis for CD11b, CD31, VEGFR-2, CXCR2, and CXCR4 surface expression on EPCs and eEPCs (fluorescence intensity of control IgG staining shown in shaded grey). b Effect of hypoxic treatment (2 % O2) on the surface expression levels of CXCR2, CXCR4, and CD74 on eEPCs. Normoxic conditions (20 % O2) (green) were compared with a 24 h (blue) and 48 h (red) hypoxic treatment and isotype control IgG staining (black). c The secretion of angiogenic EPC-derived factors/chemokines is upregulated by hypoxia. Secretion of MIF, VEGF, CXCL12, and CXCL1 from eEPCs at different time points following hypoxic stimulation was measured by ELISAs (*p < 0.05 vs. control, n = 3)
Simian virus 40-immortalized murine endothelial cells (SVECs) were cultured in Dulbecco’s modified eagle’s medium/Ham’s F-12 (PAA, Invitrogen, Karlsruhe) supplemented with 5 % FCS (Sigma-Aldrich, Schnelldorf, Germany). Cells were cultured at 37 °C and 5 % CO2.
Simian virus 40-immortalized murine endothelial cells (SVECs) were cultured in Dulbecco’s modified eagle’s medium/Ham’s F-12 (PAA, Invitrogen, Karlsruhe) supplemented with 5 % FCS (Sigma-Aldrich, Schnelldorf, Germany). Cells were cultured at 37 °C and 5 % CO2.
Hypoxic conditions
For hypoxic stimulation experiments, eEPCs or EPCs were incubated in a 37 °C hypoxic Innova CO-48 incubator (New Brunswick Scientific, Enfield, USA) at 2 % O2 and 5 % CO2 and stimulations performed as indicated in “Results” and figure legends.
Flow cytometry
To characterize the endothelial phenotype of the cells, we stained for monocyte (fluorescein isothiocyanate (FITC)-conjugated CD11b antibody, eBioscience, Frankfurt, Germany), and endothelial [phycoerythrin (PE)-conjugated VEGFR2 antibody (BD) and PE-cyanine dye7-conjugated CD31 antibody, eBioscience] markers as well as for chemokine receptors CXCR2-PE (R&D Systems, Wiesbaden-Nordenstadt, Germany), CXCR4-PE (BD), and CD74-FITC (BD). Analyses were performed using a FACS Canto flow cytometer and FLOWJO Software. Unstained cells served as controls.
Enzyme-linked immunosorbent assays (ELISAs)
VEGF, CXCL12, and CXCL1 levels in the supernatants of the cultured eEPCs were determined using DuoSet ELISA Development Kits from R&D Systems in accordance with the manufacturer’s protocol. MIF levels were detected by a modification of an established mouse MIF ELISA as previously described [39], using the anti-mouse MIF mAb clone XIV.14.3 as capture antibody and the BAF289 antibody (R&D Systems) as detection antibody.
Cell adhesion assay
The adhesion assay was performed under normoxic and hypoxic conditions in a 96-well plate applying a static adhesion format. SVECs were cultured in 24-well inserts for 24 h. Calcein-labeled eEPCs were treated with various neutralizing monoclonal antibodies (10 μg/ml anti-mouse VEGF antibody, 10 μg/ml anti-human/mouse CXCL12 antibody, 10 μg/ml anti-mouse CXCL1 antibody (all R&D), or 10 μg/ml anti-MIF (NIH/IIID.9), with chemokines/cytokines (50 ng/ml rmVEGF165, 50 ng/ml rmCXCL12, 50 ng/ml rmCXCL1 (all from PeproTech, Hamburg, Germany), 50 ng/ml recombinant murine MIF (rmMIF; prepared as described previously [7]), or isotype control immunoglobulins (10 μg/ml) and incubated for 16 h. The stimuli were added to the experimental media immediately before the hypoxic incubation was initiated. Unattached cells were washed away and adherent eEPCs on the endothelial layer were analyzed by multiple fluorescence top reading (excitation 480 nm, emission 520 nm) with a TECAN® i-control reader and i-control software. The adhesion index was calculated as percent of control (unstained monolayer).
Secretion of angiogenic factors under hypoxic and normoxic conditions and cell viability
Embryonic EPCs or EPCs were incubated overnight in 12-well plates in a normoxic or hypoxic incubator. Concentrations of angiogenic factors were determined by ELISA at the indicated time points. In situ Cell Death Detection Kit (Roche) was used for the detection of apoptotic cells, and counter-staining was performed with DAPI.
Chemotaxis assay
Chemotaxis assays were performed in 24-well cell culture chambers using Transwell devices. Calcein-labeled EPCs and eEPCs were transferred to the top of membrane inserts and allowed to migrate for 2 h towards different chemoattractants which were added immediately before the experiment started [50 ng/ml rmMIF; 50 ng/ml rmVEGF165 (PeproTech); 50 ng/ml rmCXCL12 (PeproTech); or 50 ng/ml rmCXCL1 (PeproTech)]. Migrated cells that had reached the bottom side of the Transwell chamber were quantified using a fluorescence microscope and expressed as percent of the migration of buffer-treated control cells (chemotactic index, CTX).
Transmigration assay
Transmigration assays also were performed in 24-well Transwell chambers. BrdU-labeled non-proliferating SVECs were placed in fibronectin-coated inserts and allowed to form an endothelial monolayer. Calcein-labeled eEPCs or EPCs suspended in assay medium were applied on top of the inserts and were allowed to transmigrate for 2 h against rmVEGF165, rmCXCL12, rmCXCL1, or rmMIF (all at 50 ng/ml). The cells that had migrated to the bottom side of the filter were stained with DAPI, counted using a fluorescence microscope and expressed as percent of control (transmigration index, TMX).
In vitro Matrigel assay
BD Matrigel™ basement membrane matrix was thawed overnight, placed in 24-well plates and allowed to polymerize. Due to the fact that the EPCs can form tubes only in the presence of mature endothelial cells [12], a cell suspension of SVECs and eEPCs or EPCs (1:2) in assay medium was seeded and directly stimulated with rmVEGF165, rmCXCL12, rmCXCL1, or rmMIF (all at 50 ng/ml). After 24 h of incubation with the stimuli, the calcein-labeled EPCs or eEPCs were found to integrate into the formed tubes (see Supplemental Figure 1), and tube formation was quantified by counting the total number of tube-like structures in five random microscopic fields [12].
In vivo Matrigel assay
Matrigel (500 μl per mouse; BD Matrigel™) containing different chemokines/cytokines (rmMIF, rmVEGF165, rmCXCL12 or rmCXCL1; all at 50 ng/ml) and/or calcein-labeled eEPCs, was sub-cutaneously transplanted into 8-week-old male C57BL6/6J mice (5 mice per group) after anesthesia with Ketamine (100 mg/kg) and Xylazine (10 mg/kg). The Matrigel mixture solidified to form a plug upon implantation. After 7 days, the mice were euthanized with isoflurane aerosol and the Matrigel plugs were removed. Each Matrigel plug was fixed in 10 % buffered formalin and embedded in paraffin. Five-micrometer sections were cut and used for further immunohistochemistry analysis. Animal studies were approved by the local authorities and complied with German animal protection law.
Immunohistochemistry
Serial sections (10 per mouse) of the Matrigel plugs were stained to analyze blood vessel formation (hematoxylin and eosin) and content of lymphocytes (CD3, Serotec), macrophages (Mac2, Cedarlain Lab), vessels (CD31, Santa Cruz), and myofibroblasts (α-smooth muscle actin, DAKO). Vessels and cells were counted in the total Matrigel area and expressed as number of tubes or cells/field or percentage of positive cells of the total cell number if the number of the cells was significantly reduced.
Statistical analysis
Data represent mean ± SEM. Statistical analysis was performed with Prism 4 software (Graph Pad) using unpaired Student’s t test or 1-way ANOVA followed by Newman–Keuls post-test, as appropriate. p values <0.05 were considered significant.
Results
Characterization of EPCs and upregulation of chemokine receptor expression and angiogenic chemokine/mediator secretion in EPCs
We first wished to characterize the EPCs used in this study. As shown by flow cytometry, isolated primary murine EPC (EPCs) as well as eEPCs were positive for the mononuclear-(CD11b) and endothelial-specific markers CD31 and VEGFR-2 (Fig. 1a). Moreover, EPCs expressed substantial levels of CXCR2 and CXCR4 on their surface. This confirmed prior data showing that CXCR2 and CXCR4 can serve as markers for EPCs. In contrast, resting eEPCs did not exhibit any appreciable CXCR2 or CXCR4 surface expression (Fig. 1a). However, exposing eEPCs to hypoxic conditions (2 % O2) for 24 or 48 h led to a marked upregulation of the surface expression of CXCR2 and CXCR4, whereas the third MIF receptor CD74 was neither detected on EPCs (data not shown) nor on resting or hypoxia-stimulated eEPCs (Fig. 1b). Of note, hypoxic conditions did not affect the viability or proliferation rate of eEPC within 24–48 h after hypoxic challenge. Only after 72 h, some isolated apoptotic cells were observed (Supplemental Figure 1A).
Embryonic EPCs have been shown to carry angiogenic mediators [29], but the effect of hypoxic gradients on angiogenic factor/chemokine expression by EPCs as it may occur in ischemic EPC recruitment situations in vivo is unknown. We challenged eEPCs with hypoxic conditions over a time course of 48 h and analyzed the levels of secreted MIF, CXCL1, CXCL8, and VEGF by ELISA at different time intervals upon hypoxia. MIF was abundantly secreted and the secretion profile followed a biphasic curve with maxima at 3 and 48 h which is reminiscent of the bimodal MIF secretion profile of hypoxically treated endothelial cells [42]. The secretion of the other three proteins was monophasic. CXCL1 levels peaked 1 h after hypoxia and then declined, whereas CXCL12 secretion was only detectable in a narrow window of 3–6 h. In contrast, VEGF production increased continuously over the entire time course, but significant secretion levels were not detected until 6 h after hypoxic exposure (Fig. 1c, *p < 0.05 vs. control).
Enhancement of EPC recruitment by angiogenic factors/chemokines: prominent role for MIF and VEGF
EPCs recruited into ischemic/hypoxic tissues are subject to chemotactic migration, adhesion, and transmigration processes. Also as we have shown above, EPCs also express and secrete angiogenic factors and chemokines upon hypoxic challenge. Thus, we next compared the effects of MIF, CXCL1, CXCL12, and VEGF on EPC and eEPC adhesion, chemotaxis, and transmigration in vitro. Adhesion experiments of EPCs or eEPCs on SVEC monolayers showed that MIF, VEGF, CXCL12, and CXCL1 all promoted the static adhesion of eEPC by a small, yet significant, margin (Fig. 2a). This effect did not differ between hypoxic and normoxic conditions (compare Fig. 2a, b).
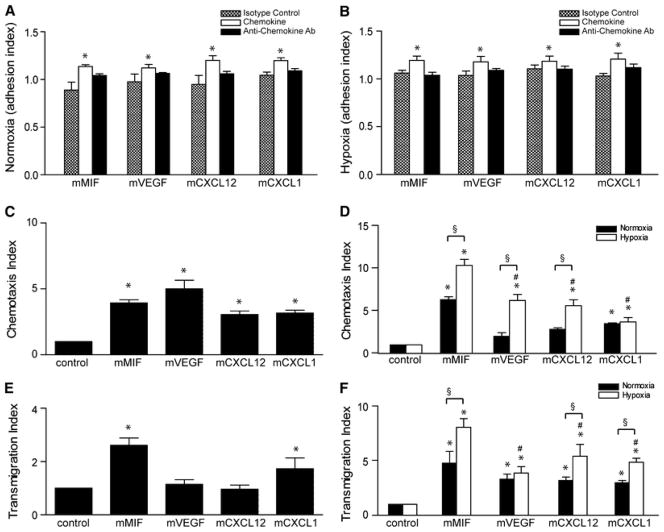
Figure 2.
Enhancement of EPC recruitment by angiogenic factors/chemokines: prominent role for MIF and VEGF. a, b Static adhesion of eEPCs on an endothelial layer under normoxic (a) and hypoxic (b) conditions (*p < 0.05 vs. isotype control, n = 3). c Chemotactic migration of calcein-labeled EPCs under normoxic conditions (*p < 0.05 vs. control, n = 3). d Chemotactic migration of calcein-labeled eEPCs under normoxic (black bars) and hypoxic (white bars) conditions towards MIF, VEGF, CXCL12, and CXCL1 (*p < 0.05 vs. control, § p < 0.05 vs. normoxia, # p < 0.05 vs. MIF under hypoxia; n = 3). e Transmigration of EPCs through an endothelial layer induced by MIF, VEGF, CXCL12, and CXCL1 (*p < 0.05 vs. control, n = 3). f Transmigration experiment as in e except that eEPCs were analyzed and normoxic versus hypoxic conditions compared for all chemokines/factors (*p < 0.05 vs. control, § p < 0.05 vs. normoxia, # p < 0.05 vs. MIF in hypoxia; n = 3)
More strikingly, all factors/chemokines markedly and significantly enhanced EPC chemotaxis (Fig. 2c), but only MIF and CXCL1 also triggered the transmigration capacity of the EPCs through an endothelial layer (Fig. 2e). Whereas for EPCs chemotaxis and transmigration were only studied under normoxic conditions, eEPC chemotaxis and transmigration was compared between normoxic and hypoxic conditions (Fig. 2d, f). MIF had the most apparent effect on both chemotaxis and transmigration of eEPCs, with chemotactic and transmigration indices of >sixfold and >fourfold observed, respectively; these effects were significantly increased under hypoxic conditions at which MIF increased eEPC chemotaxis by tenfold and transmigration by eightfold. Enhancement of chemotaxis and transmigration by the other chemokines/factors appeared somewhat weaker (2-to 3-fold under normoxia and 3- to 5-fold under hypoxia), but was also significant for most conditions, with VEGF and CXCL12 exerting the strongest chemotactic/transmigration effects on hypoxically challenged eEPCs. Thus, MIF is a highly potent angiogenic recruitment factor when compared to VEGF, CXCL12 and CXCL1 both under normoxic and hypoxic conditions, and in particular with respect to the embryonic EPCs.
Comparison of the tube formation potential of the angiogenic factors/chemokines in vitro and in vivo
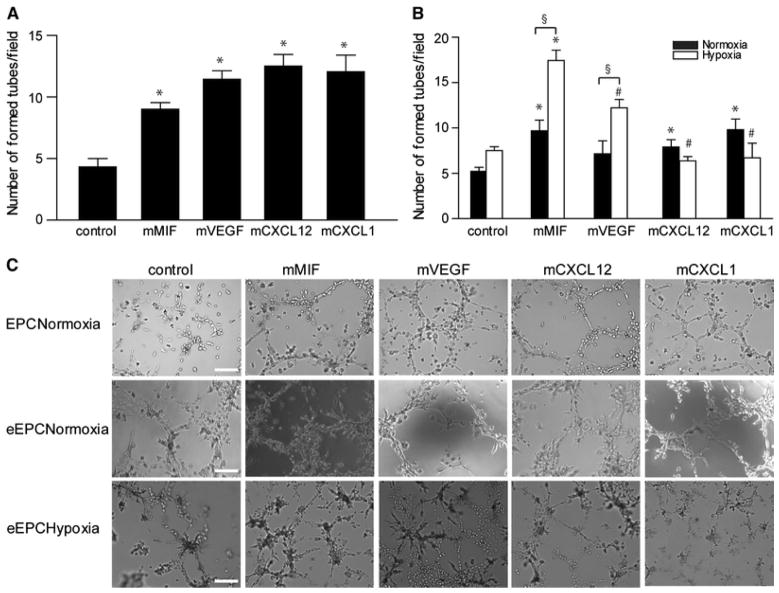
To study the role of the pro-angiogenic factors in tube formation, Matrigel experiments were performed. First, a suspension of SVECs together with EPCs or eEPCs was added on a Matrigel base and incubated for 24 h with the various angiogenic factors. All tested factors significantly improved EPC integration and tube formation on Matrigel in vitro (Fig. 3a). We have also pre-stained EPCs or eEPCs with calcein to confirm their contribution to tube formation (Supplemental Figure 1B). Tube integration of eEPCs was promoted by all three chemokines, but not VEGF, under normoxic conditions, whereas under hypoxia only MIF significantly accelerated eEPC-mediated tube formation compared to the respective hypoxic control. This effect of MIF under hypoxia also was significant compared to VEGF-, CXCL1-, or CXCL12-stimulated tube formation rates under hypoxia (Fig. 3b, c).
Figure 3.
Comparison of the tube formation potential of the angiogenic factors/chemokines in vitro. Tube formation was evaluated in Matrigel matrices containing (e)EPCs/SVECs cell suspensions. a Number of formed tubes in vitro by EPCs under normoxic conditions after 24 h (*p < 0.05 vs. control; n = 6). b Embryonal EPC-mediated tube formation assay in vitro under normoxia versus hypoxia after stimulation with MIF, VEGF, CXCL12 or CXCL1 for 24 h (*p < 0.05 vs. respective control, § p < 0.05 vs. normoxia at the respective stimulation condition, # p < 0.05 vs. MIF in hypoxia; n = 4). c Representative images from each group (scale bar 50μm)
Tube formation and differentiation of eEPC in matrigel in vivo
To directly study the differentiation capacity of eEPCs under hypoxic conditions in vivo, we subcutaneously transplanted Matrigel plugs containing mixtures of eEPCs and the different angiogenic factors/chemokines or control buffer. After 7 days, the tube formation rate in all groups was 10 times higher as in Matrigel plugs without eEPCs, suggesting a decisive role for the progenitor cells themselves in initiating and sustaining vessel formation (compare Fig. 4a with 10–20 tubes per microscopic field with Fig. 5a with 100–150 tubes per field). In addition, the CXCL12 group showed a significantly increased number of tubes compared to all other eEPC-containing groups, implying an important role for this chemokine in tube assembly, and therefore, in the later phases of angiogenesis (Fig. 5a).
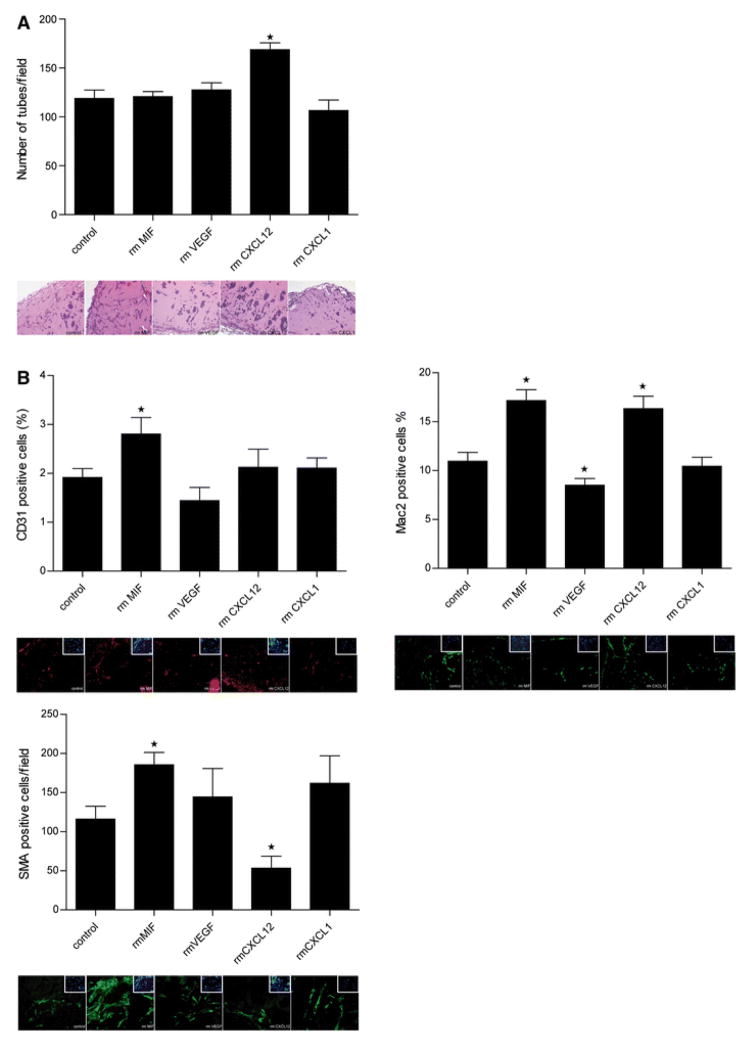
Figure 4.
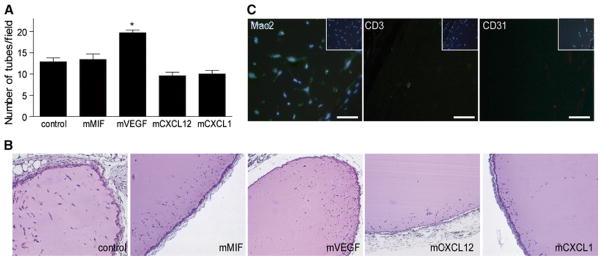
Comparison of the tube formation potential of the angiogenic factors/chemokines in vivo. a In vivo tube formation in transplanted Matrigel plugs containing MIF, VEGF, CXCL12, or CXCL1 analyzed after 1 week (*p < 0.05 vs. control; n = 5). b Representative H&E stains from a (scale bar 400 μm). c Specific immunohistochemistry stainings for macrophages (Mac-2), lymphocytes (CD3) and endothelial cells (CD31) as detected in MIF-containing Matrigel plugs (scale bar 25 μm)
Figure 5.
Tube formation and differentiation of eEPCs in Matrigel in vivo. a In vivo tube formation in transplanted Matrigel plugs containing both eEPCs and either MIF, VEGF, CXCL12, or CXCL1 analyzed after 1 week and representative H&E stains (scale bar 400 μm). Percentage of CD31-, Mac-2-, and SMA-positive cells as detected in the Matrigel plug sections; each respective bar diagram is accompanied by representative staining (*p < 0.05 vs. control; n = 6; scale bar 25 μm)
Next, we wished to find out which cell types had been preferentially recruited into the Matrigel plugs and/or which differentiation processes towards what cell type had been favored under the influence of the various angiogenic factors/chemokines. Thus, the CD31+, Mac-2+, and smooth muscle cell (SMC) content in the Matrigel implants were analyzed. As revealed by immunohistochemistry, only 2–3 % of the cells were local CD31+ cells. Despite this overall low abundance of CD31+ cells, we observed a significant differentiation of recruited cells towards an endothelial phenotype upon MIF, but not VEGF, CXCL12, or CXCL1 stimulation (Fig. 5b). More importantly, MIF also was the only factor which promoted the differentiation towards SMCs, a cell type which is decisive in the outgrowth of larger vessels and which was abundantly found in the newly formed vessels (Fig. 5b). Surprisingly, CXCL12 did not promote differentiation towards SMCs, but even inhibited this differentiation process, although it is known to play a central role in the recruitment of SMC progenitors [27]. The promoting effect of CXCL12 on overall tube formation in vivo (Fig. 5a) appears to be accounted for by an incorporation of Mac-2+ cells, as only CXCL12 and MIF enhanced Mac-2+ cells (Fig. 5b; Supplemental Figure 1C). In fact, macrophages are known to form columns and tubes in vitro and in vivo, frequently preceding neovessel formation [2].
Discussion
Angiogenesis is a central physiological process in growth and development and has been shown to be an excellent therapeutic target for the treatment of cardiovascular disease. A large number of preclinical studies have been performed in animal models using different pro-angiogenic factors with promising results, leading to the belief that such new therapeutic approaches, when successfully translated into clinical settings, could rapidly be beneficial for millions of suffering patients [17].
Unfortunately, most of the clinical trials did not achieve statistically significant improvements [43], probably due to the improper choice of molecular targets in an overall context of a complex cellular environment in vivo. Further, the fact that our knowledge about the molecular events regulating blood vessel formation is far from being understood has led to a loss of interest of such approaches in the past years. Moreover, promoting angiogenesis in an unstable and injured tissue locus could favor the development of local tumorigenic processes. Therefore, it would be dangerous to use such therapies in clinical practice without knowing the involved players and the precise mechanisms.
The most important information about the underlying angiogenic mechanisms and the involved pro-angiogenic factors has come from experiments with knock-out mice, in the context of tumor research. Mice gene-deficient in the Cxcl12 receptor Cxcr4 displays profound defects in the hematopoietic and nervous systems and die perinatally [35]. Similarly, VEGF is a critical regulator of somatic growth and is essential for survival in early postnatal life [18]. Cxcr2 −/− and Mif −/− mice display a relatively normal phenotype, but tumor growth is significantly impaired compared to wild-type littermates, mostly due to a decreased tumor microvascular density [24]. Since all these factors have proved important in neovascularization, corresponding selective antagonists were broadly used to successfully control and suppress tumor growth.
Whereas anti-angiogenic approaches against selective targets have proven successful in tumor pathology, pro-angiogenic therapies as they would be envisioned in cardiovascular disease ought to cover multiple steps of angiogenesis and should consider the complex simultaneous and/or sequential interplay of involved angiogenic factors and chemokines. Thus, only by understanding these mechanisms, vessel formation may be manipulated and controlled to improve the function of a damaged organ. We thus analyzed the multiple steps involved in angiogenesis, focusing on pivotal established factors such as CXCL1, CXCL12, and VEGF and laying a particular focus on the emerging role of the angiogenic capacity of the cytokine MIF, whose potent properties as a non-cognate chemokine and EPC-derived pro-angiogenic factor have recently been uncovered [19, 29, 42]. Endothelial progenitor cells have been assigned a crucial role in neo-angiogenesis [4]. However, since their discovery in 1997 by Asahara et al., their precise characteristics have been debated. EPCs appear to undergo different stages of differentiation and can therefore exhibit different characteristics. The so-called early outgrowth EPCs (EOCs), derived from circulating CD34-positive mononuclear cells, which additionally express CD45 and CD14, exert enhanced adhesion proprieties but surprisingly fail to proliferate in vitro [38]. In contrast, a small EPC sub-fraction, the so-called late outgrowth EPCs (LOCs), lacks hematopoietic markers but has the ability to proliferate [23]. In addition to responding to angiogenic stimuli, both of these EPC sub-populations express CD31 and secrete angiogenic factors such as VEGF and inflammatory cytokines/chemokines driving neo-angiogenesis [3]. The murine embryonic EPC cell line (the “eEPCs”) used in this study, expresses subsets of mesodermal as well as early endothelial cell markers, is able to adhere and proliferate, secretes pro-angiogenic factors and improves neovascularization and tissue recovery in vivo [20, 29]. Thus, these eEPCs exhibit characteristics of both EOCs and LOCs.
Soon after the occurrence of an acute ischemic event, the affected tissue starts to undergo apoptotic and/or necrotic cell death and local vessel structures are being destroyed. Therefore, rapid neovascularization is essential for the survival of any residual and the renewed cells and for the preservation of organ function. In such situations, circulating EPCs, likely EOCs, are being activated and adhere to the site of injury. This first step in new vessel formation is essential but not sufficient to sustain angiogenesis.
We found a marked up-regulation of the surface expression of CXCR2 and CXCR4 on EPCs and eEPCs. CXCR2 and CXCR4 are the principal receptors for CXCL1 and CXCL12, respectively, and have recently been found to serve as potent non-cognate receptors for MIF as well [6]. In the context of angiogenesis, increased MIF-triggered recruitment responses of monocytes and EPCs in a CXCR2- and CXCR4-dependent manner, respectively, are notable [19, 42].
EPCs not only respond to ischemic triggers by migration, adhesion, and differentiation, but also carry a battery of pro-angiogenic factors with them as cargo [28, 29]. We showed that EPCs not only express the mRNA of angiogenic factors [29] but also secrete critical angiogenic factors. Namely, the secretion of CXCL12, CXCL1, VEGF, and MIF was demonstrated, with MIF being the most prominent secreted factor at both early and later time points following ischemic challenge. These chemokines/angiogenic factors are also secreted by hypoxically challenged endothelium [42]. Thus, although this latter aspect was not explicitly analyzed in the current study, it appears that both locally produced EC- and EPC-derived CXCL12, CXCL1, VEGF, and MIF contribute to the production of these factors under ischemic challenge such as in myocardial infarction, where they would contribute to EPC recruitment and neo-angiogenesis. In fact, we found that CXCL12, CXCL1, VEGF, and MIF all promoted EPC adhesion in vitro, although by a small margin only. More markedly, essentially all factors promoted EPC and eEPC chemotactic migration and transmigration through endothelial layers in vitro, with MIF and VEGF being the most potent factors under hypoxic conditions. This effect nicely corresponded to an observed upregulation of surface Cxcr2 and Cxcr4 on the EPCs after hypoxic stimulation. Generally, hypoxia stimulation effects only were analyzed on eEPCs due to the low cell yields that are obtained for primary mouse blood-derived EPCs.
The angiogenic potency and EPC-activating capacity of all four studied factors also became apparent in in vitro tube formation assays in SVEC/eEPC-containing Matrigel chambers. All factors, but again most prominently MIF and VEGF following hypoxic prestimulation, promoted in vitro migration and tube formation. Assessing in vivo vascularization responses by Matrigel implants containing the angiogenic factors/chemokines either alone or in conjunction with eEPCs, then suggested that both local and recruited EPCs can contribute to an increase in tube formation in vivo, implying their role in in vivo angiogenesis. Evidence for a role of local EPCs comes from the observation that the transplantation of eEPCs alone markedly amplifies the subsequent tube formation response, while external EPC recruitment in vivo, as measurable in our model, appears to be mostly triggered by VEGF. This notion is confirmed by earlier studies showing a gross beneficial effect of early transplanted EPCs on cardiac function, while later systemic transplantation did not improve cardiac function [31]. It may be speculated that after transmigration to an (ischemic) injured site, EPCs transdifferentiate into LOCs, which in turn have the capacity to proliferate at the local site, therefore making a new import of angiogenic cells unnecessary.
From all studied factors, MIF seems to play a central role in the angiogenic response under hypoxic conditions. It is already known that hypoxia induces a biphasic pattern of MIF release from endothelial cells: early secretion from preformed MIF stores [16], predictably responsible for self-maintaining adhesion and transmigration of EPCs and later secretion of MIF, attributable to HIF-1α-driven de novo MIF synthesis [42]. This later MIF fraction could be responsible for the initiation of tube formation and differentiation towards endothelial, and SMC phenotypes. In fact, we surprisingly observed that MIF was the only factor able to favor a differentiation towards CD31-positive ECs and SMA-positive SMCs in vivo, suggesting an important role for MIF in the more advanced phases of angiogenesis. Together with CXCL12, MIF was the only factor to distinctly promote macrophage incorporation into the forming tubes. This could represent an effect on an ‘early’ incorporation event or gain a differentiation process. Macrophages are known to form columns and tubes in vitro and in vivo, frequently preceding neovessel formation [2]. As VEGF secretion from EPCs was pronounced over a broad time interval, and because VEGF had strong effects on EPC adhesion and chemotaxis, VEGF likely has an important role in the initiation of tube formation, but perhaps alone leading to immature, leaky vessels [9]. On the other hand, our results would suggest that HIF-1α-induced VEGF as well as HIF-1α-induced CXCL12 [10] synthesis under hypoxic conditions sustain tube formation and angiogenesis, processes in which SMCs are involved. This is surprising, because CXCL12 is known as the main chemoattractant for smooth muscle progenitor cells [40]. In conjunction, our in vitro and mouse model data could be suggestive of the following sequelae of events involving angiogenic chemokines and EPCs in situations of tissue/vessel injury and local hypoxia (scheme, Fig. 6): the early recruitment of EPCs is critical for the initiation of angiogenesis by local ‘acute’ release of angiogenic chemokines such as CXCL1 and MIF. Once recruited, EPCs would be able to also release angiogenic chemokines such as CXCL1, CXCL12, MIF, and VEGF on their own for a short time period, maintaining an activation circle and recruiting other cells involved in angiogenesis, including monocytes [31] and SMCs [40]. Factors like VEGF and MIF seem to be more critical in the later stages, i.e., promoting tube/vessel formation. Since EPC numbers in blood are limited, an external ‘therapeutic’ application at such a time point was thought to be beneficial for the following angiogenic processes. Our data emphasize that angiogenesis is a highly complex process, depending on several angiogenic factors and chemokines and involving multiple steps. EPCs are responsive, recruited angiogenic cells but also important sources of angiogenic factors. Furthermore, we have identified a previously unknown role for EPC-derived MIF not only in EPC recruitment and early tube formation steps, but also in EPC differentiation into ECs and SMCs as prominent steps in the later stages of the angiogenic process. Thus, our data contribute to an improved understanding of the complex angiogenic interplay between ECs, EPCs, and pivotal angiogenic factors, pinpointing and discerning key players among them and offering important mechanistic information which will be useful in planning interventional studies in preclinical models of cardiovascular disease to probe the role of these factors in modulating EPC function further.
Figure 6.
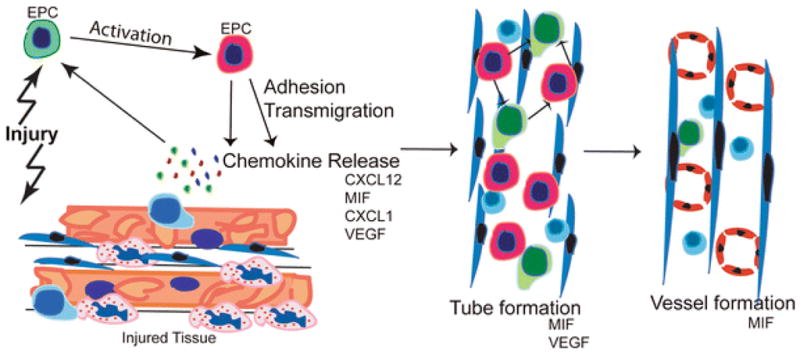
Schematic summarizing the proposed role of EPCs and the studied angiogenic factors/chemokines in neo-angiogenesis. After tissue injury or destruction of an inflammatory reaction is induced by the release of cytokines and angiogenic chemokines. These activate circulating EPCs, which upregulate their angiogenic chemokine receptors due to the hypoxic challenge, adhere and transmigrate to the site of injury. Here, together with monocytes they integrate into tube structures, and differentiate into mature endothelial cells (“vessel formation”). Our data suggest that MIF and VEGF are critical players in the earlier processes. Also MIF, but surprisingly not CXCL12, is important in the later stages including fully functional vessel formation
Supplementary Material
(A) Apoptosis of eEPCs in normal and hypoxic conditions (TUNEL staining). (B) Calcein- labelled EPCs and eEPCs integrated into tubes in matrigel assays in vitro, in normal and hypoxic conditions. (C) Mac-2 positive macrophages in the matrigel plugs in vivo.
Acknowledgments
We thank Nives Hörmann and Meike Jung for assistance with the hypoxia experiments and Kiril Bidzhekov and Christian Kupatt for providing the eEPC cell clone. This study was supported by IZKF Aachen (K1-2, K1-4) of the Faculty of Medicine, RWTH Aachen University to E.L. and J.B., by the Deutsche Forschungsgemeinschaft (DFG) grants BE1977/4-2/FOR 809 and GRK1508/1-P13 to J.B.), and the NIH (R.B.).
Source of funding
This study was supported by IZKF Aachen (K1-2, K1-4) of the Faculty of Medicine, RWTH Aachen University to E.L. and J.B., by the Deutsche Forschungsgemeinschaft (DFG) grants BE1977/4-2/FOR 809 and GRK1508/1-P13 to J.B.), and the NIH (R.B.).
Non-standard abbreviations and acronyms
- MIF
macrophage migration inhibitory factor
- eEPC
embryonic endothelial progenitor cell
- EPC
endothelial progenitor cell
- SVEC
simian virus (SV) 40-immortalized murine endothelial cell
- CVD
cardiovascular disease
- CXC
chemokine or chemokine receptor of the CXC sub-family
Footnotes
Disclosures
None.
References
- Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13:665–676. doi: 10.1089/scd.2004.13.665. [DOI] [PubMed] [Google Scholar]
- Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006;168:529–541. doi: 10.2353/ajpath.2006.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Belo AV, Leles F, Barcelos LS, Ferreira MA, Bakhle YS, Teixeira MM, Andrade SP. Murine chemokine CXCL2/KC is a surrogate marker for angiogenic activity in the inflammatory granulation tissue. Microcirculation. 2005;12:597–606. doi: 10.1080/10739680500253535. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration Inhibitory factor (MIF) Biochemistry. 1994;33:14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6:1102–1103. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–191. [PMC free article] [PubMed] [Google Scholar]
- Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- Dewor M, Steffens G, Krohn R, Weber C, Baron J, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes fibroblast migration in scratch-wounded monolayers in vitro. FEBS Lett. 2007;581:4734–4742. doi: 10.1016/j.febslet.2007.08.071. pii: S0014-5793(07)00958-1. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Flieger O, Engling A, Bucala R, Lue H, Nickel W, Bernhagen J. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett. 2003;551:78–86. doi: 10.1016/s0014-5793(03)00900-1. pii:S0014579303009001. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenic therapy of the human heart. Circulation. 1998;97:628–629. doi: 10.1161/01.CIR.97.7.628. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Grieb G, Piatkowski A, Simons D, Hormann N, Dewor M, Steffens G, Bernhagen J, Pallua N. Macrophage migration inhibitory factor is a potential inducer of endothelial progenitor cell mobilization after flap operation. Surgery. 2012;151:268–277.e261. doi: 10.1016/j.surg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Muller S, Willhauck M, Spitzweg C, Gildehaus FJ, Munzing W, Hannappel E, Bock-Marquette I, DiMaio JM, Hatzopoulos AK, Boekstegers P, Kupatt C. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004;172:2853–2860. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J Clin Investig. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Kupatt C, Bock-Marquette I, Boekstegers P. Embryonic endothelial progenitor cell-mediated cardioprotection requires Thymosin beta4. Trends Cardiovasc Med. 2008;18:205–210. doi: 10.1016/j.tcm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, Thalgott M, Buttner K, Browarzyk C, Mages J, Hoffmann R, Deten A, Lamparter M, Muller F, Beck H, Buning H, Boekstegers P, Hatzopoulos AK. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- Liehn EA, Postea O, Curaj A, Marx N. Repair after myocardial infarction, between fantasy and reality: the role of chemokines. J Am Coll Cardiol. 2011;58:2357–2362. doi: 10.1016/j.jacc.2011.08.034. http://dx.doi.org/10.1016/j.jacc.2011.08.03432. [DOI] [PubMed] [Google Scholar]
- Liehn EA, Schober A, Weber C. Blockade of keratinocyte-derived chemokine inhibits endothelial recovery and enhances plaque formation after arterial injury in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1891–1896. doi: 10.1161/01.ATV.0000143135.71440.75. [DOI] [PubMed] [Google Scholar]
- Liehn EA, Tuchscheerer N, Kanzler I, Drechsler M, Fraemohs L, Schuh A, Koenen RR, Zander S, Soehnlein O, Hristov M, Grigorescu G, Urs AO, Leabu M, Bucur I, Merx MW, Zernecke A, Ehling J, Gremse F, Lammers T, Kiessling F, Bernhagen J, Schober A, Weber C. Double-edged role of the CXCL12/CXCR4 axis in experimental myocardial infarction. J Am Coll Cardiol. 2011;58:2415–2423. doi: 10.1016/j.jacc.2011.08.033. http://dx.doi.org/10.1016/j.jacc.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449–460. doi: 10.1016/s1286-4579(02)01560-5. pii:S1286457902015605. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss J, Werner C, Lorenz D, Gensch C, Bohm M, Laufs U. The renin inhibitor aliskiren upregulates pro-angiogenic cells and reduces atherogenesis in mice. Basic Res Cardiol. 2010;105:725–735. doi: 10.1007/s00395-010-0120-5. [DOI] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.CIR.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- Schober A, Bernhagen J, Thiele M, Zeiffer U, Knarren S, Roller M, Bucala R, Weber C. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation. 2004;109:380–385. doi: 10.1161/01.CIR.0000109201.72441.09. [DOI] [PubMed] [Google Scholar]
- Schober A, Knarren S, Lietz M, Lin EA, Weber C. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein E-deficient mice. Circulation. 2003;108:2491–2497. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- Schuh A, Liehn EA, Sasse A, Hristov M, Sobota R, Kelm M, Merx MW, Weber C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol. 2008;103:69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- Simons D, Grieb G, Hristov M, Pallua N, Weber C, Bernhagen J, Steffens G. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med. 2011;15:668–678. doi: 10.1111/j.1582-4934-2010-01-041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, Laham RJ, Li W, Pike M, Sellke FW, Stegmann TJ, Udelson JE, Rosengart TK. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.CIR.102.11.e73. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Engelhardt B, Vestweber D, Hatzopoulos AK. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197:1755–1765. doi: 10.1084/jem.20021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcher D, Vasic D, Heinz P, Bach H, Durst R, Hausauer A, Hombach V, Marx N. LXR activation inhibits chemokine-induced CD4-positive lymphocyte migration. Basic Res Cardiol. 2010;105:487–494. doi: 10.1007/s00395-010-0092-5. [DOI] [PubMed] [Google Scholar]
- Walenta KL, Bettink S, Bohm M, Friedrich EB. Differential chemokine receptor expression regulates functional specialization of endothelial progenitor cell subpopulations. Basic Res Cardiol. 2011;106:299–305. doi: 10.1007/s00395-010-0142-z. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117:1594–1602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Apoptosis of eEPCs in normal and hypoxic conditions (TUNEL staining). (B) Calcein- labelled EPCs and eEPCs integrated into tubes in matrigel assays in vitro, in normal and hypoxic conditions. (C) Mac-2 positive macrophages in the matrigel plugs in vivo.